The First Crystal Structure of an Anti-Geometric Homoleptic Zinc Complex from an Unsymmetric Curcuminoid Ligand
Abstract
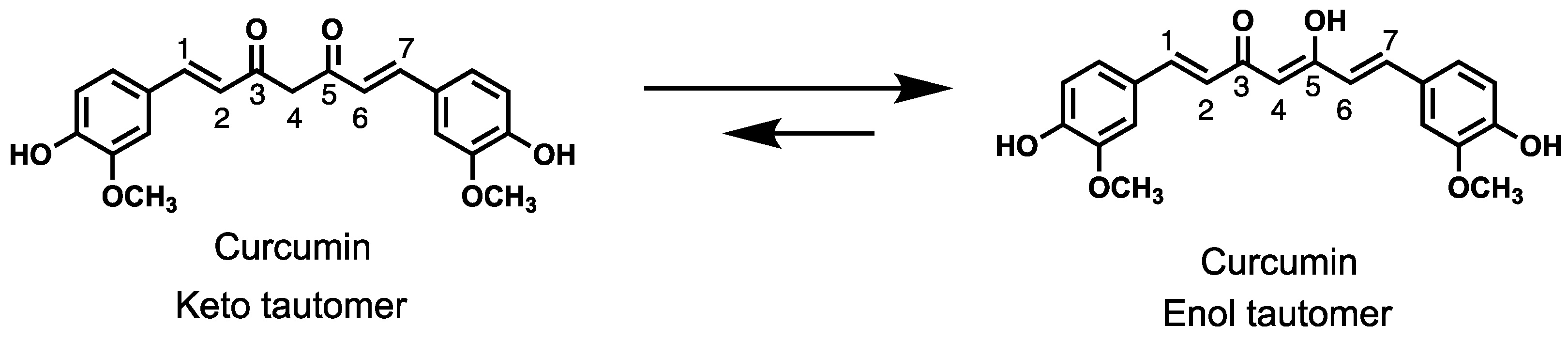
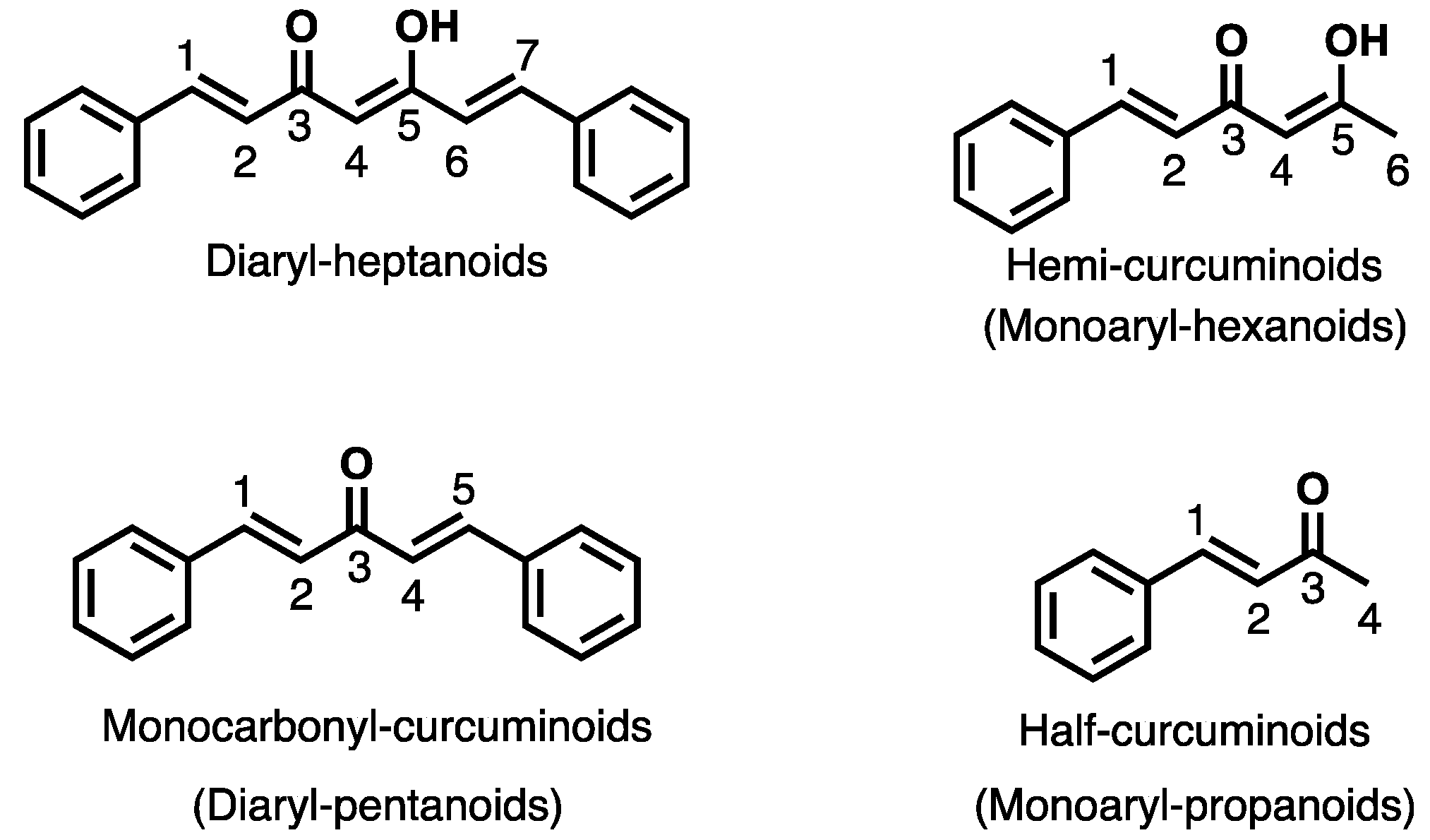
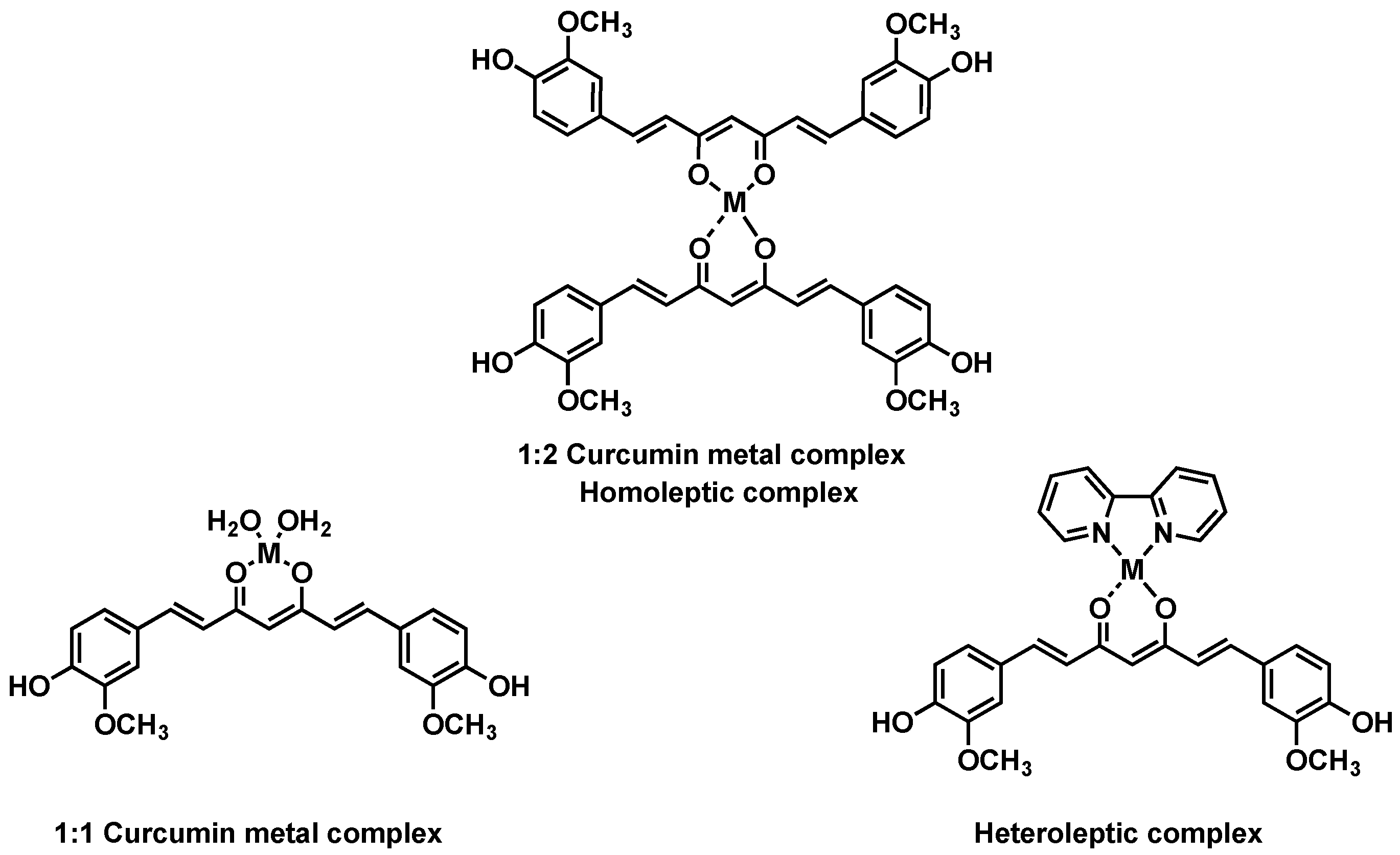
1. Introduction
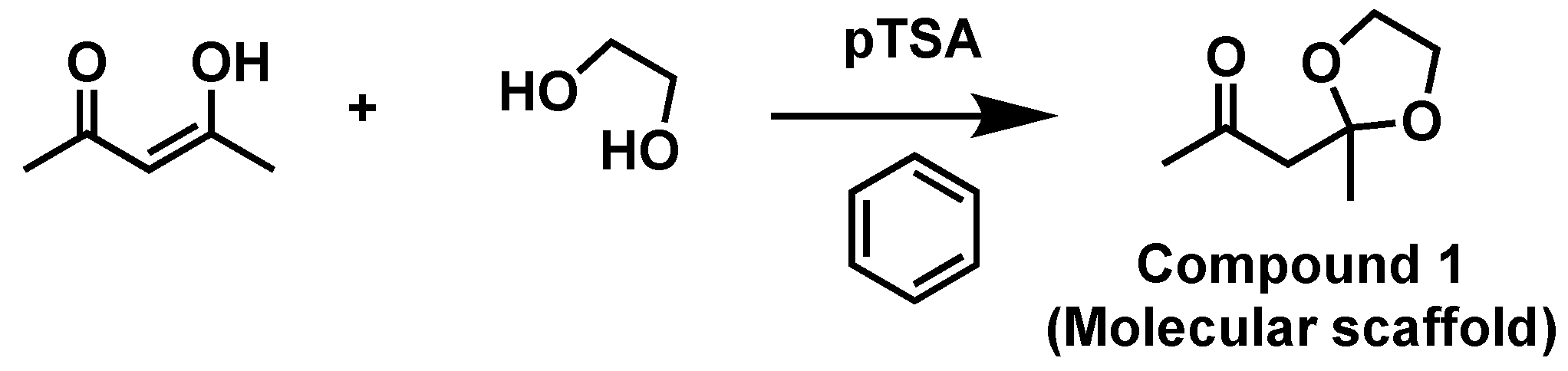
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Du, Z.; Xue, G.; Chen, Q.; Lu, Y.; Zheng, X.; Conney, A.H.; Zhang, K. Synthesis and Biological Evaluation of Unsymmetrical Curcumin Analogues as Tyrosinase Inhibitors. Molecules 2013, 18, 3948–3961. [Google Scholar] [CrossRef]
- Saladini, M.; Lazzari, S.; Pignedoli, F.; Rosa, R.; Spagnolo, F.; Ferrari, E. New Synthetic Glucosyl-Curcuminoids, and Their 1H and 13C NMR Characterization, from Curcuma longa L. Plant Foods Hum. Nutr. 2009, 64, 224–229. [Google Scholar] [CrossRef]
- Torres-Rodríguez, E.; Arias-Cedeño, Q.; Almeida-Saavedra, M.; Michalik-Michalik, M.; Vogel-Vogel, C. Study of the Keto-Enolic Equilibrium in Structures of Synthetic Curcuminoids by Means of RMN and X Rays Diffraction. Rev. Cuba. Química 2013, XXV, 206. [Google Scholar]
- Haritakun, W.; Changtam, C. Cytotoxic Activity of Curcuminoids and Curcuminoid Analogues Against Human Oral Cancer KB Cells. SDU Res. J. 2016, 9, 141–158. [Google Scholar]
- Deepthi, T.V.; Venugopalan, P. Synthesis, DNA-Binding, and Cytotoxic Studies on Three Copper(II) Complexes of Unsymmetrical Synthetic Analogues of Curcumin. J. Coord. Chem. 2016, 69, 3403–3416. [Google Scholar] [CrossRef]
