Abstract
Nanomaterials are materials at the nanometric scale that have distinctive functionalities and properties. Due to their unique properties relative to traditional materials, nanomaterials attract great interest from researchers. ZnO-based nanomaterials especially demonstrate versatility, accessibility, biocompatibility and low toxicity. In recent years, there has been a growing interest in developing eco-friendly and sustainable approaches for synthesizing nanomaterials. In the development of ecological techniques for their synthesis, using natural resources is a popular choice. Employing egg white for ZnO nanoparticle synthesis represents an environmentally method that uses a natural resource. The great advantage of green synthesis using egg white is that it is a cost-effective, renewable, and bio-degradable resource that offers biocompatibility. Egg white is rich in proteins, amino acids, and other biomolecules that possess reducing properties. These biomolecules interact with metal ions, leading to the reduction and nucleation of nanoparticles. Additionally, the proteins in egg white act as capping agents, stabilizing the nanoparticles and preventing their aggregation. The proteins of white albumen have different functional groups and maintain product attributes, such as dispersion and stability. This paper focuses on the characterization of ZnO nanoparticles obtained by the assisted synthesis of egg white. This study explores the potential of ovalbumin, the major protein in egg white, as a template for the synthesis of nanostructured ZnO. The synthesis process utilized egg white from different sources (commercially raised hens, home-raised hens, and ducks) and varying zinc nitrate concentrations (1M and 2M) to evaluate their influence on nanoparticle morphology and size. Various complementary techniques are employed to analyze the resulting nanostructures: XRD, SEM, and ATR-FTIR. Also, antibacterial properties are investigated. This study underscores the viability of different egg whites as a green resources for synthesizing nanostructured ZnO and contributes to the development of sustainable nanotechnology approaches.
1. Introduction
Biogenic synthesis is of great interest among researchers due to its non-toxicity and eco-friendliness [1]. Biogenic synthesis utilizes biological agents like plants, biomolecules, microorganisms, and enzymes as stabilizing and reducing agents [2]. Also known as the green synthesis of nanomaterials, the methods also involve algae, animal cell culture, and fungi. Compared to chemical synthesis, biosynthesis has advantages such as a lower production cost; relatively simple method; and the ability to obtain nanomaterials with improved properties, such as higher purity, better dispersion, and higher stability [1].
Biogenic synthesis is used to obtain a multitude of nanoparticles based on gold, silver, calcium carbonate, titanium oxide, etc. For example, Meydan et al. obtained CaCO3 nanoparticles through green synthesis, using Gum Arabic as a natural polymer [3]. Rasheed et al. used Artemisia vulgaris leaf extract for synthesizing silver nanoparticles without the addition of a capping agent [4]. Alarif et al. synthesized TiO2 nanoparticles via green chemistry, using marine natural extracts of two red algae [5].
Most researchers accepted the green synthesis method using bioresources to obtain ZnO nanoparticles [6] because zinc oxide has multitude of applications: the rubber industry [7], ceramics industry [8], skin treatment [9], antibacterial uses [10], sun protection [11], food additives [12], pigments [13], sensors [14], and wound dressing [15]. For instance, Zeghoud et al. used plants that were cleaned with distilled water and simply soaked to produce a proper plant extract. They used also Zn(II) salt solution to form a precipitate that acts as a precursor for nanoparticles. These ZnO nanoparticles were obtained by calcinating the precipitate at 400 °C. The morphology varies—flower, hexagonal or sphere—with a particle size between 10 and 500 nm [16]. Mahdi et al. studied bacterial-mediated synthesis to obtain ZnO nanoparticles, using Lactococcus and Bacillus. The nanopowder obtained by Lactococcus is nano-spheres with diameter size in the range of 55–60.5 nm, while the nanopowder obtained by Bacillus constitutes nano-rods with an average diameter of 99 nm. Both nanopowders have a pure hexagonal crystalline structure [17]. Raliya et al. reported the elaboration of ZnO nanoparticles with sizes between 1.2 and 6.8 nm and spherical and hexagonal structures, using extracellular secretions of Aspergillus fumigatus, TFR-8 and ZnNO3 [18].
A summary of the research that used bio resources is presented in Table 1.

Table 1.
Nanoparticles synthesized using bioresources.
Recently, researchers started to study the biogenic synthesis of ZnO using ovalbumin from egg white. Egg white is a natural, biodegradable material, reducing the environmental impact associated with chemical synthesis methods that often involve toxic chemicals and solvents. The approximate global egg production in 2018 was 78 million metric tons [19]. The leading producers of eggs are India, the USA, and China.
The proteins in egg white can act as capping and reducing agents, influencing the size, shape, and morphology of the synthesized nanomaterials. Ovalbumin, the main protein of egg white, is a water-soluble protein that has the characteristics of biocompatibility and biodegradability, good reactivity, minimal toxicity, and good stability. Ovalbumin has the CAS registration number 9006-50-2 and has the name 5,5-dimethyl-2,4-dioxo-oxazolidine-3-carboxamide. Its chemical formula is C6H8N2O4 [20].
Ovalbumin can serve as a template for nanostructured metal oxide synthesis through various mechanisms. The protein can act as a reducing agent, converting metal ions into metal nanoparticles, or it can form a metal–ovalbumin complex. In the case of Zn salt as precursors, Zn ions from a zinc salt solution interact with the functional groups (amino, carboxyl, and thiol) present in the proteins of egg white, forming Zn–ovalbumin complexes. For instance, Moalei et al. thoroughly mixed hydrated zinc nitrate with egg white; the powder obtained is pure, has a hexagonal structure, and has particle size estimated to be 29–48 nm; zinc oxide was developed for photocatalytic applications [21]. Puduky et al. used zinc nitrate, distilled water and egg white to obtain a pure hexagonal wurtzite structure of zinc oxide powder with photocatalytic applications [22]. Ahmed et al. used zinc acetate dihydrate and egg white for their experiment; the ZnO powder obtained has a wurtzite structure, is pure, and has an average particle size of around 50 nm; the zinc oxide obtained is used in antibacterial applications [23].
