Grain Growth Behavior of Alumina in Zirconia-Toughened Alumina (ZTA) Ceramics During Pressureless Sintering
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
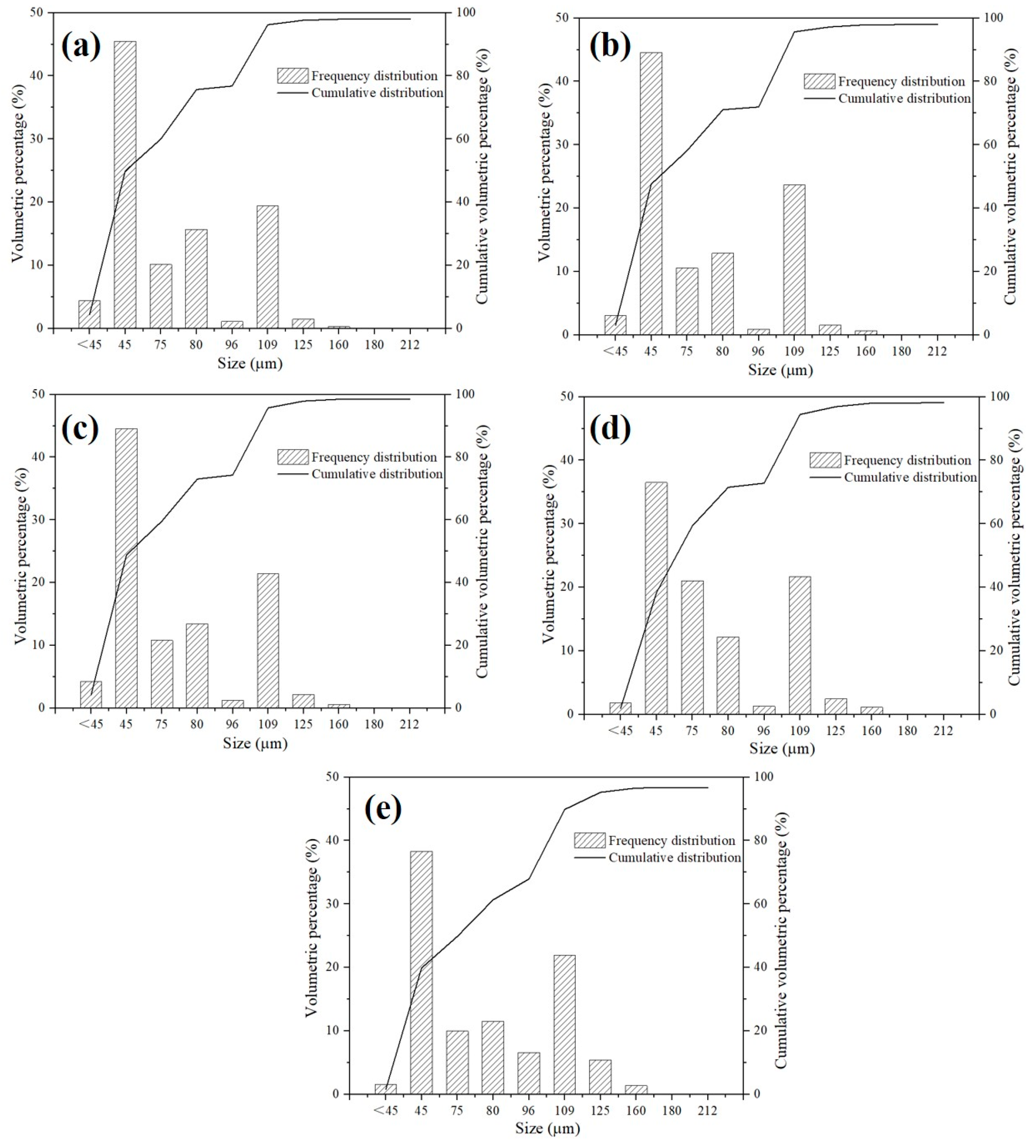
3.1. Particle Size Distribution of ZTA Granules
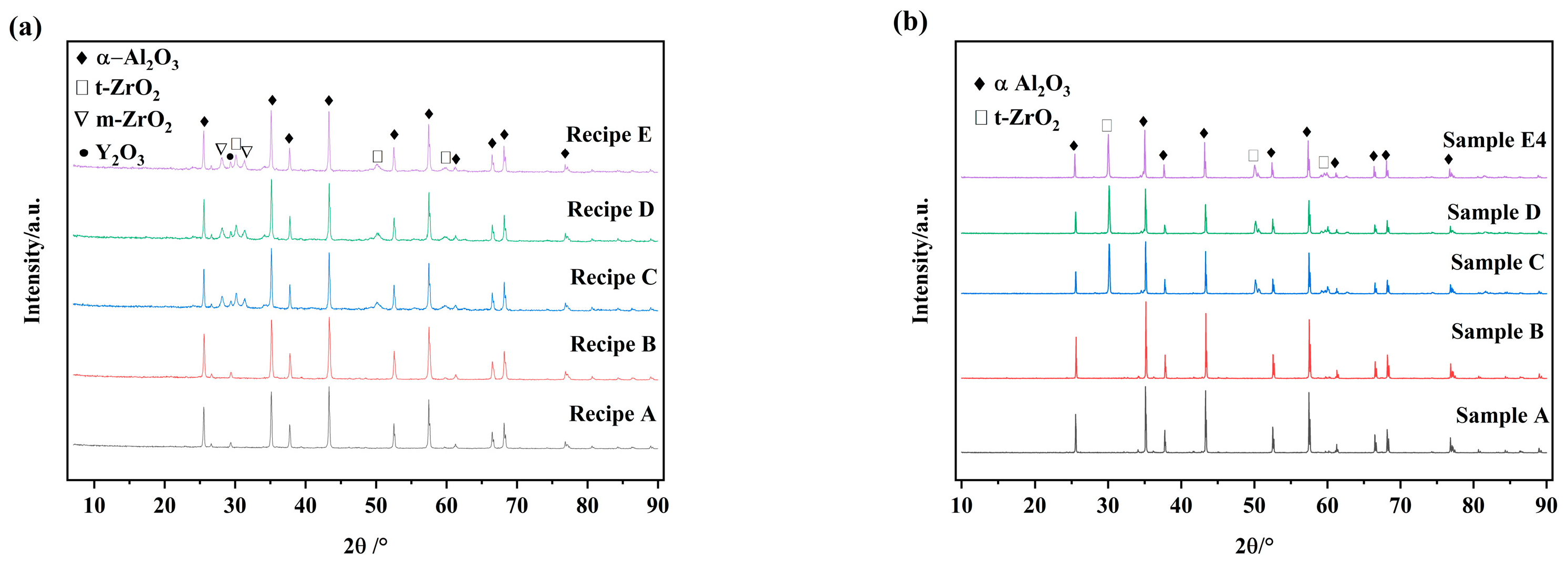
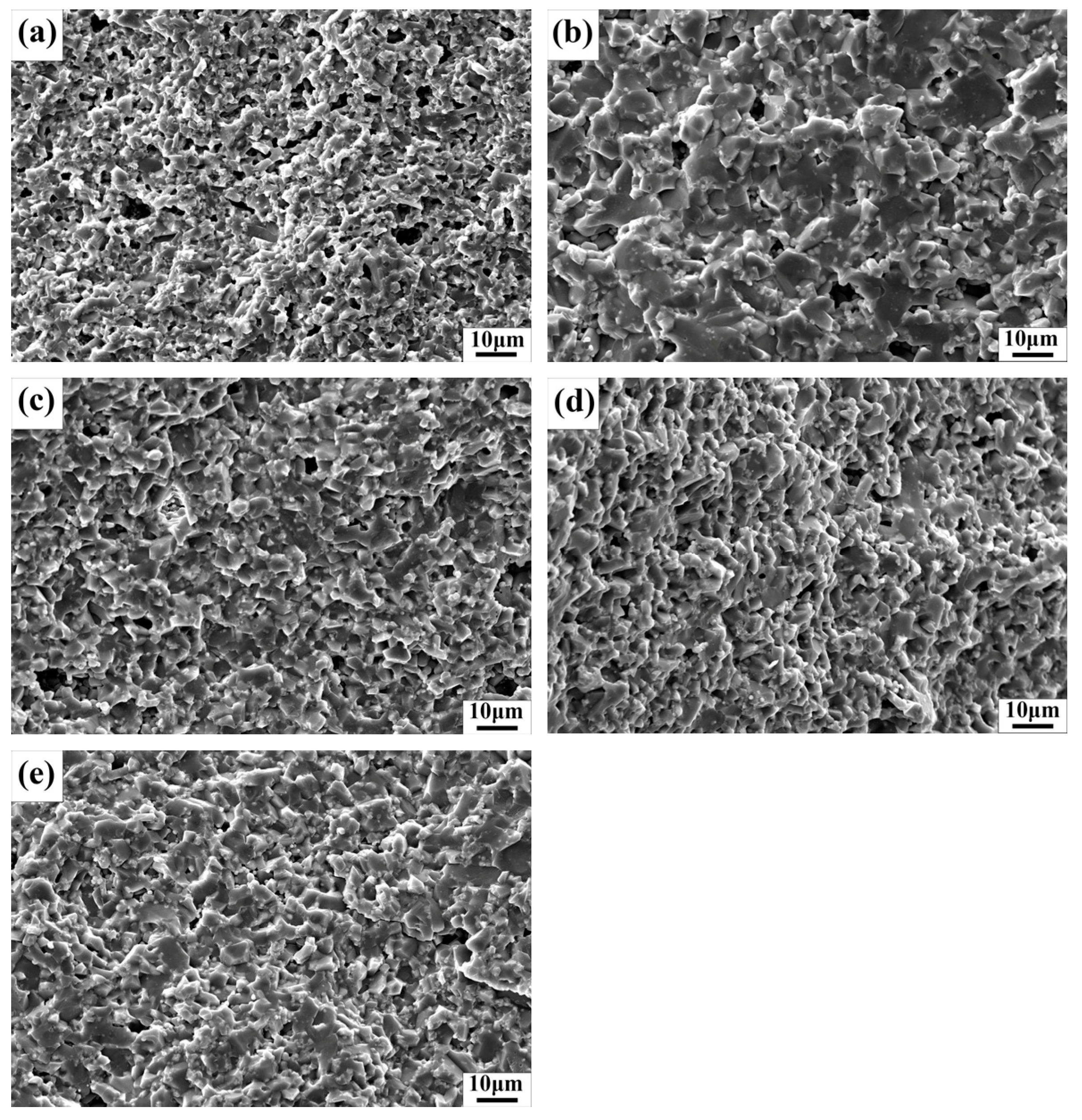
3.2. Microstructure and Densities of Sintered ZTA Ceramics
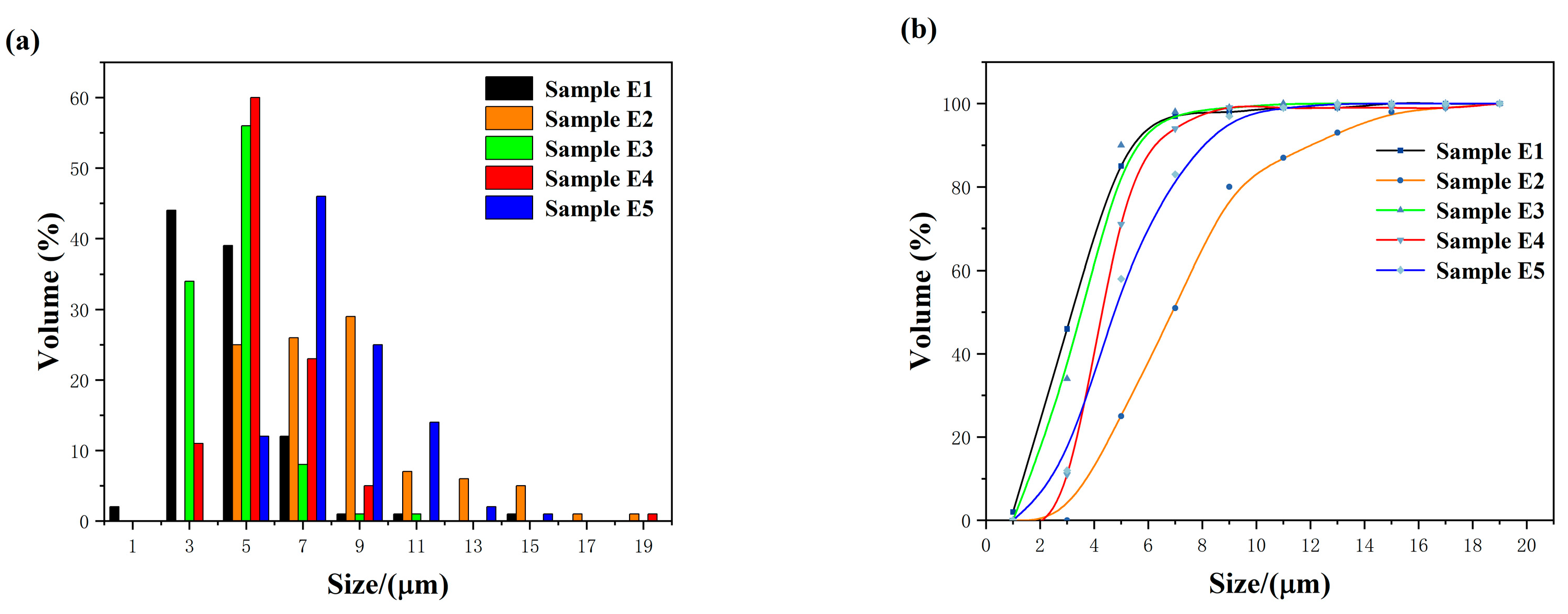
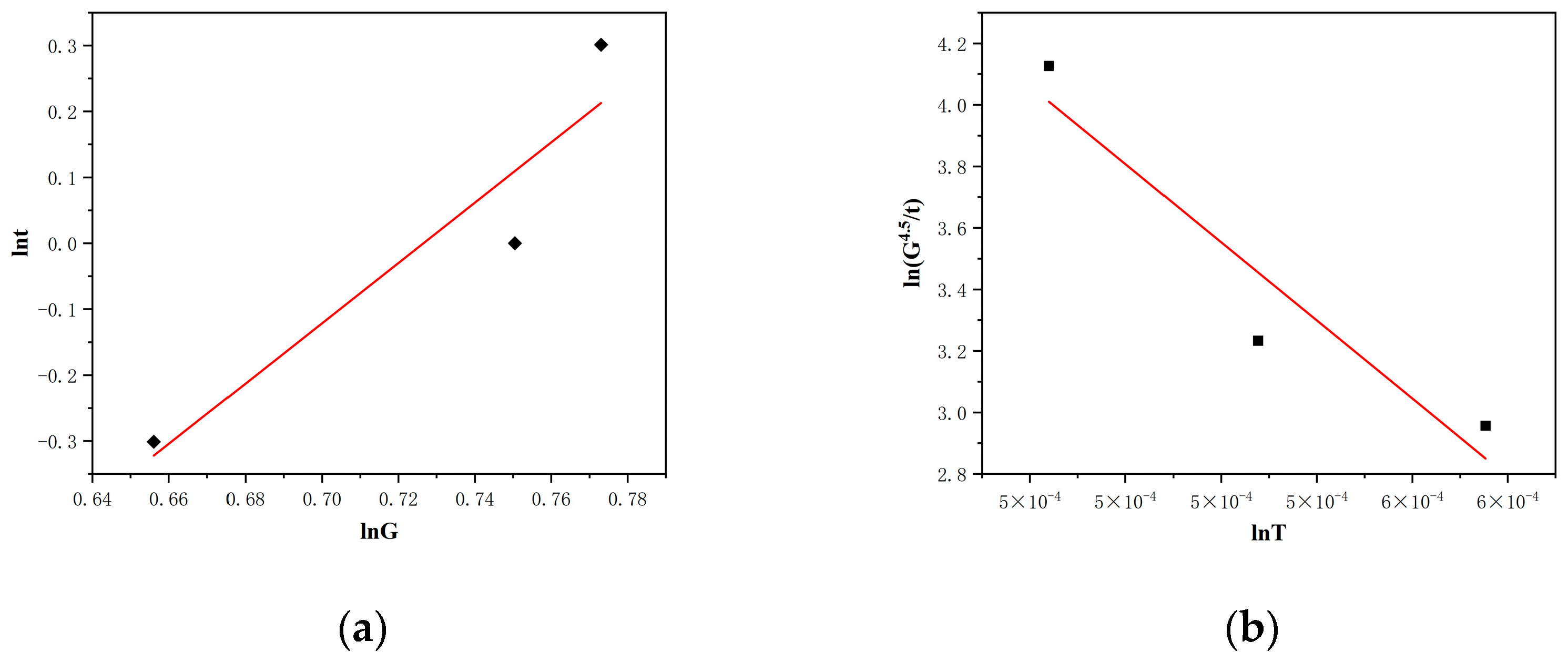
3.3. Grain Growth Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Xu, Y.D.; Liu, S.W.; Chen, H.H.; Tao, J.; Tong, X.; Li, Y.; Huang, S.; Lin, J.; Wen, C.; et al. Microstructure, mechanical properties, friction and wear performance, and cytotoxicity of additively manufactured zirconia-toughened alumina for dental applications. Compos. Part B Eng. 2023, 250, 110459. [Google Scholar] [CrossRef]
- Mangalaraja, R.V.; Chandrasekhar, B.K.; Manohar, P. Effect of ceria on the physical, mechanical and thermal properties of yttria stabilized zirconia toughened alumina. Mater. Sci. Eng. A 2003, 343, 71–75. [Google Scholar] [CrossRef]
- Kyomoto, M.; Shoyama, Y.; Saiga, K.; Moro, T.; Ishihara, K. Reducing fretting-initiated crevice corrosion in hip simulator tests using a zirconia-toughened alumina femoral head. J. Biomed. Mater. Res. Part B 2018, 106, 2815–2826. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Li, W.; Yi, Y.L. Interface characteristics and wear resistance of Ni-B4C plated ZTA reinforced high chromium cast iron composite. China Foundry 2023, 20, 253–262. [Google Scholar] [CrossRef]
