Abstract
Owing to their exceptional properties, which are usually determined by the growth conditions, 2D transition metal dichalcogenides (TMDCs) offer numerous research directions for applications in the fields of spintronics, valleytronics, and optoelectronics. Here, we focus on the chemical vapor deposition (CVD) synthesis of WSe2 (tungsten diselenide) nanoclusters/nanoflakes by using a liquid precursor for tungsten (ammonium metatungstate) on Si/SiO2, fused silica, and sapphire substrates. Various WSe2 clusters with different sizes, thicknesses, and geometries were analyzed by means of optical and atomic force microscopy (AFM) and Raman spectroscopy. The observed structures were mostly WSe2 multilayers; however, monolayer formations were also found. They showed significant morphological differences, as well as wide nucleation density and size variations, possibly related to precursor/substrate surface interactions under the same CVD synthesis conditions. The largest WSe2 domains with a lateral size of up to hundreds of micrometers were observed on sapphire, probably caused by a higher growth rate of singular nucleation sites. WSe2 domains with irregular and triangular shapes were simultaneously identified on fused silica, whereas multilayered pyramidal WSe2 structures dominated in the case of Si/SiO2 substrates. The application of polarized Raman spectroscopy to precisely determine and differentiate the characteristic vibrational modes (, , and ) enabled the unambiguous identification of 2D and/or multilayered WSe2 formations with a high crystallinity level. The presented comparative analysis of samples prepared in relatively simple synthesis conditions (moderate working temperatures and ambient pressure) provides a base for further progress of the facile metatungstate CVD method and relevant opportunities for the exploration of 2D TMDC materials.
1. Introduction
The unique 2D physical nature of transition metal dichalcogenide (TMDC) materials offers numerous research directions in the fields of spintronics, valleytronics, and optoelectronics due to the materials’ exceptional properties, which are predominantly determined by the growth conditions.
The TMDC materials’ individual electronic band structure creates a unique spectrum of topological, magnetic, and electric phenomena. Tungsten diselenide [1,2,3,4] is a widely studied material that belongs to the constantly progressing group of layered 2D materials [5]. As is typical for 2D TMDCs, the manifestation of various physical properties in WSe2 depends on the layer number due to the weak van der Waals interlayer and strong intralayer ionic/covalent bonding.
The distinctive physical characteristics of WSe2 include p-type semiconductor behaviors with strong spin–orbital coupling [6], a low thermal conductivity [7], a direct/indirect band gap [8] evolving with the number of layers [9], and corresponding intense excitonic [10] and light–matter interaction effects [11]. The combination of these properties offers perspectives for a variety of WSe2-based spintronic [12], valleytronic [13], and optoelectronic devices. In addition, the broad set of TMDC properties is highly dependent on the structural configurations and the thickness/number of layers, which are determined by the synthesis approach. Currently, the fundamental and practical WSe2 investigations [2,3,4] are dependent on the development of techniques such as chemical vapor deposition (CVD) [14,15,16] and preparation strategies such as mechanical exfoliation and transfer [17,18,19,20,21].
The CVD synthesis of W-based TMDC [20] is challenging due to the high melting (evaporation/sublimation) points, requirements for low vapor pressures, minimal electronegativity ratio with chalcogenide elements, and limited chemical reactivity (especially for Te). In order to achieve suitable growth conditions, an efficient CVD process requires the use of high-volatility precursors, an enriched H2 carrier/reactive gas atmosphere (and/or low pressure), and heterogenic approaches using additional reagents as reaction catalysts, growth and eutectics promoters [22,23,24,25]. However, until technological feasibility and scalability requirements are met, there are many remaining obstacles to precise control over all the variables of nucleation, growth, and coalescence processes toward the realization of continuous 2D films.
Generally, powdered WO3 is the most widely used precursor, along with various W-based carbonates, hexachlorides, and oxychlorides [26,27] (for metal–organic CVD). To accomplish synthesis in a 2D form, the conventional CVD method uses the vapor–solid–solid (VSS) mechanism based on WO3 adsorption on the substrate and corresponding chemical reactions facilitated through surface chalcogenide diffusion [28,29,30,31,32].
Other prospective precursor groups include sodium tungstate Na2WO4 [33] and complex ammonia compounds such as metatungstate hydrate (AMT) (NH4)6[H2W12O40]·nH2O [34,35,36,37,38,39,40]. These precursors have been used for a vapor–liquid–solid (VLS) CVD process [25], as well as low-temperature hydro(solvo)thermal and template-assisted synthesis [21] for the preparation of Mo- [41,42] and V-based TMDC systems [37,38,39].
As an alternative, metatungstate precursors are widely accessible from chemistry suppliers and are less toxic compared with the conventional W-based chloride and oxycarbonate reagents. Furthermore, we must note their suitable aqueous solubility (in contrast to WO3), which provides unique options for solution-based direct deposition over various substrates and the practical advantage of a single-step conformal TMDC coating over 3D structures such as optical fibers [43], which is not accessible in VSS CVD. This can effectively simplify the CVD process requirements regarding the thermal and hydrogen-enriched reaction atmosphere conditions.
Moreover, this approach allows for particular control of the location and size of the growing TMDC seed islands [41] for the formation of 1D structures (nanowires) [18], 2D clusters (flakes and ribbons) [44], and 3D forms (pyramids) [45]. Another advantageous aspect is the possibility of chemical modifications via precursor mixing [36,37,38,39,40,46] and more precise doping for tailoring of the electronic, optical, and magnetic properties. The unique prospects of the liquid phase precursor method [47] have also been developed toward the preparation of lateral TMDC hetero-interfaces as well as the implementation of industrial inkjet printing [48] and robust patterned deposition control [49] of 2D MoSe2, MoS2, WSe2, and SnSe2 layers [50].
One of the main synthesis challenges in liquid precursor-mediated CVD is related to the chemical complexity of the starting reagents. Principally, this could lead to a higher level of structural disorder in the final TMDC structures and limited practical performance of base electronic devices, such as the field-effect transistor (FET). Nevertheless, there are reports of prototype TMDC FET devices produced via the VLS approach that show characteristics (electron mobility, on–off ratio, and threshold voltage range) [51] comparable with those of devices fabricated using the conventional VSS precursors.
The metatungstate precursor can be utilized together with the large family of alkali metal growth promoters [52,53,54], which include organic sodium cholate (SC) hydrate (C24H39NaO5·nH2O), sodium hydroxide, and conventional salts [22,55,56]. These reagents have a positive effect on the CVD process by increasing the surface substrate/precursor adhesive adatom energy, thereby improving the eutectic and catalytic conditions for the chemical reactions by forming the necessary intermediate complexes [25,57].
To the best of our knowledge, the synthesis of WSe2 (or WS2, WTe2) using the metatungstate/sodium cholate method is mainly applied on Si/SiO2 substrates (and, very recently, on soda lime glass [48]) and is rather unexplored in the case of sapphire (using Na2WO4 [58]) and fused silica substrates. The high synthesis temperatures required for the vapor–solid CVD growth of WSe2 on quartz and sapphire [28,29,30,31,32] result in significant strain effects and an unusual step-edge-guided layer-over-layer growth process (in the latter case).
In the present study, we are focusing on the CVD synthesis of WSe2 using AMT as the main precursor, together with SC as a heterogeneous nucleation and growth promoter. Because TMDC’s growth process is determined by the dynamic eutectic interactions of the precursor/substrate interface, the experiments were performed using Si/SiO2, fused silica, and c-cut sapphire. In addition, an auxiliary study measuring the wettability (contact angle) and hydrophilic properties of the tungstate/cholate aqueous solution is presented. Following a CVD process with several facile steps (the specific details of which are presented herein), peculiar WSe2 cluster formations of different geometry, size, and thickness were obtained and analyzed by means of optical microscopy, AFM, and Raman spectroscopy. The observed sophisticated growth morphology related to the synthesis conditions and substrate/interface effects provides a base for further improvements and opens up novel directions in TMDC synthesis.
2. Materials and Methods
2.1. Precursor Deposition and CVD Synthesis Procedure
The base chemical reaction of WSe2 formation relies on the relatively low (600 °C) decomposition temperature (1) [59] of metatungstate (n = 1–22, CAS No. 12333-11-8) to stable m-WO3 (monoclinic), which then reacts with the Se vapor phase through the carrier gas (2) and intermediate gaseous precursor . The gas also acts as a reducing reaction agent for WSe2 formation via the VLS method [33].
