Growth of Spontaneous Nucleation AlN Crystals by Al-Base Alloy Evaporation in Nitrogen Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. The Sintering of Al-Base Alloy
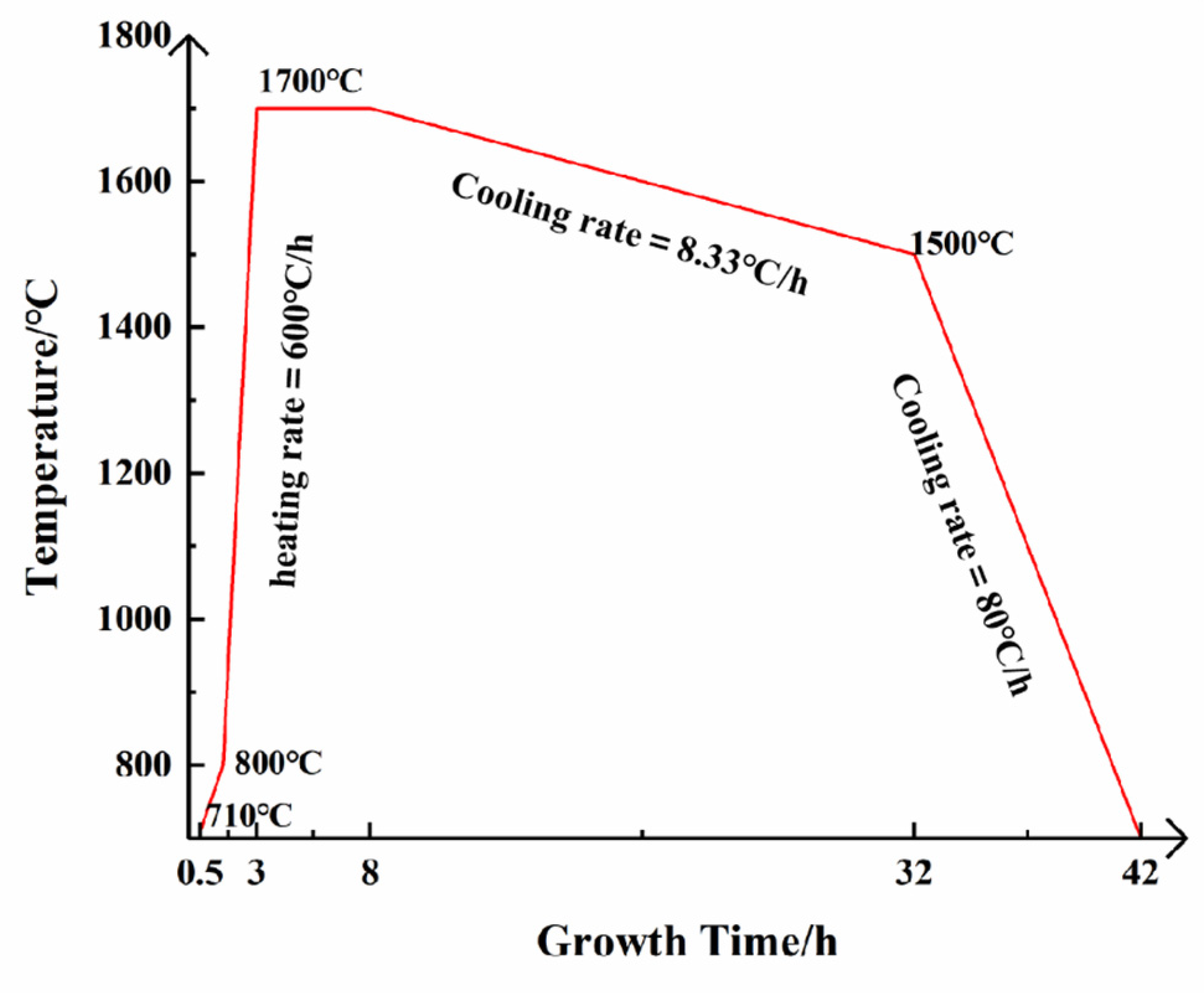
2.2. The AlN Crystals Growth by the Modified Vapor Transport Method
2.3. The Cleanliness of AlN Crystals
2.4. Characterizations
3. Results and Discussions
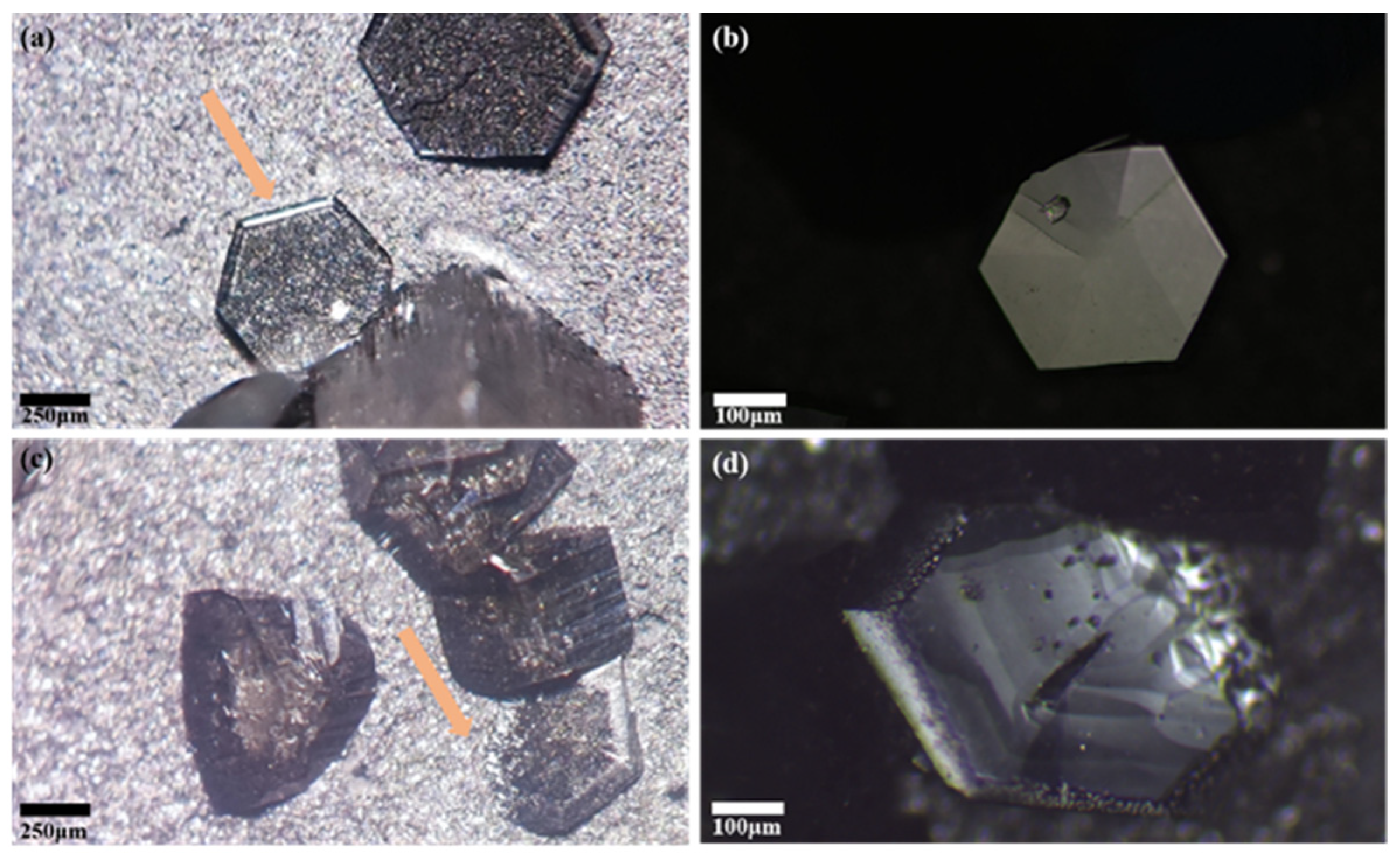
3.1. Surface Morphology of Spontaneous Nucleation AlN Crystals
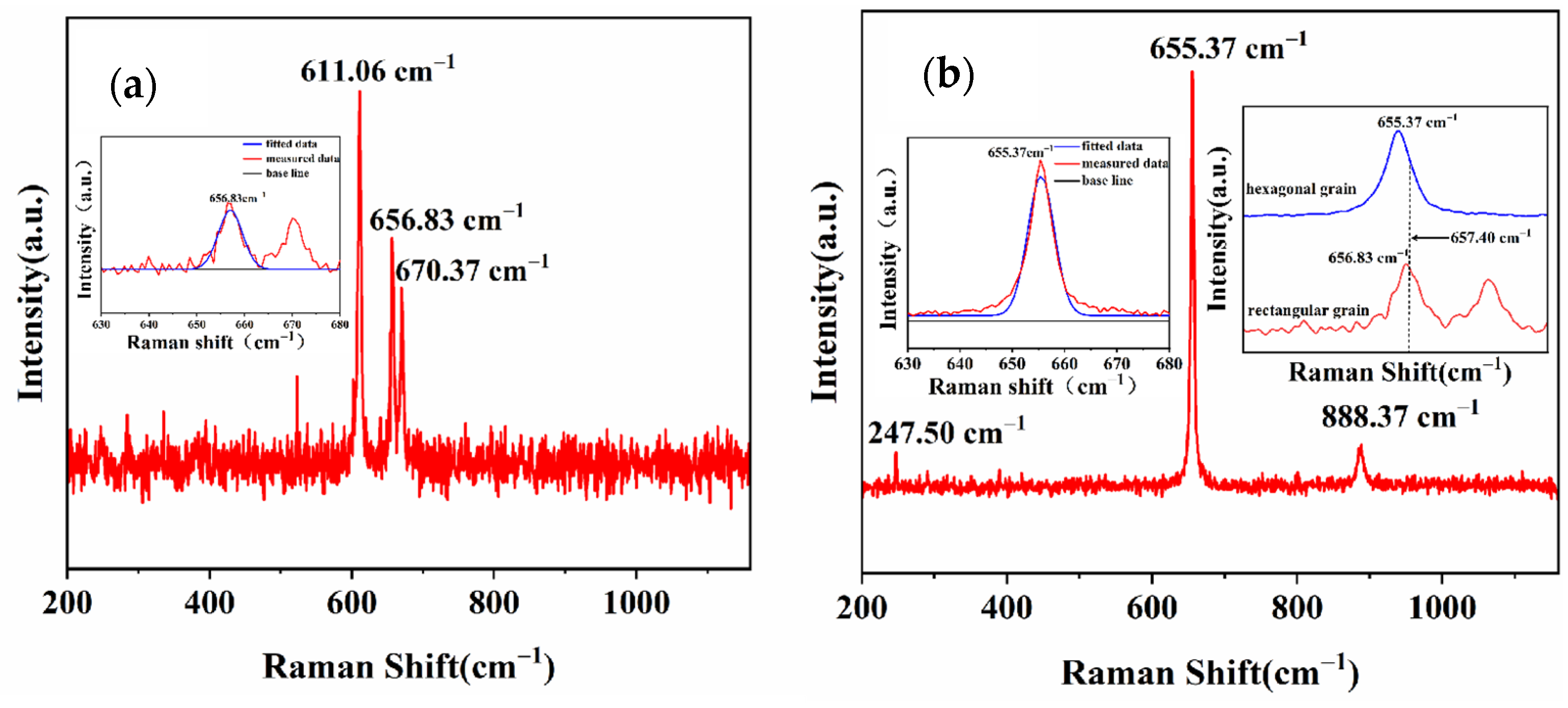
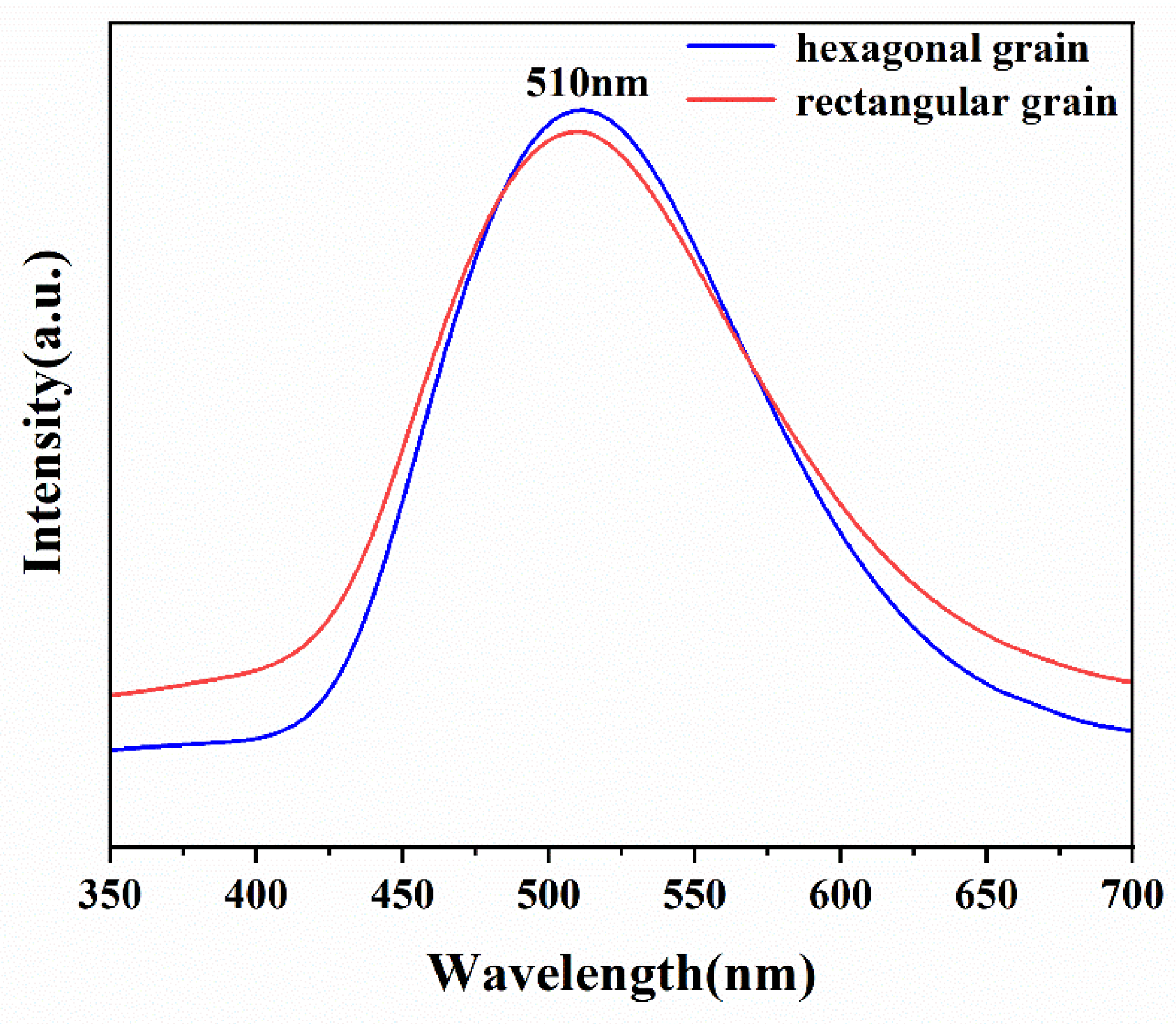
3.2. Crystalline Quality of Spontaneous Nucleation AlN Crystals
3.3. Growth Mechanism of Spontaneous Nucleation AlN Crystals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strite, S.; Morkoç, H. GaN, AlN, and InN: A review. J. Vac. Sci. Technol. B 1992, 10, 1237–1266. [Google Scholar] [CrossRef]
- Grandusky, J.R.; Chen, J.; Gibb, S.R.; Mendrick, M.C.; Moe, C.G.; Rodak, L.; Garrett, G.A.; Wraback, M.; Schowalter, L.J. 270nm Pseudomorphic Ultraviolet Light-Emitting Diodes with Over 60 mW Continuous Wave Output Power. Appl. Phys. Express 2013, 6, 032101. [Google Scholar] [CrossRef]
- Slack, G.A.; Bartram, S.F. Thermal Expansion of Some Diamondlike Crystals. J. Appl. Phys. 1975, 46, 89–98. [Google Scholar] [CrossRef]
