Mechanism of Aluminium Electrochemical Oxidation and Alumina Deposition Using a Carbon Sphere Electrode
Abstract
1. Introduction
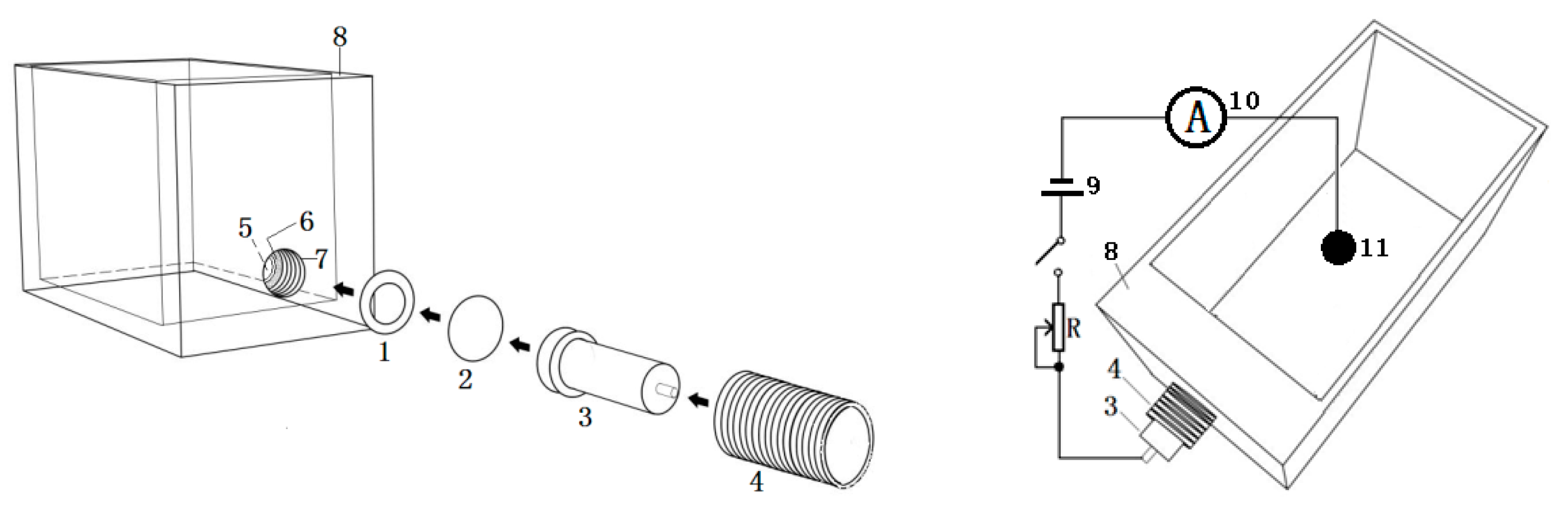
2. Materials and Methods
- Ring-shaped adhesive pad;
- Circular aluminium foil;
- Reducer copper electrode;
- Inner fastening screw;
- Small cylindrical hole;
- Small cylindrical hole annular enclosure;
- Large cylindrical hole;
- Electrolyser tank wall (acrylic material);
- Voltage source (0–40 V);
- Ammeter (0–3 A);
- Carbon sphere electrode.
