Low Temperature Raman Spectroscopy of Tetrahydrofuran: Phonon Spectra Compared to Matrix Isolation Spectra in Air
Abstract
1. Introduction
2. Experimental
3. Computational Details
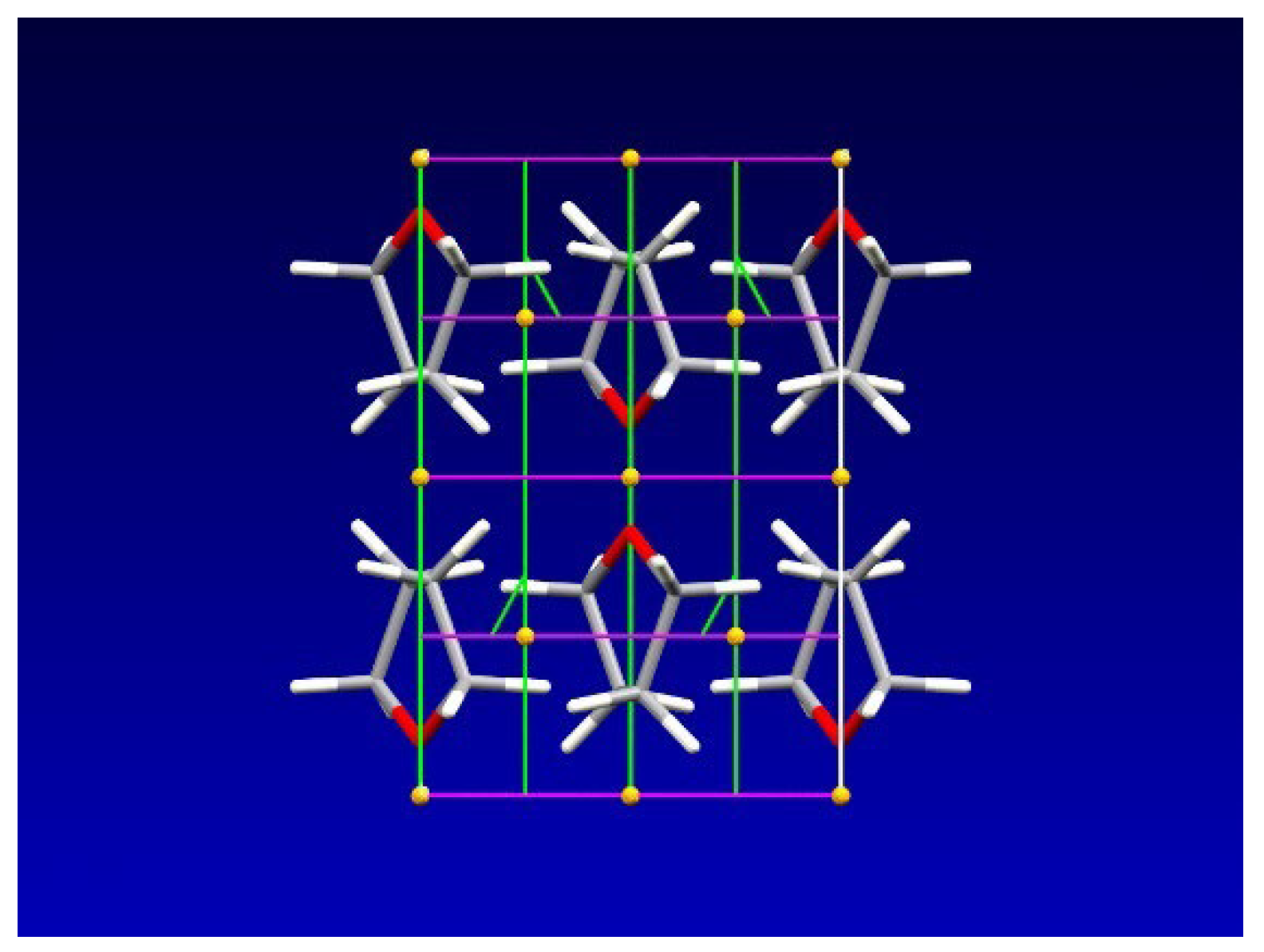
4. Results and Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grdadolnik, J.; Mohacek-Grosev, V.; Baldwin, R.L.; Avbelj, F. Populations of the three major backbone conformations in 19 amino acid dipeptides. Proc. Natl. Acad. Sci. USA 2011, 108, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Angyal, S.J. The Composition of Reducing Sugars in Solution. Adv. Carboh. Chem. Biochem. 1984, 42, 15–68. [Google Scholar] [CrossRef]
- Arukawa, Y.; Komatsu, K.; Feng, J.; Zhu, C.; Tsuji, H. Distinct twist-bend nematic phase behaviors associated with the esther-linked liquid crystal dimers. Mater. Adv. 2021, 2, 261–272. [Google Scholar] [CrossRef]
- Man Park, S.; Ho Kwon, S. Deciphering the conformational preference and ionization dynamics of tetrahydrofuran: Insights from advanced spectroscopic techniques. J. Chem. Phys. 2024, 160, 114308. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.D.; Boese, R. Tetrahydrothiophene and Tetrahydrofuran, Computational and X-ray Studies in the Crystalline Ph/ase. Cryst. Growth Des. 2015, 15, 1073–1081. [Google Scholar] [CrossRef]
- Bowron, D.T.; Finney, J.L.; Soper, A.K. The structure of liquid tetrahydrofuran. J. Am. Chem. Soc. 2006, 128, 5119–5126. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.-W.; Kim, D.Y.; Park, J.; Seo, Y.-T.; Zeng, H.; Moudrakovski, I.L.; Ratcliffe, C.I.; Ripmeester, J.A. Tuning clathrate hydrates for hydrogen storage. Nature 2005, 434, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.A. The evolution of the structural, vibrational and electronic properties of the cyclic ethers—On ring size. An ab initio study. J. Mol. Struct. 2014, 1073, 125–133. [Google Scholar] [CrossRef]
- Yang, T.; Su, G.; Ning, C.; Deng, J.; Wang, F.; Zhang, S.; Ren, X.; Huang, Y. New Diagnostic of the Most Populated Conformer of Tetrahydrofuran in the Gas Phase. J. Phys. Chem A 2007, 111, 4927–4933. [Google Scholar] [CrossRef]