- Kneissl, M.; Yang, Z.; Teepe, M.; Knollenberg, C.; Schmidt, O.; Kiesel, P.; Johnson, N.M.; Schujman, S.; Schowalter, L.J. Ultraviolet Semiconductor Laser Diodes on Bulk AlN. J. Appl. Phys. 2007, 101, 123103. [Google Scholar] [CrossRef]
- Herro, Z.G.; Zhuang, D.; Schlesser, R.; Sitar, Z. Growth of AlN Single Crystalline Boules. J. Cryst. Growth 2010, 312, 2519–2521. [Google Scholar] [CrossRef]
- Selvaduray, G.; Sheet, L. Aluminium Nitride: Review of Synthesis Methods. Mater. Sci. Technol. 2013, 9, 463–473. [Google Scholar] [CrossRef]
- Li, S.; Adachi, M.; Ohtsuka, M.; Fukuyama, H. Synthesis of AlN Single Crystal by Solution Growth Method Using Type 430 Ferritic Stainless Steel Flux. AIP Adv. 2023, 13, 085105. [Google Scholar] [CrossRef]
- Witzke, H.D. Über Wachstum von AIN-Einkristallen aus der Dampfphase. Phys. Status Solidi 2006, 2, 1109–1114. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, B.; Hu, H.; Wang, Y.; Kong, Z.; Wu, Y.; Shao, Y.; Hao, X. State-of-the-Art and Prospective Progress of Growing AlN Substrates by Physical Vapor Transport. J. Cryst. Growth 2023, 617, 127276. [Google Scholar] [CrossRef]
- Drum, C.M. Axial Imperfections in Filamentary Crystals of Aluminum Nitride. I. J. Appl. Phys. 1965, 36, 816–823. [Google Scholar] [CrossRef]