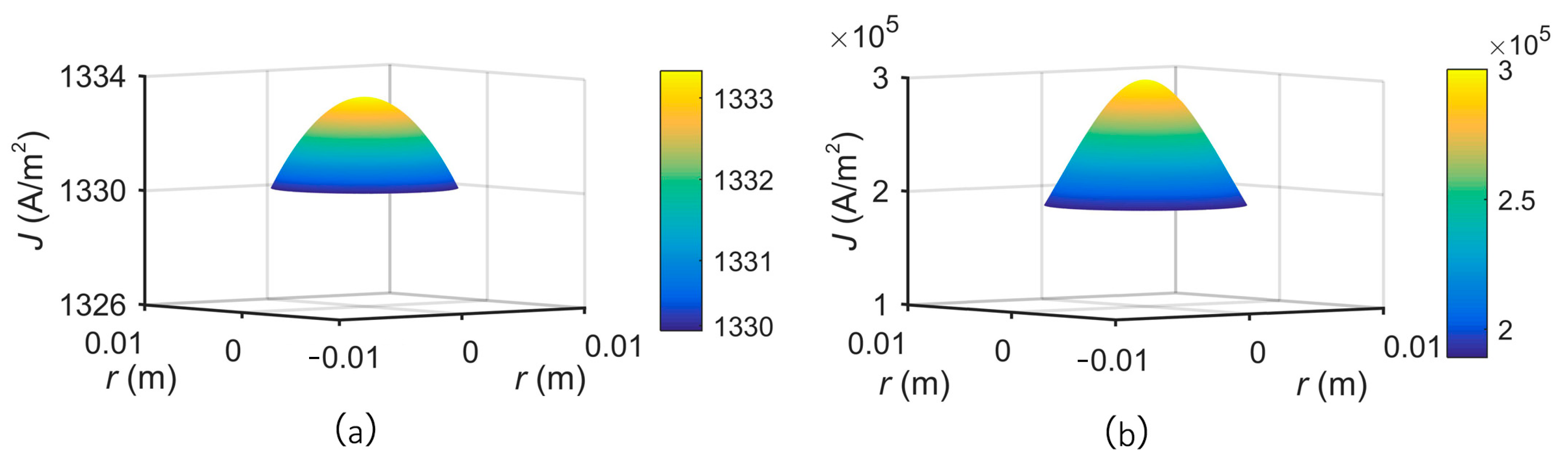
3. Theory and Simulation
4. Results and Discussion
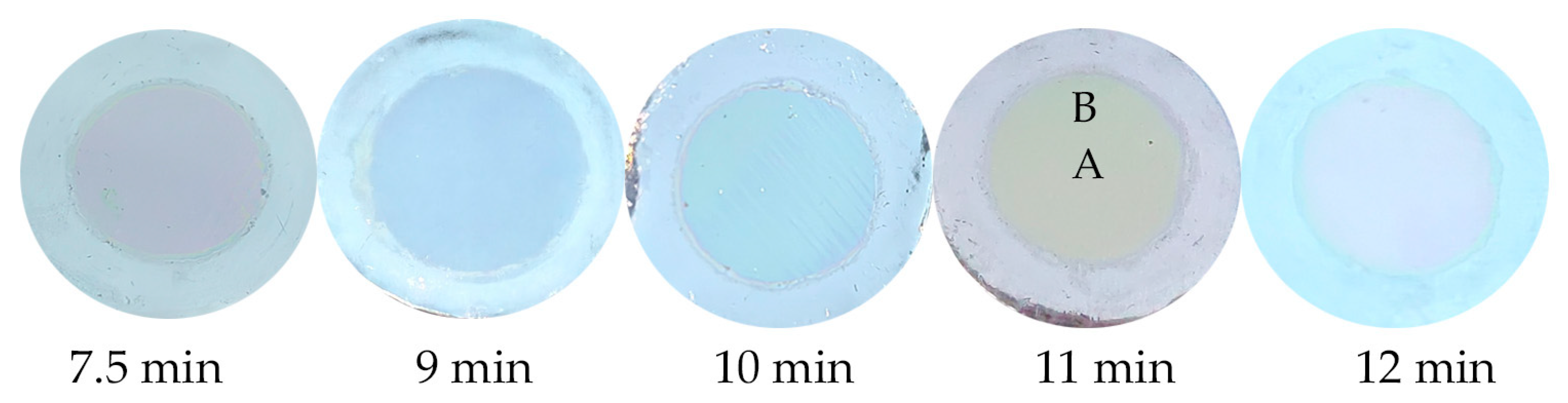
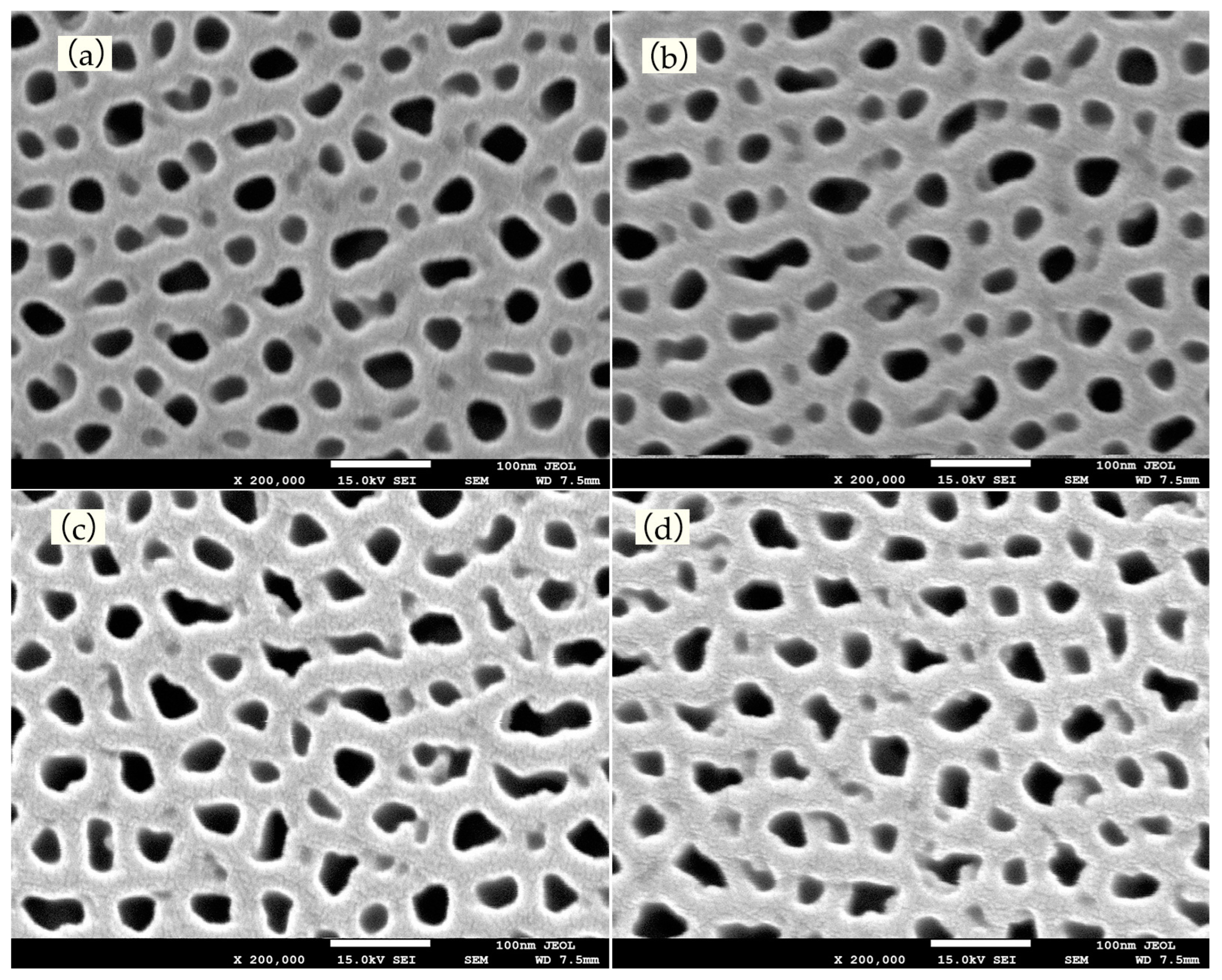
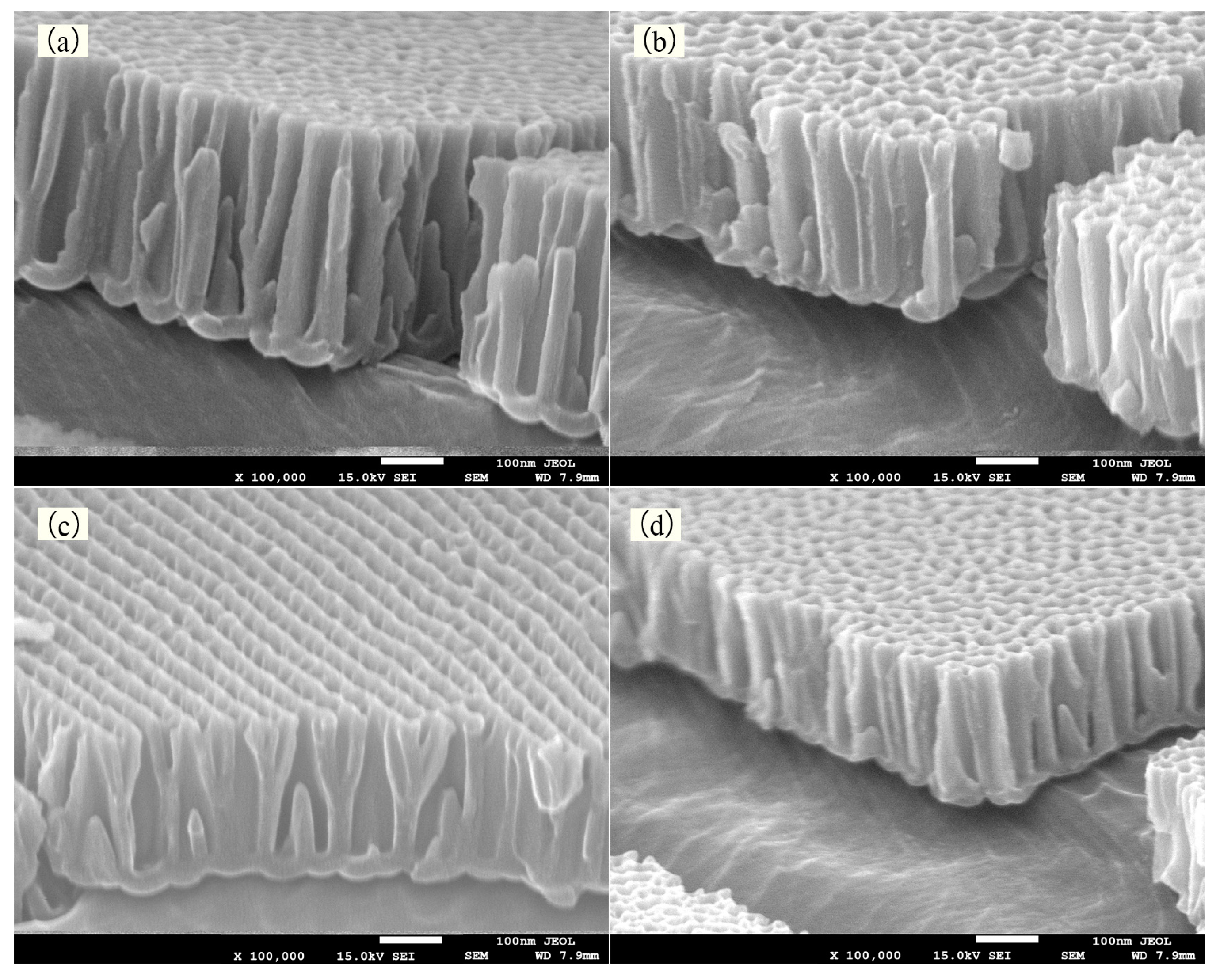
4.1. Experimental Results and Discussion of Anodic Oxidation
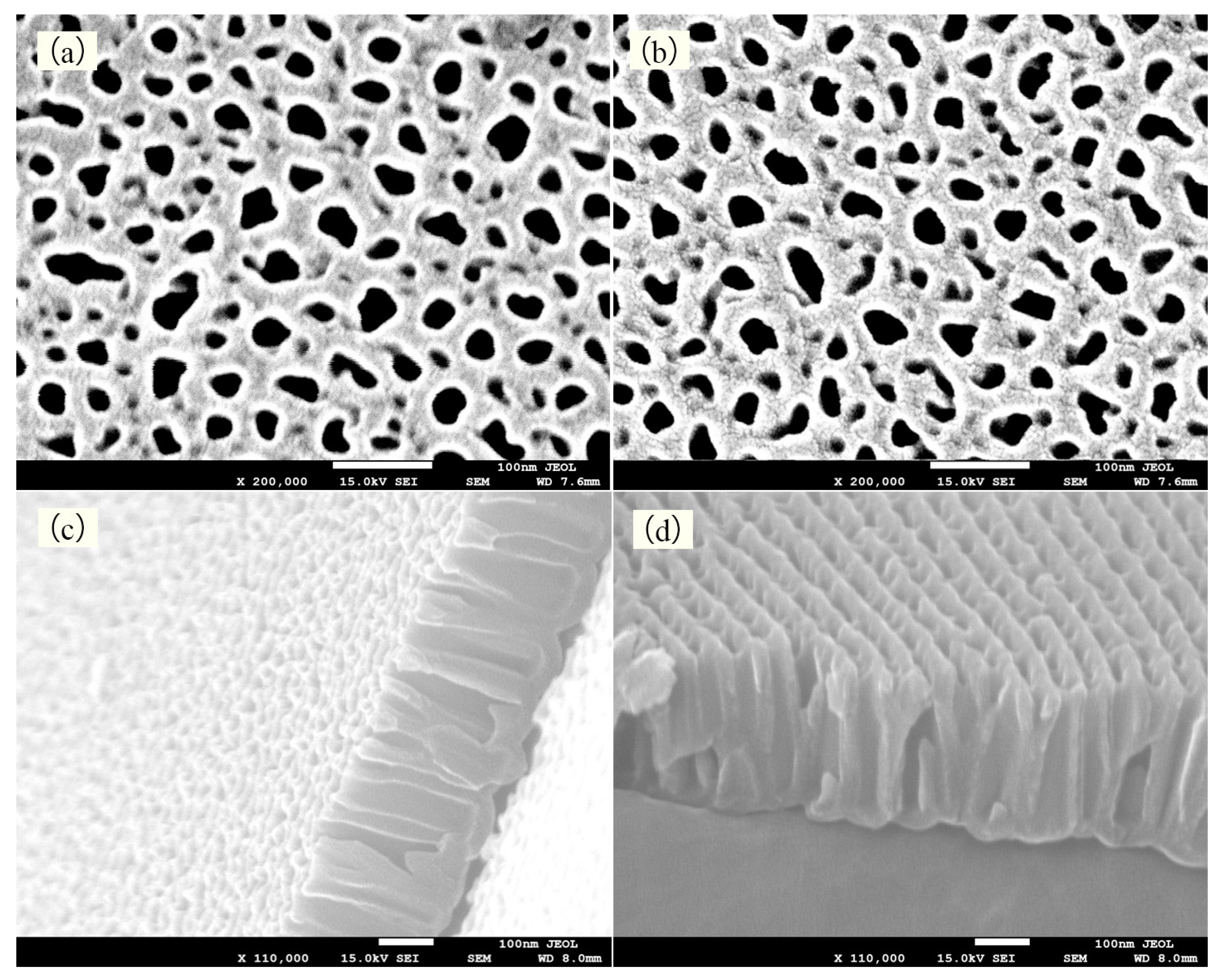
4.2. Experimental Results and Discussion of Electrodeposition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kushnir, S.E.; Napolskii, K.S. Thickness-dependent iridescence of one-dimensional photonic crystals based on anodic alumina. Mater. Des. 2018, 144, 140–150. [Google Scholar] [CrossRef]
- Liu, S.X.; Tian, J.L.; Zhang, W. Fabrication and application of nanoporous anodic aluminum oxide: A review. Nanotechnology 2021, 32, 222001. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, M.A.; Ferré-Borrull, J.; Marsal, L.F. An optical biosensor for the determination of cathepsin B as a cancer-associated enzyme using nanoporous anodic alumina modified with human serum albumin-thionine. Microchim. Acta 2020, 187, 230. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.D.; Lin, D.; Wang, X.; Lennon, A. Laser doping through anodic aluminium oxide silicon solar cell. Sol. Energy Mater. Sol. Cells 2016, 145, 349–357. [Google Scholar] [CrossRef]