- Rayon, V.M.; Sordo, J.A. Pseudorotation motion in tetrahydrofuran: An ab initio study. J. Chem. Phys. 2005, 122, 204303. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J. Molecular orbital theory of the electronic structure of organic compounds. XXIII. Pseudorotation in saturated five-membered ring compounds. J. Amer. Chem. Soc. 1975, 97, 1358–1367. [Google Scholar] [CrossRef]
- Sont, W.N.; Wieser, H. The radial transtion in tetrahydrofuran. J. Raman Spectrosc. 1981, 11, 334–338. [Google Scholar] [CrossRef]
- Greenhouse, J.A.; Strauss, H.L. Spectroscopic Evidence for Pseudorotation. II. The Far-Infrared Spectra of Tetrahydrofuran and 1,3-Dioxolane. J. Chem. Phys. 1969, 50, 124–134. [Google Scholar] [CrossRef]
- Engerholm, G.G.; Luntz, A.C.; Gwinn, W.D.; Harris, D.O. Ring Puckering in Five-Membered Rings. II. The Microwave Spectrum, Dipole Moment, and Barrier to Pseudorotation in Tetrahydrofuran. J. Chem. Phys. 1969, 50, 2446–2457. [Google Scholar] [CrossRef]
- Mamleev, A.H.; Gunderova, L.N.; Galeev, R.V. Microwave Spectrum and Hindered Pseudorotation of Tetrahydrofuran. J. Struct. Chem. 2001, 42, 365–370. [Google Scholar] [CrossRef]
- Melnik, D.G.; Gopalakrishnan, S.; Miller, T.A.; de Lucia, F.C. The absorption spectroscopy of the lowest pseudorotational states of tetrahydrofuran. J. Chem. Phys. 2003, 118, 3589–3599. [Google Scholar] [CrossRef]
- Luger, P.; Buschmann, J. Twist conformation of Tetrahydrofuran in the Crystal. Angew. Chem. Int. Ed. Engl. 1983, 22, 410. [Google Scholar] [CrossRef]
- David, W.I.F.; Ibberson, R.M. A reinvestigation of the structure of tetrahydrofuran by high-resolution neutron powder diffraction. Acta Cryst. C 1992, 48, 301–303. [Google Scholar] [CrossRef]
- Aleksanyan, V.T.; Antipov, B.G. On the intramolecular dynamics of five-membered ring compounds in solid state. In Raman Spectroscopy Linear and Nonlinear; Lascombe, J., Huong, P., Eds.; John Wiley & Sons: New York, NY, USA, 1982; pp. 605–606. [Google Scholar]
- Cadioli, B.; Gallinella, E.; Coulombeau, C.; Jobic, H.; Berthier, G. Geometric Structure and Vibrational Spectrum of Tetrahydrofuran. J. Phys. Chem. 1993, 97, 7844–7856. [Google Scholar] [CrossRef]
- Gallinella, E.; Cadioli, B.; Flament, J.P.; Berthier, G. A scaled force field for tetrahydrofuran and its isotopomers from quantum mechanical calculations and infrared and Raman spectra. J. Mol. Struct. 1994, 315, 137–148. [Google Scholar] [CrossRef]