Sodium cholate is an anionic, amphipathic complex consisting of both hydrophilic and hydrophobic radicals; thus, it is utilized as a stabilizing surfactant (such as in liquid phase exfoliation [60]) with the important role of enhancing the precursor’s adsorption on the substrate [61] and facilitating TMDC growth. Reaction (2) is also possibly mediated by SC, leading to the formation of intermediate Na-based byproduct compounds [62]. The exact nature of the entire set of chemical, crystallization, and growth processes of the liquid precursor-intermediated CVD approach is not yet fully understood [44,63]. We presume that it is similar to the model proposed recently for Na2WO4 [33] (Mo [64,65], Nb [66]) complexes within the framework of the vapor–liquid–solid mechanism for TMDC, where the eutectic precursor phase/substrate interface [66] and thermodynamic nucleation evolution [67,68] play key roles.
To prepare the aqueous solution precursor, we used 0.025 g of metatungstate (Alfa Aesar, CAS No. 12333-11-8) and 0.1 g of sodium cholate (Alfa Aesar CAS No. 206986-87-0) in 5 mL of deionized water, giving a mass ratio of 0.4:1:50, which is close to the principal (3.1 mM) solubility limit of the metatungstate [39]. We observed that after one week, the solution formed coagulated particles (probable SC micelle aggregation), which is detrimental for the next stages of the synthesis process; therefore, the aqueous precursor mixture was prepared shortly before the synthesis. In addition, the wettability and corresponding contact angles of the precursor solution were preliminary determined using a drop shape analyzer (via the sessile drop technique). The measured contact angle values are presented in Figure 1a and were as follows: for Si/SiO2, for fused silica, and for sapphire. These values indicate suitable wettability due to the salinity of the solution. This indirectly suggests a moderate surface energy as a key parameter determining the level of precursor molecule adhesion over the substrates. The spin coating uniformity is also limited by the low solubility of the tungstate/cholate mixture. Correspondingly, we performed several experiments to determine the optimal parameters for homogenous deposition over the substrate, achieved by means of dynamic spin coating (for 60 sec) in the range of 6000–8000 rpm. The precursor solution was applied in microliter droplet amounts (after the initial spinning acceleration), considering the type of the selected substrate (Si/SiO2, sapphire, and fused silica), to a final amount of approximately 10 ÷ 25 µL/cm2 (shown in Figure 1b for Si/SiO2).
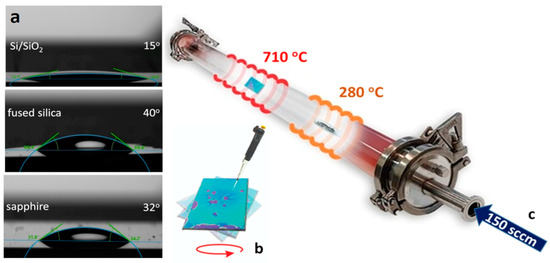
Figure 1.
(a) Contact angles of the metatungstate/cholate aqueous solution over the substrates; (b) spin-coated precursor over Si/SiO2; (c) schematic and parameters of the CVD process.
The main parameters and a schematic of the CVD synthesis process in a three-zone quartz tube reactor are presented in Figure 1c. The substrates covered with tungstate/cholate were placed in the central furnace zone (~710 °C), and the direct chemical selenization reaction was maintained by using ultra-high-purity Se (5 N/1 ÷ 1.5 g) shots in a quartz boat in the upstream thermal zone (~280 °C). The heating rates for the Se zone (18 °C/min) and WSe2 growth zone (44 °C/min) were synchronized to reach the target temperatures simultaneously. This was followed by a 2 h dwell stage at 710 °C for the selenization and growth (nucleation) process. The third (empty) zone was set to 730 °C to improve the thermal plateau in the reaction zone. The CVD process was mediated under a 150 sccm flow of an Ar (90%)/H2 (10%) reactive carrier gas mixture. The pre-synthesis reactor atmosphere purge procedure was performed with the same gas mixture for 45 min.
2.2. Experimental Techniques
The growth features, shape formations, and layer thickness of deposited WSe2 were analyzed by means of optical and atomic force microscopy and polarized Raman spectroscopy.
The following standard substrates were used: Si/SiO2 (with a 300 nm oxide layer), fused silica (JGS2, with both sides polished and an rms roughness of <1 nm), and c-cut sapphire (synthetic standard with an rms roughness of ~0.3 nm) supplied by Ossila. Before the spin coating deposition of the precursor solution, the substrates were cleaned by standard wet chemistry protocols. No additional pre-treatments to modify the substrate surface were applied. The substrates’ wettability regarding the aqueous (DI) tungstate/cholate solution was determined by measuring the contact angle using a Krüss DSA25S drop shape analyzer.
Optical microscopy images of WSe2 clusters were observed using an Olympus BX53 microscope with reflected and scattered light in the dark field (DF) mode.
The Raman spectrum characterization was performed using a HORIBA Jobin Yvon Labram HR visible spectrometer in a backscattering geometry with a He-Ne laser (excitation wavelength at 633 nm). A high-resolution 1800 lines mm−1 grating was used, which was needed particularly to distinguish the close-lying WSe2 Raman peaks. Si standard and neon lines were used to calibrate the frequency, and the Raman line parameters were determined by means of fitting to Voigt profiles. The scattered radiation was captured using a CCD detector. The laser power was kept below 0.5 mW to avoid heating damage to the layers and corresponding measurement artifacts.
The nanoscale surface topography and thickness profile were analyzed using an Asylum Research MFP-3D atomic force microscope in the standard AC tapping mode (AC160TS-R3 tips).
3. Characterization and Analysis
3.1. Metatungstate Method for WSe2 Deposition on Si/SiO2
TMDC cluster synthesis is related to the crystal lattice symmetry of the given compound (hexagonal 2H for WSe2 or, for instance, orthorhombic 1T for WTe2) and can occur in typical triangle, hexagon [69], or nanobelt flake patterns [70]. The lateral size and shape of the clusters depend on the chemical potentials of the reacting elements and a favorable edge growth energy [71], which is determined by the CVD synthesis parameters, precursor types, and substrate effect.
In the case of WSe2 cluster formation on Si/SO2 substrate via the metatungstate approach, the optical microcopy observations show the expected equilateral triangular structures (Figure 2a,b), with sizes ranging between 3 and 10 µm.
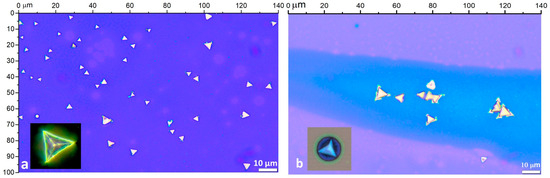
Figure 2.
Optical images of WSe2 on Si/SiO2 under reflected illumination: 100× magnification of equilateral flakes (a,b) with an enlarged view in DF mode ((a), inset) and local substrate corrosive effects ((b), inset) around the WSe2 pyramids.
However, the coverage and distribution density over the substrate area are relatively low, and clusters mostly formed in scattered groups within the zones with higher precursor accumulation during the spin coating deposition process. The kinks formations at the vertices and edges of some of the triangular domains (Figure 2b) are typical morphological features of VLS growth. These are usually developed as a crystallization irregularity due to a non-uniform molten precursor concentration at the liquid–solid interface [66,72]. In addition, the VLS process of binding the precursor’s nucleus onto the silicon oxide layer can cause local corrosive effects [54] (in the formed cluster’s vicinity), as shown in the inset image of Figure 2b.
The observed faceted morphology of the majority of the clusters (in the enlarged DF image), together with the AFM height profiles and the Raman data (presented in Figure 3a,b), reveals a predominantly vertical growth of thick multilayered WSe2 in a pyramidal form. The WSe2 pyramid apex thickness can reach up to hundreds of nanometers depending on the particular cluster size. Both the edge/side wall cross-sections are presented as profile graphs in Figure 3b.
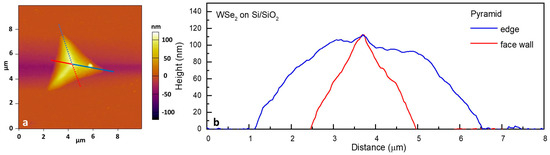
Figure 3.