- Singh, R.; Tønnesen, H.H.; Vogensen, S.B.; Loftsson, T.; Másson, M. Studies of Curcumin and Curcuminoids. XXXVI. The Stoichiometry and Complexation Constants of Cyclodextrin Complexes as Determined by the Phase-Solubility Method and UV-Vis Titration. J. Incl. Phenom. Macrocycl. Chem. 2010, 66, 335–348. [Google Scholar] [CrossRef]
- Cornago, P.; Cabildo, P.; Sanz, D.; Claramunt, R.M.; Torralba, M.C.; Torres, M.R.; Elguero, J. Structures of Hemi-Curcuminoids in the Solid State and in Solution. Eur. J. Org. Chem. 2013, 2013, 6043–6054. [Google Scholar] [CrossRef]
- Shetty, D.; Kim, Y.J.; Shim, H.; Snyder, J.P. Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs). Molecules 2015, 20, 249–292. [Google Scholar] [CrossRef]
- Dong, L.; Zheng, S.; Zhang, Y.; Jiang, X.; Wu, J.; Zhang, X.; Shan, X.; Liang, D.; Ying, S.; Feng, J.; et al. Design, Synthesis, and Evaluation of Semi-Conservative Mono-Carbonyl Analogs of Curcumin as Anti-Inflammatory Agents against Lipopolysaccharide-Induced Acute Lung Injury. Medchemcomm 2015, 6, 1544–1553. [Google Scholar] [CrossRef]
- Obregón-Mendoza, M.A.; Arias-Olguín, I.I.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Chávez, M.I.; Toscano, R.A.; Cassani, J.; Enríquez, R.G. Expected and Unexpected Products in Half Curcuminoid Synthesis: Crystal Structures of but-3-En-2-Ones and 3-Methylcyclohex-2-Enones. Crystals 2021, 11, 404. [Google Scholar] [CrossRef]
- Masuda, T.; Matsumura, H.; Oyama, Y.; Takeda, Y.; Jitoe, A.; Kida, A.; Hidaka, K. Synthesis of (+/−)-Cassumunins A and B, New Curcuminoid Antioxidants Having Protective Activity of the Living Cell against Oxidative Damage. J. Nat. Prod. 1998, 61, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal Complexes of Curcumin—Synthetic Strategies, Structures and