- Ding, Z.Z.; Zreiqat, H.; Mirkhalaf, M. Rationally-designed self-shaped ceramics through heterogeneous green body compositions. Mater. Horiz. 2022, 9, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Wade-Zhu, Y.; Wade-Zhu, J.; Wu, H.; Binner, J.; Vaidhyanathan, B. The ballistic impact performance of nanocrystalline zirconia-toughened alumina (nZTA) and alumina ceramics. J. Eur. Ceram. Soc. 2021, 41, 1427–1437. [Google Scholar] [CrossRef]
- Chen, J.Q.; Wang, W.L.; Sun, X.N.; Sun, G.X.; Liang, Y.J. Microstructure and mechanical properties of zirconia toughened nacre-like alumina ceramics. Mater. Sci. Eng. A 2022, 855, 143908. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Z.; Feng, G.; Cao, J.; Zhang, H.; Deng, D. Characterization of ZTA composite ceramic/Ti6Al4V alloy joints brazed by AgCu filler alloy reinforced with one-dimensional Al18B4O33 single crystal. Crystals 2022, 12, 933. [Google Scholar] [CrossRef]
- Olhero, S.M.; Ganesh, I.; Torres, P.M.C.; Alves, F.J.; Ferreira, J.M.F. Aqueous colloidal processing of ZTA composites. J. Am. Ceram. Soc. 2010, 92, 9–16. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Yu, X.; Shan, Y.; Liu, Z.; Yin, Y. Effect of microstructure on the fracture behavior of micro–nano ZTA composite. Mater. Chem. Phys. 2008, 112, 213–217. [Google Scholar] [CrossRef]