This study explores the eco-friendly synthesis of zinc oxide (ZnO) nanoparticles using egg white as a natural and biodegradable precursor. The novelty of this paper is in the determination of optimal biogenic synthesis parameters for ZnO nanoparticles using different types of eggs and at different concentrations of zinc salt. Leveraging the reducing and stabilizing properties of ovalbumin, the major protein in egg white, ZnO nanoparticles with tailored structural and functional properties were synthesized.
2. Materials and Methods
For this research, six experiments were carried out as follows:
- The precursors used for the first and second experiments are as follows: 50 mL zinc nitrate of concentrations 1M and 2M, and 50 mL ovalbumin from eggs of hens raised on commercial farms.
- The precursors used for the third and fourth experiments are as follows: 50 mL zinc nitrate of concentrations 1M and 2M, and 50 mL ovalbumin from eggs of home-raised hens.
- The precursors used for the fifth and sixth experiment are as follows: 50 mL zinc nitrate of concentrations 1M and 2M, and 50 mL ovalbumin from duck eggs.
The choice of different types of egg is justified by the fact that different types of egg can lead to obtaining nanoparticles with various sizes, shapes, and antibacterial properties, due to different protein content. No such studies have been reported so far. By understanding how egg white composition influences the nanoparticle formation process, we can optimize synthesis and create superior-performing materials for a wide range of applications. For example, Table 2 presents the amount of protein from both hen and duck egg whites.

Table 2.
Amount of egg white protein [24,25].
The equipment and materials used in the elaboration of ZnO by biogenic hydrolytic synthesis are Berzelius glass, an oven, mortar, pestle, burette, pH meter, magnetic stirrer, and cylinder. Zinc nitrate hexahydrate, Zn(NO3)2 · 6H2O, 99.8% purity (Sigma Aldrich purchase, St. Louis, MI, USA) was used to obtain 1M and 2M solution in 50 mL deionized water. After mixing the Zn(II) solutions and the egg white, the initial Zn foam is subjected to ultrasonic action for 10 min and then left for 10 h at room temperature (22 °C) for drying. The next step is drying the Zn foam at 120 °C for 4 h in the oven. The final Zn foam is ground and washed with distilled water to remove all protein and then filtered. After that, the filtrate is dried for 3 h at 120 °C, resulting in a yellow powder. Then, the powder is washed with demineralized water and ethyl alcohol, filtered, and mortared after the first drying and grinding. The final process is calcination for 2 h at 550 °C until a white powder is obtained. The process schema is presented in Figure 1.
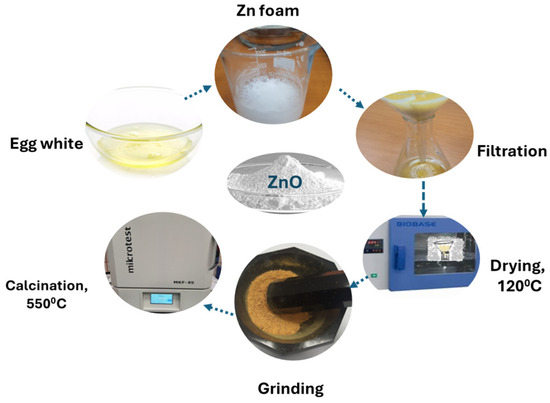
Figure 1.
The elaboration of ZnO by biogenic hydrolytic synthesis process schema.
The methods used in the characterization of the obtained powders are X-ray diffraction (XRD), electron microscopy coupled with energy-dispersive spectroscopy (SEM-EDS) and attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR).
The X-ray diffraction analysis of calcined powders was carried out using the Rigaku Ultima IV X-ray diffractometer, Japan, equipped with a D/teX Ultra detector, graphite monochromator for kβ radiation and a vertical goniometer, focusing on Bragg–Brentano. X-ray diffractograms were recorded using CuKα radiation in the measurement range 2θ with a step of 0.020°, the scan speed being 1.5°/min and a scan range of 22.00–85.00°.
The scanning electron microscope used for the examination of particle morphology was a Hitachi SU 5000, Hitachi, Japan, with secondary electron detectors (SE—secondary electrons) and coupled with the X-ray fluorescence spectrometry module (EDS—energy-dispersive spectrometry). The particles were spread on a carbon type and analyzed at variable pressure, with a 25 kV acceleration voltage and 127 µA emission current. The EDS analysis was performed on the sample area.
The equipment used to determine the ATR-FTIR spectra is Bruker Tensor 27, BrukerOptik GmbH, Ettlingen, Germany, in the range 4.000–350 cm−1, with a resolution of 4 cm−1. ATR-FTIR analysis of the foams formed after the hydrolysis of 1M and 2M zinc nitrate with ovalbumin by different egg types, was performed every hour, for 4 h at 120 °C. After the foam washing and drying for 3 h at 120 °C, the resulting powder was collected, to be calcinated at 550 °C for 2 h.