AFM analysis of WSe2 on Si/SiO2 with a thickness map (a) and profiles of the pyramidal clusters (b).
As noted in the Introduction, the various TMDC growth structures often demonstrate unique physical properties [73,74,75]. For instance, pyramidal MoS2 structures [76] show sophisticated helical electrical current flow [77] and, additionally, high energy harvesting capabilities through an electrocatalytic hydrogen evolution reaction [78]. Among the TMDC clusters with similar 3D geometry, altered optical, magnetic, catalytic, and electrical properties were also found for MoS2 (strong excitonic absorption and cathodoluminescence [79]), WS2 (complex Raman spectrum [80], non-linear optical response, and catalytic activity [81,82]), WSe2 [83,84] (plasmonic effects), and in anti-pyramidal MoS2/WS2 vertical heterostructures [58] prepared via a VLS growth process.
A record non-linear optical (second and third harmonic) response and strong light–matter interactions were observed for nanopyramidal MoTe2 [45] (obtained via liquid precursor-mediated CVD) with an edge-rich structure. In addition, higher optical harmonics generation was investigated for WS2 with enhanced intensity as the thickness increased [82], reaching several orders of magnitude for a thickness (~100 nm) comparable with that in our case.
The Raman signal sensitivity regarding peak positions, shifts, and intensity relations provides quite reliable indications of the TMDC thickness (and, consequently, the layer number) [85,86,87,88]. Typically, for WSe2, the main Raman modes are the mode, caused by out-of-phase vibrations of the Se sublattice perpendicular to the layer planes, and the weaker peak, attributed to in-plane oscillations of both tungsten and selenium atoms; however, their spectral positions are very close to each other, at around 250 cm−1.
These first-order phonons appear within a broad band consisting of second-order features, dominated by the exceptionally intense mode, appearing typically at 633 nm excitation due to excitonic resonance. For single-layer WSe2, the mode is expected to be virtually degenerate with —both lying between 249.5 and 250 cm−1 [88]. With an increasing layer number, slightly hardens while softens, and the frequency distance between these modes saturates at about 3 cm−1 for thicknesses approaching those of bulk samples [88]. As can be expected from the different vibrational patterns of both modes, the Raman intensity ratio sharply increases with the layer number. The band undergoes a downshift from ~261 cm−1 to ~257 cm−1 with increasing WSe2 thickness from monolayers to trilayers [85,86,87,88]. Taken together, these dependencies can enable a semiquantitative estimate of the WSe2 thickness. The possible presence of a faint peak close to 308 cm−1 could be considered as an additional indication. This peak, which is assigned to the forbidden mode (modulating the vertical W-Se bond) by some authors, is reportedly activated in the Raman spectrum of multilayered WSe2 by van der Waals interactions between adjacent layers [88]. In order to accurately identify the corresponding spectral tendencies for the WSe2 layers, polarized backscattering Raman spectroscopy was implemented using the opposite polarization properties of the and modes, with being allowed only in parallel and only in crossed polarization geometry. Figure 4a,b shows the two polarized Raman spectra for a selected WSe2 pyramid cluster.
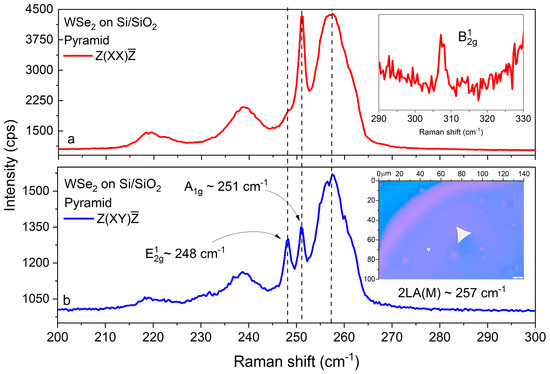
Figure 4.
Raman characterization of WSe2 on Si/SiO2: (a) parallel- and (b) cross-polarized Raman spectra of a pyramidal WSe2 domain, with main peaks identification and an enlarged view (inset (a)) of . Inset (b): optical image of the studied pyramidal cluster.
We observed the peak at ~248 cm−1, the peak at ~251 cm−1, and a redshifted band close to ~257 cm−1 (see Figure 4a,b), as expected for a multilayered structure [85,86,87,88] and in accordance with the AFM results (Figure 3). Furthermore, there was a well-formed peak at 308 cm−1. The intensity exceeds that of the line by an order of magnitude, and its forbidden appearance in the cross-polarized spectra with an intensity comparable with that of the allowed peak facilitates the precise determination of the frequency distance for these modes.
3.2. Metatungstate Method for WSe2 Deposition on Fused Silica
The WSe2 produced via metatungstate deposition on fused silica formed different complex structures. An optical microscopy gallery of the different formations is presented in Figure 5a–c. The majority of the clusters had circular/oval shapes with lateral size ranging within 10–15 µm and a relatively dense and homogeneous distribution (Figure 5a). There were also areas where highly crystalline equilateral domains with a smaller size (~5–10 µm) formed (Figure 5b). In addition, we observed more sophisticated patterns, usually labeled as TMDC fractal and “kirigami” shapes [75,89], such as hollow pyramids, spiral and dent rings, and propellers (Figure 5c enlarged) with both AA(A-) and AB(A-) symmetric layer stacking arrangements [90]. This complexity shows that cluster nucleation is probably affected by a broad set of factors, such as the local structural disorder of the substrate and irregular precursor accumulation [19] with variations in the W/Se stoichiometric ratio. We can also consider possible re-evaporation [91] and chemically driven etching effects during the CVD process [92].

Figure 5.
Optical images of WSe2 on fused silica under reflected illumination and 100× magnification of the dominant cluster forms (a), symmetrical triangular and ring structures (b), and an enlarged view of kirigami-type “ring” and “propeller” WSe2 structures (c).
For selected triangular WSe2 clusters on fused silica, AFM images and topographic profiles are shown in Figure 6a–f. In order to express the morphological formations and growth relief in sharper contrast, 2D maps of the AFM height and tapping mode phase variations are also presented.
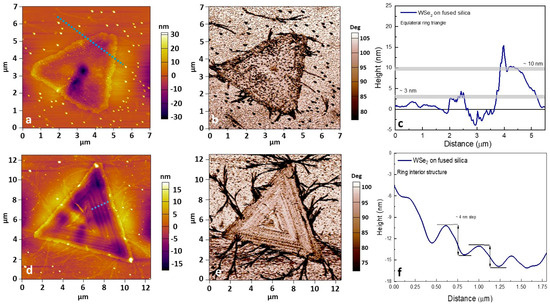
Figure 6.
AFM (a–f) height and phase retrace maps for WSe2 on fused silica, thickness profiles of selected triangular flakes, and the complex step-like internal relief of the WSe2 “rings”.
The AC-mode phase shifts are related to the viscoelastic mechanical properties and allow us to differentiate the deposited TMDC layers from the substrate. The thickness profile identifies ~3–10 nm multilayered equilateral WSe2 with observed inner concavities, probably due to the corrosive reaction [54] between the molten precursor and the silica oxide substrate during VLS crystallization (similar to that for Si/SiO2). The complexity of the WSe2 ring dent structure is also revealed by ~3–4 nm step-like formations in the interior (Figure 6f).
The cross- and parallel-polarization Raman spectra of WSe2 on fused silica are presented in Figure 7a–d for two selected triangular clusters: a flat one (left panel) and one with a supposed step-like ring structure (right panel).
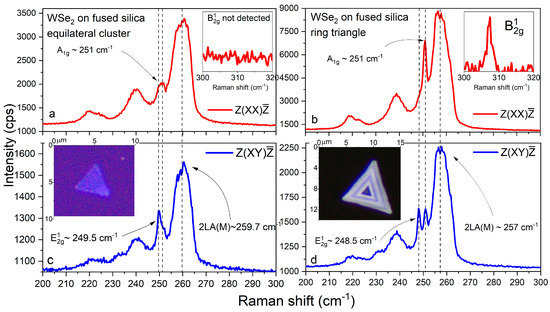
Figure 7.
Cross- and parallel-polarized Raman spectra (a–d) of mono- and multilayer WSe2 clusters on fused silica. Insets: optical images of investigated WSe2 domains.