- Li, J.; Cai, Q.; Luo, G.; Zhong, X.; Shen, Q.; Tu, R.; Guo, X.; Ding, R. Zirconia toughened alumina ceramics via forming intragranular structure. Materials 2024, 17, 1309. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Cai, D.L.; Yang, Z.H.; Duan, X.M.; Sun, Y.; Li, H.; Duan, W.; Jia, D.; Zhou, Y. Anisotropies in structure and properties of hot-press sintered h-BN-MAS composite ceramics: Effects of raw h-BN particle size. J. Eur. Ceram. Soc. 2019, 39, 539–546. [Google Scholar] [CrossRef]
- O, Y.T.; Koo, J.B.; Hong, K.J.; Park, J.S.; Shin, D.C. Effect of grain size on transmittance and mechanical strength of sintered alumina. Mater. Sci. Eng. A 2004, 374, 191–195. [Google Scholar] [CrossRef]
- Basha, S.A.; Sarkar, D. Grain growth suppression of ZTA by multi-step sintering. Mater. Today Proc. 2020, 26, 1226–1230. [Google Scholar] [CrossRef]
- Yang, X.; Guo, J.; Xie, H.; Li, Y.; Yang, X. Preparation of ZTA composite ceramics derived from sol-gel method by DIW printing. Int. J. Appl. Ceram. Technol. 2024, 21, 3851–3862. [Google Scholar] [CrossRef]
- Fan, J.Y.; Lin, T.T.; Hu, F.X.; Yu, Y.; Ibrahim, M.; Zheng, R.; Huang, S.; Ma, J. Effect of sintering temperature on microstructure and mechanical properties of zirconia-toughened alumina machinable dental ceramics. Ceram. Int. 2017, 43, 3647–3653. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, B.; Shao, G.; Zhang, R. Preparation of large size ZTA ceramics with eccentric circle shape by microwave sintering. J. Adv. Ceram. 2016, 5, 291–297. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.A.; Tan, J.L.; Sun, J.X.; Guo, H.; Bai, J.; Zhou, P.; Zhang, D.; Liu, G. Effect of sintering temperature on the microstructures and mechanical properties of ZrO2 ceramics fabricated by additive manufacturing. Ceram. Int. 2024, 50, 11392–11399. [Google Scholar] [CrossRef]
- Chen, I.W.; Wang, X.H. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature 2000, 404, 168–171. [Google Scholar] [CrossRef]
- Golieskardi, M.; Satgunam, M.; Ragurajan, D. Effect of holding time on the mechanical properties and microstructural evaluation of 3Y-TZP ceramics. J. Aust. Ceram. Soc. 2017, 53, 1001–1006. [Google Scholar] [CrossRef]
- Tahir, A.; Rasche, S.; Könke, C. Discrete element model development of ZTA ceramic granular powder using micro computed tomography. Adv. Powder Technol. 2018, 29, 3471–3482. [Google Scholar] [CrossRef]
- Mullot, J.; Lecompte, J.P.; Montanaro, L.; Negro, A. Influence of powder drying route on the mechanical properties of alumina-zirconia composites. J. Eur. Ceram. Soc. 1993, 11, 309–313. [Google Scholar] [CrossRef]
- Alexander, K.B.; Becher, P.F.; Waters, S.B.; Bleier, A. Grain growth kinetics in alumina-zirconia (CeZTA) composites. J. Am. Ceram. Soc. 1994, 77, 939–946. [Google Scholar] [CrossRef]
