Electrosynthesis of Co-ZIF Using Bio-Derived Solvents: Electrochemical Evaluation of Synthesised MOFs as a Binder-Free Supercapacitor Electrode in Alkaline Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Synthesis
2.2. Electrochemical Synthesis of Co-ZIF MOF Coating
2.3. Characterisation of MOF Coatings
3. Results
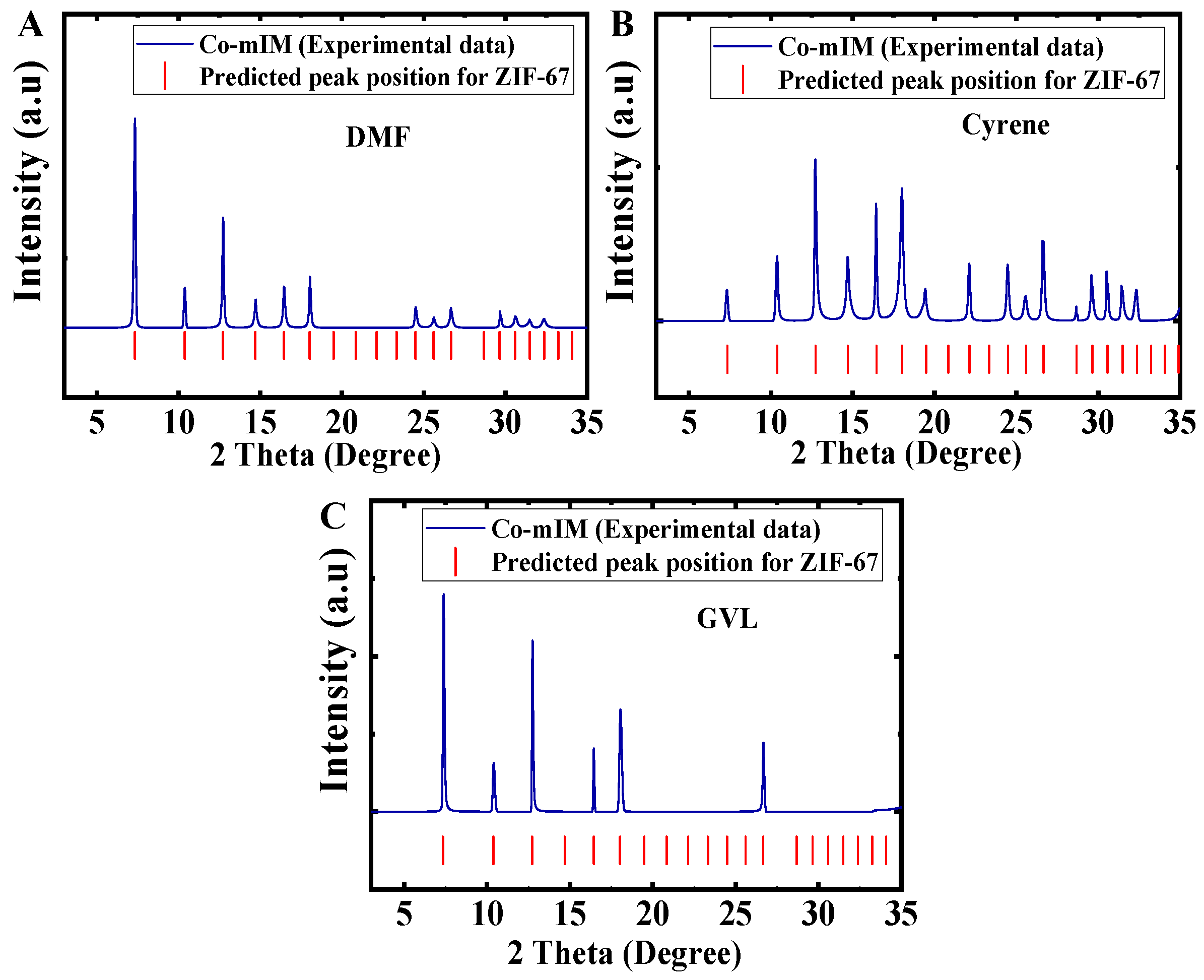
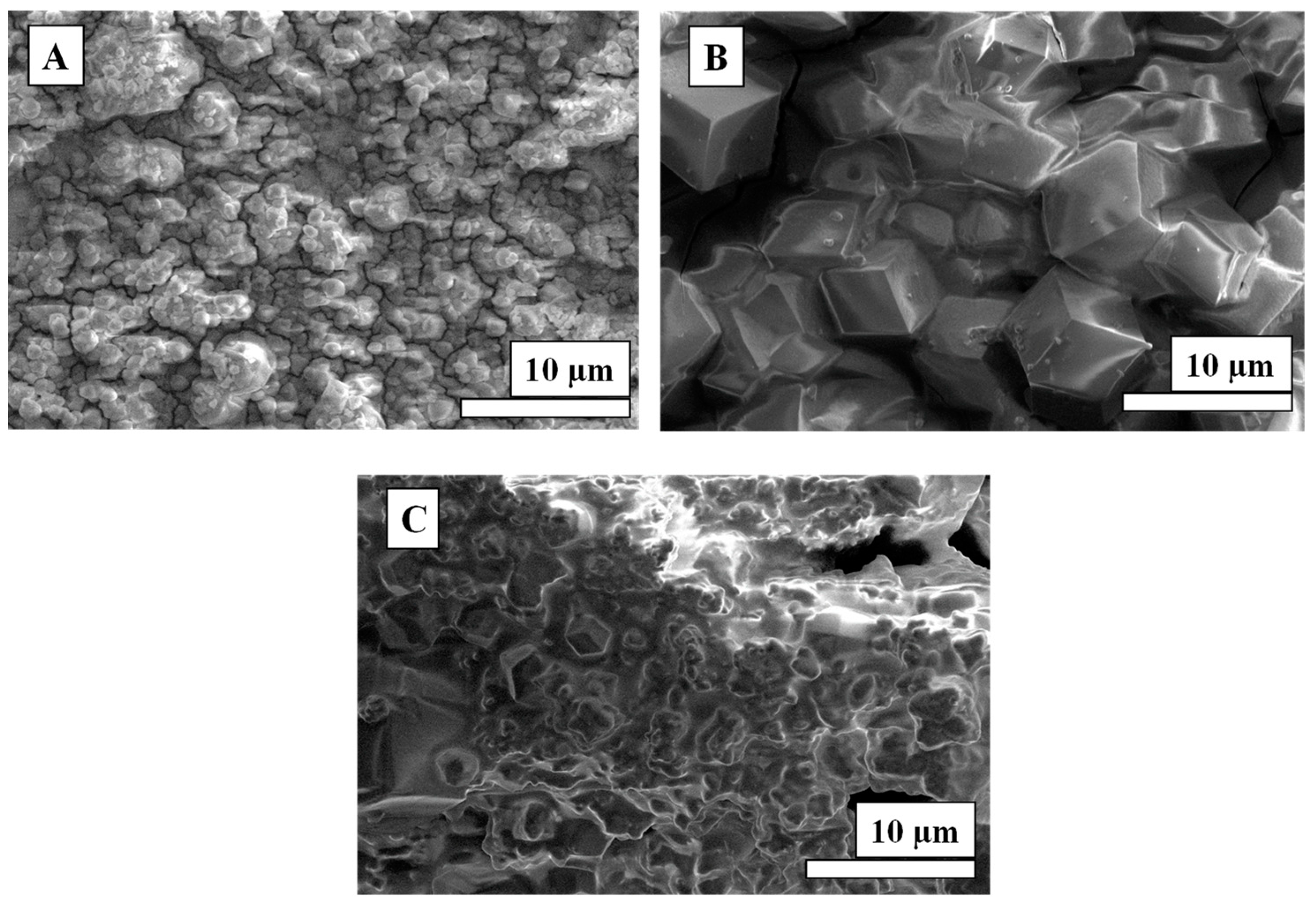
3.1. Synthesis of Co-mIM ZIFs Using Various Solvents
3.2. Synthesis of Co-bIM Using Various Solvents
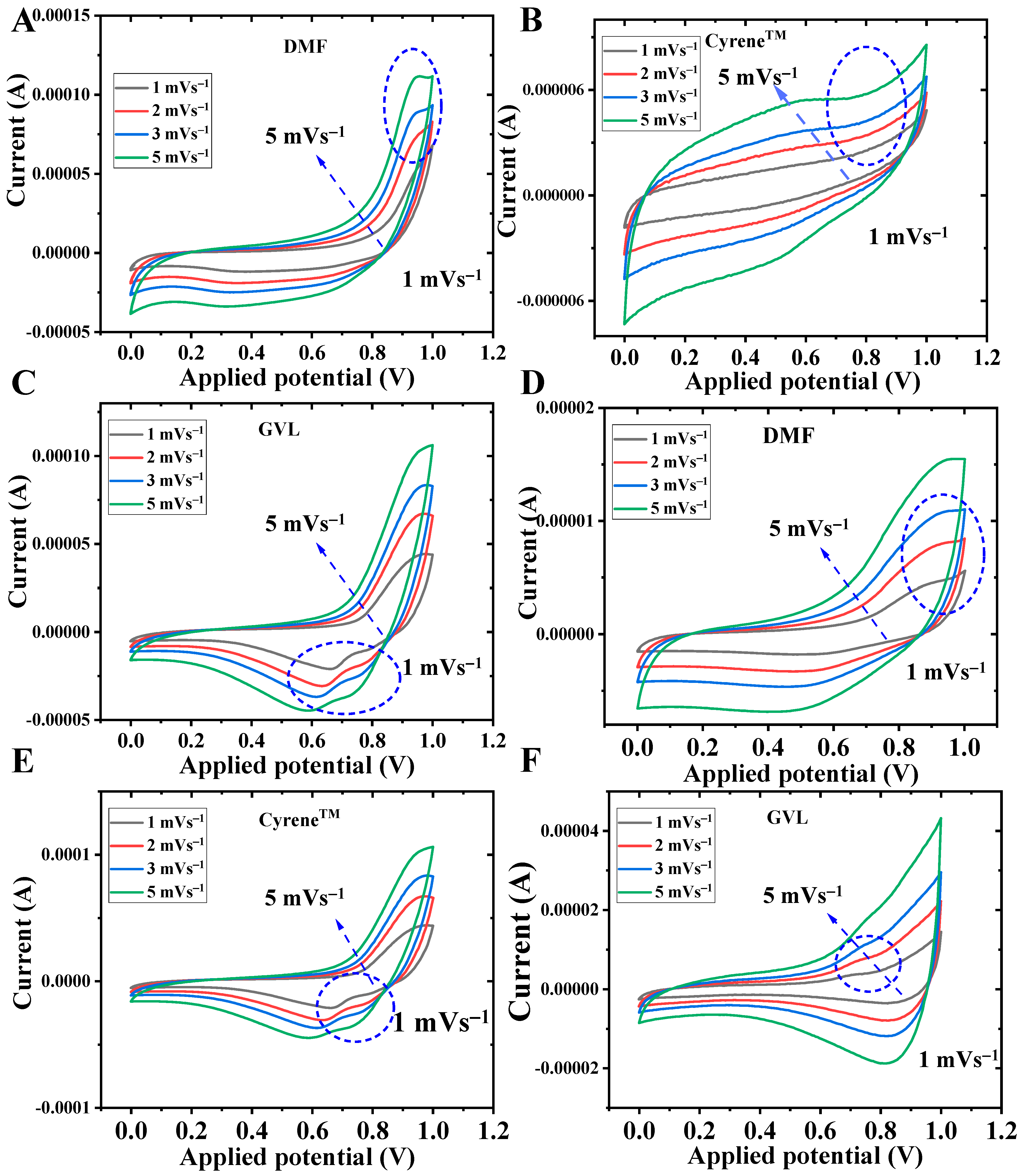
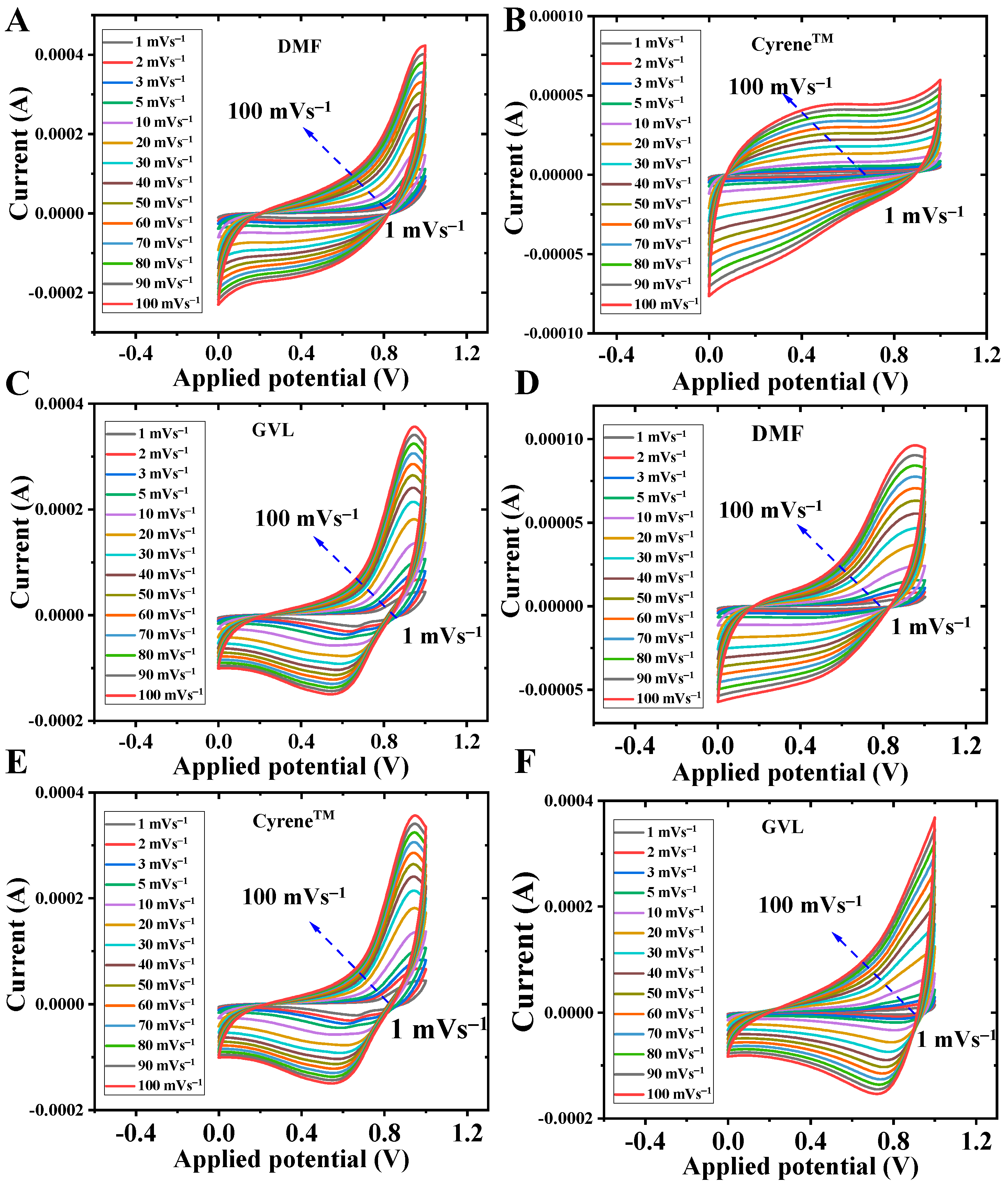
3.3. Cyclic Voltammetry
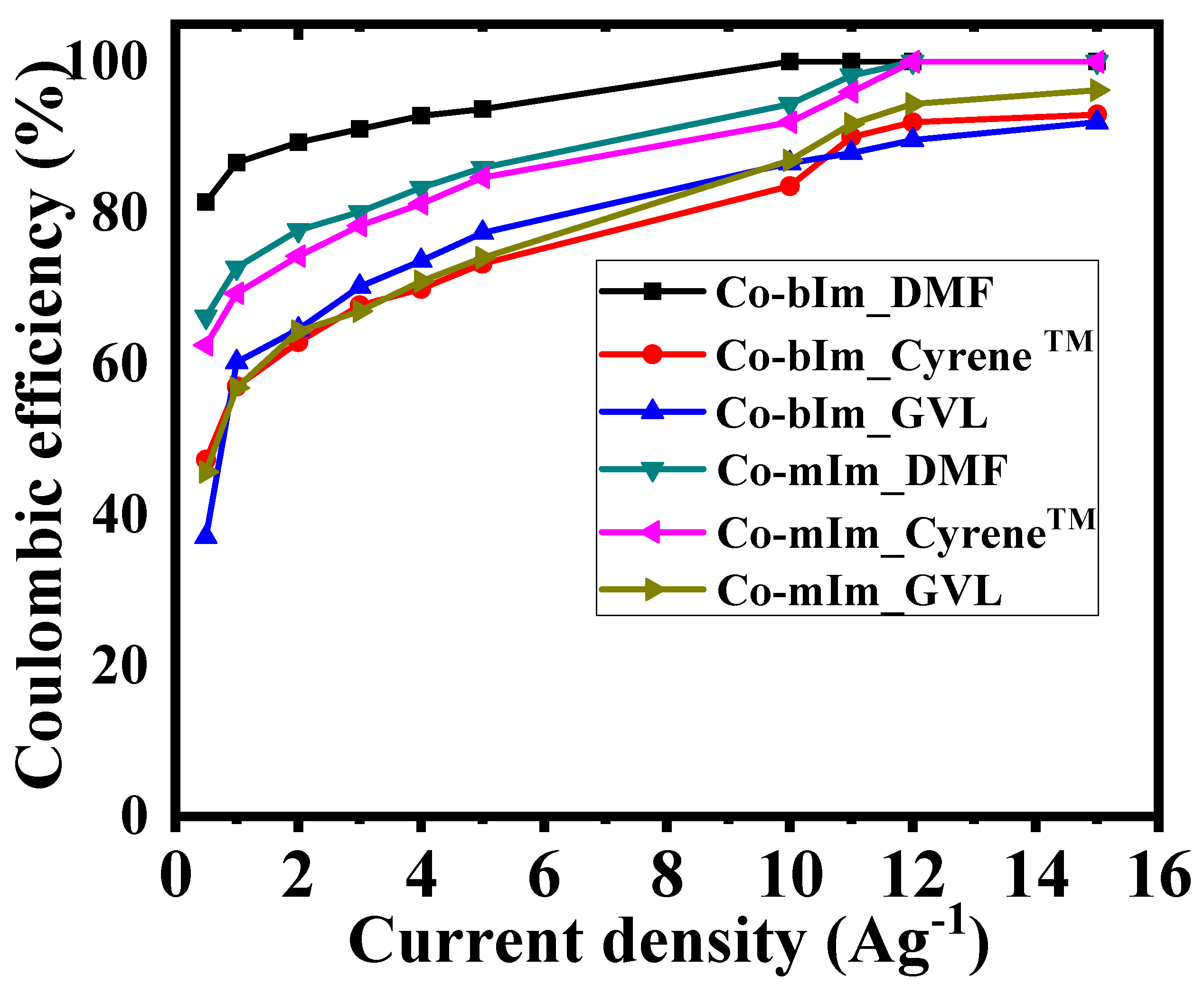
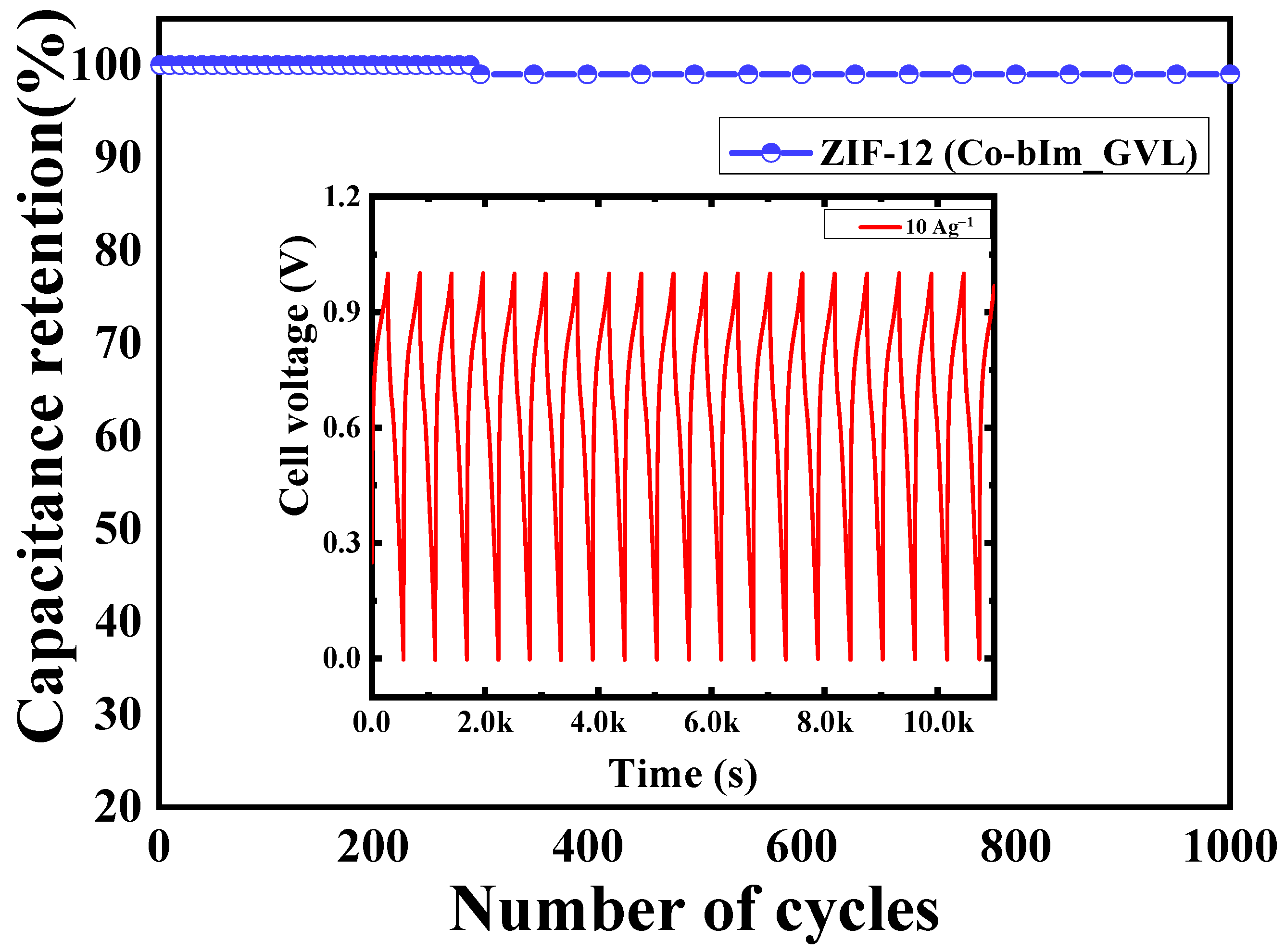
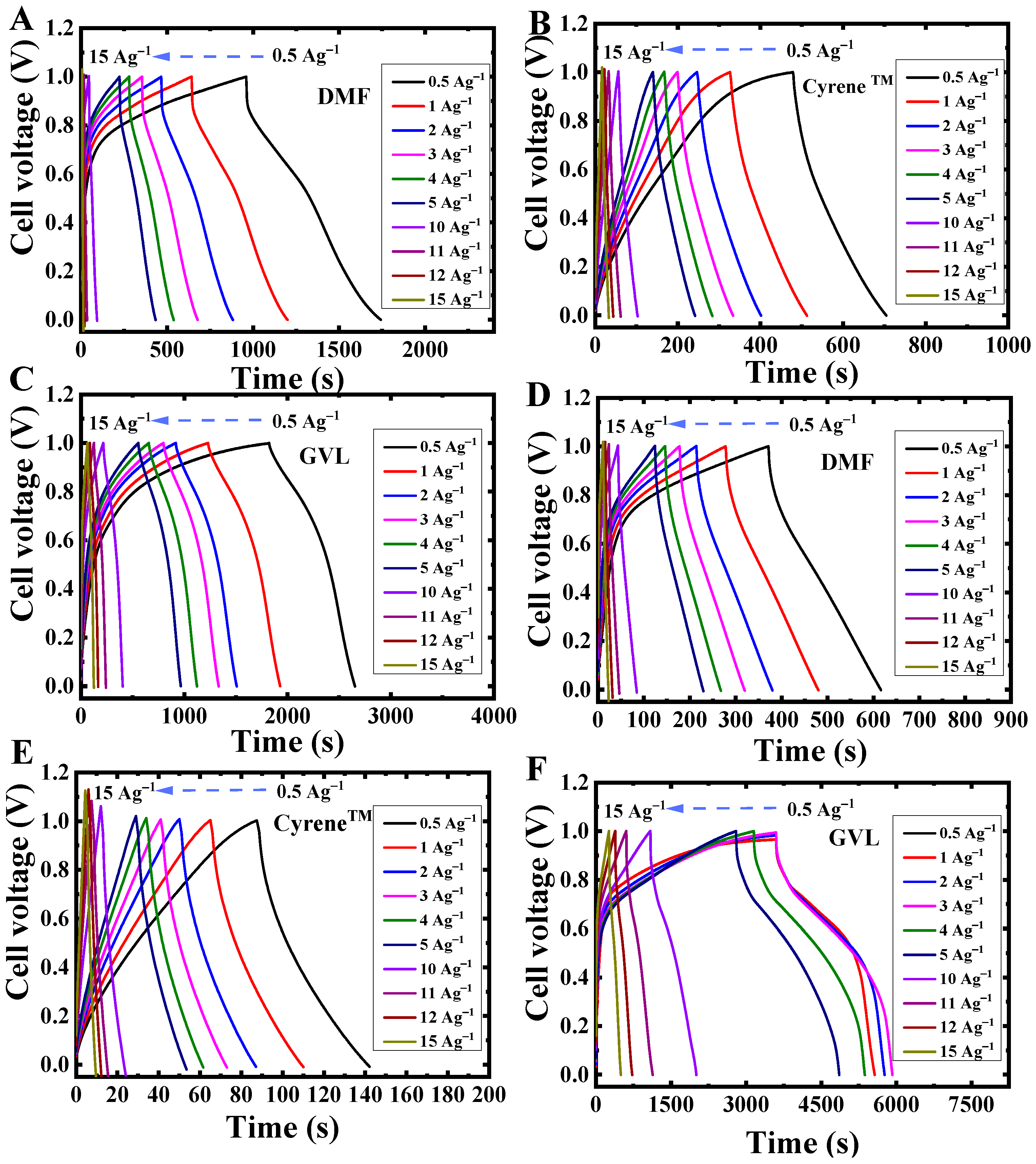
3.4. Galvanostatic Charge-Discharge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Citarella, A.; Amenta, A.; Passarella, D.; Micale, N. Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials. Int. J. Mol. Sci. 2022, 23, 15960. [Google Scholar] [CrossRef] [PubMed]
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: A bio-based novel and sustainable solvent for organic synthesis. Green Chem. 2022, 24, 6435–6449. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Brohl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Winterton, N. The green solvent: A critical perspective. Clean. Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-Garcia, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef] [PubMed]