The – distance for the flat cluster amounts to 1.5 cm−1, the band is centered near 260 cm−1, and no peak was detected near 308 cm−1. This implies that the flat cluster consists of monolayer and bilayer regions. The mode positions for the ring cluster ( at 248.5 cm−1, at 251 cm−1, and at ~257 cm−1) evidence a significantly greater thickness, additionally confirmed by the presence of the forbidden peak around 308 cm−1 [85,86,88,93].
3.3. Metatungstate Method for WSe2 Deposition on Sapphire
The growth of WSe2 on sapphire also led to a rich set of cluster geometries and sizes, presented in Figure 8a–c. In this case, large polygonal formations with “saw-tooth” [91] edges were observed (Figure 8a). Their lateral size reached dozens of micrometers, and the domains comprised numerous multilayered bulk islands, possibly induced by singular nucleation sites. On the microscale, individual triangular flakes were also observed (Figure 8b), together with larger polygonal formations. Their equilateral geometry usually marks a highly crystalline structure with a relative lateral size of 10 µm. Like in the case of Si/SiO2, on sapphire substrate, pyramidal structures that were 10–40 µm in size with a typical faceted morphology were observed (Figure 8c). This particular growth is probably mediated by localized vertical precursor accumulations [49] or substrate defects that act as seeding centers [94]. The rounded edges of the peripheral layer also usually develop because of an irregular precursor distribution during WSe2 formation. The DF illumination (enlarged inset image) demonstrates intensive light-scattering effects from the pyramid walls. As noted, such 3D TMDC nanoarchitectures show extreme optical second and third harmonic generation with the potential for non-linear photonic applications [82].

Figure 8.
Optical images of WSe2 on sapphire (under reflected light and ×100 magnification) showing (a) formations with the largest lateral size, (b) equilateral triangular clusters, and (c) pyramidal clusters of WSe2 with DF illumination (inset).
AFM scan maps of a selected cluster are presented in Figure 9a–c. We determined the topographic profile of one selected equilateral triangular WSe2 (~10 µm) cluster. To express the flake/substrate differences, the nanometer topological gradient map (a) and an AFM phase contrast image (b) are also presented.
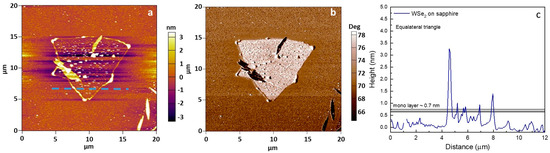
Figure 9.
AFM height (a) and phase retrace (b) 2D maps for WSe2 on sapphire with the thickness profile (c) of the monolayer cluster.
The topographic profile in Figure 9c marks a cluster thickness of ~0.7 nm, close to that expected for a WSe2 monolayer [16]. The observed height increase (a few nanometers) is possibly related to multilayer formation at the edges as a consequence of inhomogeneous extra precursor at the faceted domain borders [95]. Alternatively, this could also be affected by the particular substrate morphology and localized defects [30,31]. The edge area feature was identified for all observed clusters (Figure 9a,b).
The polarized Raman spectra for selected mono- and multilayer WSe2 clusters on sapphire are presented in Figure 10a–d. In the former case, the virtually identical spectral positions of the (~249.7 cm−1) and (~249.8 cm−1) vibrational modes in the corresponding crossed and parallel polarization, the upshift of the band above 260 cm−1, and the absence of a peak unambiguously indicate that the cluster thickness is within the monolayer limit [85,86,87,88,93]. For the thicker cluster, the expected larger separation between (~249 cm−1) and (~251 cm−1) was observed, together with a small redshift of (~258 cm−1) and a discernible peak close to 308 cm−1. Still, these data imply a relatively thin, few-layered structure in contrast to the thicker multilayered clusters, for which the Raman data are shown in Figure 4a,b and 7a,b. Note the typical sharp peak structure of the main modes (as well as for Si/SiO2 and fused silica), which is characteristic of objects with a high quality of crystalline ordering.
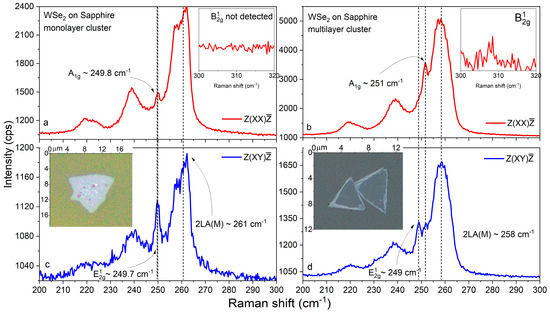
Figure 10.
Cross- and parallel-polarized Raman spectra (a–d) of mono- and multilayer WSe2 clusters on sapphire. Insets: optical images of the investigated WSe2 domains.
The Raman spectrum and, specifically, the out-of-plane mode , are very susceptible to possible doping effects, for instance, due to plausible Na inclusions (from sodium cholate) [96]. However, the spectral data for all samples on Si/SiO2, sapphire, and fused silica substrates fit the peak positions indicative of pure WSe2.
4. General Remarks
Overall, the presented data regarding the variety of WSe2 clusters in terms of their size, thickness, and shape illustrate the significant differences associated with the studied substrates. These differences can be explained within the framework of the vapor–liquid–solid phenomenological model [33,64,65,66,67,68], where the growth mechanism is dependent on the eutectic vapor–liquid and liquid–solid interfaces. Importantly, the interfacial free energy of the molten W precursor nucleus specific for the particular substrate (Si/SiO2, fused silica, or sapphire) determines its contact area and the volume profile of the grown nanocluster.
The eutectic intermediates [49,62] formed by the fusion of WO3 and alkali metal complexes (the final products of thermal decomposition of the metatungstate and the cholate promoter) strongly affect the growth kinetics. More precisely, the lowered melting point of the mixture facilitates thermodynamically favorable edge expansion on the substrate and aggregation into larger clusters. This is followed by crystallization into multilayer (vertical) or monolayer (lateral) clusters, also depending on the interfacial free energy [49]. The final WSe2 formation stages with chemical conversion are determined by Se super-saturation and dissolution into the molten WO3/alkali metal mixture [49] and hydrogen-induced reduction.
The simultaneous formation of monolayer and multilayer WSe2 (observed in the case of the sapphire substrate) and, especially, the presence of more composite clusters fractions can be ascribed to localized perturbations in the above growth process under a number of concurrent factors. This random morphological variation was observed for all substrate types. Nucleation into self-assembled multilayer structures could be caused by precursor accumulation and/or substrate defects acting as seed centers, promoting multilayer growth (pyramidal formations). Deviations from the highly crystalline triangular geometry can be associated with local stoichiometric imbalance (propeller flakes) and/or etching and substrate corrosion effects (for Si/SiO2 and fused silica) creating numerous ring dent clusters.
Another important factor is the adsorption effect of the precursors, utilized by the sodium cholate hydrate. The purpose of the surfactant seed promoter is to provide favorable heterogeneous nucleation sites. This is correspondingly determined by the interaction and anchoring of its polar (hydrophilic) and nonpolar (hydrophobic) parts with the precursor solvent and the substrate surface [52,54,61]. However, the intensity of these interactions depends on the different adhesive energies of the particular substrate materials and also influences the consequent CVD multilayer/monolayer formation process of WSe2. In this context, further optimization of the method is required to allow for precise thickness control and uniform planar growth. Nevertheless, it shows opportunities for the synthesis and engineering of TMDC structures with particular forms and applications. Based on the unique excitonic [10] and spintronic [12] characteristics of monolayer WSe2, together with the valleytronic [97] and memristive [98] functionalities of multilayered structures, the implemented liquid precursor-mediated CVD approach opens up many practical possibilities.
The presented variety of Raman spectra resulting from the challenging mono- and multilayered morphology of the WSe2 formations reveals the informative capabilities of polarized Raman spectroscopy for the analysis of TMDC. This is well demonstrated by tracking the spectral behavior (frequency shifts and intensity change) of the fundamental and vibration modes together with the second-order and the occasional presence of forbidden peaks, exploiting their high sensitivity to the layer number. In addition, the spectral parameters of the and Raman lines, such as the FWHM (full width of half-maximum) and their distance and intensity ratio, are summarized in Table 1 for all studied clusters.