Medicinal Applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Dubourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal–Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Nagpal, M.; Vashistha, V.K.; Arora, R.; Issar, U. Recent Advances in the Antioxidant Activity of Metal-Curcumin Complexes: A Combined Computational and Experimental Review. Free Radic. Res. 2024, 58, 11–26. [Google Scholar] [CrossRef]
- Figueroa-Depaz, Y.; Pérez-Villanueva, J.; Soria-Arteche, O.; Martínez-Otero, D.; Gómez-Vidales, V.; Ortiz-Frade, L.; Ruiz-Azuara, L. Casiopeinas of Third Generations: Synthesis, Characterization, Cytotoxic Activity and Structure–Activity Relationships of Mixed Chelate Compounds with Bioactive Secondary Ligands. Molecules 2022, 27, 3504. [Google Scholar] [CrossRef] [PubMed]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Grapin, M.; Fierascu, R.C.; Raut, I.; Constantin, M. Functionalized Palygorskite as a Delivery Platforms for Bioactive Asymmetric Beta-Diketone Dyes. Crystals 2024, 14, 659. [Google Scholar] [CrossRef]
- Zimnitskiy, N.S.; Korotaev, V.Y.; Barkov, A.Y.; Kochnev, I.A.; Sosnovskikh, V.Y. Hemicurcuminoids (1-Styryl-1,3-Diketones)—Valuable Multi-Faceted Building Blocks for Organic Synthesis. New J. Chem. 2023, 47, 5110–5149. [Google Scholar] [CrossRef]
- Venugopalan, P.; Krishnankutty, K. Metal Chelates of 6-Aryl-5-Hexene-2,4-Diones. J. Indian Chem. Soc. 2001, 78, 472–473. [Google Scholar]
- Paul, M.; Krishnankutty, K. Synthesis and Characterisation of Co(II), Ni(II) and Cu(II) Complexes of Some 6-Aryl-5-Hexene-2,4-Diones. Asian J. Chem. 2002, 14, 949–954. [Google Scholar]
