Designing Continuous Crystallization Protocols for Curcumin Using PAT Obtained Batch Kinetics
Abstract
1. Introduction
2. Theory
3. Experimental
3.1. Materials
3.2. Crystal Growth Experiments
3.3. Seed Crystals
4. Results and Discussions
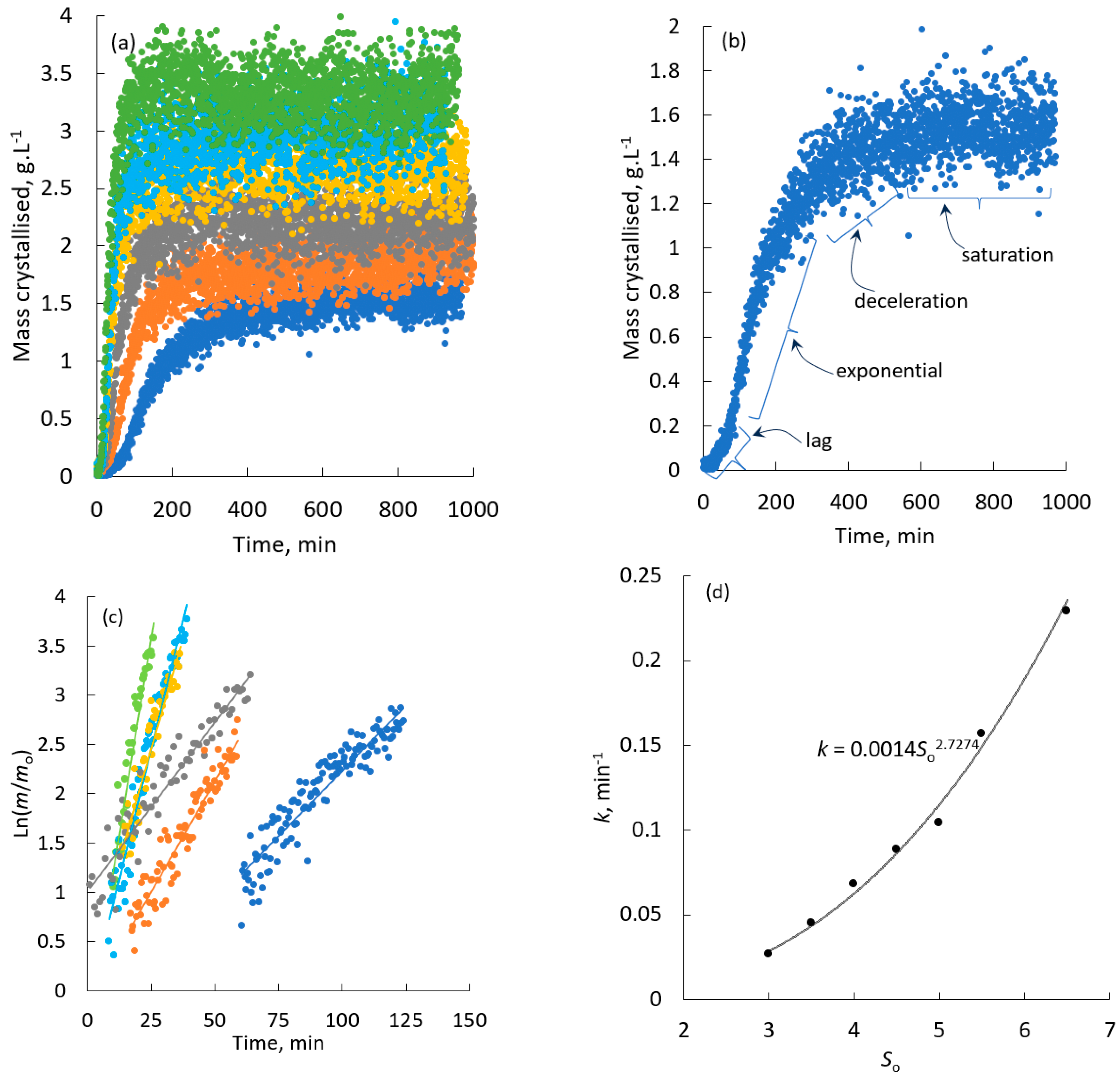
4.1. Batch Crystallisation Kinetics
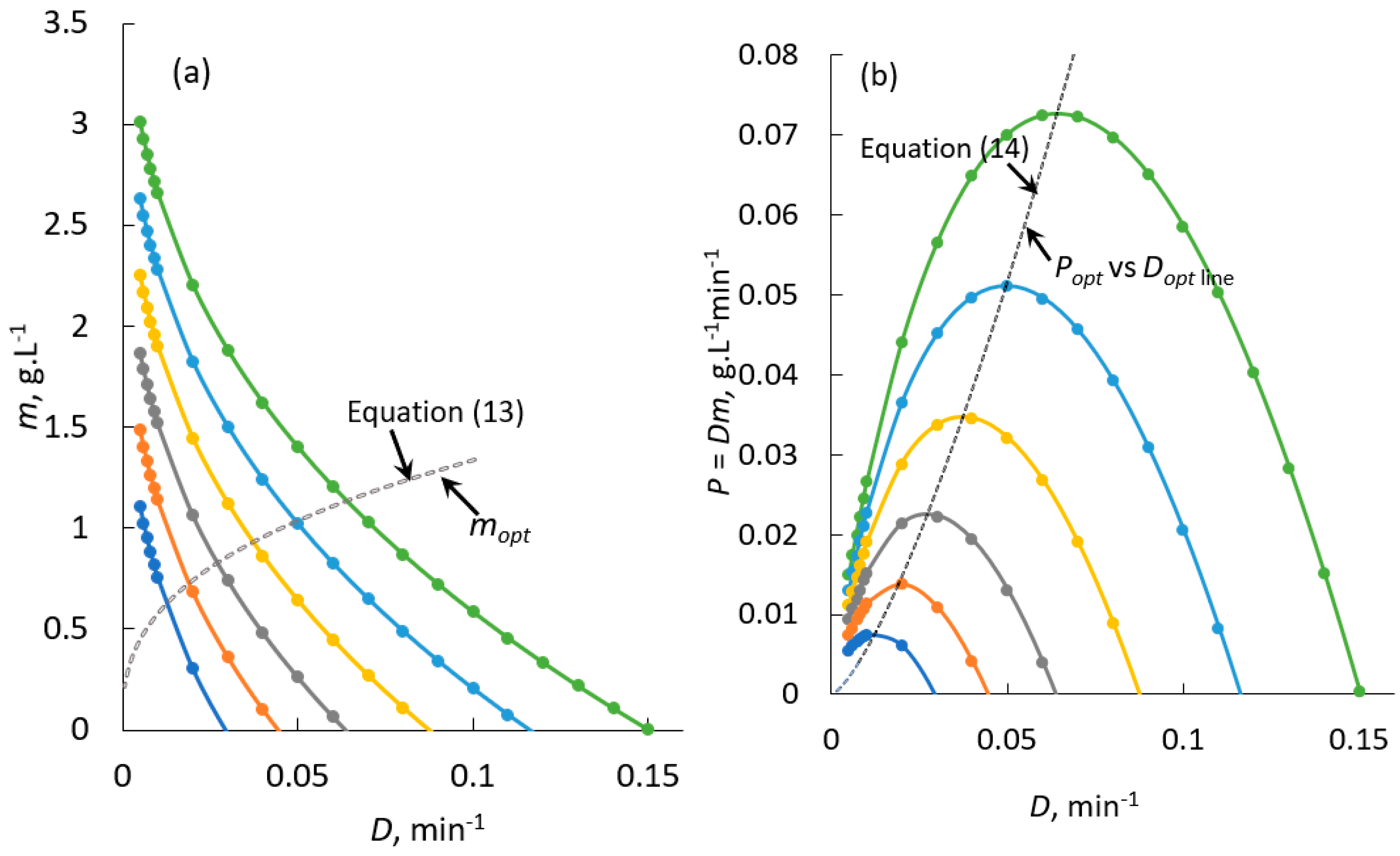
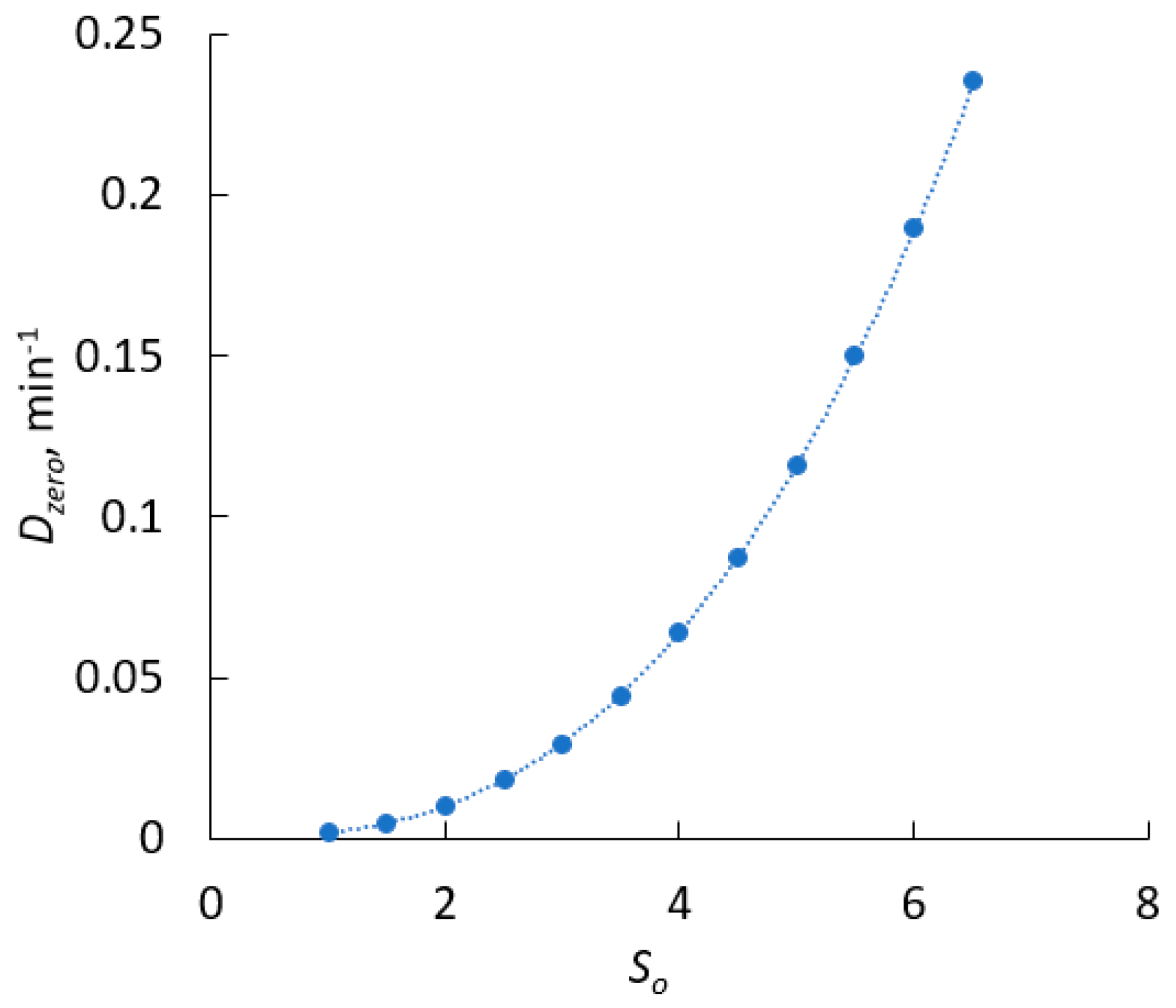
4.2. Continuous Crystallisation of Curcumin: Theoretical Prediction
4.3. Theoretical Procedure for the Continuous Production of Curcumin
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ginde, R.M.; Myerson, A.S. Effect of Impurities on Cluster Growth and Nucleation. J. Cryst. Growth 1993, 126, 216–222. [Google Scholar] [CrossRef]