- Taylor, K.M.; Lenie, C. Some Properties of Aluminum Nitride. J. Electrochem. Soc. 1960, 107, 308–314. [Google Scholar] [CrossRef]
- Schlesser, R.; Sitar, Z. Growth of Bulk AlN Crystals by Vaporization of Aluminum in a Nitrogen Atmosphere. J. Cryst. Growth 2002, 234, 349–353. [Google Scholar] [CrossRef]
- Kobayashi, R.; Fukutomi, Y.; Takagi, T. Synthesis of AlN Needles by Nitridation of Al-Si Melt. J. Ceram. Soc. Jap. 2016, 124, 1161–1163. [Google Scholar] [CrossRef]
- Wu, P.; Funato, M.; Kawakami, Y. Environmentally Friendly Method to Grow Wide-Bandgap Semiconductor Aluminum Nitride Crystals: Elementary Source Vapor Phase Epitaxy. Sci. Rep. 2015, 5, 17405. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Mutharasan, R.; Koczak, M. Feasibility of Aluminium Nitride Formation in Aluminium Alloy. Mater. Sci. Eng. A 1995, 195, 121–129. [Google Scholar] [CrossRef]
- Song, Y.; Kawamura, F.; Taniguchi, T.; Shimamura, K.; Ohashi, N. Conditions for growth of AlN single crystals in Al-Sn flux. J. Am. Ceram. Soc. 2018, 101, 876–4879. [Google Scholar] [CrossRef]
- Yonemura, M.; Kamei, K.; Munetoh, S. Precipitation of Single Crystalline AlN from Cu-Al-Ti Solution under Nitrogen Atmosphere. J. Mater. Sci. Mater. Electron. 2005, 16, 197–201. [Google Scholar] [CrossRef]