Table 1.
Raman line characteristics for the studied WSe2 clusters: FWHM and peak intensity ratio calculated by Voigt fitting.
Our data are in very good agreement with the corresponding numerical values and trends presented in the pioneering work of Zhao et al. [88]. The slightly larger and linewidths in our spectra can be explained by the difference in growth methods. Our WSe2 clusters were obtained by means of direct CVD synthesis, similar to that used for the deposition of continuous layers, while the polarized Raman analysis in Ref. [88] was performed on exfoliated flakes from WSe2 crystals grown by means of chemical vapor transport.
5. Conclusions
In conclusion, we investigated the growth prospects for multi- and monolayer WSe2 formation (on Si/SiO2, fused silica, and sapphire substrates) using a facile CVD method based on the utilization of an ammonium metatungstate precursor and sodium cholate as a growth promoter. WSe2 synthesis was achieved via a simple preparation procedure at moderate working temperatures (~710 °C) and ambient pressure.
Various WSe2 formations with different sizes, layer numbers, and structures were observed and characterized via optical microscopy, AFM, and polarized Raman spectroscopy. It was shown that under the applied synthesis conditions, the metatungstate/cholate method resulted in the formation of both monolayer and multilayer WSe2 clusters.
A comprehensive polarized Raman analysis to accurately identify the first-( and ) and second-order () vibrational modes enabled us to obtain a reliable semiquantitative estimate of the WSe2 thickness for the variety of WSe2 structures observed on the different substrates.
A higher distribution density and coverage of the synthesized flakes were observed in the case of fused silica and sapphire. For sapphire, the maximal lateral size of the WSe2 flakes reached hundreds of micrometers. Meanwhile, in the case of Si/SiO2, most of the clusters were scattered pyramidal forms with heights of up to ~100 nm and lateral sizes of tens of micrometers. The presented results provide a base for further development of this simplistic metatungstate synthesis method towards improved growth control and reproducibility.
Author Contributions
Conceptualization, K.B., P.R., V.M. and D.D.; methodology, K.B., P.R., N.M., V.V., V.S., T.L., D.D. and V.M.; software, K.B.; validation, K.B. and P.R.; formal analysis, K.B. and P.R.; investigation, K.B., P.R., N.M., V.V., V.S., T.L., D.D. and V.M.; resources, D.D., V.M. and P.R.; data curation, K.B.; writing—original draft preparation, K.B.; writing—review and editing, K.B., V.M., D.D. and P.R.; visualization, K.B.; supervision, D.D. and V.M.; project administration, D.D. and V.M.; funding acquisition, D.D. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by the Bulgarian Science Fund under the grant numbers DFNI KП-06-ДO 02/2 and DFNI KП-06-ДO 02/3 within the framework of the M-ERA program project “Functional 2D materials and heterostructures for hybrid spintronic memristive devices”. Research equipment of the Distributed Research Infrastructure INFRAMAT, part of the Bulgarian National Roadmap for Research Infrastructures and supported by Bulgarian Ministry of Education and Science, was used in this investigation.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
N.M.: V.M., and P.R. acknowledge financial support from the European Regional Development Fund within the Operational Programme “Science and Education for Smart Growth 2014–2020” under the Project CoE “National Center of Mechatronics and Clean Technologies” BG05M2OP001-1.001-0008-C01.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohl, M.; Rautio, A.-R.; Asres, G.A.; Wasala, M.; Patil, P.D.; Talapatra, S.; Kordas, K. 2D Tungsten Chalcogenides: Synthesis, Properties and Applications. Adv. Mater. Interfaces 2020, 7, 2000002. [Google Scholar] [CrossRef]
- Eftekhari, A. Tungsten dichalcogenides (WS2, WSe2, and WTe2): Materials chemistry and applications. J. Mater. Chem. A 2017, 5, 18299–18325. [Google Scholar] [CrossRef]
- Cheng, Q.; Pang, J.J.; Sun, D.; Wang, J.J.; Zhang, S.; Liu, F.; Chen, Y.; Yang, R.; Liang, N.; Lu, X.; et al. WSe2 2D p-type semiconductor-based electronic devices for information technology: Design, preparation, and applications. InfoMat 2020, 2, 656–697. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, J.; Xu, K.; Chow, E.K.C.C.; Zhu, W. Material Synthesis and Device Aspects of Monolayer Tungsten Diselenide. Sci. Rep. 2018, 8, 5221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lin, J.; Huang, X.; Zhou, Y.; Chen, Y.; Xia, J.; Wang, H.; Xie, Y.; Yu, H.; Lei, J.; et al. A library of atomically thin metal chalcogenides. Nature 2018, 556, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shen, J.; Long, G.; Wu, Z.; Bao, Z.Q.; Liu, C.C.; Xiao, X.; Han, T.; Lin, J.; Wu, Y.; et al. Odd-Integer Quantum Hall States and Giant Spin Susceptibility in p-type Few-Layer WSe2. Phys. Rev. Lett. 2017, 118, 067702. [Google Scholar] [CrossRef]
- Wang, J.; Xie, F.; Cao, X.H.; An, S.C.; Zhou, W.X.; Tang, L.M.; Chen, K.Q. Excellent Thermoelectric Properties in monolayer WSe2 Nanoribbons due to Ultralow Phonon Thermal Conductivity. Sci. Rep. 2017, 7, 41418. [Google Scholar] [CrossRef]
- Desai, S.B.; Seol, G.; Kang, J.S.; Fang, H.; Battaglia, C.; Kapadia, R.; Ager, J.W.; Guo, J.; Javey, A. Strain-induced indirect to direct bandgap transition in multilayer WSe2. Nano Lett. 2014, 14, 4592–4597. [Google Scholar] [CrossRef]
- Zhao, W.; Ghorannevis, Z.; Chu, L.; Toh, M.; Kloc, C.; Tan, P.H.; Eda, G. Evolution of electronic structure in atomically thin sheets of WS2 and WSe2. ACS Nano 2013, 7, 791–797. [Google Scholar] [CrossRef]
- Courtade, E.; Semina, M.; Manca, M.; Glazov, M.M.; Robert, C.; Cadiz, F.; Wang, G.; Taniguchi, T.; Watanabe, K.; Pierre, M.; et al. Charged excitons in monolayer WSe2: Experiment and theory. Phys. Rev. B Condens. Matter Mater. Phys. 2017, 96, 085302. [Google Scholar] [CrossRef]
- Robert, C. When bright and dark bind together. Nat. Nanotechnol. 2018, 13, 982–983. [Google Scholar] [CrossRef]