- Acosta, L.K.; Law, C.S.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F. Tuning intrinsic photoluminescence from light-emitting multispectral nanoporous anodic alumina photonic crystals. Marsal 2022, 7, 026108. [Google Scholar] [CrossRef]
- Sachiko, O.; Hidetaka, A. A new perspective on pore growth in anodic alumina films. Electrochem. Commun. 2021, 124, 106972. [Google Scholar]
- Pappas, J.M.; Thakur, A.R.; Dong, X. Effects of zirconia doping on additively manufactured alumina ceramics by laser direct deposition. Mater. Des. 2020, 192, 108711. [Google Scholar] [CrossRef]
- Szwachta, G.; Januszewska, B.; Włodarski, M.; Norek, M. From gradient-index to step-index filters: A switch between the two types of photonic crystals induced by the amplitude and period of sinusoidal function applied during high-temperature anodisation of aluminium. Appl. Surf. Sci. 2023, 607, 155031. [Google Scholar] [CrossRef]
- Domínguez-Adame, F.; Martín-González, M.; Sánchez, D.; Cantarero, A. Nanowires: A route to efficient thermoelectric devices. Phys. E 2019, 113, 213–225. [Google Scholar] [CrossRef]
- Manzano, C.V.; Martin-Gonzalez, M. Electrodeposition of V-VI Nanowiresand Their Thermoelectric Properties. Front. Chem. 2019, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Revisiting anodic alumina templates: From fabrication to applications. Nanoscale 2021, 13, 2227–2265. [Google Scholar] [CrossRef] [PubMed]
- Evertsson, J.; Vinogradov, N.A.; Harlow, G.S. Self-organization of porous anodic alumina films studied in situ by grazing-incidence transmission small-angle X-ray scattering. RSC Adv. 2018, 8, 18980–18991. [Google Scholar] [CrossRef] [PubMed]
- Kima, B.; Youn, Y.; Park, Y.-S. Impurity-driven formation of branched pores in porous anodic alumina. Scr. Mater. 2016, 122, 102–105. [Google Scholar] [CrossRef]
- Roslyakova, I.V.; Gordeeva, E.O.; Napolskii, K.S. Role of Electrode Reaction Kinetics in Self-Ordering of Porous Anodic Alumina. Electrochim. Acta 2017, 241, 362–369. [Google Scholar] [CrossRef]
- Białek, E.; Włodarski, M.; Norek, M. Influence of Anodization Temperature on Geometrical and Optical Properties of Porous Anodic Alumina(PAA)-Based Photonic Structures. Materials 2020, 13, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Kant, K.; Low, S.P.; Marshal, A.; Shapter, J.G.; Losic, D. Nanopore gradients on porous aluminum oxide generated by nonuniform anodization of aluminum. Appl. Mater. 