- Kazuma, T.; Hiroyuki, O.; Noriyuki, H.; Takeshi, K. Decomposition of the Anisotropic Strain in 3D-Sturcture GaN Layers Using Raman Spectroscopy. Sci. Rep. 2023, 14, 3330. [Google Scholar]
- Zheng, W.; Zheng, R.; Huang, F.; Wu, H.; Li, F. Raman Tensor of AlN Bulk Single Crystal. Photonics Res. 2015, 3, 38. [Google Scholar] [CrossRef]
- Cros, A.; Wang, J.; Demangeot, F.; Péchou, R.; Daudin, B. Splitting of the Surface Phonon Modes in Wurtzite Nanowires. Jpn. J. Appl. Phys. 2013, 52, 08JL1. [Google Scholar] [CrossRef]
- Liu, L.; Liu, B.; Edgar, J.H.; Rajasingam, S.; Kuball, M. Raman Characterization and Stress Analysis of AlN Grown on SiC by Sublimation. J. Appl. Phys. 2002, 92, 5183–5188. [Google Scholar] [CrossRef]
- Prokofyeva, T.; Seon, M.; Vanbuskirk, J.; Holtz, M.; Nikishin, S.A.; Faleev, N.N.; Temkin, H.; Zollner, S. Vibrational Properties of AlN Grown on (111)-Oriented Silicon. Phys. Rev. B 2001, 63, 125313. [Google Scholar] [CrossRef]
- Kuball, M.; Hayes, J.M.; Prins, A.D.; van Uden, N.W.A.; Dunstan, D.J.; Shi, Y.; Edgar, J.H. Raman Scattering Studies on Single-Crystalline Bulk AlN under High Pressures. Appl. Phys. Lett. 2001, 78, 724–726. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Wang, Z.; He, G.; Lei, D.; Gong, J.; Wu, L. Anisotropic Three-Dimensional Thermal Stress Modeling and Simulation of Homoepitaxial AlN Single Crystal Growth by the Physical Vapor Transport Method. Cryst. Growth Des. 2018, 18, 2998–3007. [Google Scholar] [CrossRef]
- Payman, K.; Antoinette, M.M. Modeling micromechanical response to thermal history in bulk grown aluminum nitride. Phys. Status Solidi C 2015, 12, 345–348. [Google Scholar]
- Kuball, M.; Hayes, J.M.; Shi, Y.; Edgar, J.H.; Prins, A.D.; van Uden, N.W.A.; Dunstan, D.J. Raman Scattering Studies on Single-Crystalline Bulk AlN: Temperature and Pressure Dependence of the AlN Phonon Modes. J. Cryst. Growth 2001, 231, 391–396. [Google Scholar] [CrossRef]
- Sarua, A.; Kuball, M.; Van Nostrand, J.E. Deformation Potentials of the E2(high) Phonon Mode of AlN. Appl. Phys. Lett. 2002, 81, 1426–1428. [Google Scholar] [CrossRef]
- Akiyama, T.; Nakamura, K.; Ito, T. Ab Initio-Based Study for Adatom Kinetics on AlN (0001) Surfaces during Metal-Organic Vapor-Phase Epitaxy Growth. Appl. Phys. Lett. 2012, 100, 251601. [Google Scholar] [CrossRef]
- Hartmann, C.; Wollweber, J.; Dittmar, A.; Irmscher, K.; Kwasniewski, A.; Langhans, F.; Neugut, T.; Bickermann, M. Preparation of Bulk AlN Seeds by Spontaneous Nucleation of Freestanding Crystals. Jpn. J. Appl. Phys. 2013, 52, 8JA06. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, X.; Zhao, Q.; Wu, J.; Yu, T. Oxygen Reduction Through Specific Surface Area Control of AlN Powder for AlN Single-Crystal Growth by Physical Vapor Transport. Semicond. Sci. Technol. 2024, 39, 025006. [Google Scholar] [CrossRef]
- Slack, G.A.; Schowalter, L.J.; Morelli, D.; Freitas, J.A. Some Effects of Oxygen Impurities on AlN and GaN. J. Cryst. Growth 2002, 246, 287–298. [Google Scholar] [CrossRef]
- Kamei, K.; Shirai, Y.; Tanaka, T.; Okada, N.; Yauchi, A.; Amano, H. Solution Growth of AlN Single Crystal Using Cu Solvent under Atmospheric Pressure Nitrogen. Phys. Status Solidi C 2007, 4, 2211–2214. [Google Scholar] [CrossRef]
- Chen, W.; Qin, Z.; Tian, X.; Zhong, X.; Sun, Z.; Li, B.; Zheng, R.; Guo, Y.; Wu, H. The Physical Vapor Transport Method for Bulk AlN Crystal Growth. Molecules 2019, 24, 1562. [Google Scholar] [CrossRef] [PubMed]
- Drum, C.M.; Mitchell, J.W. Electron Microscopic Examination of Role of Axial Dislocations in Growth of AlN Whiskers. Appl. Phys. Lett. 1964, 4, 164–165. [Google Scholar] [CrossRef]