- Cheng, Y.J.; Li, C.W.; Kuo, C.L.; Shih, T.L.; Chen, J.J. Improved Synthesis of Asymmetric Curcuminoids and Their Assessment as Antioxidants. Molecules 2022, 27, 2547. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc Deficiency and Zinc Supplementation in Allergic Diseases. Biomolecules 2024, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.; Bharati, P.; Chaudhari, U.K.; Singh, A.; Kushawaha, S.K.; Singh, N.K.; Bharty, M.K. Syntheses, Crystal Structures and Photoluminescent Properties of New Homoleptic and Heteroleptic Zinc(II) Dithiocarbamato Complexes. Polyhedron 2015, 85, 712–719. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Mirian Estévez-Carmona, M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-Level Cytotoxicity in Vitro with Minimal Acute Toxicity In Vivo. Molecules 2019, 24, 1598. [Google Scholar] [CrossRef] [PubMed]
- Meza-Morales, W.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Arenaza-Corona, A.; Ramírez-Apan, M.T.; Toscano, R.A.; Poveda-Jaramillo, J.C.; Enríquez, R.G. Three New Coordination Geometries of Homoleptic Zn Complexes of Curcuminoids and Their High Antiproliferative Potential. RSC Adv. 2023, 13, 8577–8585. [Google Scholar] [CrossRef]
- Obregón-Mendoza, M.A.; Estévez-Carmona, M.M.; Alvarez-Ricardo, Y.; Meza-Morales, W.; Escobedo-Martínez, C.; Soriano-García, M.; Enríquez, R.G. Crystal Structure, Synthesis and Biological Activity of Ether and Ester Trans-Ferulic Acid Derivatives. Int. J. Org. Chem. 2018, 8, 359–377. [Google Scholar] [CrossRef]
- Mestrelab Research. MNova Software v15.0.1-35756. Available online: https://mestrelab.com/learn_support/mnova/ (accessed on 10 March 2024).
