Abstract
Paracetamol-4,4′-bipyridine cocrystals were synthesized using a solution method, reflux method, grinding method, and ultrasonic method. The structures and properties were characterized through the utilization of single-crystal X-ray diffraction (SXRD), powder X-ray diffraction (PXRD), polarized light microscopy (PLM), thermogravimetric analysis (TGA), elemental analysis (EA), and infrared spectroscopy (IR). The results show that the four methods synthesized different cocrystal morphologies, but the same structure and properties coupled with a notably high purity level. All featured strong hydrogen bonds formed between the paracetamol,4,4′-bipyridine and water molecules. An additional notable feature is the presence of π...π stacking interactions between the pyridine rings of adjacent 4,4′-bipyridine molecules. The solubility of paracetamol (active pharmaceutical ingredient, API) and the cocrystal was measured and discussed. In the dissolution experiment, the cocrystal showed a much faster dissolution rate than the API in simulated gastric fluid media (pH = 1.2). Furthermore, the pharmacokinetic (PK) behavior of the cocrystal and the API was investigated to evaluate the effectiveness of this strategy for enhancing the oral absorption of paracetamol. The in vitro and in vivo studies revealed that the paracetamol-4,4′-bipyridine cocrystal possessed an excellent dissolution behavior and an improved pharmacokinetic profile.
1. Introduction
Paracetamol (acetyl-p-aminophenol, APAP) is a commonly used over-the-counter medication that is primarily used to relieve mild to moderate pain and reduce fever [1,2]. The mechanism of action entails inhibiting the activity of cyclooxygenase enzymes, selectively targeting the body’s hypothalamic thermoregulatory center, and suppressing the synthesis of prostaglandins, a process which, in turn, induces peripheral vasodilation and facilitates sweating to alleviate fever [3,4]. Furthermore, due to its ability to suppress the production and release of prostaglandins, it can alleviate mild to moderate pain by raising the pain tolerance. Nevertheless, the limited solubility of the compound, the vulnerability of the phenolic hydroxyl group to oxidation, which undermines its stability, and the particle size play a crucial role in affecting the solubility and in vitro dissolution rate of the medication. This often leads to diminished in vivo bioavailability of solid oral dosage forms [5,6]. Clinical studies have indicated that administering high doses or engaging in long-term use of APAP may result in liver damage [7,8,9]. In 2012, the FDA advised that adults should not consume more than 3 g of APAP daily, with no more than 650 mg every 6 h as necessary. Exceeding this recommended dosage could result in potentially fatal liver damage [10,11,12]. Some researchers have synthesized a series of N-methyl-N-alk oxymethamine-modified acetaminophen derivatives that can be slowly converted into acetaminophen, but their water solubility is lower than that of the API [13]. When glycine is directly applied to the phenolic hydroxyl group, it has been demonstrated to inhibit the depletion of hepatic glutathione and mitigate hepatotoxicity. The therapeutic efficacy of glycine is comparable to that of acetaminophen [14]. However, there is a scarcity of reported data concerning its water solubility. The development of acetaminophen-based medications with enhanced water solubility, rapid drug release, and high bioavailability while ensuring safety and stability holds significant potential for practical applications.
Pharmaceutical cocrystals represent an innovative solid-state form of medication that has seen progressive development [15]. A cocrystal that is designed with reason can significantly improve the physical, chemical, and biological properties of an API. This includes improvements in solubility, dissolution rate, permeability, bioavailability, stability, and mechanical processing characteristics [16,17,18,19,20]. Green and sustainable technologies offer significant potential within the sphere of pharmaceutical development [21]. Because they necessitate fewer synthetic steps and employ minimal solvent usage, solvent-free operation is often possible [22]. A variety of drug cocrystals have been introduced to the market as new chemical entities [23], such as sacubitril–valsartanl [24,25] and tramadol–celecoxib hydrochloride [26]. Compared with new drugs, the development cycle for cocrystals is significantly shortened, offering a novel approach to the creation of new medications and generics. This method is characterized by its low risk, low cost, and high reward, while maintaining the inherent activity of a drug [27]. Furthermore, it is entirely possible to significantly improve the physicochemical and biopharmaceutical properties of medications through the inclusion of appropriate therapeutic agents, thereby eliminating the need for chemical modifications. A cocrystal can enhance the compressibility of a solid API [28]. This approach simplifies the process of developing new drugs and paves a new path for treating complex diseases [29,30].
As illustrated in Scheme 1, APAP (active pharmaceutical ingredient, API) comprises several functional groups, such as -OH, -C=O, and -NH-. The -OH group can serve as either a hydrogen bond donor or acceptor, the -C=O group can act as a hydrogen bond acceptor by binding hydrogen atoms, and the -NH- group can function as either a hydrogen bond donor or acceptor, readily participating in hydrogen bonding to form supramolecular compounds. 4,4′-bipyridine (cocrystal formation, CCF) is a widely utilized pharmaceutical intermediate with the molecular formula C10H8N2; it is characterized by its steric hindrance and absence of branching or bridging effects and is a linear, rigid, double-base ligand. The two pyridine rings in its structure are planar and are linearly connected, enabling potential interplane π-π stacking interactions with the aromatic ring of the API.
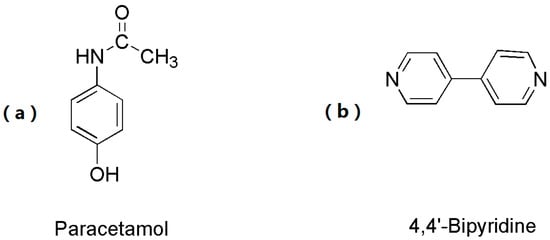
Scheme 1.
Molecular structures of the API and CCF. (a) API: Paracetamol, (b) CCF: 4,4′-bipyridine.