- Zhao, H.L.; Shen, J.Y.; Lu, W.H. Study on the structural characteristics of phase diagram of Al2O3-ZrO2-SiO2 system. J. Chin. Ceram. Soc. 1991, 3, 280–288. [Google Scholar]
- Pan, X.; Mayer, J.; Rühle, M.; Niihara, K. Silicon nitride based ceramic nanocomposites. J. Am. Ceram. Soc. 1996, 79, 585–590. [Google Scholar] [CrossRef]
- Orlovská, M.; Húlek, L.; Bača, Ľ.; Kovár, V.; Tomanová, K.; Kitzmantel, M.; Janek, M.; Neubauer, E. Neubauer. Study of the alumina sintering process with a low zirconia content. Ceram. Int. 2022, 48, 2736–2743. [Google Scholar] [CrossRef]
- Du, W.Y.; Ai, Y.; He, W.; Chen, W.; Liang, B.; Lv, C. Formation and control of “intragranular” ZrO2 strengthened and toughened Al2O3 ceramics. Ceram. Int. 2020, 46, 8452–8461. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Wu, H.B.; Liu, X.J.; Huang, Z.R. Influence of particle-level pairing on solid-phase sintered silicon carbide ceramics. J. Inorg. Mater. 2018, 33, 1167–1172. (In Chinese) [Google Scholar] [CrossRef]
- Lóh, N.J.; Simão, L.; Jiusti, J.; De Noni, A., Jr.; Montedo, O.R.K. Effect of temperature and holding time on the densification of alumina obtained by two-step sintering. Ceram. Int. 2017, 43, 8269–8275. [Google Scholar] [CrossRef]
- Chen, X.; Lu, T.; Wei, N.; Hua, T.; Zeng, Q.; Wu, Y. Fabrication and microstructure development of Yb: YAG transparent ceramics from co-precipitated powders without additives. J. Am. Ceram. Soc. 2019, 102, 7154–7167. [Google Scholar] [CrossRef]