- Farran, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernaiz, M.J.; Linhardt, R.J. Green solvents in carbohydrate chemistry: From raw materials to fine chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef]
- Clark, J.H.; El Deswarte, F.I.; Farmer, T.J. The integration of green chemistry into future biorefineries. Biofuels Bioprod. Biorefining 2008, 3, 72–90. [Google Scholar] [CrossRef]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Jordan, A.; Hall, C.G.J.; Thorp, L.R.; Sneddon, H.F. Replacement of Less-Preferred Dipolar Aprotic and Ethereal Solvents in Synthetic Organic Chemistry with More Sustainable Alternatives. Chem. Rev. 2022, 122, 6749–6794. [Google Scholar] [CrossRef]
- Camp, J.E. Bio-available Solvent Cyrene: Synthesis, Derivatization, and Applications. ChemSusChem 2018, 11, 3048–3055. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, M.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. Preparation and Application of Green Sustainable Solvent Cyrene. Chemistry 2023, 5, 2322–2346. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Adduru, J.; Rao, T.N.; Karthik, M. Conversion of Solar Energy into Electrical Energy Storage: Supercapacitor as an Ultrafast Energy-Storage Device Made from Biodegradable Agar-Agar as a Novel and Low-Cost Carbon Precursor. Glob. Chall. 