- Stocka, J.; Čeponkus, J.; Šablinskas, V.; Rodziewicz, P. Conformational diversity of the THF molecule in N2 matrix by means of FTIR matrix isolation experiment and Car-Parrinello molecular dynamics simulations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 238, 118425. [Google Scholar] [CrossRef] [PubMed]
- Mohaček-Grošev, V.; Furić, K.; Vujnović, V. Raman study of water deposited in solid argon matrix. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 269, 120770. [Google Scholar] [CrossRef]
- Furić, K.; Volovšek, V. Water ice at low temperatures and pressures: New Raman results. J. Mol. Struct. 2010, 976, 174–180. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C109999&Mask=4&Type=ANTOINE&Plot=on (accessed on 19 April 2024).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. (Eds.) Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Berenblut, B.J.; Dawson, P.; Wilkinson, G.R. The Raman spectrum of gypsum. Spectrochim. Acta 1971, 27A, 1849–1863. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Cavagnat, D.; Lascombe, J.; Lassegues, J.C.; Horsewill, A.J.; Heidemann, A.; Suck, J.B. Neutron and Raman scattering studies of the methyl dynamics in solid toluene and nitromethane. J. De Phys. 1984, 45, 97–105. [Google Scholar] [CrossRef]
- Stein, A.; Lehmann, C.W.; Luger, P. Crystal Structure of Cyclobutane at 117 K. J. Am. Chem. Soc. 1992, 114, 7684–7687. [Google Scholar] [CrossRef]
- Durig, J.R.; Sullivan, J.F.; Durig, D.T. Low-frequency vibrational spectra of molecular crystals. XXII: Cyclobutane-d0 and cyclobutane-d8. Mol. Cryst. Liq. Cryst. 1979, 54, 51–58. [Google Scholar] [CrossRef]
- Sherwood, J.N. (Ed.) The Plastically Crystalline State, Orientationally-Disordered Crystals; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Angell, C.A.; Busse, L.E.; Cooper, E.I.; Kadiyale, R.K.; Dworkin, A.; Ghelfenstein, M.; Szwarc, H.; Vassal, A. Glasses and glassy crystals from molecular and molecular ionic systems. J. Chim. Phys. Paris 1985, 82, 267–274. [Google Scholar] [CrossRef]
- NIH National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetrahydrofuran (accessed on 1 May 2024).