Molaei et al. studied ZnO NPs obtained from egg white and zinc nitrate at different calcination temperatures (450 °C, 600 °C, and 700 °C) for 3 h. The best reported calcination temperature was 600 °C [21]. Ahmed et al. studied different calcination temperatures (400 °C, 550 °C, and 650 °C) for 3 h to obtain ZnO NPs using egg white and zinc acetate; the best crystallinity was observed at 550 °C and 650 °C [23]. Extreme temperatures associated with long calcination times can lead to excessive sintering of the particles, which can lead to a decrease in the specific surface area and a decrease in the reactivity of the material. The temperature of 550 °C represents an optimal compromise between ensuring good crystallinity and preventing unwanted sintering.
Zinc oxide powders have been coded for easier recognition, as shown in Table 3.

Table 3.
The codes for ZnO powders.
As shown in Table 2, the mass losses, observed before and after the calcination process, reflect the elimination of organic compounds and the formation of pure ZnO nanoparticles. The samples from eggs of home-raised hens (ZnO_EHH_1 and ZnO_EHH_2) show slightly higher losses than those from commercial farms (ZnO_EHC_1 and Zno_EHC_2), suggesting a higher content of ovalbumin. The duck egg samples (ZnO_ED_1 and ZnO_ED_2) have the highest relative losses, which may indicate a larger amount of ovalbumin being degraded. The high percentage of mass loss may indicate a lower impurity content and a purer final material. Duck egg samples (ED) can generate smaller nanoparticles due to the extremely high percentage of removal of ovalbumin, similar to organic material.
3. Results
3.1. Compositional Characterization with ATR-FTIR
ATR-FTIR analysis of the foam formed after the hydrolysis of 1M and 2M zinc nitrate with ovalbumin from different egg types, was performed each hour for 4 h, drying at 120 °C. The real time analysis aims to identify the time required to eliminate the protein.
ATR-FTIR analysis of the Zn foam formed after the hydrolysis of Zn (II) 1M and 2M with ovalbumin from eggs from both hens raised in commercial farms and home-raised hens was performed every hour for 4 h at 120 °C. Since no significant differences between spectra were observed during the process, we selected the spectra of the final particles, as shown in Figure 2.
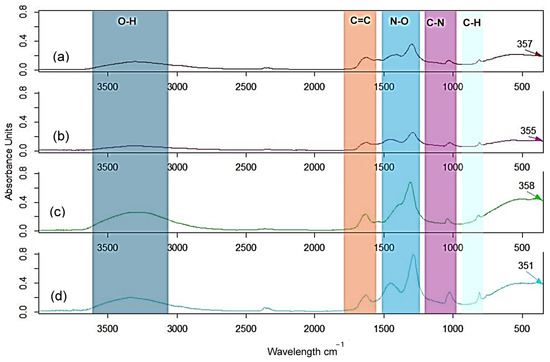
Figure 2.
ATR-FTIR spectra of the Zn foam 1M with ovalbumin (a) from eggs of raised hens in commercial farms and (b) from home-raised hens, and Zn foam 2M with ovalbumin (c) from eggs of raised hens in commercial farms and (d) from home-raised hens, after 4 h at 120 °C.
ATR-FTIR analysis of the foam formed after the hydrolysis of 1M and 2M zinc nitrate solution with ovalbumin from duck eggs was performed every hour for 4 h. The spectra after 4 h are presented in Figure 3.
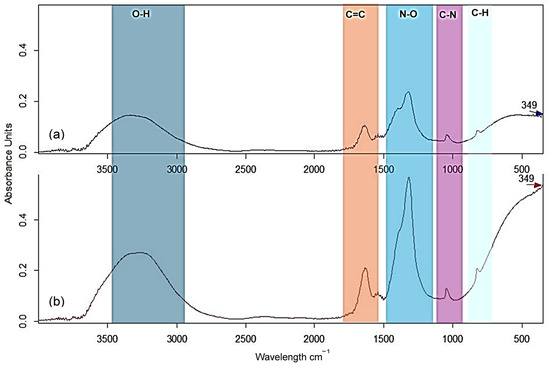
Figure 3.
ATR-FTIR spectra of the Zn foam with ovalbumin from duck eggs: (a) 1M and (b) 2M after 4 h at 120 °C.
Thermal degradation of ovalbumin at 12 °C involves the breaking of existing chemical bonds in amino acid chains and the formation of new ones. This leads to significant changes in the FTIR spectrum. A peptide bond is formed between the carboxyl group of one amino acid and the amino group of another amino acid, with the elimination of a water molecule. This is the “backbone” of ovalbumin. The region around 1500–1200 cm−1 indicates the breaking of peptide bonds under Zn(II) solution influence and the formation of new C-N bonds. A decrease in the intensity of the bands may be associated with the loss of protein structure. Zinc can form coordination bonds with nitrogen or oxygen in the side chain of amino acids. This affects protein function.
The maximum observed difference between 3200 and 3550 cm−1 is attributed to O-H stretching. The fingerprint region (1400–400 cm−1) contains complex vibrational modes that can be used to identify specific functional groups N-O, C-N, C-H and Zn-O compound. The difference between spectra is the peak region corresponding to Zn-O bond. It observed a slight displacement of vibration due to presence of protein on the surface of ZnO nanoparticles.
The ZnO nanoparticles obtained from egg white of duck eggs showed a faster elimination of proteins compared to those from hen eggs, due to the different protein content. The spectra of the nanoparticles obtained from higher Zn(II) concentrations (2M) indicated a stronger interaction with the functional groups of ovalbumin, which resulted in a more efficient synthesis process.
Because the protein is still confirmed in ATR-FTIR spectra, the samples were washed with demineralized water and ethyl alcohol, filtered and grinded. Another process of drying for 2 h at 120 °C was carried out to eliminate the water and the remaining protein. All samples were calcinated at 550 °C for 2 h. The ATR-FTIR spectra after calcination presents only the vibration of Zn-O around 380–395 cm−1. The confirmation is carried out by comparation with spectra from commercial ZnO, with the vibration of Zn-O around 358 cm−1, as shown in Figure 4.