- Obregón-Mendoza, M.A.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Estévez-Carmona, M.M.; Enríquez, R.G. High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach. Molecules 2023, 28, 289. [Google Scholar] [CrossRef]
- Arenaza-Corona, A.; Obregón-Mendoza, M.A.; Meza-Morales, W.; Ramírez-Apan, M.T.; Nieto-Camacho, A.; Toscano, R.A.; Pérez-González, L.L.; Sánchez-Obregón, R.; Enríquez, R.G. The Homoleptic Curcumin–Copper Single Crystal (ML2): A Long Awaited Breakthrough in the Field of Curcumin Metal Complexes. Molecules 2023, 28, 6033. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction: Yarnton, U. CrysAlisPRO Software System. 2024. Available online: https://rigaku.com/products/crystallography/x-ray-diffraction/crysalispro (accessed on 10 August 2024).
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Coffin, A.; Ready, J.M. Selective Synthesis of (+)-Dysoline. Org. Lett. 2019, 21, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Zawadiak, J.; Mrzyczek, M. Correlation of Substituted Aromatic β-Diketones’ Characteristic Protons Chemical Shifts with Hammett Substituent Constants. Magn. Reson. Chem. 2013, 51, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, S.; Feng, J.; Xiao, Y.; Xue, X.; Zhang, H.; Wang, Y.; Liang, X. Structure Elucidation and NMR Assignments for Curcuminoids from the Rhizomes of Curcuma Longa. Magn. Reson. Chem. 2009, 47, 902–908. [Google Scholar] [CrossRef]
- Jȩdrzkiewicz, D.; Marszałek-Harych, A.; Ejfler, J. Serendipitous Synthesis Found in the Nuances of Homoleptic Zinc Complex Formation. Inorg. Chem. 2018, 57, 8169–8180. [Google Scholar] [CrossRef]
- Rachwalski, M. Special Issue: Asymmetry and Symmetry in Organic Chemistry. Symmetry 2023, 15, 1363. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Y.; Ge, J.; Lin, X.; Liu, D.; Wang, S.; Zhang, J.; Zhou, G.; Li, S. Synthesis, Characterization, DNA/BSA Interactions and in Vitro Cytotoxicity Study of Palladium(II) Complexes of Hispolon Derivatives. J. Inorg. Biochem. 2020, 202, 110857. [Google Scholar] [CrossRef]