- Sierra, J.F.; Fabian, J.; Kawakami, R.K.; Roche, S.; Valenzuela, S.O. Van der Waals heterostructures for spintronics and opto-spintronics. Nat. Nanotechnol. 2021, 16, 856–868. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Dong, B.; Wang, H.; Wang, H.; Zhang, Y.; Han, Z.; Zhang, H. Valley manipulation in monolayer transition metal dichalcogenides and their hybrid systems: Status and challenges. Rep. Prog. Phys. 2021, 84, 026401. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Tang, L.; Tan, J.; Nong, H.; Liu, B.; Cheng, H.-M. Chemical Vapor Deposition Growth of Two-Dimensional Compound Materials: Controllability, Material Quality, and Growth Mechanism. Acc. Mater. Res. 2020, 2, 36–47. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, M.; Wang, L.; Chen, Y.; Xing, Z.; Zhang, T.; Liu, Z.; Zuo, J.; Nan, F.; Mendes, R.G.; et al. Ultrafast Self-Limited Growth of Strictly Monolayer WSe2 Crystals. Small 2016, 12, 5741–5749. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, J.; Ding, F. Strategies, Status, and Challenges in Wafer Scale Single Crystalline Two-Dimensional Materials Synthesis. Chem. Rev. 2021, 121, 6321–6372. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.T.; Qu, K.; Chen, X.; Ahn, J.H. Large-area synthesis of transition metal dichalcogenides via CVD and solution-based approaches and their device applications. Nanoscale 2021, 13, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Ji, E.; Capasso, A.; Lee, G.-H.H. Recent Progresses in the Growth of Two-dimensional Transition Metal Dichalcogenides. J. Korean Ceram. Soc. 2019, 56, 24–36. [Google Scholar] [CrossRef]
- Brent, J.R.; Savjani, N.; O’Brien, P. Synthetic approaches to two-dimensional transition metal dichalcogenide nanosheets. Prog. Mater. Sci. 2017, 89, 411–478. [Google Scholar] [CrossRef]
- Han, J.H.; Kwak, M.; Kim, Y.; Cheon, J. Recent Advances in the Solution-Based Preparation of Two-Dimensional Layered Transition Metal Chalcogenide Nanostructures. Chem. Rev. 2018, 118, 6151–6188. [Google Scholar] [CrossRef]
- Xie, C.; Yang, P.; Huan, Y.; Cui, F.; Zhang, Y. Roles of salts in the chemical vapor deposition synthesis of two-dimensional transition metal chalcogenides. Dalt. Trans. 2020, 49, 10319–10327. [Google Scholar] [CrossRef]
- Wong, S.L.; Liu, H.; Chi, D. Recent progress in chemical vapor deposition growth of two-dimensional transition metal dichalcogenides. Prog. Cryst. Growth Charact. Mater. 2016, 62, 9–28. [Google Scholar] [CrossRef]
- You, J.; Hossain, M.D.; Luo, Z. Synthesis of 2D transition metal dichalcogenides by chemical vapor deposition with controlled layer number and morphology. Nano Converg. 2018, 5, 26. [Google Scholar] [CrossRef]
- Li, S. Salt-assisted chemical vapor deposition of two-dimensional transition metal dichalcogenides. iScience 2021, 24, 103229. [Google Scholar] [CrossRef]
- Bosi, M. Growth and synthesis of mono and few-layers transition metal dichalcogenides by vapour techniques: A review. RSC Adv. 2015, 5, 75500–75518. [Google Scholar] [CrossRef]
- Lan, S.; Zhang, Z.; Hong, Y.; She, Y.; Pan, B.; Xu, Y.; Wang, P. Judicious Selection of Precursors with Suitable Chemical Valence State for Controlled Growth of Transition Metal Chalcogenides. Adv. Mater. Interfaces 2023, 10, 2300713. [Google Scholar] [CrossRef]
- Alahmadi, M.; Mahvash, F.; Szkopek, T.; Siaj, M. A two-step chemical vapor deposition process for the growth of continuous vertical heterostructure WSe2/h-BN and its optical properties. RSC Adv. 2021, 11, 16962–16969. [Google Scholar] [CrossRef]
- Zhang, X.; Choudhury, T.H.; Chubarov, M.; Xiang, Y.; Jariwala, B.; Zhang, F.; Alem, N.; Wang, G.C.; Robinson, J.A.; Redwing, J.M. Diffusion-Controlled Epitaxy of Large Area Coalesced WSe2 Monolayers on Sapphire. Nano Lett. 2018, 18, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Aljarb, A.; Liu, S.; Li, P.; Ma, C.; Xue, F.; Lopatin, S.; Yang, C.W.; Huang, J.K.; Wan, Y.; et al. Growth of 2H stacked WSe2 bilayers on sapphire. Nanoscale Horiz. 2019, 4, 1434–1442. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Ge, M.; Ma, Y.; Abbas, A.N.; Zhou, C. Step-Edge-Guided Nucleation and Growth of Aligned WSe2 on Sapphire via a Layer-over-Layer Growth Mode. ACS Nano 2015, 9, 8368–8375. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ahn, G.H.; Cho, J.; Amani, M.; Mastandrea, J.P.; Groschner, C.K.; Lien, D.H.; Zhao, Y.; Ager, J.W.; Scott, M.C.; et al. Synthetic WSe2 monolayers with high photoluminescence quantum yield. Sci. Adv. 2019, 5, eaau4728. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.R.; Mehmood, N.; Çakiroǧlu, O.; Serkan Kasirga, T. Real time optical observation and control of atomically thin transition metal dichalcogenide synthesis. Nanoscale 2019, 11, 7317–7323. [Google Scholar] [CrossRef] [PubMed]
- Naylor, C.H.; Parkin, W.M.; Gao, Z.; Kang, H.; Noyan, M.; Wexler, R.B.; Tan, L.Z.; Kim, Y.; Kehayias, C.E.; Streller, F.; et al. Large-area synthesis of high-quality monolayer 1T’-WTe2 flakes. 2D Mater. 2017, 4, 021008. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, S.V.; Zhao, M.Q.; Masih Das, P.; Zhang, Q.; Price, C.C.; Gao, Z.; Shenoy, V.B.; Drndić, M.; Johnson, A.T.C. Controlled Growth of Large-Area Bilayer Tungsten Diselenides with Lateral P-N Junctions. ACS Nano 2019, 13, 10490–10498. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Joon Yun, S.; Jong Yu, W.; Hee Lee, Y.; Fan, S.; Yun, S.J.; Lee, Y.H.; Yu, W.J. Tailoring Quantum Tunneling in a Vanadium-Doped WSe2/SnSe2 Heterostructure. Adv. Sci. 2020, 7, 1902751. [Google Scholar] [CrossRef]
- Joon Yun, S.; Loc Duong, D.; Manh Ha, D.; Singh, K.; Luan Phan, T.; Choi, W.; Kim, Y.-M.; Hee Lee, Y.; Yun, S.J.; Duong, D.L.; et al. Ferromagnetic Order at Room Temperature in Monolayer WSe2 Semiconductor via Vanadium Dopant. Adv. Sci. 2020, 7, 1903076. [Google Scholar] [CrossRef]
- Duong, D.L.; Yun, S.J.; Kim, Y.; Kim, S.G.; Lee, Y.H. Long-range ferromagnetic ordering in vanadium-doped WSe2 semiconductor. Appl. Phys. Lett. 2019, 115, 242406. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, B.; Sebastian, A.; Olson, D.H.; Liu, M.; Fujisawa, K.; Pham, Y.T.H.; Jimenez, V.O.; Kalappattil, V.; Miao, L.; et al. Monolayer Vanadium-Doped Tungsten Disulfide: A Room-Temperature Dilute Magnetic Semiconductor. Adv. Sci. 2020, 7, 2001174. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Jimenez, V.; Pham, Y.T.H.; Liu, M.; Zhang, F.; Yu, Z.; Kalappattil, V.; Muchharla, B.; Eggers, T.; Duong, D.L.; Terrones, M.; et al. Light-Controlled Room Temperature Ferromagnetism in Vanadium-Doped Tungsten Disulfide Semiconducting Monolayers. Adv. Electron. Mater. 2021, 7, 2100030. [Google Scholar] [CrossRef]
- Han, G.H.; Kybert, N.J.; Naylor, C.H.; Lee, B.S.; Ping, J.; Park, J.H.; Kang, J.; Lee, S.Y.; Lee, Y.H.; Agarwal, R.; et al. Seeded growth of highly crystalline molybdenum disulphide monolayers at controlled locations. Nat. Commun. 2015, 6, 6128. [Google Scholar] [CrossRef]
- Naylor, C.H.; Parkin, W.M.; Ping, J.; Gao, Z.; Zhou, Y.R.; Kim, Y.; Streller, F.; Carpick, R.W.; Rappe, A.M.; Drndić, M.; et al. Monolayer single-crystal 1T′-MoTe2 grown by chemical vapor deposition exhibits weak antilocalization effect. Nano Lett. 2016, 16, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yu, W.; Liu, C.; Cheng, X.; Qiao, R.; Liang, J.; Zhou, X.; Wang, J.; Wu, M.; Zhao, Y.; et al. Optical fibres with embedded two-dimensional materials for ultrahigh nonlinearity. Nat. Nanotechnol. 2020, 15, 987–991. [Google Scholar] [CrossRef]