2018, 2, 1800037. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Bharathi Sankar, A.; Sri Rohita, D.; Nanaji, K.; Narasinga Rao, T.; Karthik, M. Achieving High Voltage and Excellent Rate Capability Supercapacitor Electrodes Derived From Bio-renewable and Sustainable Resource. ChemistrySelect 2020, 5, 8759–8772. [Google Scholar] [CrossRef]
- Tahir, M.A.; Arshad, N.; Akram, M. Recent advances in metal organic framework (MOF) as electrode material for super capacitor: A mini review. J. Energy Storage 2022, 47, 103530. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Fu, H.; Zong, H.; Guo, H. Metal-Organic Frameworks and Their Derivatives-Based Nanostructure with Different Dimensionalities for Supercapacitors. Small 2023, 19, e2303911. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Ahmad, F.; Fatima, M.; Shahzad, A.; Javed, M.S.; Atiq, S.; Khan, M.A.; Danish, M.; Munir, O.; Arif, S.M.B.; et al. Exploring new frontiers in supercapacitor electrodes through MOF advancements. J. Energy Storage 2024, 76, 109822. [Google Scholar] [CrossRef]
- Wang, K.-B.; Xun, Q.; Zhang, Q. Recent progress in metal-organic frameworks as active materials for supercapacitors. EnergyChem 2020, 2, 100025. [Google Scholar] [CrossRef]