- Caruso, F.; Subbaraju, G.V.; Ramani, M.V.; Gariboldi, M.; Marras, E.; Kloer, C.; Sulovari, A.; Kaur, S.; Rossi, M. Synthesis, X-ray Diffraction and Anti-Proliferative Biological Activity of Hispolon Derivatives and Their (H6-p-Cymene)(Hispolonato)Ruthenium[II] Chloride Complexes. Inorg. Chim. Acta 2022, 542, 121099. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]

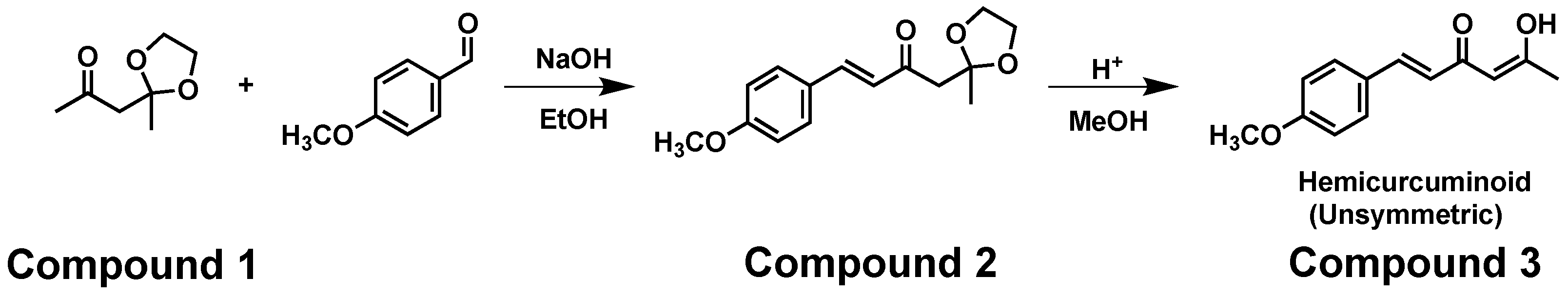
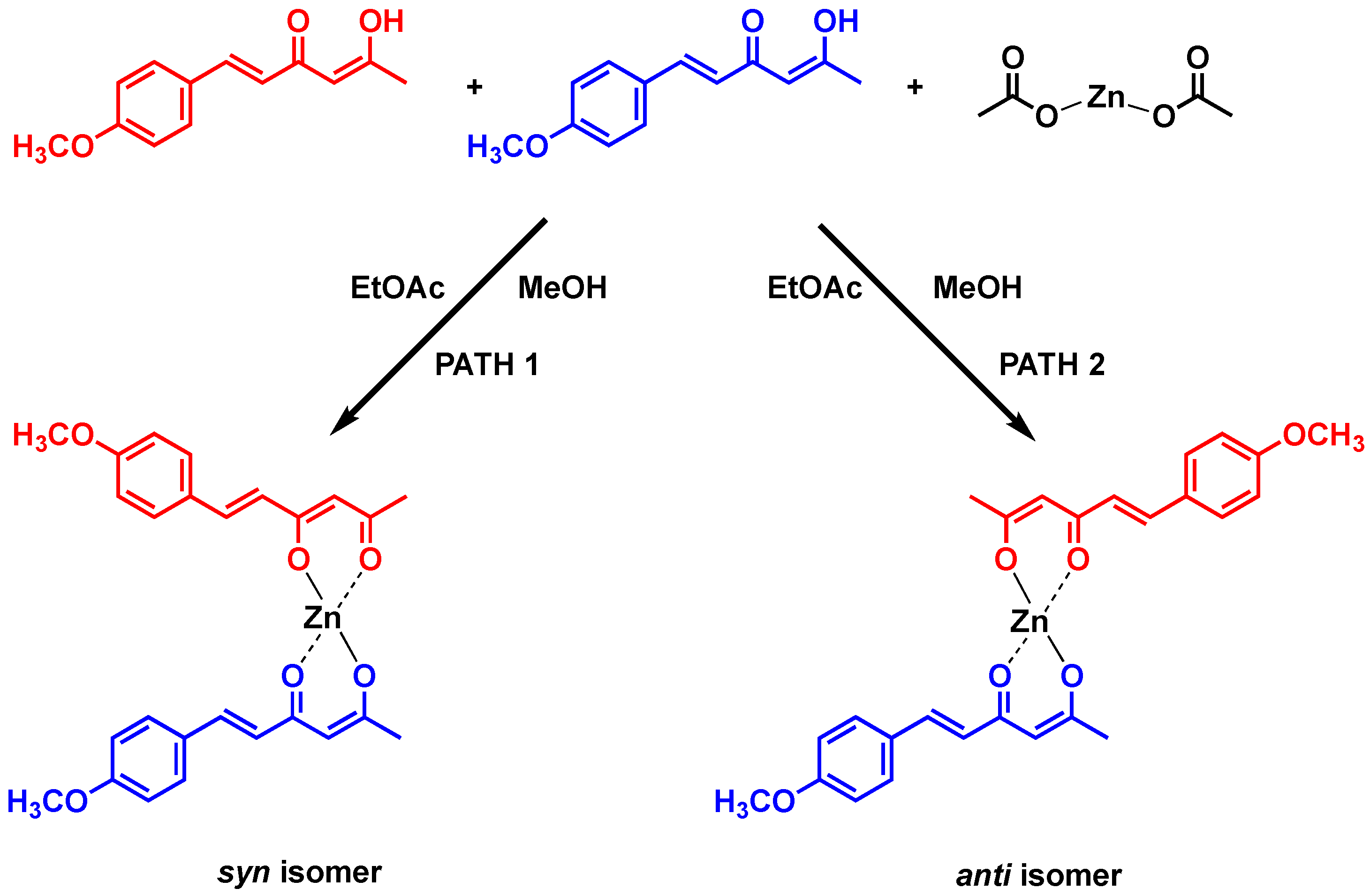
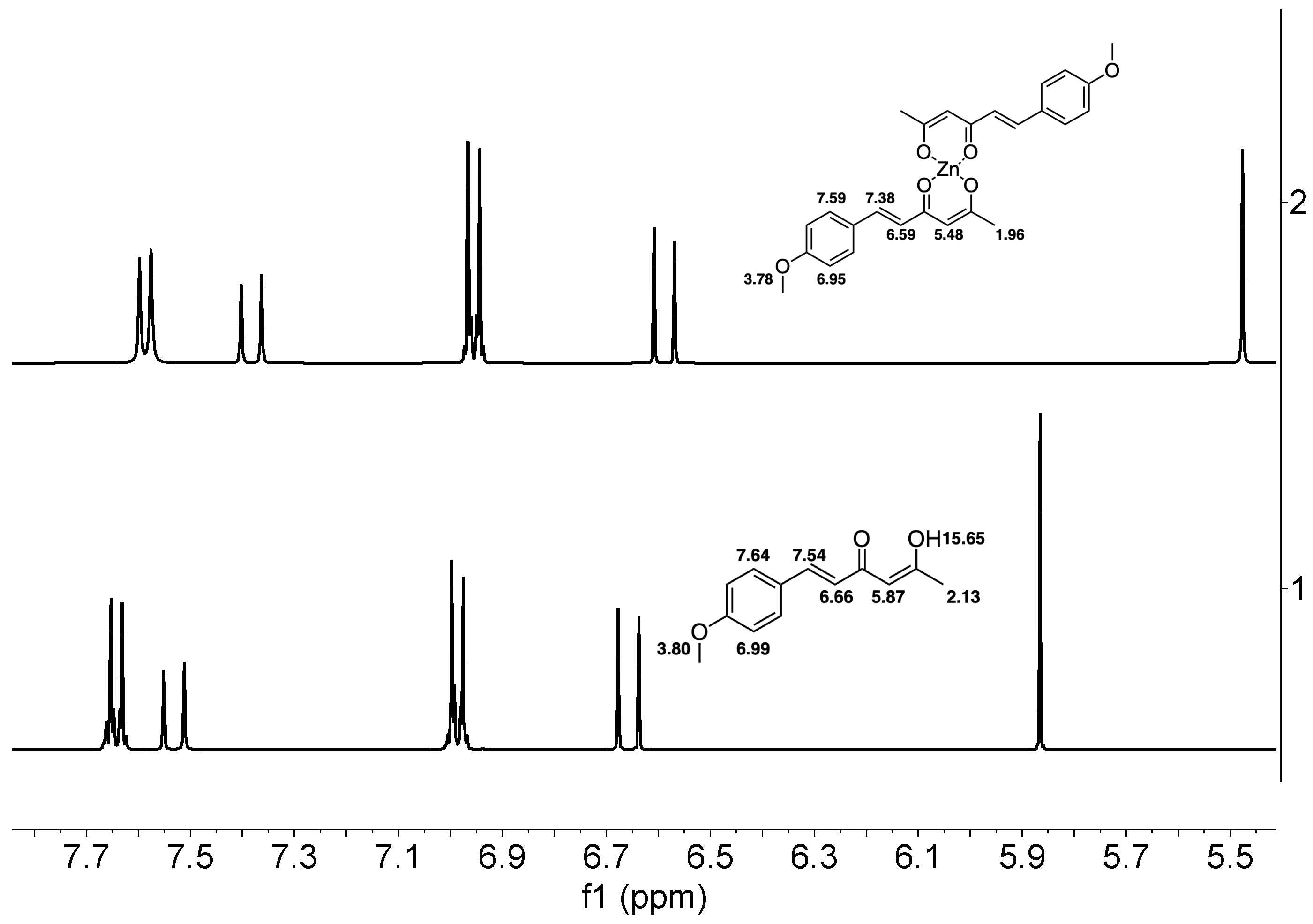


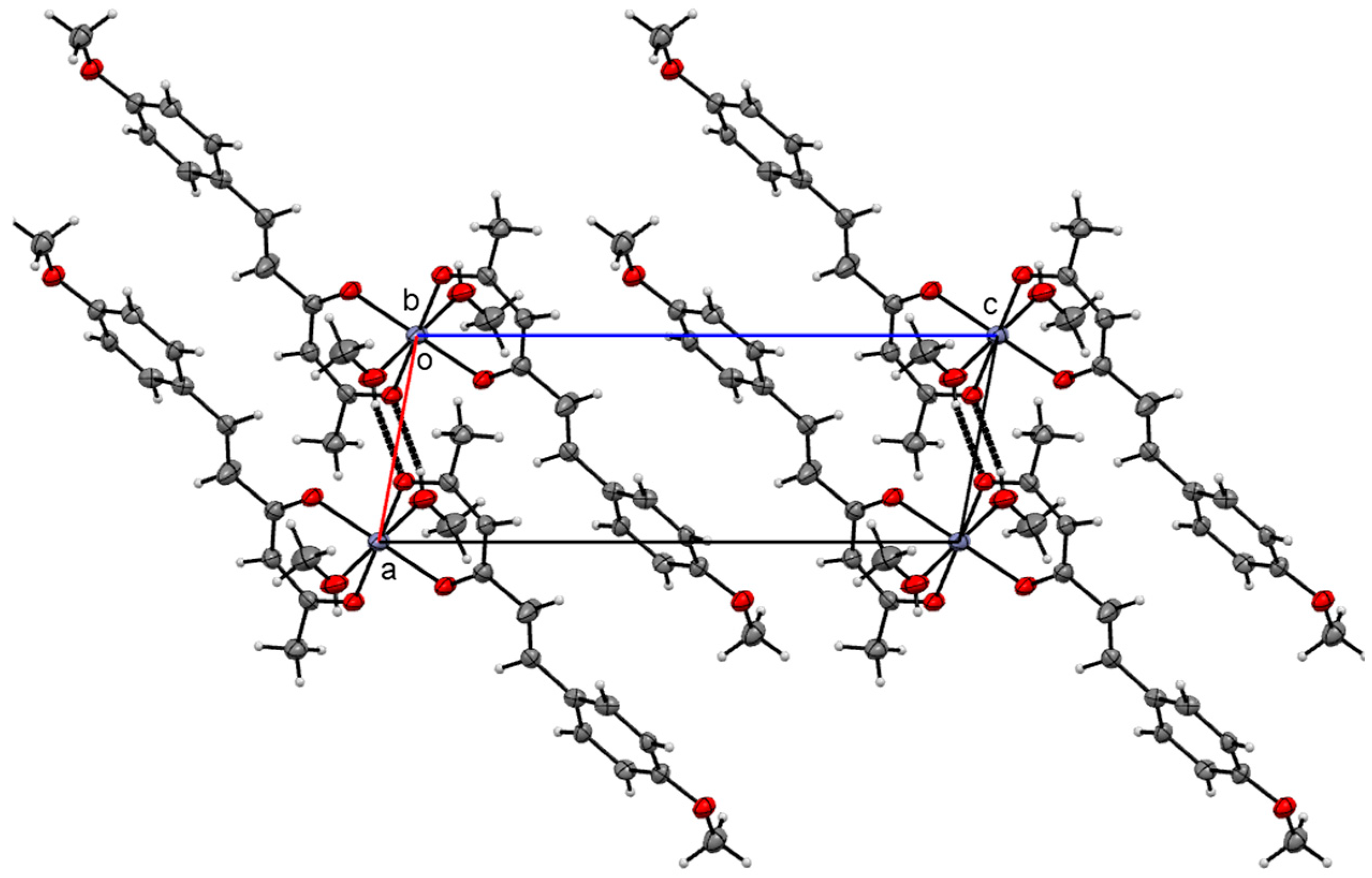
| Hydrogen | Compound 3, δ = ppm 1 | Compound 4, δ = ppm 1 |
|---|---|---|
| -CH3 | 2.13 (singlet) | 1.96 (singlet) |
| =C-H (α) | 6.66 (doublet) | 6.59 (doublet) |
| =C-H (β) | 7.54 (doublet) | 7.38 (doublet) |
| -CH (Methine) | 5.87 (singlet) | 5.48 (singlet) |
| Ar-H (AA′) | 6.99 (multiplet) | 6.95 (multiplet) |
| Ar-H (BB′) | 7.64 (multiplet) | 7.59 (multiplet) |
| -O-CH3 | 3.80 (singlet) | 3.78 (singlet) |
| -OH (enol) | 15.65 (broad) | - |
| -OOC-CH3 (acetate) | - | 2.08 (singlet) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obregón Mendoza, M.A.; Escamilla, G.M.; Tavera-Hernández, R.; Sánchez-Obregón, R.; Toscano, R.A.; Enríquez, R.G. The First Crystal Structure of an Anti-Geometric Homoleptic Zinc Complex from an Unsymmetric Curcuminoid Ligand. Crystals 2024, 14, 751. https://doi.org/10.3390/cryst14090751
Obregón Mendoza MA, Escamilla GM, Tavera-Hernández R, Sánchez-Obregón R, Toscano RA, Enríquez RG. The First Crystal Structure of an Anti-Geometric Homoleptic Zinc Complex from an Unsymmetric Curcuminoid Ligand. Crystals. 2024; 14(9):751. https://doi.org/10.3390/cryst14090751
Chicago/Turabian StyleObregón Mendoza, Marco A., Gabriela Marmolejo Escamilla, Rosario Tavera-Hernández, Rubén Sánchez-Obregón, Rubén A. Toscano, and Raúl G. Enríquez. 2024. "The First Crystal Structure of an Anti-Geometric Homoleptic Zinc Complex from an Unsymmetric Curcuminoid Ligand" Crystals 14, no. 9: 751. https://doi.org/10.3390/cryst14090751
APA StyleObregón Mendoza, M. A., Escamilla, G. M., Tavera-Hernández, R., Sánchez-Obregón, R., Toscano, R. A., & Enríquez, R. G. (2024). The First Crystal Structure of an Anti-Geometric Homoleptic Zinc Complex from an Unsymmetric Curcuminoid Ligand. Crystals, 14(9), 751. https://doi.org/10.3390/cryst14090751