2010, 2, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Han, W.; Gu, J.J.; Li, H.T.; Qi, Y.K. Preparation and study of anodic alumina thin films with rainbow rings. Acta Phys. Sin. 2015, 64, 076102. (In Chinese) [Google Scholar] [CrossRef]
- Qi, Y.K.; Yang, S.M.; Li, X.; Wang, H.M.; Xu, Q.; Gu, J.J. Design of alumina composite films with gradual structural color and magnetism using non-uniform electric field. Thin Solid Film. 2021, 740, 138999. [Google Scholar] [CrossRef]
- Qi, Y.K.; Gu, J.J.; Shi, G.C.; Li, X.; Wang, A.; Yang, S.M. Symmetrically Controlled Design of Twin Alumina-Co Composite Thin Films. Coatings 2022, 12, 223. [Google Scholar] [CrossRef]
- Thompson, G.E.; Wood, G.C. Treatise on Materials and Technology; Academic Press: New York, NY, USA, 1983; Volume 23, pp. 269–271. [Google Scholar]


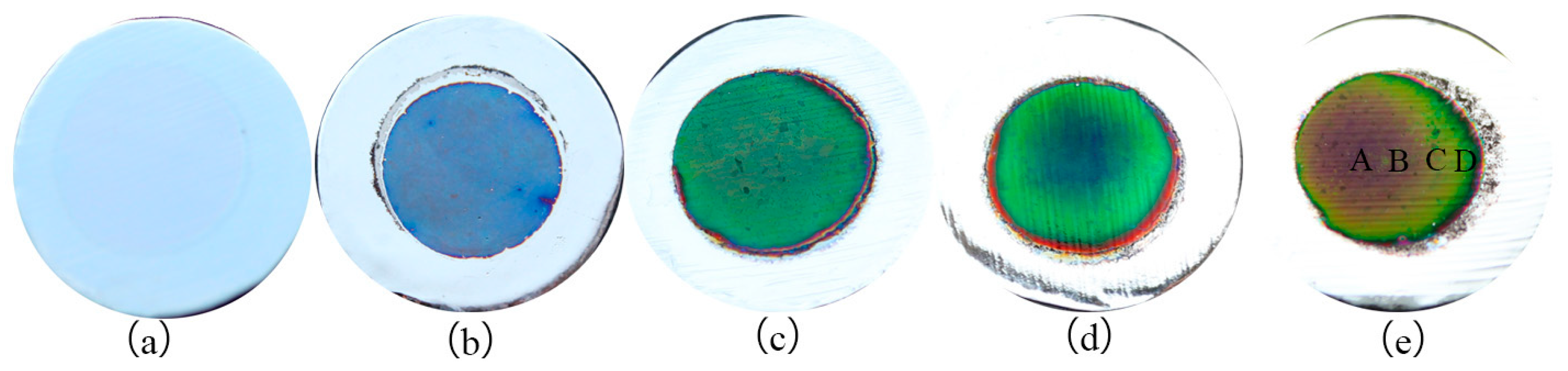


| Position | A | B |
|---|---|---|
| Aperture radius/(nm) | 10 | 9 |
| Aperture pitch/(nm) | 53 | 46 |
| Porosity (%) | 13 | 14 |
| Refractive index | 1.57 | 1.56 |
| Interference levels | 1 | 1 |
| Thickness/(nm) | 290 | 280 |
| Wavelengths/(nm) | 607 | 582 |