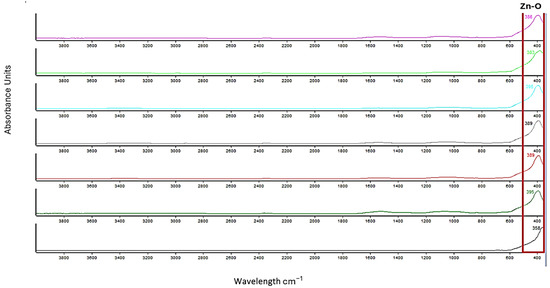
Figure 4.
ATR-FTIR spectra of final powders: ZnO_EHC_1 (magenta) and ZnO_EHC_2 (bright green), ZnO_EHH_1 (turquoise) ZnO_EHH_2 (gray), ZnO_ED_1 (red) ZnO_ED_2 (green) and commercial ZnO (black).
Analyzing the spectra, we can conclude that the temperature of 120 °C was chosen to allow the gradual removal of proteins, and calcination at 550 °C ensured the formation of pure ZnO crystals. The disappearance of the band at 1500–1200 cm−1 indicates the degradation of protein structures, and the appearance of a distinct band around 380–395 cm−1 confirms the formation of Zn-O bonds.
After ATR-FTIR analysis, it can be concluded that the formation of ZnO using ovalbumin consists of the following:
- Dehydration and degradation of the Zn–ovalbumin complex began at 120 °C. As the temperature rises, the proteins vibrate more intensely. This increased thermal energy can disrupt the weak interactions (hydrogen bonds, hydrophobic interactions) that maintain the folded structure of the protein. This unfolding may expose zinc-binding sites and weaken the interaction between zinc and amino acid side chains.
- Dissociation of zinc: When the protein unfolds due to heat, the zinc binding bridges may become less favorable for zinc coordination. This can lead to the dissociation of zinc from the complex. Free zinc ions can interact with oxygen and become less functional.
- Oxidation of zinc: High temperatures can accelerate oxidation reactions.
Varying the source of ovalbumin and the concentration of the Zn(NO3)2 precursor provides a way to control the quality of the resulting ZnO nanoparticles.
3.2. Structural Analysis
The XRD spectra of the final powders, after calcination, show a single pure ZnO phase regardless of the origin of the ovalbumin from eggs of home-raised hens, eggs of hens raised in commercial farms, and duck eggs, but at different concentrations of Zn (II) 1M and 2M. The phase is zincite no. 04-005-4711 according to PDF5+2024-DB.
In the case of the XRD structural analysis of the powders obtained by biogenic synthesis of Zn (II), 1 M and 2M, using ovalbumin from hens raised in commercial farms and home-raised, the spectra observable in Figure 5 are obtained. The absence of other crystalline phases or impurities indicates the purity of the synthesized powders.
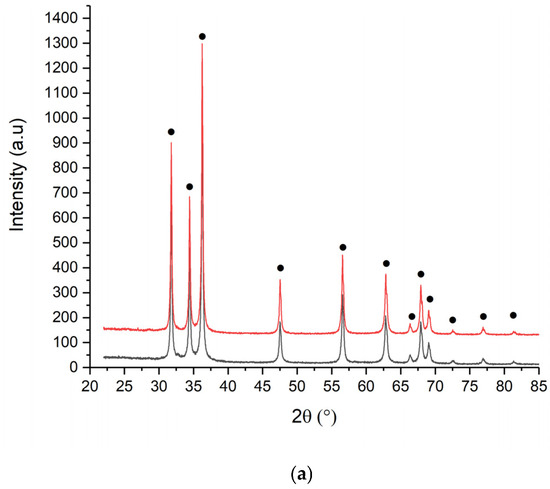
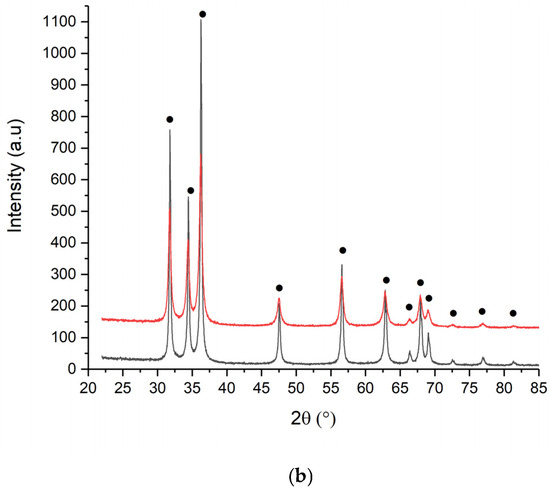
Figure 5.
XRD spectra of (a) ZnO_EHC and (b) EHH 1M (black) and 2M (red).
Calcination at 550 °C favors crystallization processes, leading to the formation of better-defined ZnO crystals and a controlled increase in particle size.
The structural analysis of the powders obtained by biogenic synthesis of Zn(II), 1M and 2M, using albumin from duck egg, is presented in Figure 6. The spectra present only zincite peaks, without impurities, are in agreement with ATR-FTIR analysis, thus demonstrating the complete removal of ovalbumin from the surface of the processed ZnO particles.