- Lebedev, B.V.; Rabinovich, I.B.; Milov, I.V.; Lityagor, V.Y. Thermodynamic properties of tetrahydrofuran from 8 K to 322 K. J. Chem. Thermodyn. 1978, 10, 321–329. [Google Scholar] [CrossRef]
- Rong-Ri, T.; Xin, S.; Lin, H.; Feng-Shon, Z. Liquid glass transition of tetrahydrofuran and 2-methyltetrahydrofuran. Chin. Phys. B 2012, 21, 086402. [Google Scholar] [CrossRef]
- Mohaček Grošev, V.; Stelzer, F.; Jocham, D. Internal rotation dynamics of nitromethane at low temperatures. J. Mol. Struct. 1999, 476, 181–189. [Google Scholar] [CrossRef]
- Cavagnat, D.; Brom, H.; Nugteren, P.R. Methyl internal rotation in partially deuterated molecular solids: The NO2CHD2 and NO2CH2D systems. J. Chem. Phys. 1987, 87, 801–808. [Google Scholar] [CrossRef]
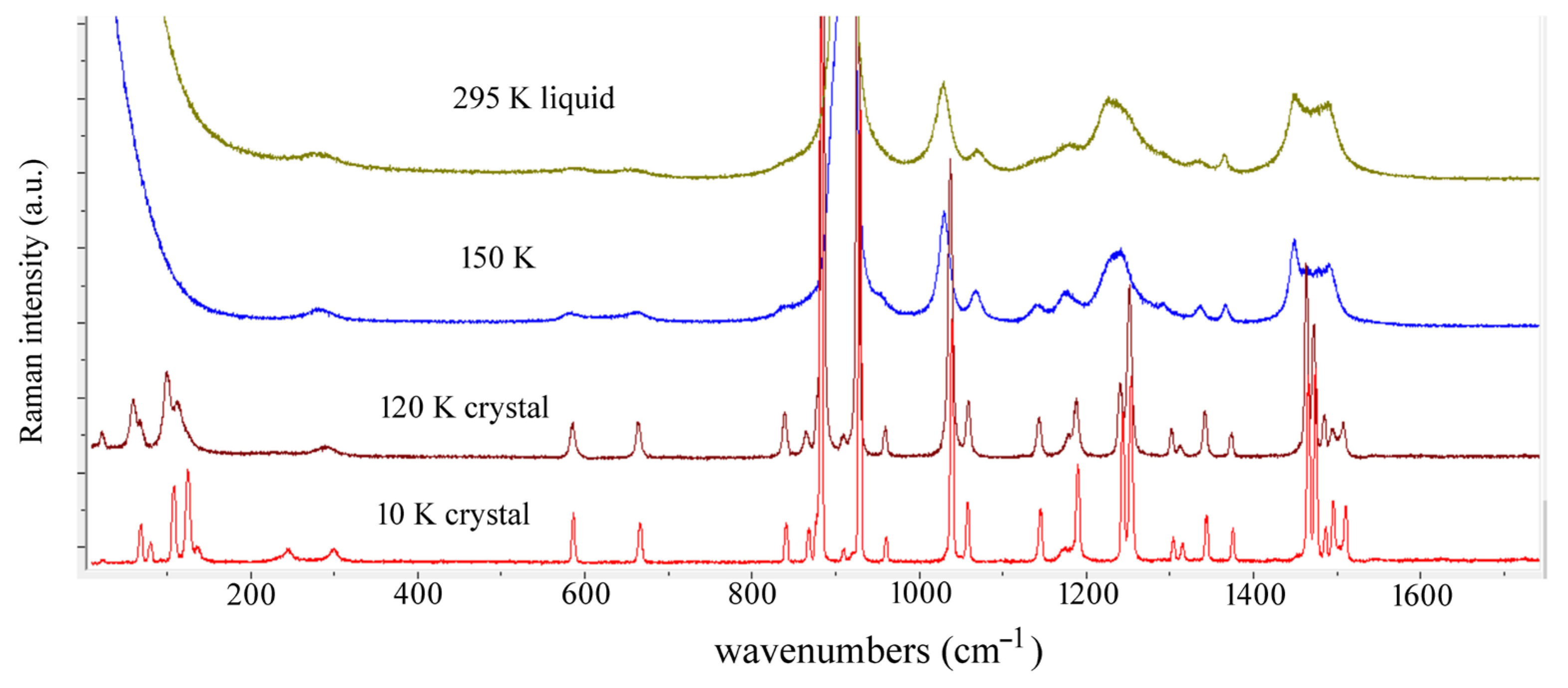

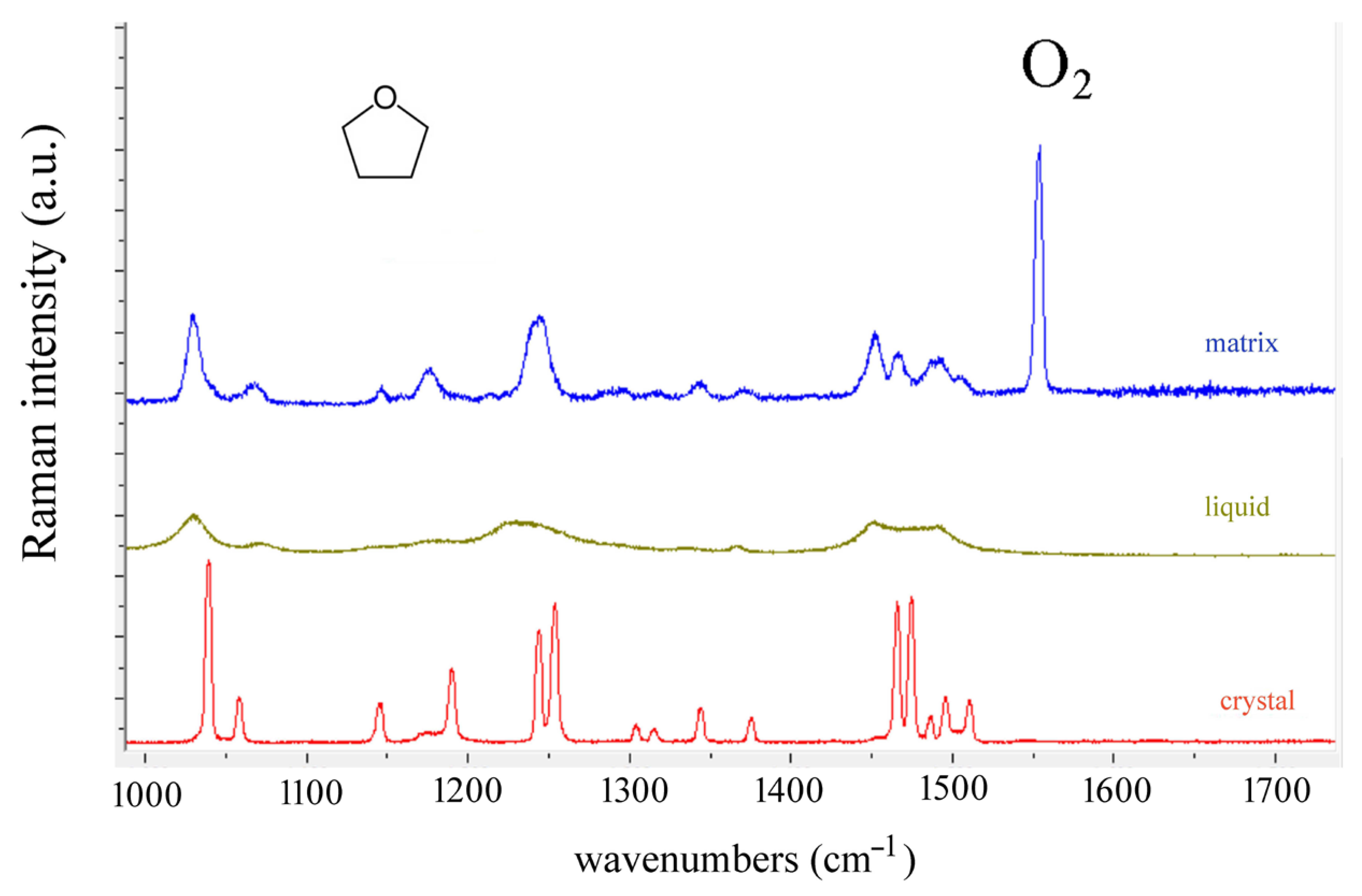
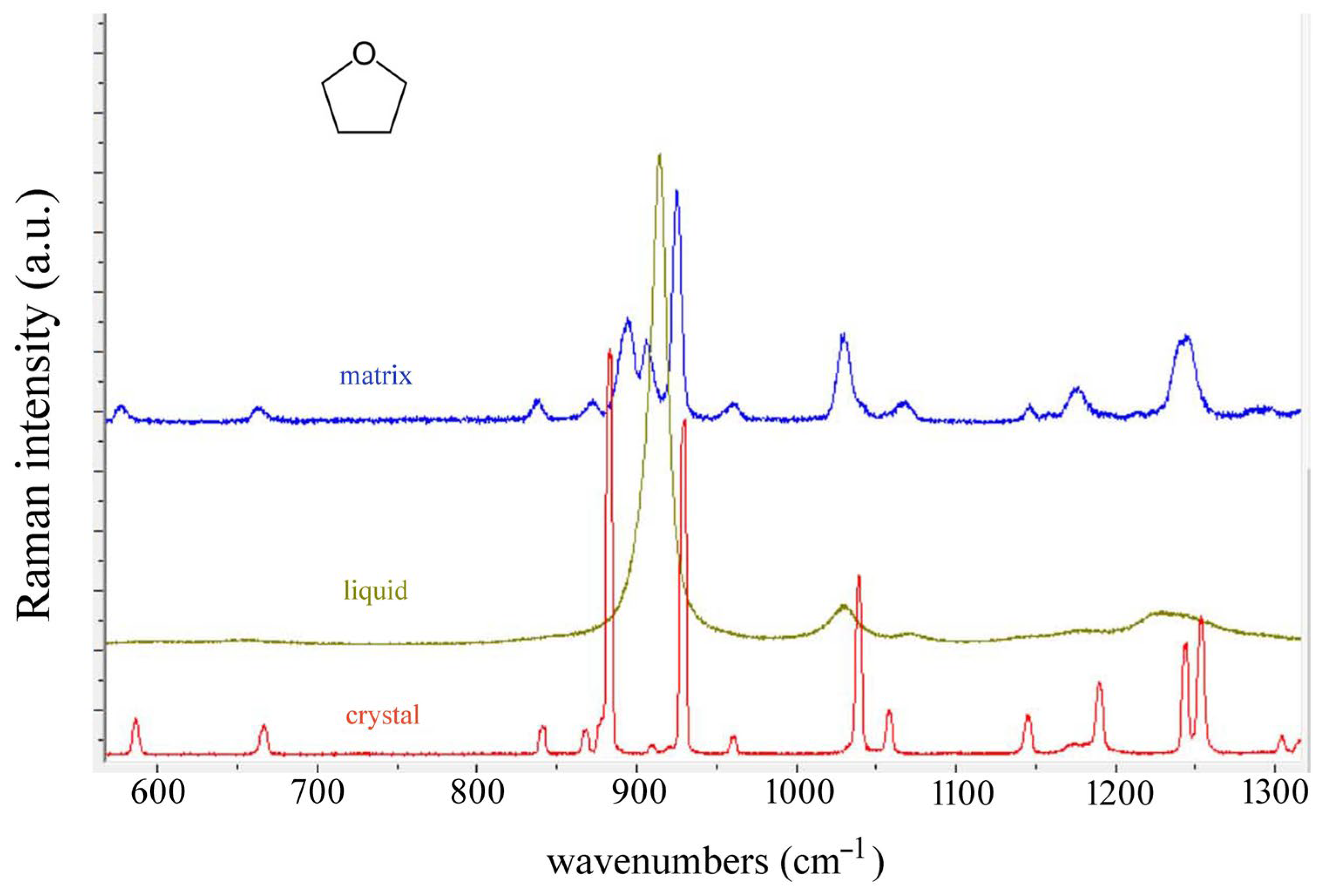



| Observed Bands in Raman Spectra of Tetrahydrofuran | ||||
|---|---|---|---|---|