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Hsi, K.H.; Kenny, M.; Simi, A.; Myerson, A.S. Purification of Structurally Similar Compounds by the Formation of Impurity Co-Former Complexes in Solution. Cryst. Growth Des. 2013, 13, 1577–1582. [Google Scholar] [CrossRef]
- Kuvadia, Z.B.; Doherty, M.F. Effect of Structurally Similar Additives on Crystal Habit of Organic Molecular Crystals at Low Supersaturation. Cryst. Growth Des. 2013, 13, 1412–1428. [Google Scholar] [CrossRef]
- Mascia, S.; Heider, P.L.; Zhang, H.; Lakerveld, R.; Benyahia, B.; Barton, P.I.; Braatz, R.D.; Cooney, C.L.; Evans, J.M.B.; Jamison, T.F.; et al. End-to-End Continuous Manufacturing of Pharmaceuticals: Integrated Synthesis, Purification, and Final Dosage Formation. Angew. Chem. Int. Ed. 2013, 52, 12359–12363. [Google Scholar] [CrossRef]
- Wood, B.; Girard, K.P.; Polster, C.S.; Croker, D.M. Progress to Date in the Design and Operation of Continuous Crystallization Processes for Pharmaceutical Applications. Org. Process Res. Dev. 2019, 23, 122–144. [Google Scholar] [CrossRef]
- Pfizer & Siemens: Continuous Manufacturing in Pharma—YouTube. Available online: https://www.youtube.com/watch?v=AFSo8qaY5EU&t=48s (accessed on 2 August 2023).