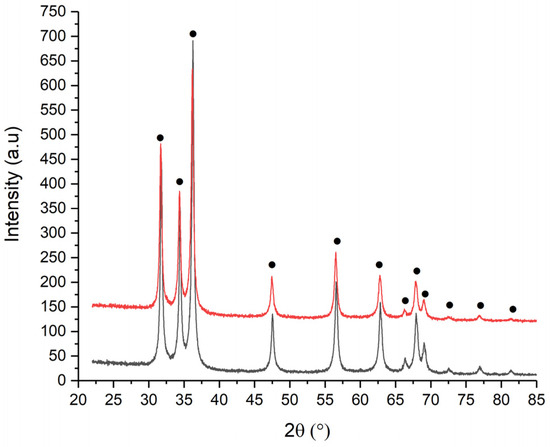
Figure 6.
XRD spectra of ZnO_ED_1 (black) and ZnO_ED_2 (red).
The crystallite size for all samples is calculated using the Williams Hall method. It is observed that the crystallite size varies from one type of powder to another, depending on the concentration of Zn(II) and type of albumin. Table 4 shows the average crystallite sizes calculated using the previously mentioned method and the qualitive results.

Table 4.
Qualitive phase analysis.
From the data analysis, it is observed that the crystallite sizes increase depending on the concentration of the Zn(II), for the same type of ovalbumin, from 48.3 nm to 52.2 nm, for ovalbumin from eggs of raised hens in commercial farms. At same concentration of Zn(II) for different source of ovalbumin can assume a diminution of crystallite grain size.
For ZnO elaborated with ovalbumin from duck eggs, a decrease in size is observed from 42.7 nm to 27.7 nm, from which we can draw the conclusion that the type of ovalbumin determines the obtaining of smaller ZnO particles.
3.3. Morphological and Compositional Analysis (SEM-EDS)
The SEM micrographs reveal various particle morphologies depending on Zn (II) concentration and the type of ovalbumin provenience. The size of a particle from SEM is measured using the ImageJ program. The shapes of particles and the histograms were analyzed, also EDS spectra which show the quantity of each component element of ZnO elaborated powder, as shown in Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12.
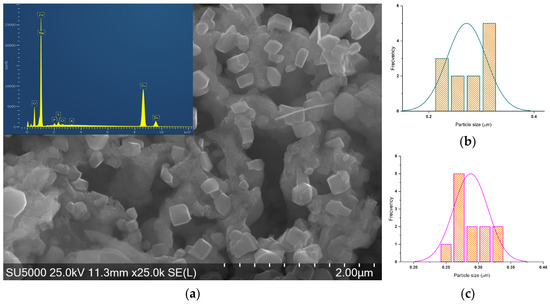
Figure 7.
(a) SEM-EDS micrograph of ZnO_EHC_1 and histograms of (b) hexagonal and (c) parallelepipedal particles.
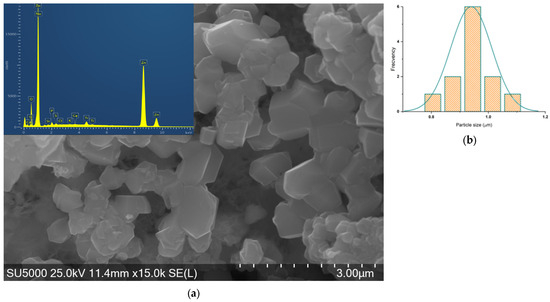
Figure 8.
(a) SEM-EDS micrograph of ZnO_EHC_2 and (b) histogram of asymmetrical particles.
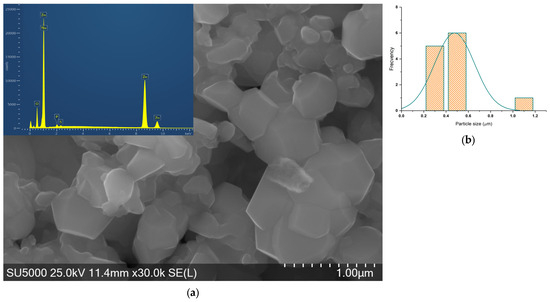
Figure 9.
(a) SEM-EDS micrograph of ZnO_EHH_1 and (b) histogram of asymmetrical particles.
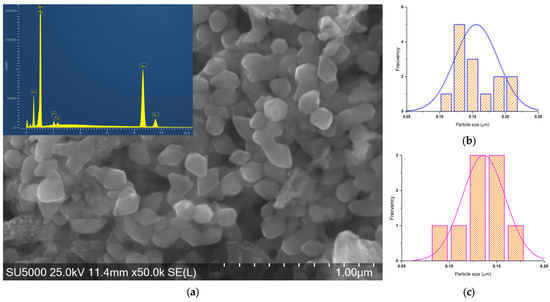
Figure 10.
(a) SEM-EDS micrograph of ZnO_EHH_2 and histograms of (b) rhombic particles and (c) hexagonal.
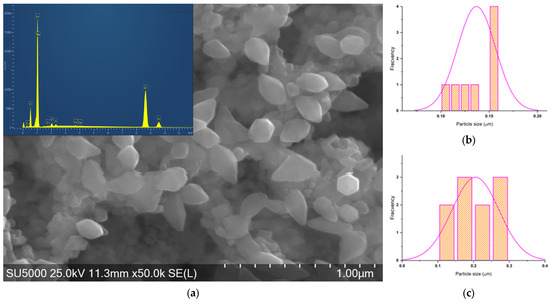
Figure 11.
(a) SEM of ZnO_ED_1 micrograph and histograms of (b) hexagonal particles and (c) elongated hexagonal particles.