| Matrix Isolated 10 K | Assignment C2 Conformer | Crystal 10 K C2 Conformer | Liquid 295 K | Assignment from [20] |
| 3019 w | ||||
| 3003 mw, sh | ||||
| 2992 s | 2988 s, sh, br | 2 ν5 | ||
| 2984 s, br | ν1 | 2983 m | ||
| 2972 mw, sh | ||||
| 2964 s, br | ν1 | |||
| 2947 s, br | ν2 | 2951 ms | ||
| 2943 m | 2943 s, br | ν2 | ||
| 2935 ms, br | ν3 | 2935 m, sh | ||
| 2924 ms, sh | ||||
| 2915 s, br | ν3 | |||
| 2881 s, br | ν4 | 2880 m | 2878 s | ν4 |
| 2860 vvs | 2864 s, sh | |||
| 2729 w | ν22 + ν26 | 2732 m | 2721 mw | ν22 + ν26 |
| 2676 w | ν5 + ν10 | 2676 w | 2661 w | ν5 + ν10 |
| 2328 vs. N2 | ||||
| 1553 s, O2 | ||||
| 1506 mw | ν15 + ν16 | 1511 m | ||
| 1492 m | ν5 | 1496 m | ||
| 1487 m, sh | ν22 | 1487 w | 1489 m, br | ν5 |
| 1474 ms | 1475 m, br, sh | ν22 | ||
| 1466 m | ν6 | 1466 ms | ||
| 1452 m | ν23 | 1453 vw | 1451 m, br | ν23 |
| 1371 w | ν7 | 1375 mw | 1367 w | ν7 |
| 1343 w | ν24 | 1344 m | 1337 w | ν24 |
| 1317 vw | ν8 | 1315 w | ||
| 1296 w | ν25 | 1304 w | ||