- Zhang, D.; Xu, S.; Du, S.; Wang, J.; Gong, J. Progress of Pharmaceutical Continuous Crystallization. Engineering 2017, 3, 354–364. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous Crystallization Processes in Pharmaceutical Manufacturing: A Review. Org. Process Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Wang, T.; Lu, H.; Wang, J.; Xiao, Y.; Zhou, Y.; Bao, Y.; Hao, H. Recent Progress of Continuous Crystallization. J. Ind. Eng. Chem. 2017, 54, 14–29. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, S.; Macaringue, E.G.J.; Zhang, T.; Gong, J.; Wang, J. Recent Progress in Continuous Crystallization of Pharmaceutical Products: Precise Preparation and Control. Org. Process Res. Dev. 2020, 24, 1785–1801. [Google Scholar] [CrossRef]
- Çelik, M. Quality by Design, Process Analytical Technology, GMP and Regulatory Affairs. Pharm. Dev. Technol. 2018, 23, 553. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, G.M.; Sakhare, S.S.; Chavan, R.D.; Hamand, V.G.; Hamand, V.G. Quality Control and Quality Assurance in Pharmaceutical Industry. Int. J. Adv. Res. Sci. Commun. Technol. 2023, 2, 501–510. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, R.; Chaurasia, D.K.; Shukla, D.T.P. A Review: Quality Assurance and Quality Control. Int. J. Res. Appl. Sci. Eng. Technol. 2023, 11, 45. [Google Scholar] [CrossRef]
- Pharmaceutical Industry Wastes $50 Billion a Year Due to Inefficient Manufacturing—The Source—Washington University in St. Louis. Available online: https://source.wustl.edu/2006/10/pharmaceutical-industry-wastes-50-billion-a-year-due-to-inefficient-manufacturing/ (accessed on 2 August 2023).
- Center for Drug Evaluation and Research. Q13 Continuous Manufacturing of Drug Substances and Drug Products Guidance for Industry; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2023.
- Domokos, A.; Nagy, B.; Gyürkés, M.; Farkas, A.; Tacsi, K.; Pataki, H.; Liu, Y.C.; Balogh, A.; Firth, P.; Szilágyi, B.; et al. End-to-End Continuous Manufacturing of Conventional Compressed Tablets: From Flow Synthesis to Tableting through Integrated Crystallization and Filtration. Int. J. Pharm. 2020, 581, 119297. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Nagy, Z.K.; Ni, X.W. Recent Advances in Continuous Crystallization. Chem. Eng. Res. Des. 2022, 186, 610–613. [Google Scholar] [CrossRef]
- Kshirsagar, S.; Szilagyi, B.; Nagy, Z.K. Experimental Design for the Efficient Determination of the Crystallization Kinetics of a Polymorphic System in Combined Cooling and Antisolvent Crystallization. Cryst. Growth Des. 2023, 23, 1486–1499. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Fujiwara, M.; Braatz, R.D. Modelling and Control of Combined Cooling and Antisolvent Crystallization Processes. J. Process Control 2008, 18, 856–864. [Google Scholar] [CrossRef]
- Su, Q.; Nagy, Z.K.; Rielly, C.D. Pharmaceutical Crystallisation Processes from Batch to Continuous Operation Using MSMPR Stages: Modelling, Design, and Control. Chem. Eng. Process. Process Intensif. 2015, 89, 41–53. [Google Scholar] [CrossRef]
- Li, J.; Trout, B.L.; Myerson, A.S. Multistage Continuous Mixed-Suspension, Mixed-Product Removal (MSMPR) Crystallization with Solids Recycle. Org. Process Res. Dev. 2016, 20, 510–516. [Google Scholar] [CrossRef]
- Garslde, J.; Shah, M.B. Crystallization Kinetics from MSMPR Crystallizers. Ind. Eng. Chem. Process Des. Dev. 1980, 19, 509–514. [Google Scholar] [CrossRef]
- Du, X.; Xie, C.; Liu, B.; Yuan, P.; Sun, H. Continuous Crystallization Process of Cefminox Sodium in MSMPR Crystallizer. Cryst. Res. Technol. 2023, 58, 2200256. [Google Scholar] [CrossRef]