There are many methods used for cocrystal synthesis, solvent evaporation, flash-freezing, freeze-drying, anti-solvent addition, dry grinding, liquid-assisted grinding, thermal cocrystallization, rapid precipitation, spray drying, electrospray crystallization, and microspacing in-air sublimation [31,32,33,34]. The most common way to obtain multi-component crystals involving APIs is through solution crystallization, but, for a specific API, some cocrystals can sometimes be obtained only by changing the preparation method [35]. Given the compact size of 4,4′-bipyridine and the steric hindrance of paracetamol, it was chosen as the template for synthesizing paracetamol-4,4′-bipyridine through solution, reflux, grinding, and ultrasonic methods. This is the first time that paracetamol cocrystals have been synthesized in four ways.
2. Materials and Methods
2.1. Materials
Paracetamol was kindly supplied by the Huameihua Technology Group Wuhan Co., Ltd. (Wuhan, China). 4,4′-bipyridine, with a purity of 99%, was procured from Aladdin Reagent Co., Ltd(Shanghai, China). All other chemicals utilized in this study were of analytical grade and were employed directly without any further purification. Throughout the research, distilled water, which was prepared using demineralized water, was consistently used.
2.2. Equipment
2.2.1. Polarized Light Microscopy (PLM)
Photos of the cocrystals were taken with a CX40P polarized light microscope (Shun yu optical, Ningbo, China).
2.2.2. Single-Crystal X-Ray Diffraction (SXRD)
A single crystal was selected and mounted for comprehensive X-ray structural analysis on a state-of-the-art Bruker SMART CCD diffractometer utilizing Mo-Kα radiation with a wavelength of 0.71073 Å. The intricate structures were methodically deciphered through direct methods and subsequently refined with the utmost precision using full-matrix least-squares calculations based on F2 values facilitated by the SHELXL-97 software. Non-hydrogen atoms underwent anisotropic refinement to ensure accuracy, while hydrogen atoms were strategically positioned at their calculated locations and refined by utilizing a riding mode for efficiency. A concise summary of the cocrystals’ parameters is presented in Table 1.

Table 1.
Crystallographic data and structure refinement parameters for cocrystal.
2.2.3. Powder X-Ray Diffraction (PXRD)
Crystals were characterized utilizing a Scintag X1 diffractometer equipped with Cu-Kα radiation (λ = 1.5418 Å) while operating at 40 kV and 35 mA. Data acquisition encompassed an angular span of 4° to 40° for 2θ, and it was executed in the continuous scan mode. The process involved a step size of 0.05° for 2θ and a scanning velocity of 1.0°/min, ensuring precise and comprehensive data collection.
2.2.4. Thermal Gravity Analysis (TGA)
Thermogravimetric analysis (TGA) was performed in an N2 atmosphere at 1 atm with a heating rate of 10 °C/min in the temperature range of 30–400 °C on a Perkin–Elmer diamond TGA.
2.2.5. Elemental Analysis (EA)
Elemental analysis (C, H, and N) was carried out on a Perkin–Elmer 240 analyzer (PerkinElmer, Inc., Waltham, American).
2.2.6. Infrared Spectral Analysis (IR)
Infrared spectral analysis was conducted in the range of 3700–1000 cm−1 using KBr pellets and a SHIMADZU IRPrestige-21 Fourier-transform infrared spectrometer (Shimadzu Corporation, Kyoto, Japan).
2.2.7. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
An LC-MS/MS system consisting of an Agilent 1100 Series HPLC system (Agilent Technologies, Palo Alto, CA, USA) and a Q-Trap 2000 mass spectrometer (Applied Biosystems MDS Sciex, Toronto, ON, Canada) was utilized to conduct the analysis. Chromatographic separation was achieved efficiently through the use of an MP-C18 column (100 mm × 4.6 mm I.D., 5 μm particle size, Agilent Technologies) that was operated at 35 °C with a mobile phase consisting of acetonitrile and ammonium acetate (5 mM) in a ratio of 80:20 (v/v), flowing at a rate of 1 mL/min.
The analysis was performed with an electrospray ionization (ESI) source while operating in the positive ion (ESI+) mode. The instrument parameter settings were carefully adjusted as follows: the ion spray voltage (IS) was set to 5500 V, the declustering potential (DP) was set to 55 V, the collision energy (CE) was set to 35 eV, the collision cell exit potential (CXP) was set to 15 V, the channel electron multiplier (CEM) was set to 2000 V, the nitrogen curtain gas was set to 20 psi, gas 1 (GS1) and gas 2 (GS2) were set to 40 and 55 psi, respectively, the source temperature was maintained at 450 °C, and the dwell time was set to 200 ms.
Data acquisition was facilitated by the Analyst Software 1.3.2 (Applied Biosystems MDS Sciex, Toronto, ON, Canada).
2.2.8. Pharmacokinetic Study (PK)
A pharmacokinetic study of the active pharmaceutical ingredient (API) and its co-crystal was conducted on beagle dogs. Following an overnight fast, 12 male beagle dogs were randomly assigned to four groups before administration. To standardize the gastric pH variability, 35 mL of an HCl-KCl buffer solution (0.1 M/L) was orally administered to the beagle dogs fifteen minutes prior to dosing. The first group received gelatin capsules containing 4 mg of the API, without any excipients or additives. Groups 2 through 4 were orally administered gelatin capsules with cocrystals, each containing an equivalent amount of 4 mg of the API, also without excipients or additives. Blood samples were drawn from the jugular vein before oral administration and at 0.183 h, 0.167 h, 0.333 h, 0.5 h, 0.75 h, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h, 8 h, 10 h, and 12 h post-dosing. Plasma was isolated through centrifugation at 3500 rpm for 5 min and subsequently stored at −20 °C until analysis by LC-MS/MS. For the analysis, 100 μL of each plasma sample was transferred to a 10 mL glass tube, to which 100 μL of an internal standard solution was added. After vortexing the mixture, the sample was extracted with 3 mL of petroleum ether for 10 min. Following centrifugation at 3500 rpm for 5 min, the organic phase was collected and evaporated under nitrogen at 40 °C. The residue was then re-dissolved in 150 μL of mobile phase, and 20 μL of the sample was taken for LC-MS/MS analysis. Pharmacokinetic parameters, such as maximum plasma concentration (Cmax), time to reach Cmax (Tmax), area under the plasma concentration–time curve (AUC0-∞), and half-life (t1/2), were evaluated.