- Witek, S.R.; Butler, E.P. Zirconia particle coarsening and the effects of zirconia additions on the mechanical properties of certain commercial aluminas. J. Am. Ceram. Soc. 1986, 69, 523–529. [Google Scholar] [CrossRef]
- Smith, C.S. Grains, phases, and interfaces an interpretation of microstructure. Metal. Technol. 1948, 175, 15–51. [Google Scholar]
- Hellman, P.; Hillert, M. On the effect of second-phase particles on grain growth. Scand. J. Metall. 1975, 4, 211–219. [Google Scholar]
- Hillert, M. Inhibition of grain growth by second-phase particles. Acta Metall. 1988, 36, 3177–3181. [Google Scholar] [CrossRef]
- Hassold, G.N.; Holm, E.A.; Srolovitz, D.J. Effects of particle size on inhibited grain growth. Scripta Metal. Mater. 1990, 24, 101–106. [Google Scholar] [CrossRef]
- Okada, K.; Sakuma, T. The role of Zener’s pinning effect on the grain growth in Al2O3-ZrO2. J. Ceram. Soc. Jpn. 1992, 100, 382–386. [Google Scholar] [CrossRef]
- Gonzalez, J.L.; Piumbini, C.K.; Scopel, W.L.; Deleprani, F.; Gomes, A.; Cunha, A. Pore structure dependence with the sintering time for dense ceramic bulk YBa2Cu3Oy. Ceram. Int. 2013, 39, 3001–3006. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, W.S.; Yao, S.W.; Wang, J.; Ma, Y.Z. Kinetics and mechanisms for the densification and grain growth of the α-alumina fibers isothermally sintered at elevated temperatures. Ceram. Int. 2022, 48, 21756–21762. [Google Scholar] [CrossRef]