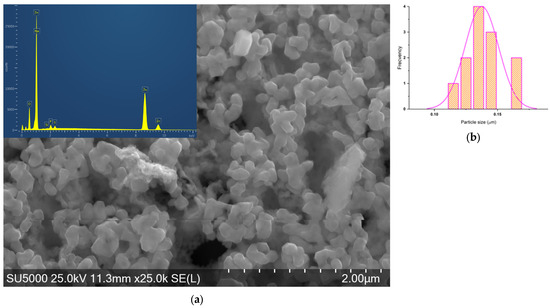
Figure 12.
(a) SEM-EDS micrograph of ZnO_ED_2 and (b) histogram of hexagonal particles.
The shapes of the particles are determined first, and then as many measurements as possible are made for each shape. ZnO_EHC_1 exhibits hexagonal and parallelepipedal particle shapes with a relatively narrow size distribution.
ZnO_EHC_2 particles have one asymmetrical shape with a wider size distribution compared to the previous sample.
ZnO_EHH_1 particles have one asymmetrical shape with a wider size distribution.
For ZnO_EHH_2 two shapes of the particles can be found: rhombus and hexagonal with a relatively narrow size distribution.
For ZnO_ED_1 two shapes of the particles can be found: hexagonal and elongated hexagonal, with a moderate size distribution.
ZnO_ED_2 particles have one hexagonal shape with a narrow size distribution.
The particles synthesized with ovalbumin from duck eggs showed smaller mean sizes and a more uniform distribution compared to those from hen eggs. The SEM analysis demonstrated that the use of different egg whites, from different sources, significantly influences the size and morphology of ZnO particles, contributing to the optimization of biogenic synthesis.
All the EDS spectra show prominent peaks corresponding to Zn and O, confirming the presence of zinc oxide in all samples. In addition to Zn and O, several other peaks are observed in some spectra, indicating the presence of impurities. In the Zn–ovalbumin complex, this can lead to the formation of disulfide bridges between cysteine or methionine residues or Zn–amino acid side chains. While cysteine can bind zinc, these disulfide bridges can reduce the availability of cysteine for zinc binding, potentially contributing to complex degradation. Therefore, due to degradation of ovalbumin some impurities, including Na, Si, P, S, Cl, K, Ca, and Ti, can occur. These are in accordance with the elemental analysis data, presented in Table 5. More intense Zn peaks of ZnO synthesized at Zn (II) 1M with ovalbumin from hens suggest a higher concentration of zinc, comparable with ZnO synthesized by ovalbumin from duck eggs at Zn (II) 2M.

Table 5.
The quantity of component elements for obtained ZnO powders.
As expected, Zn is the most abundant element in all samples, making up roughly 73–75% of the total weight. Oxygen is also present in significant quantities, around 16–18%, consistent with its presence in zinc oxide. The high oxygen content in duck egg samples may indicate a more complete oxidation process compared to chicken eggs. The concentrations of Zn and O vary depending on the source of ovalbumin and the concentration of the precursor.
Several other elements, including Na, Si, P, S, Cl, K, Ca, and Ti, are present in varying amounts. Their presence is observed in the ZnO powders obtained using ovalbumin from eggs of hens raised in commercial farms. S may indicate the presence of ovalbumin sulfide protein residues. The higher content in EHC samples may reflect differences in ovalbumin composition in commercial eggs. Na, Si, Ca, and P may reflect the presence of residual from ovalbumin proteins. A conclusion would be that the nutrients and vitamins used in poultry farms are the source of these.
4. The Antibacterial Properties of Obtained ZnO
Zinc oxide (ZnO) has been extensively studied for its antibacterial properties against a wide range of bacteria. Escherichia coli, a Gram-negative bacterium, is a common cause of urinary tract infections, diarrhea, and other gastrointestinal illnesses. Some strains of E. coli are highly pathogenic and can lead to severe infections. Enterococcus faecalis is Gram-positive bacterium and is a common cause of hospital-acquired infections, particularly urinary tract infections, wound infections, and endocarditis. It is known for its ability to develop resistance to antibiotics, making it a significant public health concern.
ZnO NPs exhibit promising antibacterial properties, making them potential candidates for various applications, including wound dressings, water treatment, and food packaging. The synthesis method plays a crucial role in determining the antibacterial activity of ZnO NPs. By carefully selecting the synthesis method, it is possible to tailor the properties of ZnO NPs to optimize their antibacterial efficacy. Table 6 presents synthesis methods and their advantages, disadvantages, and antibacterial activity.

Table 6.
Advantages, disadvantages, and antibacterial activity of the synthesis methods.
In recent years, researchers have presented the properties of the obtained zinc oxide in their papers, emphasizing the antibacterial properties [23,24,25,26,27]. Thus, Ahmed et al. performed antibacterial tests through the diffusion technique, using ZnO nanorods elaborated with egg white and zinc acetate. The antibacterial activity was better on Pseudomonas aeruginosa than on Staphylococcus aureus and Escherichia coli [23]. Singh et al. carried out the antibacterial test of textile clothes impregnated with ZnO NPs elaborated through hydrolysis starting from zinc acetate and ammonium hydroxide. The effect was a 99% reduction in the growth of Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus [26]. Babayevska et al. demonstrated the antibacterial properties of ZnO nano- and micro-particles obtained using chemical and physical methods. S. aureus was more susceptible to NP treatment than E. coli strain. [27]. Thus, it is observed that for the elaboration of ZnO NPs, the chemical and physical elaboration methods that use zinc acetate have been more intensively studied than the biogenic methods with egg white, using the same zinc salt. There are no results presented on the synthesis of egg white using zinc nitrate and no studies on the properties of elaborated ZnO NPs.
By testing ZnO against both Gram-negative and Gram-positive bacteria, its broad-spectrum of antibacterial activity can be determined.