2.3. Synthesis
2.3.1. Ultrasonic Method
First, 15.1 mg (0.1 mmol) of paracetamol and 15.6 mg (0.1 mmol) of 4,4′-bipyridine was accurately weighed into a 25 mL glass vial. Subsequently, 8 mL of deionized water was precisely measured and added to the vial. The vial was covered with a layer of tin foil, and the cap was securely tightened. The vial was positioned in a water bath set at 80 °C and sonicated for a duration of 1 h. Following this, the vial was removed and placed in an oven at a constant temperature of 80 °C for a period of 6 days. After the 6-day period, the vial was removed from the oven and allowed to cool to room temperature. At this point, a small quantity of crystals began to precipitate. The vial was allowed to stand for an additional day, during which time a significant number of crystals formed. These crystals constituted the paracetamol-4,4′-bipyridine cocrystal, achieving a yield of 90%.
2.3.2. Solution Method
First, 15.1 mg (0.1 mmol) of paracetamol and 15.6 mg (0.1 mmol) of 4,4′-bipyridine was accurately measured and weighed into a 25 mL glass vial. Subsequently, 8 mL of deionized water was added to the vial. A magnetic stir bar was placed inside the vial, and it was ensured that it was securely in place. The mouth of the vial was covered with a layer of tin foil, and the container was tightly sealed with a lid to maintain an internal pressure of 101.325 KPa. The glass vial was positioned on a magnetic stirrer, and the temperature was set to 80 °C. The mixture was allowed to stir continuously for 3 h. Following this, the magnetic stir bar was promptly removed from the vial and placed in an oven at 80 °C. After 7 days, the stir bar was removed from the oven and allowed to cool to room temperature over the course of an additional 7 days. During this period, pale yellow, needle-like crystals gradually precipitated out, forming the desired paracetamol-4,4′-bipyridine cocrystal. The yield of this cocrystal was approximately 90%.
2.3.3. Condensation Reflux Method
First, 15.1 mg (0.1 mmol) of paracetamol and 15.6 mg (0.1 mmol) of 4,4′-bipyridine was accurately weighed into a 25 mL round-bottom flask. A mixed solvent of dehydrated ethanol and water (10 mL (vol 1:1)) was added, a reflux condenser was set up, the cooling water was started, and reflux occurred at 90–100 °C for 3 h. Then, it was filtered and allowed to stand at room temperature for 1 day to volatilize; transparent needle-like crystals precipitated, and these were the paracetamol-4,4′-bipyridine cocrystal that was described, with a yield of about 90%.
2.3.4. Grinding Method
First, 15.1 mg (0.1 mmol) of paracetamol and 15.6 mg (0.1 mmol) of 4,4′-bipyridine was accurately measured and weighed into an agate mortar. Then, the mixture was ground for precisely 10 min, ensuring that it achieved a fine and uniform consistency. Subsequently, the ground mixture was sieved to obtain a micro-powder with a 200 mesh fineness. Then, 50 μL of a mixed solvent (vol 1:1) of dehydrated ethanol and water was accurately dispensed onto the sieved powder, followed by thorough mixing and grinding for an additional minute. This process yielded an amorphous powder with a 100% productivity rate.
3. Results and Discussion
3.1. PLM Photograph
To examine the morphology of the cocrystals, a polarizing microscope was employed to capture images of the cocrystals produced through the grinding, ultrasonic, solution, and reflux methods, as shown in Figure 1. The cocrystals resulting from grinding were fragmented, with a pronounced crystal fragmentation; those produced through sonication manifested as clusters; the cocrystals synthesized via the solution and reflux methods adopted a rod-like shape, featuring fine, high-purity crystals with a favorable morphology, excellent transparency, and a smooth crystal surface. Among the crystals synthesized using these four methods, solely those from the solution and reflux methods fulfilled the test criteria for single-crystal diffraction.
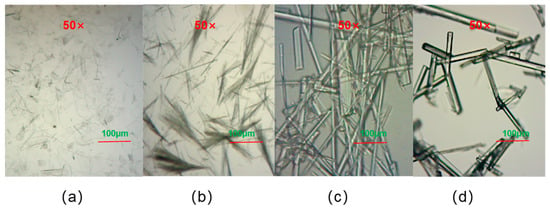
Figure 1.
Polarized micrographs of the cocrystals: (a) ultrasonic method; (b) grinding method; (c) solution method; (d) condensation reflux method.
3.2. Crystal Structures
The cocrystal (paracetamol-4,4′-bipyridine-H2O) was categorized within the triclinic crystal system, specifically in the space group P-1. The asymmetric unit contained two paracetamol molecules, two 4,4′-bipyridine molecules, and five water molecules; the paracetamol and the 4,4′-bipyridine were connected via water molecules, and this system was the ternary cocrystal paracetamol-4,4′-bipyridine-H2O (Figure 2a). In the crystal structure of the paracetamol-4,4′-bipyridine-H2O cocrystal, the paracetamol molecule was connected to a water molecule through O-H···O and N-H···O interactions, the 4,4′-bipyridine molecule was connected to a water molecule through O-H···N interactions, and two adjacent water molecules were connected by O-H···O interactions; these were connected via hydrogen bonds to produce a supramolecular structural unit (Figure 2b).