| Corresponding colour | Yellow | Yellow |
| Time/(min) | 7.5 | 9 | 10 | 11 | 12 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position | A | B | A | B | A | B | A | B | A | B |
| Thickness/(nm) | 198 | 191 | 238 | 230 | 264 | 255 | 290 | 280 | 317 | 306 |
| Wavelengths/(nm) | 414 | 397 | 498 | 478 | 553 | 530 | 607 | 582 | 664 | 636 |
| Specific surface area (m2/g) | 9.15 | 8.74 | 8.55 | 8.39 | 8.25 | |||||
| Corresponding colour | Purple | Purple | Blue | Blue | Green | Green | Yellow | Yellow | Red | Red |
| Position | A | B | C | D |
|---|---|---|---|---|
| Aperture radius/(nm) | 20 | 18 | 16 | 14 |
| Aperture pitch/(nm) | 56 | 60 | 68 | 77 |
| Porosity | 0.46 | 0.33 | 0.20 | 0.12 |
| Refractive index | 1.41 | 1.43 | 1.52 | 1.57 |
| Interference levels | 1 | 1 | 1 | 1 |
| Thickness/(nm) | 387 | 283 | 250 | 215 |
| Wavelengths/(nm) | 728 | 540 | 507 | 440 |
| Corresponding colour | Red | Yellow | Green | Blue |
| Refractive Index | M Layer: n air-Al2O3 = 1.57 | N Layer: n Co-Al2O3 = 1.67 | ||
|---|---|---|---|---|
| Position | A | B | C | D |
| Thickness/(nm) | 313 | 300 | 269 | 269 |
| Thickness of air-Al2O3/(nm) | 119 | 150 | 165 | 194 |