- Voytovych, R.; MacLaren, I.; Gülgün, M.A.; Cannon, R.M.; Rühle, M. The effect of yttrium on densification and grain growth in α-alumina. Acta Mater. 2002, 50, 3453–3463. [Google Scholar] [CrossRef]
- Du, W.Y. Formation control and growth kinetics of “endocrystalline” ZrO2/Al2O3 composite ceramics. Master’s Thesis, Nanchang Hangkong University, Nanchang, China, 2020. (In Chinese). [Google Scholar]
- Lange, F.F.; Hirlinger, M.M. Hindrance of grain growth in Al2O3 by ZrO2 inclusions. J. Am. Ceram. Soc. 1984, 67, 164–168. [Google Scholar] [CrossRef]


| Recipe | Reactive Alumina (wt.%) | Calcined Alumina (wt.%) | YSZ (wt.%) | Nano Alumina (wt.%) |
|---|---|---|---|---|
| A | 100 | 0 | 0 | 0 |
| B | 99 | 0 | 0 | 1 |
| C | 85 | 0 | 15 | 0 |
| D | 68 | 17 | 15 | 0 |
| E | 84 | 0 | 15 | 1 |
| Sample | Raw Material Formula | Sintering Temperature (°C) | Holding Time (h) |
|---|---|---|---|
| A | A | 1600 | 1 |
| B | B | 1600 | 1 |
| C | C | 1600 | 1 |
| D | D | 1600 | 1 |
| E1 | E | 1520 | 1 |
| E2 | E | 1680 | 1 |
| E3 | E | 1600 | 0.5 |
| E4 | E | 1600 | 1 |
| E5 | E | 1600 | 2 |
| Recipe | D10 (μm) | D50 (μm) | D90 (μm) | D90/D10 | Span |
|---|---|---|---|---|---|
| A | 48.7 | 75.1 | 119.9 | 2.46 | 0.95 |
| B | 48.9 | 75.6 | 120.7 | 2.47 | 0.95 |
| C | 49.7 | 76.1 | 121.2 | 2.44 | 0.94 |
| D | 51.7 | 77.8 | 121.7 | 2.35 | 0.90 |
| E | 51.6 | 80.3 | 126.2 | 2.45 | 0.93 |