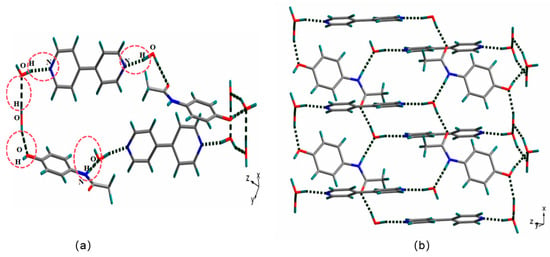
Figure 2.
Supramolecular structural unit of paracetamol-4,4′-bipyridine cocrystals formed via hydrogen bonds (a); structure of the cocrystal supramolecular space network formed via hydrogen bonding (b).
The supramolecular steric structure of the cocrystals via π···π conjugation (Figure 3a) and C-H···π interactions were investigated (Figure 3b). The pyridine rings of two neighboring 4,4′-bipyridine molecules were linked through π···π conjugation, while the benzene rings of two adjacent paracetamol molecules were connected via C-H···π interactions. In the supramolecular space network structure, the C-H on the 4,4′-bipyridine heterocycle extended through C-H···π interactions with the benzene ring in the adjacent paracetamol molecule, which included dislocated parallel stacking and edge-to-face T-shaped stacking.
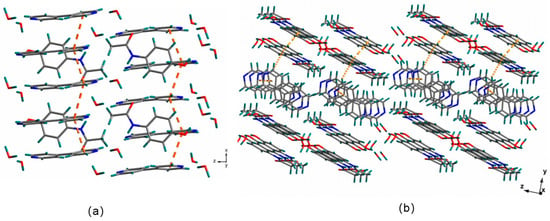
Figure 3.
Supramolecular steric structure of the cocrystals via π···π conjugation (a) and C-H···π interactions (b).
3.3. PXRD Analysis
Figure 4 shows the XRD comparison of the 4,4′-bipyridine ligand (a), the raw material (paracetamol) (b), and the cocrystals synthesized using the four different methods ((c), (d), (e), and (f)). As it can be seen in the figure, the cocrystals synthesized using the four methods had similar crystal diffraction peaks, with the main diffraction peaks appearing at 4.62°, 9.37°, 18.42°, and 21.33°, respectively, indicating that the cocrystals synthesized with the four methods were the same crystal. The characteristic diffraction peaks of the cocrystals were completely different from those of the raw material (paracetamol) and the ligand (4,4′-bipyridine), indicating that the cocrystal was a new phase.
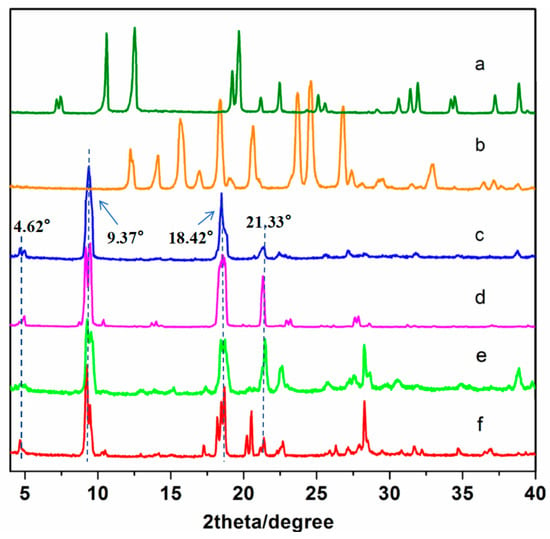
Figure 4.
The PXRD patterns of the API, CCF, and cocrystals: (a) API, (b) CCF, (c) ultrasonic method, (d) solution method, (e) condensation reflux method, and (f) grinding method.
As shown in Figure 5, the PXRD patterns of the cocrystals were compared with those simulated from the single-crystal structures. The experimental PXRD patterns of each crystal exhibited good agreement with the simulated one, indicating that the cocrystals were successfully obtained as a pure crystalline phase.
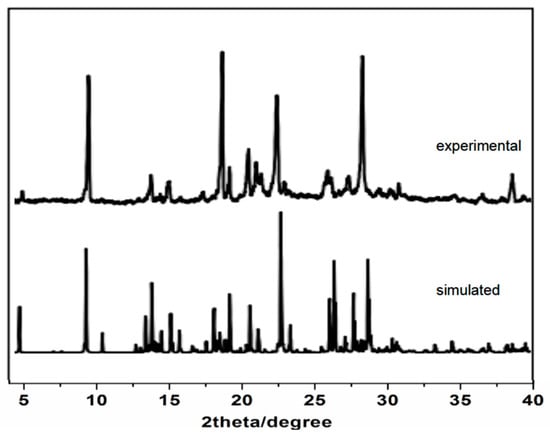
Figure 5.
The experimental and simulated PXRD patterns.
3.4. Thermal Analysis
The TGA curves for the API (paracetamol), CCF (4,4-bipyridine), and cocrystal are shown in Figure 6. The thermal decomposition processes of the three compounds exhibited distinct differences. The cocrystals underwent complete decomposition at 293 °C, whereas 4,4′-bipyridine reached this point at 216 °C, and paracetamol decomposed fully at 287 °C. Consequently, this underscores the notion that the cocrystals represent a novel entity possessing unique thermal characteristics that are dissimilar to those of both the API drug substance and the CCF. Furthermore, the cocrystals exhibited a significantly higher thermal stability than that of both paracetamol and 4,4′-bipyridine, indicating that cocrystals can improve the thermal stability of paracetamol. The cocrystals were resolved by 12.78% at 163 °C due to the loss of water by the cocrystal molecules (paracetamol-4,4′-bipyridine-H2O). The water molecules in the cocrystals were hydrogen-bonded to paracetamol and 4,4′-bipyridine, and their full release temperature was 163 °C.