- Guan, H.; Zhao, B.; Zhao, W.; Ni, Z. Liquid-precursor-intermediated synthesis of atomically thin transition metal dichalcogenides. Mater. Horiz. 2023, 10, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Tong, X.; Liu, S.; Wang, J.; Zhao, Y.; Liu, R.; Zhao, X.; Zhang, N.; Cao, F.; Liu, Y.; et al. Superior Nonlinear Optical Response in Non-Centrosymmetric Stacking Edge-Rich Spiral MoTe2 Nanopyramids. Adv. Funct. Mater. 2022, 32, 2113052. [Google Scholar] [CrossRef]
- Lee, E.; Kim, J.; Yun, S.J.; Kim, Y. Near-field visualization of charge transfer at MoSe2/WSe2 lateral heterojunction. Opt. Mater. Express 2019, 9, 1864–1871. [Google Scholar] [CrossRef]
- Zhang, T.; Fujisawa, K.; Zhang, F.; Liu, M.; Lucking, M.C.; Gontijo, R.N.; Lei, Y.; Liu, H.; Crust, K.; Granzier-Nakajima, T.; et al. Universal In Situ Substitutional Doping of Transition Metal Dichalcogenides by Liquid-Phase Precursor-Assisted Synthesis. ACS Nano 2020, 14, 4326–4335. [Google Scholar] [CrossRef]
- Wan, X.; Miao, X.; Yao, J.; Wang, S.; Shao, F.; Xiao, S.; Zhan, R.; Chen, K.; Zeng, X.; Gu, X.; et al. In Situ Ultrafast and Patterned Growth of Transition Metal Dichalcogenides from Inkjet-Printed Aqueous Precursors. Adv. Mater. 2021, 33, 2100260. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, X.; Chen, R.; Sun, J.; Kang, H.; Ji, D.; Liu, Y.; Wei, D. Self-Expanding Molten Salt-Driven Growth of Patterned Transition-Metal Dichalcogenide Crystals. J. Am. Chem. Soc. 2022, 144, 8746–8755. [Google Scholar] [CrossRef]
- Patil, B.; Bernini, C.; Marré, D.; Pellegrino, L.; Pallecchi, I. Ink-jet printing and drop-casting deposition of 2H-phase SnSe2 and WSe2 nanoflake assemblies for thermoelectric applications. Nanotechnology 2021, 33, 035302. [Google Scholar] [CrossRef]
- Abbas, O.A.; Zeimpekis, I.; Wang, H.; Lewis, A.H.; Sessions, N.P.; Ebert, M.; Aspiotis, N.; Huang, C.C.; Hewak, D.; Mailis, S.; et al. Solution-Based Synthesis of Few-Layer WS2 Large Area Continuous Films for Electronic Applications. Sci. Rep. 2020, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Lee, Y.H.; Lin, Y.; Fang, W.; Yu, L.; Dresselhaus, M.S.; Kong, J. Role of the seeding promoter in MoS2 growth by chemical vapor deposition. Nano Lett. 2014, 14, 464–472. [Google Scholar] [CrossRef]
- Yun, S.J.; Han, G.H.; Kim, H.; Duong, D.L.; Shin, B.G.; Zhao, J.; Vu, Q.A.; Lee, J.; Lee, S.M.; Lee, Y.H. Telluriding monolayer MoS2 and WS2 via alkali metal scooter. Nat. Commun. 2017, 8, 2163. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Han, G.H.; Yun, S.J.; Zhao, J.; Keum, D.H.; Jeong, H.Y.; Ly, T.H.; Jin, Y.; Park, J.H.; Moon, B.H.; et al. Role of alkali metal promoter in enhancing lateral growth of monolayer transition metal dichalcogenides. Nanotechnology 2017, 28, 36LT01. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Liu, K.; Yang, S.; Wang, F.; Su, J.; Jin, B.; Li, H.; Zhai, T. Salt-assisted chemical vapor deposition of two-dimensional materials. Sci. China Chem. 2019, 62, 1300–1311. [Google Scholar] [CrossRef]
- Huang, L.; Hu, Z.; Jin, H.; Wu, J.; Liu, K.; Xu, Z.; Wan, J.; Zhou, H.; Duan, J.; Hu, B.; et al. Salt-Assisted Synthesis of 2D Materials. Adv. Funct. Mater. 2020, 30, 1908486. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Tang, D.M.; Zhao, W.; Xu, H.; Chu, L.; Bando, Y.; Golberg, D.; Eda, G. Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Appl. Mater. Today 2015, 1, 60–66. [Google Scholar] [CrossRef]
- Bai, X.; Li, S.; Das, S.; Du, L.; Dai, Y.; Yao, L.; Raju, R.; Du, M.; Lipsanen, H.; Sun, Z. Single-step chemical vapour deposition of anti-pyramid MoS2/WS2 vertical heterostructures. Nanoscale 2021, 13, 4537–4542. [Google Scholar] [CrossRef]
- Hunyadi, D.; Sajó, I.; Szilágyi, I.M. Structure and thermal decomposition of ammonium metatungstate. J. Therm. Anal. Calorim. 2013, 116, 329–337. [Google Scholar] [CrossRef]
- Griffin, A.; Nisi, K.; Pepper, J.; Harvey, A.; Szydłowska, B.M.; Coleman, J.N.; Backes, C. Effect of Surfactant Choice and Concentration on the Dimensions and Yield of Liquid-Phase-Exfoliated Nanosheets. Chem. Mater. 2020, 32, 2852–2862. [Google Scholar] [CrossRef]
- Ko, H.; Kim, H.S.; Ramzan, M.S.; Byeon, S.; Choi, S.H.; Kim, K.K.; Kim, Y.H.; Kim, S.M. Atomistic mechanisms of seeding promoter-controlled growth of molybdenum disulphide. 2D Mater. 2019, 7, 015013. [Google Scholar] [CrossRef]
- Wang, P.; Lei, J.; Qu, J.; Cao, S.; Jiang, H.; He, M.; Shi, H.; Sun, X.; Gao, B.; Liu, W. Mechanism of Alkali Metal Compound-Promoted Growth of Monolayer MoS2: Eutectic Intermediates. Chem. Mater. 2019, 31, 873–880. [Google Scholar] [CrossRef]
- Kim, M.; Son, M.; Seo, D.B.; Kim, J.; Jang, M.; Kim, D.I.; Lee, S.; Yim, S.; Song, W.; Myung, S.; et al. Dual Catalytic and Self-Assembled Growth of Two-Dimensional Transition Metal Dichalcogenides Through Simultaneous Predeposition Process. Small 2023, 19, 2206350. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Y.C.; Liu, X.Y.; Hu, Z.; Wu, J.; Nakajima, H.; Liu, S.; Okazaki, T.; Chen, W.; Minari, T.; et al. Wafer-scale and deterministic patterned growth of monolayer MoS2 via vapor–liquid–solid method. Nanoscale 2019, 11, 16122–16129. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.C.; Zhao, W.; Wu, J.; Wang, Z.; Hu, Z.; Shen, Y.; Tang, D.M.; Wang, J.; Zhang, Q.; et al. Vapour–liquid–solid growth of monolayer MoS2 nanoribbons. Nat. Mater. 2018, 17, 535–542. [Google Scholar] [CrossRef]
- Cheng, Z.; He, S.; Han, X.; Wang, M.; Zhang, S.; Liu, S.; Liang, G.; Zhang, S.; Xia, M. Interfaces determine the nucleation and growth of large NbS2 single crystals. CrystEngComm 2021, 23, 1312–1320. [Google Scholar] [CrossRef]
- Qiang, X.; Iwamoto, Y.; Watanabe, A.; Kameyama, T.; He, X.; Kaneko, T.; Shibuta, Y.; Kato, T. Non-classical nucleation in vapor–liquid–solid growth of monolayer WS2 revealed by in-situ monitoring chemical vapor deposition. Sci. Rep. 2021, 11, 22285. [Google Scholar] [CrossRef]
- Li, C.; Kameyama, T.; Takahashi, T.; Kaneko, T.; Kato, T. Nucleation dynamics of single crystal WS2 from droplet precursors uncovered by in-situ monitoring. Sci. Rep. 2019, 9, 12958. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Liu, Y.; Tang, W.; Nai, C.T.; Li, L.; Zheng, J.; Gao, L.; Zheng, Y.; Shin, H.S.; et al. Chemical Vapor Deposition of Large-Sized Hexagonal WSe2 Crystals on Dielectric Substrates. Adv. Mater. 2015, 27, 6722–6727. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Q. A simple method for understanding the triangular growth patterns of transition metal dichalcogenide sheets. AIP Adv. 2015, 5, 107105. [Google Scholar] [CrossRef]