- He, H.; Li, R.; Yang, Z.; Chai, L.; Jin, L.; Alhassan, S.I.; Ren, L.; Wang, H.; Huang, L. Preparation of MOFs and MOFs derived materials and their catalytic application in air pollution: A review. Catal. Today 2021, 375, 10–29. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, X.; Shi, Y.; Peng, Y.; Zhou, H.; Wu, X.; Ma, J.; Jin, J.; Pi, Y.; Pang, H. Recent Progress in 2D Metal-Organic Framework-Related Materials. Small 2024, 20, e2305548. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Xie, L.-H.; Li, X.; Li, J.-R. Construction and application of base-stable MOFs: A critical review. Chem. Soc. Rev. 2022, 51, 6417–6441. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Matzger, A.J. Metal–Organic Frameworks: Examples, Counterexamples, and an Actionable Definition. Cryst. Growth Des. 2017, 17, 4043–4048. [Google Scholar] [CrossRef]
- Soni, S.; Bajpai, P.K.; Arora, C. A review on metal-organic framework: Synthesis, properties and application. Charact. Appl. Nanomater. 2020, 3, 87–106. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Selvaraj, S.C.; Kim, B.-S. Morphology engineering of Co-MOF nanostructures to tune their electrochemical performances for electrocatalyst and energy-storage applications supported by DFT studies. Appl. Surf. Sci. 2022, 605, 154691. [Google Scholar] [CrossRef]