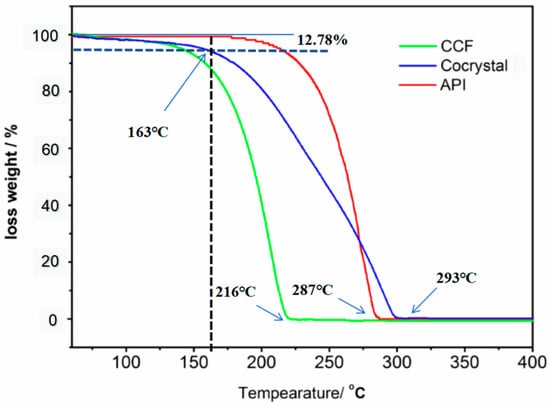
Figure 6.
The TGA curves of the original API, CCF, and cocrystals.
The TGA curves for the cocrystals synthesized with the four methods are presented in Figure 7. As is evident in the figure, the thermal decomposition temperature for the coproducts prepared with these four methods remained consistent at 293 °C, and the weight loss curves exhibited a fundamentally similar trend. This reinforces the conclusion that the cocrystal structures synthesized through the four methods are identical.
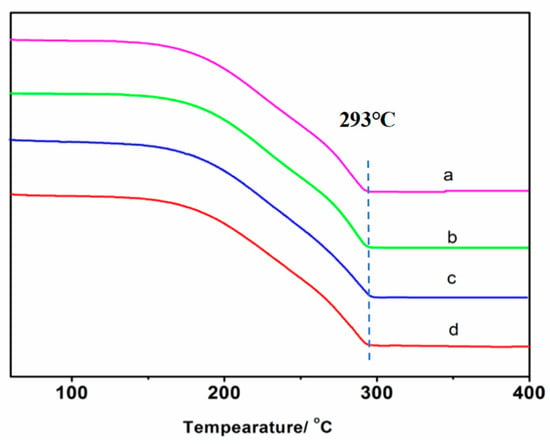
Figure 7.
The TGA curves of cocrystals synthesized with different methods: (a) ultrasonic method, (b) solution method, (c) condensation reflux method, and (d) grinding method.
The differential thermal analysis (DTA) curves of paracetamol, 4,4-bipyridine, and the cocrystals are depicted in Figure 8. The DTA curve of 4,4-bipyridine exhibited two distinct endothermic peaks at 116 °C and 210 °C, respectively. Consequently, the melting point of 4,4-bipyridine was identified as 116 °C, as is evident in Figure 8a. Similarly, the differential heat curve of paracetamol displayed heat absorption peaks at 172 °C and 273 °C, thereby indicating that the melting point of paracetamol was 172 °C, as shown in Figure 8b. The DTA curves corresponding to the four different synthesis methods for the cocrystals displayed a high degree of consistency, featuring two endothermic peaks at 138 °C and 290 °C, respectively. This consistency verifies that the cocrystals synthesized via these four methods are identical, as depicted in Figure 8c–f. Notably, the melting point of the cocrystals was 138 °C, which is between those of the active pharmaceutical ingredient (API) and cocrystal former (CCF), signifying an enhancement in the thermal stability of paracetamol through the formation of these cocrystals.
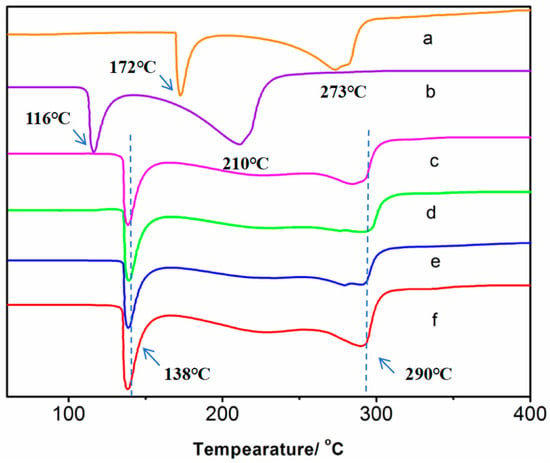
Figure 8.
The DTA curves of the original API, CCF, and cocrystals: (a) CCF, (b) API, (c) ultrasonic method, (d) solution method, (e) condensation reflux method, and (f) grinding method.
3.5. IR
To further confirm the cocrystal structure (Figure 9), IR spectral data for the cocrystals, original API, and CCF in the range of 3500–1600 cm−1 were examined in detail for relevant chemical information on these solid forms. Figure 7 displays a comparison of the infrared spectra of the cocrystals, paracetamol, and 4-bipyridine. As is evident in the illustration, the infrared absorption curve of the cocrystal distinctly diverged from that of the API and CCF. Specifically, the sharp and pronounced spectral peak at 3310 cm−1 signifies the expansion and vibrational absorption peak of the N-H bond within the acylamino; the peak moved in the high-frequency direction (3489 cm−1) in the cocrystal due to the influence of hydrogen bonding. Furthermore, the peak at 1680 cm−1 corresponds to the vibrational absorption of carbonyl. Meanwhile, the peaks observed at 1600 cm−1 and 1500 cm−1 are indicative of the backbone structure of the aromatic ring, thereby confirming its presence within the crystal.