- Wang, S.; Rong, Y.; Fan, Y.; Pacios, M.; Bhaskaran, H.; He, K.; Warner, J.H. Shape evolution of monolayer MoS2 crystals grown by chemical vapor deposition. Chem. Mater. 2014, 26, 6371–6379. [Google Scholar] [CrossRef]
- Momeni, K.; Ji, Y.; Zhang, K.; Robinson, J.A.; Chen, L.Q. Multiscale framework for simulation-guided growth of 2D materials. npj 2D Mater. Appl. 2018, 2, 27. [Google Scholar] [CrossRef]
- Lv, R.; Terrones, H.; Elías, A.L.; Perea-López, N.; Gutiérrez, H.R.; Cruz-Silva, E.; Rajukumar, L.P.; Dresselhaus, M.S.; Terrones, M. Two-dimensional transition metal dichalcogenides: Clusters, ribbons, sheets and more. Nano Today 2015, 10, 559–592. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Feng, H.; Wang, L.; Chen, S.; Zhou, Z.; Wang, S.; Liu, Q. Shape-dependent close-edge 2D-MoS2 nanobelts. RSC Adv. 2020, 10, 33544–33548. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, S.; Boyle, L.; Zeng, H.; Huang, S. Controlled fractal growth of transition metal dichalcogenides. Nanoscale 2019, 11, 17065–17072. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Benitez, J.E.; Mendoza-Cruz, R.; Bazán-Díaz, L.; Garcia-Garcia, A.; Arellano-Jimenez, M.J.; Perez-Robles, J.F.; Plascencia-Villa, G.; Velázquez-Salazar, J.J.; Ortega, E.; Favela-Camacho, S.E.; et al. Synthesis and structural characterization of MoS2 micropyramids. J. Mater. Sci. 2020, 55, 12203–12213. [Google Scholar] [CrossRef]
- Ly, T.H.; Zhao, J.; Kim, H.; Han, G.H.; Nam, H.; Lee, Y.H. Vertically Conductive MoS2 Spiral Pyramid. Adv. Mater. 2016, 28, 7723–7728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Su, S.; Cheng, P.; Hu, X.; Gao, X.; Zhang, Z.; Liu, J.M. Vertically conductive MoS2 pyramids with a high density of active edge sites for efficient hydrogen evolution. J. Mater. Chem. C 2020, 8, 3017–3022. [Google Scholar] [CrossRef]
- Negri, M.; Francaviglia, L.; Kaplan, D.; Swaminathan, V.; Salviati, G.; Fontcuberta, I.; Morral, A.; Fabbri, F. Excitonic absorption and defect-related emission in three-dimensional MoS2 pyramids. Nanoscale 2022, 14, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Komen, I.; van Heijst, S.E.; Conesa-Boj, S.; Kuipers, L. Morphology-induced spectral modification of self-assembled WS2 pyramids. Nanoscale Adv. 2021, 3, 6427–6437. [Google Scholar] [CrossRef]
- Sarma, P.V.; Kayal, A.; Sharma, C.H.; Thalakulam, M.; Mitra, J.; Shaijumon, M.M. Electrocatalysis on Edge-Rich Spiral WS2 for Hydrogen Evolution. ACS Nano 2019, 13, 10448–10455. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Wang, K.; Wei, C.; Zhang, W.; Yan, Y.; Li, Y.J.; Yao, J.; Zhao, Y.S. Two-Dimensional Pyramid-like WS2 Layered Structures for Highly Efficient Edge Second-Harmonic Generation. ACS Nano 2018, 12, 689–696. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Abbas, A.N.; Ma, Y.; Fang, X.; Liu, Y.; Zhou, C. Screw-Dislocation-Driven growth of Two-Dimensional few-layer and pyramid-like WSe2 by sulfur-assisted Chemical Vapor Deposition. ACS Nano 2014, 8, 11543–11551. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Wang, K.; Liu, X.; Yan, Y.; Li, Y.J.; Yao, J.; Zhao, Y.S. Hybrid three-dimensional spiral WSe2 plasmonic structures for highly efficient second-order nonlinear parametric processes. Research 2018, 2018, 4164029. [Google Scholar] [CrossRef]
- Shi, W.; Lin, M.L.; Tan, Q.H.; Qiao, X.F.; Zhang, J.; Tan, P.H. Raman and photoluminescence spectra of two-dimensional nanocrystallites of monolayer WS2 and WSe2. 2D Mater. 2016, 3, 025016. [Google Scholar] [CrossRef]
- Tonndorf, P.; Schmidt, R.; Böttger, P.; Zhang, X.; Börner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.; et al. Photoluminescence emission and Raman response of monolayer MoS2, MoSe2, and WSe2. Opt. Express 2013, 21, 4908–4916. [Google Scholar] [CrossRef] [PubMed]
- Tangi, M.; Mishra, P.; Tseng, C.C.; Ng, T.K.; Hedhili, M.N.; Anjum, D.H.; Alias, M.S.; Wei, N.; Li, L.J.; Ooi, B.S. Band Alignment at GaN/Single-Layer WSe2 Interface. ACS Appl. Mater. Interfaces 2017, 9, 9110–9117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ghorannevis, Z.; Amara, K.K.; Pang, J.R.; Toh, M.; Zhang, X.; Kloc, C.; Tan, P.H.; Eda, G. Lattice dynamics in mono- and few-layer sheets of WS2 and WSe2. Nanoscale 2013, 5, 9677–9683. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Zhang, X.; Li, Z.Y.; Li, J. Kirigami/origami: Unfolding the new regime of advanced 3D microfabrication/nanofabrication with “folding”. Light Sci. Appl. 2020, 9, 75. [Google Scholar] [CrossRef]
- Shinde, S.M.; Dhakal, K.P.; Chen, X.; Yun, W.S.; Lee, J.; Kim, H.; Ahn, J.H. Stacking-controllable interlayer coupling and symmetric configuration of multilayered MoS2. NPG Asia Mater. 2018, 10, e468. [Google Scholar] [CrossRef]
- Cai, L.; Shearer, M.J.; Zhao, Y.; Hu, Z.; Wang, F.; Zhang, Y.; Eliceiri, K.W.; Hamers, R.J.; Yan, W.; Wei, S.; et al. Chemically Derived Kirigami of WSe2. J. Am. Chem. Soc. 2018, 140, 10980–10987. [Google Scholar] [CrossRef] [PubMed]
- Kastl, C.; Chen, C.T.; Kuykendall, T.; Shevitski, B.; Darlington, T.P.; Borys, N.J.; Krayev, A.; Schuck, P.J.; Aloni, S.; Schwartzberg, A.M. The important role of water in growth of monolayer transition metal dichalcogenides. 2D Mater. 2017, 4, 021024. [Google Scholar] [CrossRef]
- Terrones, H.; Del Corro, E.; Feng, S.; Poumirol, J.M.; Rhodes, D.; Smirnov, D.; Pradhan, N.R.; Lin, Z.; Nguyen, M.A.T.; Elías, A.L.; et al. New First Order Raman-active Modes in Few Layered Transition Metal Dichalcogenides. Sci. Rep. 2014, 4, 4215. [Google Scholar] [CrossRef] [PubMed]
- Degregorio, Z.P.; Myers, J.C.; Campbell, S.A. Rational control of WSe2 layer number via hydrogen-controlled chemical vapor deposition. Nanotechnology 2020, 31, 315604. [Google Scholar] [CrossRef]
- An, G.H.; Yun, S.J.; Lee, Y.H.; Lee, H.S. Growth Mechanism of Alternating Defect Domains in Hexagonal WS2 via Inhomogeneous W-Precursor Accumulation. Small 2020, 16, 2003326. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Shahzad, K.; Akbar, R.; Hussain, G. A review on Raman finger prints of doping and strain effect in TMDCs. Microelectron. Eng. 2020, 219, 111152. [Google Scholar] [CrossRef]
- Guan, H.; Tang, N.; Huang, H.; Zhang, X.; Su, M.; Liu, X.; Liao, L.; Ge, W.; Shen, B. Inversion Symmetry Breaking Induced Valley Hall Effect in Multilayer WSe2. ACS Nano 2019, 13, 9325–9331. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ren, J.; Li, J.; Chen, Y.; Lan, S.; Wang, J.; Wang, H.; Li, D. Multistate Memory Enabled by Interface Engineering Based on Multilayer Tungsten Diselenide. ACS Appl. Mater. Interfaces 2020, 12, 58428–58434. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).