- Jindal, V.; Shahedipour-Sandvik, F. Density Functional Theoretical Study of Surface Structure and Adatom Kinetics for Wurtzite AlN. J. Appl. Phys. 2009, 105, 084902. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Jiang, S.; Ning, L.; Wang, Y.; Chen, X.; Xu, X.; Wang, J.; Jiang, M. Relationship between Appearance Crystalline Planes and Growth Temperatures during Sublimation Growth of AlN Crystals. J. Cryst. Growth 2006, 293, 93–96. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Xu, Y.; Chen, J.; Yu, Y.; Qi, X.; Ma, W.; Hu, Z. Growth of Spontaneous Nucleation AlN Crystals by Al-Base Alloy Evaporation in Nitrogen Atmosphere. Crystals 2024, 14, 331. https://doi.org/10.3390/cryst14040331
Tao X, Xu Y, Chen J, Yu Y, Qi X, Ma W, Hu Z. Growth of Spontaneous Nucleation AlN Crystals by Al-Base Alloy Evaporation in Nitrogen Atmosphere. Crystals. 2024; 14(4):331. https://doi.org/10.3390/cryst14040331
Chicago/Turabian StyleTao, Xiaochun, Yongkuan Xu, Jianli Chen, Yonggui Yu, Xiaofang Qi, Wencheng Ma, and Zhanggui Hu. 2024. "Growth of Spontaneous Nucleation AlN Crystals by Al-Base Alloy Evaporation in Nitrogen Atmosphere" Crystals 14, no. 4: 331. https://doi.org/10.3390/cryst14040331
APA StyleTao, X., Xu, Y., Chen, J., Yu, Y., Qi, X., Ma, W., & Hu, Z. (2024). Growth of Spontaneous Nucleation AlN Crystals by Al-Base Alloy Evaporation in Nitrogen Atmosphere. Crystals, 14(4), 331. https://doi.org/10.3390/cryst14040331