- Yazdanpanah, N.; Nagy, Z.K. The Handbook of Continuous Crystallization; Royal Society of Chemistry: London, UK, 2020. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2001; ISBN 9780750648332. [Google Scholar]
- Som, S.; Singh, S.K.; Khatik, G.L.; Kapoor, B.; Gulati, M.; Kuppusamy, G.; Anandhakrishnan, N.K.; Kumar, B.; Yadav, A.K.; Kumar, R.; et al. Quality by Design-Based Crystallization of Curcumin Using Liquid Antisolvent Precipitation: Micromeritic, Biopharmaceutical, and Stability Aspects. Assay Drug Dev. Technol. 2020, 18, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Ukrainczyk, M.; Hodnett, B.K.; Rasmuson, Å.C. Process Parameters in the Purification of Curcumin by Cooling Crystallization. Org. Process Res. Dev. 2016, 20, 1593–1602. [Google Scholar] [CrossRef]
- Heffernan, C.; Ukrainczyk, M.; Gamidi, R.K.; Hodnett, B.K.; Rasmuson, Å.C. Extraction and Purification of Curcuminoids from Crude Curcumin by a Combination of Crystallization and Chromatography. Org. Process Res. Dev. 2017, 21, 821–826. [Google Scholar] [CrossRef]
- Vashistha, M.; Cliffe, C.; Murphy, E.; Palanisamy, P.; Stewart, A.; Gadipelli, S.; Howard, C.A.; Brett, D.J.L.; Kumar, K.V. Dotted Crystallisation: Nucleation Accelerated, Regulated, and Guided by Carbon Dots. CrystEngComm 2023, 25, 4729–4744. [Google Scholar] [CrossRef]
- Kumar, K.V.; Ramisetty, K.A.; Devi, K.R.; Krishna, G.R.; Heffernan, C.; Stewart, A.A.; Guo, J.; Gadipelli, S.; Brett, D.J.L.; Favvas, E.P.; et al. Pure Curcumin Spherulites from Impure Solutions via Nonclassical Crystallization. ACS Omega 2021, 6, 23884–23900. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Particle Formation Pathways and Polymorphism of Curcumin Induced by Ultrasound and Additives during Liquid Antisolvent Precipitation. CrystEngComm 2014, 16, 11102–11114. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.; Gao, T.; Nagy, Z.K. Integrated Upstream and Downstream Application of Wet Milling with Continuous Mixed Suspension Mixed Product Removal Crystallization. Cryst. Growth Des. 2015, 15, 5879–5885. [Google Scholar] [CrossRef]
- Novick, A.; Szilard, L. Description of the Chemostat. Science 1950, 112, 715–716. [Google Scholar] [CrossRef]
| So, g·L−1 | Dopt, min−1 | F = V × D, mL·min−1 | V, mL | Mopt, g·L−1 | Pmax, mg·L−1min−1 | s,/g·L−1 |
|---|---|---|---|---|---|---|
| 5.5 | 0.064 | 6.411 | 100 | 1.133 | 72.63 | 3.603 |
| 5 | 0.050 | 4.957 | 100 | 1.031 | 51.10 | 3.325 |
| 4.5 | 0.037 | 3.732 | 100 | 0.929 | 34.66 | 3.047 |
| 4 | 0.027 | 2.718 | 100 | 0.827 | 22.47 | 2.769 |
| 3.5 | 0.019 | 1.898 | 100 | 0.725 | 13.76 | 2.491 |
| 3 | 0.013 | 1.256 | 100 | 0.623 | 7.82 | 2.213 |
| 2.5 | 0.008 | 0.771 | 100 | 0.521 | 4.02 | 1.935 |
| 2 | 0.004 | 0.426 | 100 | 0.419 | 1.79 | 1.657 |
| 1.5 | 0.002 | 0.199 | 100 | 0.317 | 0.632 | 1.379 |
| 1 | 0.001 | 0.069 | 100 | 0.215 | 0.149 | 1.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vashishtha, M.; Ranjbar, M.; Walker, G.; Kumar, K.V. Designing Continuous Crystallization Protocols for Curcumin Using PAT Obtained Batch Kinetics. Crystals 2024, 14, 1069. https://doi.org/10.3390/cryst14121069
Vashishtha M, Ranjbar M, Walker G, Kumar KV. Designing Continuous Crystallization Protocols for Curcumin Using PAT Obtained Batch Kinetics. Crystals. 2024; 14(12):1069. https://doi.org/10.3390/cryst14121069
Chicago/Turabian StyleVashishtha, Mayank, Mahmoud Ranjbar, Gavin Walker, and K. Vasanth Kumar. 2024. "Designing Continuous Crystallization Protocols for Curcumin Using PAT Obtained Batch Kinetics" Crystals 14, no. 12: 1069. https://doi.org/10.3390/cryst14121069
APA StyleVashishtha, M., Ranjbar, M., Walker, G., & Kumar, K. V. (2024). Designing Continuous Crystallization Protocols for Curcumin Using PAT Obtained Batch Kinetics. Crystals, 14(12), 1069. https://doi.org/10.3390/cryst14121069