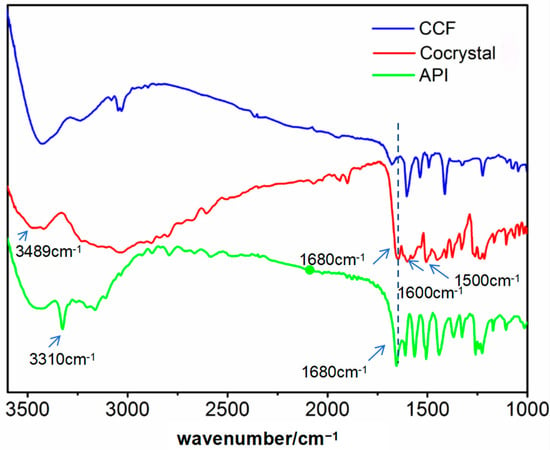
Figure 9.
The IR curves of the API, CCF, and cocrystals.
3.6. EA
The obtained cocrystal (paracetamol-4,4′-bipyridine-H2O) was tested through EA. According to the molecular formula of the cocrystal (C36H44N6O9, Mr 704.77), the theoretical content of C, H, and N was calculated and compared with the analytical values. The results of the elemental analysis (%) were as follows: calculated for C36H44N6O9 (Mr 704.77): C, 61.2; H, 6.21; N, 11.5; found: C, 61.3; H, 6.24; N, 11.9. The theoretical value was basically consistent with the measured values. The composition agreed with the solved crystal structure, which contains water molecules.
3.7. Dissolution Analysis
The primary objective of this research is to synthesize cocrystals based on paracetamol, with the ultimate goal of enhancing their solubility. Consequently, it is imperative to meticulously investigate the solubility characteristics of both the cocrystals and pure paracetamol. Figure 10 depicts the release profiles of the cocrystals and paracetamol within simulated gastric juice. Conventionally, we assess the solubility of the drug by measuring its dissolution in simulated human gastric fluid. Hence, the current section describes in vitro dissolution tests for the synthetic cocrystal and active pharmaceutical ingredient (API).
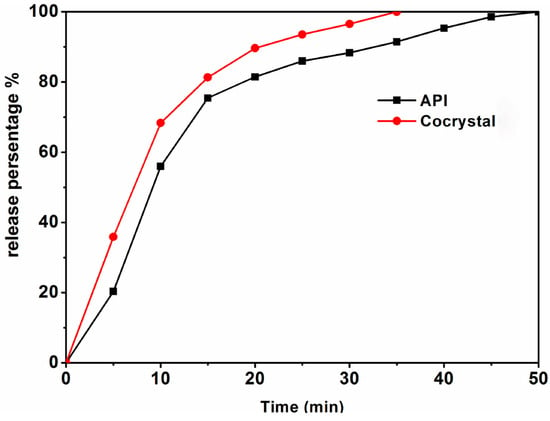
Figure 10.
Dissolution profiles in simulated gastric fluid media for the API and cocrystals.
Dissolution assays were performed by employing the basket method, as stipulated by the United States Pharmacopeia, for both the active pharmaceutical ingredient (API) and the cocrystals. A hydrochloric acid solution with a pH of 1.2 was prepared to simulate gastric fluid, and the test temperature was maintained at 37 ± 0.5 °C. The basket rotation speed was set to 100 rpm, and samples were drawn at 5 min intervals up to 50 min, with 5 mL of sample solution being collected each time. The samples were filtered through a 0.45 μm filter membrane and analyzed using a high-performance liquid chromatography–tandem mass spectrometry system (HPLC-MS/MS). Following each sample collection, an equal volume of the original solution at the same temperature was promptly added to the dissolution medium to minimize experimental errors. A comparison of the dissolution curves is depicted in Figure 8.
The dissolution rate of the cocrystals was markedly superior to that of the active pharmaceutical ingredient (API). Within 10 min, over 68% of the cocrystals had dissolved, and by 20 min, a nearly 90% dissolution was achieved. Complete dissolution of the cocrystals occurred at the 35 min mark, whereas the API’s dissolution rate reached only 90% at that time. By 55 min, the API was nearly fully dissolved. These findings indicate that the cocrystals release rate is significantly faster than that of the API, enhancing the solubility of paracetamol.
3.8. Pharmacokinetics (PK)
In pharmacokinetic studies, the main kinetic parameters for the API and cocrystals are as follows: the API’s Cmax is 6.55 ± 0.5 ng/mL, t1/2 is 4.5 ± 0.09 h, Tmax is 0.7 ± 0.00 h, and AUC0−∞ is 28.12 ± 0.10 ng h/mL; for the cocrystal, Cmax is 7.58 ± 0.5 ng/mL, t1/2 is 4.3 ± 0.30 h, Tmax is 0.83 ± 0.05 h, and AUC0−∞ is 30.33 ± 0.12 ng h/mL. As shown in Figure 11, the results indicate that the cocrystal’s AUC0−∞ and Cmax values were 1.07 times and 1.16 times higher than those of the API, respectively. This observation suggests that cocrystals with an enhanced solubility can potentially favorably affect the gastrointestinal (GI) absorption and onset of action of paracetamol.
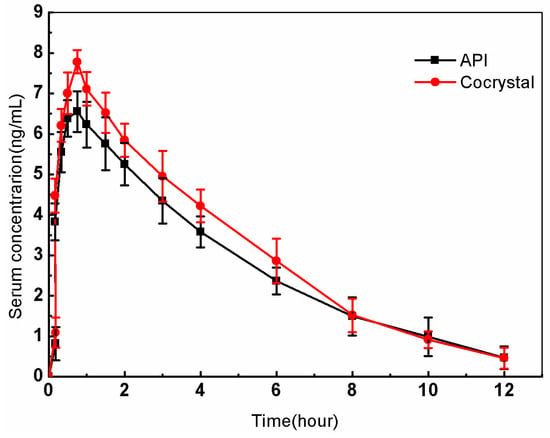
Figure 11.