| Thickness of Co-Al2O3/(nm) | 194 | 150 | 104 | 75 |
| Interference levels | 1 | 1 | 1 | 1 |
| Wavelengths/(nm) | 681 | 648 | 599 | 573 |
| Corresponding colour | Red | Red | Orange–yellow | Green |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Wang, A.; Lin, X.; Qi, Y.; Shi, G.; Han, W.; Gu, J. Mechanism of Aluminium Electrochemical Oxidation and Alumina Deposition Using a Carbon Sphere Electrode. Crystals 2024, 14, 1102. https://doi.org/10.3390/cryst14121102
Yang S, Wang A, Lin X, Qi Y, Shi G, Han W, Gu J. Mechanism of Aluminium Electrochemical Oxidation and Alumina Deposition Using a Carbon Sphere Electrode. Crystals. 2024; 14(12):1102. https://doi.org/10.3390/cryst14121102
Chicago/Turabian StyleYang, Shumin, Ao Wang, Xuemei Lin, Yunkai Qi, Guochao Shi, Wei Han, and Jianjun Gu. 2024. "Mechanism of Aluminium Electrochemical Oxidation and Alumina Deposition Using a Carbon Sphere Electrode" Crystals 14, no. 12: 1102. https://doi.org/10.3390/cryst14121102
APA StyleYang, S., Wang, A., Lin, X., Qi, Y., Shi, G., Han, W., & Gu, J. (2024). Mechanism of Aluminium Electrochemical Oxidation and Alumina Deposition Using a Carbon Sphere Electrode. Crystals, 14(12), 1102. https://doi.org/10.3390/cryst14121102