An artificial sample enriched with Escherichia coli ATCC2592 and Enterococcus faecalis ATCC19433 was carried out to test the bactericidal and bacteriostatic effect of the obtained ZnO powders. The number of microorganisms estimated to grow on the control media was approximately 50 colonies of Enterococcus faecalis and approximately 50 colonies of Escherichia coli/samples. Before being immersed in the test tubes with 18.1 mL artificial sample each, the ZnO powders were sterilized under the UV lamp in the microbiological niche for 15 min. The artificial sample consists of 18 mL sterile distilled water and 0.1 mL bacterial suspension. The working samples were coded (ZnO-1,2,3,4,5,6). The control sample (M) consists of 18 mL of sterile distilled water and 0.1 mL of bacterial suspension.
After immersing the powders in the test tubes with microorganisms, an immediate transition was made from the six samples and the control to the CCA selective medium (for the growth of E. coli bacteria), the contact time of the ZnO powders with the microorganisms being less than 30 min. The plates were incubated at a temperature of 37 °C for 24 h, the results demonstrating the bactericidal action of the ZnO powders.
After incubation at a thermostat at 37 °C, E. coli colonies did not develop and E. faecalis had significant inhibition. The whole process is summarized in Table 7.

Table 7.
The antibacterial properties of the obtained ZnO powders.
After 30 min, the most effective sample was ZnO_EHC_2, with only 26 CFU/0.5 mL, followed by ZnO_ED_2 (28 CFU/0.5 mL) when tested with E.coli. Samples from home-raised hens and duck (ED, EHH) show high efficiency. In all samples treated with ZnO NPs, there are no detectable colonies (0 CFU/1 mL), indicating complete antibacterial activity against this microorganism in the short term.
No colonies (0 CFU/1 mL) were observed in ZnO-treated samples, demonstrating the complete elimination of bacteria within 24 h for both bacteria (E. coli and E. faecalis):
Compared to the control sample, which maintains a high number of bacteria (~100 CFU for E. coli and ~103 CFU for E. faecalis), all ZnO nanoparticles samples have exceptional antibacterial activity. After a contact time of 4 h (significant bacterial load) In all samples treated with ZnO, there are no detectable colonies (0 CFU/1 mL), indicating a significant inhibition of the bacteria even under conditions of bacterial stress.
Elaborated ZnO NPs act effectively both in the short term (<30 min) and in the long term (24 h) against Gram-negative (E. coli) and Gram-positive (E. faecalis) bacteria. Efficiency probably increases due to the release of zinc ions and the formation of reactive oxygen species (ROS), which cause bacterial cell damage. Samples ZnO_ED_2 and ZnO_EHC_2 demonstrate slightly superior performance, being ideal for antibacterial applications.
ZnO NPs proved to be an excellent antibacterial agent against the tested bacteria, regardless of the ovalbumin source used in the synthesis. The synthesized ZnO nanoparticles show considerable potential in antibacterial applications, such as wound treatment, food preservation or water purification, due to their effectiveness against most known Gram-negative and Gram-positive bacteria.
5. Conclusions
Egg white synthesis for ZnO nanoparticles is a biogenic method of synthesis. This method is relatively simple and can be performed at low temperatures, which makes it attractive for industrial applications. Egg white is used as a natural precursor for obtaining ZnO nanopowders. It offers several advantages over traditional methods such as the use of bio resources and low cost. The specific effects of egg white on ZnO processing will depend on various factors, including the concentration of egg white, the type of proteins present, and the concentration of Zn salt. The novelty of the work is the comparative analysis of zinc oxide powders obtained from albumin from eggs of home-raised hens, eggs of hens raised in commercial farms, and eggs of ducks.
The variation in ovalbumin source and precursor concentration directly influenced the ovalbumin decomposition and the final characteristics of ZnO. After 2 h of calcination at 550 °C, the ATR-FTIR plots show only a Zn-O vibration. The differences between the ATR-FTIR spectra before and after calcination highlight the complete removal of proteins and the formation of pure ZnO. The ATR-FTIR results are supported by XRD analysis, which confirms the formation of a single pure ZnO phase.
XRD spectra identified zincite pure phase, regardless of the origin of the ovalbumin. Thus, using eggs from different sources yields zinc oxide powders with different crystallite sizes (between 23.6 and 52.2 nm). By using ovalbumin as a natural precursor, this study promotes environmentally friendly methods for ZnO nanoparticle synthesis, reducing reliance on toxic chemical processes.
Using SEM, we demonstrated the morphology of zinc oxide particles. The shapes vary—hexagonal, parallelepiped, asymmetric, or rhomboid—depending on ovalbumin source, while the sizes are between 0.1 and 3.0 µm. EDS analysis shows the amount of each component element of the ZnO powder. The analysis revealed that the origin of the egg white and the concentration of Zn(II) influence the particle size and morphology.
Antibacterial tests demonstrated the significant inhibition of Escherichia coli and Enterococcus faecalis, confirming the potential for biomedical and environmental applications. These results highlight the viability of egg white as an ecological resource for the synthesis of ZnO nanoparticles, demonstrating the versatility of egg white as a biogenic precursor. In future research, this process could be extended to include other bioresources and the optimization of synthesis parameters, providing sustainable solutions for the development of nanotechnology.