Mean plasma concentration–time profiles of the API and cocrystals in beagle dogs after oral administration.
4. Conclusions
Cocrystals were synthesized using four methods. Their morphology and properties were characterized using polarized light microscopy (PLM), single-crystal X-ray diffraction (SXRD), powder X-ray diffraction (PXRD) analysis, elemental analysis (EA), thermogravimetric analysis (TGA), differential thermal analysis (DTA), elemental analysis (EA), and infrared spectral analysis (IR). The results indicate that the cocrystal structures acquired exhibited identical properties, despite variations in their appearance when synthesized with the four different methods. The formation of cocrystals was mainly caused by hydrogen bonding interactions and π-π* stacking interactions. Cocrystal forms were successfully obtained as a pure crystalline phase. Additionally, the dissolution and pharmacokinetics (PK) of the API and cocrystal are discussed. As a result, the dissolution profiles of the cocrystals were significantly improved compared with those of the original API in simulated gastric fluid. The cocrystals exhibited improved dissolution rates compared with those of the original paracetamol, making them very advantageous for the use of paracetamol without side effects on the gastrointestinal tract. The impact of pharmaceuticals on the body can differ substantially, influenced by the chemical structure of the drug. Even slight alterations to this structure can result in varied biological effects. There exists considerable latitude for the modification of molecular structures to enhance pharmacokinetic characteristics. Due to the special structure of the cocrystal (paracetamol-4,4′-bipyridine-H2O) which differs from that of API, the solubility and bioavailability of paracetamol molecules were improved. Cocrystals with an enhanced solubility would potentially be favorable for their effects on gastrointestinal (GI) absorption and the onset of action of paracetamol.
Author Contributions
Conceptualization, methodology and writing—review and editing, X.Z.; software, Y.H.; validation, J.L. (Jinliang Li); formal analysis and investigation, J.L. (Jialing Lian); data curation and writing—original draft preparation, Y.C.; supervision, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Preparation of Soft Tissue Trauma Repair and Adhesive Materials and Its Biological Evaluation, a key or major project of the Guangdong Provincial Department of Education (Grant Number: 2021ZDJS139), and the Guangdong Provincial Engineering Technology Research Center of Green Polyurethane Adhesives at Zhuhai College of Science and Technology (Grant Number: 2021GCZX010).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Acknowledgments
All individuals included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, X.; Li, M.; Deng, S.; Yu, T.; Ma, Y.; Yang, H.; Ma, L. A network pharmacology-integrated metabolomics strategy for clarifying the action mechanisms of Schisandrae Chinensis Fructus for treating drug-induced liver injury by acetaminophen. Bioorg. Med. Chem. 2021, 31, 115992. [Google Scholar] [CrossRef] [PubMed]
- Prescott, L.F. Paracetamol: Past, present, and future. Am. J. Ther. 2000, 7, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Freo, U.; Ruocco, C.; Valerio, A.; Scagnol, I.; Nisoli, E. Paracetamol: A review of guideline recommendations. J. Clin. Med. 2021, 10, 3420. [Google Scholar] [CrossRef]
- Cao, Z.; Han, K.; Lu, H.; Illangamudalige, S.; Shaheed, C.A.; Chen, L.; Mathieson, S. Paracetamol Combination Therapy for Back Pain and Osteoarthritis: A Systematic Review and Meta-Analyses. Drugs 2024, 84, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; Prinderre, P.; Eouani, C.; Joachim, J.; Reynier, J.P.; Piccerelle, P.H. The preparation of orally disintegrating tablets using a hydrophilic waxy binder. Int. J. Pharm. 2004, 278, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Seager, H. Drug-delivery products and the Zydis fast-dissolving dosage form. J. Pharm. Pharmacol. 1998, 50, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Prescott, L.F. Paracetamol (acetaminophen) poisoning: The early years. Br. J. Clin. Pharmacol. 2024, 90, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Xu, Z.; Qiao, Z.; Wang, X.; Yang, C. Flaxseed lignan alleviates the paracetamol-induced hepatotoxicity associated with regulation of gut microbiota and serum metabolome. Nutrients 2024, 16, 295. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singh, T.; Singh, V.; Singh, B.; Kaur, S.; Ahmad, S.F.; Singh, B. Ehretia laevis mitigates paracetamol-induced hepatotoxicity by attenuating oxidative stress and inflammation in rats. Int. Immunopharmacol. 2024, 143, 113565. [Google Scholar] [CrossRef]
- Cousin, G.; Bruna, E.; Gendrot, E. Rapidly Disintegratable Multiparticular Tablet. U.S. Patent 5,464,632, 7 November 1995. [Google Scholar]
- Mallet, C.; Desmeules, J.; Pegahi, R.; Eschalier, A. An updated review on the metabolite (AM404)-mediated central mechanism of action of paracetamol (acetaminophen): Experimental evidence and potential clinical impact. J. Pain Res. 2023, 16, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Chidiac, A.S.; Buckley, N.A.; Noghrehchi, F.; Cairns, R. Paracetamol (acetaminophen) overdose and hepatotoxicity: Mechanism, treatment, prevention measures, and estimates of burden of disease. Expert Opin. Drug Metab. Toxicol. 2023, 19, 297–317. [Google Scholar] [CrossRef]