| 1287 w, sh | ν25 second site | 1288 mw, vbr, sh | ν25 | |
| 1246 m | ν26 | 1254 ms | 1253 m, vbr, sh | ν26 |
| 1239 m, sh | ν9 | 1244 ms | ||
| 1214 vw | ν9 second site | 1227 m | ν9 | |
| 1190 m | ||||
| 1181 w | 1179 w, br | ν10 | ||
| 1176 w | ν10 | 1173 w | ||
| 1147 w | ν11 | 1145 m | 1140 w, sh | ν11 |
| 1066 w | ν28 | 1058 m | 1072 mw | ν28 |
| 1030 m | ν12 | 1040 s | 1030 m, br | ν12 |
| 961 w | ν29 | 961 mw | 949 w, sh | ν29 |
| 925 s | ν13 | 929 vs | ||
| 920 w | ||||
| 906 m | ν30 | 910 w | 914 vs | ν13 |
| 894 m | ν14 | 883 vs | 902 s, sh | ν30 |
| 872 w | ν31 | 878 m, sh | ||
| 868 m | ||||
| 848 m | 844 w, sh | ν15 | ||
| 838 w | ν15 | 841 m | ||
| 662 w | ν16 | 667 m | 654 w, br | ν16 |
| 577 w | ν32 | 586 m | 598 w, br | ν32 |
| 299 mw, br | ν17 radial mode | |||
| 283 vw, br | ν17 | 284 w | ν17 radial mode | |
| 245 mw, asym, br | ν33 quasi pseudorotational mode | |||
| 136 mw | lattice mode | |||
| 125 m | lattice mode | |||
| 108 m | lattice mode | |||
| 94 w | lattice mode | |||
| 80 mw | lattice mode | |||
| 69 mw | lattice mode | |||
| 24 w | lattice mode | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohaček-Grošev, V. Low Temperature Raman Spectroscopy of Tetrahydrofuran: Phonon Spectra Compared to Matrix Isolation Spectra in Air. Crystals 2024, 14, 468. https://doi.org/10.3390/cryst14050468
Mohaček-Grošev V. Low Temperature Raman Spectroscopy of Tetrahydrofuran: Phonon Spectra Compared to Matrix Isolation Spectra in Air. Crystals. 2024; 14(5):468. https://doi.org/10.3390/cryst14050468
Chicago/Turabian StyleMohaček-Grošev, Vlasta. 2024. "Low Temperature Raman Spectroscopy of Tetrahydrofuran: Phonon Spectra Compared to Matrix Isolation Spectra in Air" Crystals 14, no. 5: 468. https://doi.org/10.3390/cryst14050468
APA StyleMohaček-Grošev, V. (2024). Low Temperature Raman Spectroscopy of Tetrahydrofuran: Phonon Spectra Compared to Matrix Isolation Spectra in Air. Crystals, 14(5), 468. https://doi.org/10.3390/cryst14050468