- Yap, M.H.; Fow, K.L.; Chen, G.Z. Synthesis and applications of MOF-derived porous nanostructures. Green Energy Environ. 2017, 2, 218–245. [Google Scholar] [CrossRef]
- Bhindi, M.; Massengo, L.; Hammerton, J.; Derry, M.J.; Worrall, S.D. Structure Control Using Bioderived Solvents in Electrochemical Metal-Organic Framework Synthesis. Appl. Sci. 2023, 13, 720. [Google Scholar] [CrossRef]
- Flügel, E.A.; Ranft, A.; Haase, F.; Lotsch, B.V. Synthetic routes toward MOF nanomorphologies. J. Mater. Chem. 2012, 22, 10119–10133. [Google Scholar] [CrossRef]
- Li, W.-J.; Tu, M.; Cao, R.; Fischer, R.A. Metal–organic framework thin films: Electrochemical fabrication techniques and corresponding applications & perspectives. J. Mater. Chem. A 2016, 4, 12356–12369. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal-organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331. [Google Scholar] [CrossRef]
- Al Obeidli, A.; Ben Salah, H.; Al Murisi, M.; Sabouni, R. Recent advancements in MOFs synthesis and their green applications. Int. J. Hydrogen Energy 2022, 47, 2561–2593. [Google Scholar] [CrossRef]
- He, Q.; Zhan, F.; Wang, H.; Xu, W.; Wang, H.; Chen, L. Recent progress of industrial preparation of metal–organic frameworks: Synthesis strategies and outlook. Mater. Today Sustain. 2022, 17, 100104. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Guo, X.; Xing, T.; Lou, Y.; Chen, J. Controlling ZIF-67 crystals formation through various cobalt sources in aqueous solution. J. Solid State Chem. 2016, 235, 107–112. [Google Scholar] [CrossRef]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- He, M.; Yao, J.; Liu, Q.; Zhong, Z.; Wang, H. Toluene-assisted synthesis of RHO-type zeolitic imidazolate frameworks: Synthesis and formation mechanism of ZIF-11 and ZIF-12. Dalton Trans. 2013, 42, 16608–16613. [Google Scholar] [CrossRef] [PubMed]
- Duong, A.T.A.; Nguyen, H.V.; Tran, M.V.; Ngo, Q.N.; Luu, L.C.; Doan, T.L.H.; Nguyen, H.N.; Nguyen, M.V. Influence of ZIF-9 and ZIF-12 structure on the formation of a series of new Co/N-doped porous carbon composites as anode electrodes for high-performance lithium-ion batteries. RSC Adv. 2023, 13, 17370–17383. [Google Scholar] [CrossRef]
- Cao, W.; Han, M.; Qin, L.; Jiang, Q.; Xu, J.; Lu, Z.; Wang, Y. Synthesis of zeolitic imidazolate framework-67 nanocube wrapped by graphene oxide and its application for supercapacitors. J. Solid State Electrochem. 2018, 23, 325–334. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, Y.; Gao, Y.; Wu, J.; Hu, J.; Stein, A.; Tang, B. Nanocomposites of zeolitic imidazolate frameworks on graphene oxide for pseudocapacitor applications. J. Appl. Electrochem. 