- Majumdar, S.; Sloan, K.B. Synthesis and topical delivery of N-alkyl-N-alkyloxycarbonylaminomethyl prodrugs of a model phenolic drug: Acetaminophen. Int. J. Pharm. 2007, 337, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Devarajan-Ketha, H.; Sloan, K.B. N,N′-Dialkylaminoalkylcarbonyl (DAAC) prodrugs and aminoalkylcarbonyl (AAC) prodrugs of 4-hydroxyacetanilide and naltrexone with improved skin permeation properties. Bioorg. Med. Chem. Lett. 2011, 21, 4078–4082. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.; Kolekar, K.; Khot, C.; Dabhole, S.; Salawi, A.; Sabei, F.Y.; Patravale, V. Co-crystal nanoarchitectonics as an emerging strategy in attenuating cancer: Fundamentals and applications. J. Control. Release 2023, 353, 1150–1170. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tang, Y.; Wu, Q.; Li, W.; Zhou, L.; Wang, M.; Zou, F. A drug–drug cocrystal strategy to regulate stability and solubility: A case study of temozolomide/caffeic acid. J. Mol. Struct. 2024, 1312, 138577. [Google Scholar] [CrossRef]
- Alvani, A.; Shayanfar, A. Cocrystallization for Improving Anticancer Activity of Drugs. Cryst. Res. Technol. 2024, 59, 2300253. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Patel, A.; Satapathy, B.S.; Mallick, S. Formulation development and characterization of lamotrigine-salicylic acid crystalline product: A strategy to improve oral release of drug for better management of epilepsy. Indian J. Chem. Technol. 2021, 28, 356–362. [Google Scholar]
- Wang, X.; Wang, L.; Yao, C.; Xie, G.; Song, S.; Li, H.; Tao, X. Novel formulations of the antiviral drug favipiravir: Improving permeability and tabletability. Cryst. Growth Des. 2021, 21, 3807–3817. [Google Scholar] [CrossRef]
- Liang, X.; Liu, S.; Li, Z.; Deng, Y.; Jiang, Y.; Yang, H. Efficient cocrystal coformer screening based on a Machine learning Strategy: A case study for the preparation of imatinib cocrystal with enhanced physicochemical properties. Eur. J. Pharm. Biopharm. 2024, 196, 114201. [Google Scholar] [CrossRef] [PubMed]
- Mezaal, E.N.; Sadiq, K.A.; Jabbar, M.M.; Al-Noor, T.H.; Azooz, E.A.; Al-Mulla, E.A.J. Green methods for determination of paracetamol in drug samples: A comparative study. Green Anal. Chem. 2024, 10, 100123. [Google Scholar] [CrossRef]
- Chettri, A.; Subba, A.; Singh, G.P.; Bag, P.P. Pharmaceutical co-crystals: A green way to enhance drug stability and solubility for improved therapeutic efficacy. J. Pharm. Pharmacol. 2024, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, X.R. Synthesis of two novel azilsartan cocrystals: Preparation, physicochemical characterization and solubility studies. Crystals 2020, 10, 739. [Google Scholar] [CrossRef]
- Fala, L. Entresto: First-in-class angiotensin receptor neprilysin inhibitor FDA approved for patients with heart failure. Am. Health Drug Benefits 2015, 8, 330. [Google Scholar] [PubMed]
- Berg, D.D.; Braunwald, E.; DeVore, A.D.; Lala, A.; Pinney, S.P.; Duffy, C.I.; Morrow, D.A. Efficacy and safety of sacubitril/valsartan by dose level achieved in the PIONEER-HF trial. Heart Fail. 2020, 8, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Gascom, N.; Almansa, C.; Merlos, M.; Miguel Vela, J.; Encina, G.; Morte, A.; Smith, K.; Plata-Salaman, C. Co-crystals of tramadol-celecoxib: Preclinical and clinical evaluation of a novel analgesic. Expert Opin. Investig. Drugs 2019, 28, 399–409. [Google Scholar] [CrossRef]
- Yousef, M.A.; Vangala, V.R. Pharmaceutical cocrystals: Molecules, crystals, formulations, medicines. Cryst. Growth Des. 2019, 19, 7420–7438. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Fabián, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving Mechanical Properties of Crystalline Solids by Cocrystal Formation: New Compressible Forms of Paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Wang, X.; Du, S.; Zhang, R.; Jia, X.; Yang, T.; Zhang, X. Drug-drug cocrystals: Opportunities and challenges. Asian J. Pharm. Sci. 2021, 16, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liu, J.; Ran, L. A review of pharmaceutical nano-cocrystals: A novel strategy to improve the chemical and physical properties for poorly soluble drugs. Crystals 2021, 11, 463. [Google Scholar] [CrossRef]
- Mirocki, A.; Conterosito, E.; Palin, L.; Sikorski, A.; Milanesio, M.; Lopresti, M. Crystal structure of a new 1: 1 acridine-diclofenac salt, obtained with high yield by a mechanochemical approach. Crystals 2022, 12, 1573. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Ye, X.; Yao, C.; Song, S.; Qu, Y.; Tao, X. Efficient Screening of Pharmaceutical Cocrystals by Microspacing In-Air Sublimation. J. Am. Chem. Soc. 2024, 146, 11592–11598. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.J.; Sugden, P.; Wilson, C.C.; Castro-Dominguez, B. Elusive Seed Formation via Electrical Confinement: Control of a Novel Cocrystal in Cooling Crystallization. Cryst. Growth Des. 2021, 21, 3310–3315. [Google Scholar] [CrossRef]
- Ismail, K.M.; Hassan, S.S.; Medany, S.S.; Hefnawy, M.A. A facile sonochemical synthesis of the Zn-based metal–organic framework for electrochemical sensing of paracetamol. Mater. Adv. 2024, 5, 5870–5884. [Google Scholar] [CrossRef]
- Acebedo-Martínez, F.J.; Alarcón-Payer, C.; Barrales-Ruiz, H.M.; Niclós-Gutiérrez, J.; Domínguez-Martín, A.; Choquesillo-Lazarte, D. Towards the Development of Novel Diclofenac Multicomponent Pharmaceutical Solids. Crystals 2022, 12, 1038. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).