| Sample Number | Shrinkage (%) | Relative Density (%) | Average Grain Size (μm) | Standard Deviation of Grain Size (μm) |
|---|---|---|---|---|
| A | 18.24 | 95.21 | 11.73 | 0.31 |
| B | 18.78 | 94.61 | 10.85 | 0.30 |
| C | 17.52 | 94.33 | 8.25 | 0.23 |
| D | 16.86 | 95.13 | 5.47 | 0.11 |
| E1 | 17.94 | 93.17 | 4.54 | 0.23 |
| E2 | 18.64 | 94.11 | 8.26 | 0.19 |
| E3 | 18.67 | 94.90 | 5.55 | 0.13 |
| E4 | 18.51 | 94.98 | 5.63 | 0.23 |
| E5 | 18.19 | 94.79 | 5.96 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Deng, J.; Li, W.; Liu, J.; Yuan, W. Grain Growth Behavior of Alumina in Zirconia-Toughened Alumina (ZTA) Ceramics During Pressureless Sintering. Crystals 2025, 15, 89. https://doi.org/10.3390/cryst15010089
Zhao Y, Deng J, Li W, Liu J, Yuan W. Grain Growth Behavior of Alumina in Zirconia-Toughened Alumina (ZTA) Ceramics During Pressureless Sintering. Crystals. 2025; 15(1):89. https://doi.org/10.3390/cryst15010089
Chicago/Turabian StyleZhao, Yi, Jiang Deng, Wen Li, Jianhong Liu, and Wenjie Yuan. 2025. "Grain Growth Behavior of Alumina in Zirconia-Toughened Alumina (ZTA) Ceramics During Pressureless Sintering" Crystals 15, no. 1: 89. https://doi.org/10.3390/cryst15010089
APA StyleZhao, Y., Deng, J., Li, W., Liu, J., & Yuan, W. (2025). Grain Growth Behavior of Alumina in Zirconia-Toughened Alumina (ZTA) Ceramics During Pressureless Sintering. Crystals, 15(1), 89. https://doi.org/10.3390/cryst15010089