2016, 46, 441–450. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, Y.; Dong, Q.; Hong, Y.; Liu, Y.; Wang, W.; Shao, J.; Si, W.; Dong, X. Metal Organic Framework Derived Core–Shell Structured Co9S8@N–C@MoS2 Nanocubes for Supercapacitor. ACS Appl. Energy Mater. 2018, 1, 3513–3520. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Hu, H. Recent progress on synthesis of ZIF-67-based materials and their application to heterogeneous catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Dai, J.; Xiao, S.; Liu, J.; He, J.; Lei, J.; Wang, L. Fabrication of ZIF-9@super-macroporous microsphere for adsorptive removal of Congo red from water. RSC Adv. 2017, 7, 6288–6296. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Shen, K.; Huo, J.; Liu, Y.; Guo, S. Ion-matching porous carbons with ultra-high surface area and superior energy storage performance for supercapacitors. J. Mater. Chem. A 2019, 7, 9163–9172. [Google Scholar] [CrossRef]
- Lobato, B.; Suárez, L.; Guardia, L.; Centeno, T.A. Capacitance and surface of carbons in supercapacitors. Carbon 2017, 122, 434–445. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]



| Linker Concentration (mol dm−3) | Solvent | Temperature (℃) |
|---|---|---|
| bIM 0.12 | DMF | 85 |
| bIM 0.12 | CyreneTM | 100 |
| bIM 0.12 | GVL | 85 |
| mIM 3.0 | DMF | 85 |
| mIM 3.0 | CyreneTM | 100 |