Author Contributions
Conceptualization, A.-G.S. and M.O.; methodology, E.A.V.; software, M.O.; validation, A.-G.S.; formal analysis, S.G.M.; investigation, E.A.V., E.M.M., D.A.N. and D.I.; resources, E.A.V.; data curation, A.-G.S.; writing—original draft preparation, E.A.V.; writing—review and editing, A.-G.S. and M.O.; visualization, M.O.; supervision, A.-G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chaudhary, R.G. Biogenic Synthesis of Nanoscale Materials: Green and Simple Concepts Leading to Advanced Applications. In Proceedings of the International Conference on Advanced Sustainable Futuristic Materials-ASFM-2024, Nagpur, India, 26–27 April 2024. [Google Scholar]
- Kumari, M.; Sadhu, P.; Talele, C.; Shah, N. Green Nanotechnology: How Plants Can Help Synthesize Nanoparticles for Biomedical and Environmental Purposes. J. Nat. Remedies 2024, 24, 1021–1034. [Google Scholar] [CrossRef]
- Meydan, T.G.S.; Moosavi, S.S.; Sabouri, Z.; Darroudi, M. Green synthesis of CaCO3 nanoparticles for photocatalysis and cytotoxicity. Bioprocess Biosyst. Eng. 2023, 46, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Li, C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf. B Biointerfaces 2017, 158, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Alarif, W.M.; Shaban, Y.A.; Orif, M.I.; Ghandourah, M.A.; Turki, A.J.; Alorfi, H.S.; Tadros, H.R.Z. Green Synthesis of TiO2 Nanoparticles Using Natural Marine Extracts for Antifouling Activity. Mar. Drugs 2023, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Melese, A.; Wubet, W.; Husse, A.; Mulate, K.; Hailekiros, A. A review on biogenic synthesized zinc oxide nanoparticles: Synthesis, characterizations, and its applications. Rev. Iorg. Chem. 2024, 44, 303–321. [Google Scholar] [CrossRef]
- Porter, F. Zinc Handbook: Properties, Processing and Use in Design, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Harding, F.J. Breast Cancer: Cause-Prevention-Cure; Tekline Publishing: Barnstaple, UK, 2007. [Google Scholar]
- Lynch, R.J.M. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011, 61, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mrinal, G.; Mahajan, V.K.; Mehta, K.S.; Chauha, P.S. Zinc Therapy in Dermatology: A Review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar]
- Su-Min, Y.; Soo-Jin, C. Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. Int. J. Mol. Sci. 2022, 23, 6074. [Google Scholar] [CrossRef] [PubMed]
- Osmond, G. Zinc White: A review of zinc oxide pigment properties and implication for stability in oil-based paintings. AICCM Bull. 2012, 33, 1. [Google Scholar] [CrossRef]
- Constatinoiu, I.; Viespe, C.; Busuioc, C.; Jinga, S.I. ZnO thin films by pulsed laser depositions with applications in sensors. U.P.B. Sci. Bull. Ser. B 2024, 86, 119–134. [Google Scholar]
- Stoica, A.E.; Barca, A.C.; Pitigoi, M.L.; Grumezescu, A.M.; Vasile, B.S.; Iordache, F.; Ficai, A.; Andronescu, E. Nanostructured zinc oxide for wound dressings. U.P.B. Sci. Bull. Ser. B 2024, 86, 161–174. [Google Scholar]
- Zeghoud, S.; Hemmami, H.; Seghir, B.B.; Amor, I.B.; Kouadri, I.; Rebiai, A.; Messaoudi, M.; Ahmed, S.; Pohl, P.; Simal-Gandara, J. A review on biogenic green synthesis of ZnO nanoparticles by plant biomass and their applications. Mater. Today Commun. 2022, 33, 104747. [Google Scholar] [CrossRef]
- Mahdi, Z.; Nikzad, M.; Exoji, H.; Talebnia, F. Biosynthesis of zinc oxide nanoparticles using bacteria: A study on the characterization and application for electrochemical determination of bisphenol A. Inorg. Nano-Met. Chem. 2020, 51, 1249–1257. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Nanografi. Available online: https://nanografi.com/blog/10-uses-of-calcium-oxide-in-daily-life/?srsltid=AfmBOopArKtBXpcUuAivPWdyz4_ptqSZvQ9cp0vTFK2z9l1aawqF72tL (accessed on 8 November 2024).
- Guha, S.; Majumder, K.; Mine, Y. Egg Proteins. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Molaei, P.; Rahimi-Moghadam, F. Optimized synthesis of ZnO nanostructures by egg-white content ratio manipulation for photocatalytic applications. Mater. Res. Express 2019, 6, 1250h7. [Google Scholar] [CrossRef]
- Puddukudy, M.; Yaakob, Z. Facile Synthesis of Quasi Spherical ZnO Nanoparticles with/excellent Photocatalytic Activity. J. Clust. Sci. 2014, 26, 1187–1201. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Jeong, Y.S.; Anwar, M.S.; Dwivedi, S.; Alsharaeh, E.; Koo, B.H. Novel Biomimatic Synthesis of ZnO Nanorods Using Egg White (Albumen) and Their Antibacterial Studies. J. Nanosci. Nanotechnol. 2016, 16, 5959–5965. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.F.; Dong, R.; Shuang, W.; Wei, C.; Weiguang, X.; Chuntian, Z. Nutritional requirements of meat-type and egg-type ducks: What do we know? J. Anim. Sci. Biotechnol. 2018, 9, 1. [Google Scholar]
- Desert, C.; Guerin-Dubiard, C.; Nau, F.; Jan, G.; Val, F.; Mallard, J. Comparison of Different Electrophoretic Separations of Hen Egg White Proteins. J. Agric. Food 2001, 49, 4553–4561. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Beddow, J.; Joyce, E. Evaluation of antibacterial activity of ZnO nanoparticles coated sonochemically onto textile fabrics. World J. Microbiol. Biotechnol. 2012, 2, 106–120. [Google Scholar]
- Babayevska, N.; Przysiecka, N.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).