| mIM 3.0 | GVL | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manavalan, V.; Coward, B.; Najdanovic-Visak, V.; Worrall, S.D. Electrosynthesis of Co-ZIF Using Bio-Derived Solvents: Electrochemical Evaluation of Synthesised MOFs as a Binder-Free Supercapacitor Electrode in Alkaline Electrolyte. Crystals 2024, 14, 700. https://doi.org/10.3390/cryst14080700
Manavalan V, Coward B, Najdanovic-Visak V, Worrall SD. Electrosynthesis of Co-ZIF Using Bio-Derived Solvents: Electrochemical Evaluation of Synthesised MOFs as a Binder-Free Supercapacitor Electrode in Alkaline Electrolyte. Crystals. 2024; 14(8):700. https://doi.org/10.3390/cryst14080700
Chicago/Turabian StyleManavalan, Vijayakumar, Brad Coward, Vesna Najdanovic-Visak, and Stephen D. Worrall. 2024. "Electrosynthesis of Co-ZIF Using Bio-Derived Solvents: Electrochemical Evaluation of Synthesised MOFs as a Binder-Free Supercapacitor Electrode in Alkaline Electrolyte" Crystals 14, no. 8: 700. https://doi.org/10.3390/cryst14080700
APA StyleManavalan, V., Coward, B., Najdanovic-Visak, V., & Worrall, S. D. (2024). Electrosynthesis of Co-ZIF Using Bio-Derived Solvents: Electrochemical Evaluation of Synthesised MOFs as a Binder-Free Supercapacitor Electrode in Alkaline Electrolyte. Crystals, 14(8), 700. https://doi.org/10.3390/cryst14080700








