Surface Cladding of Mild Steel Coated with Ni Containing TiO2 Nanoparticles Using a High-Temperature Arc from TIG Welding
Abstract
1. Introduction
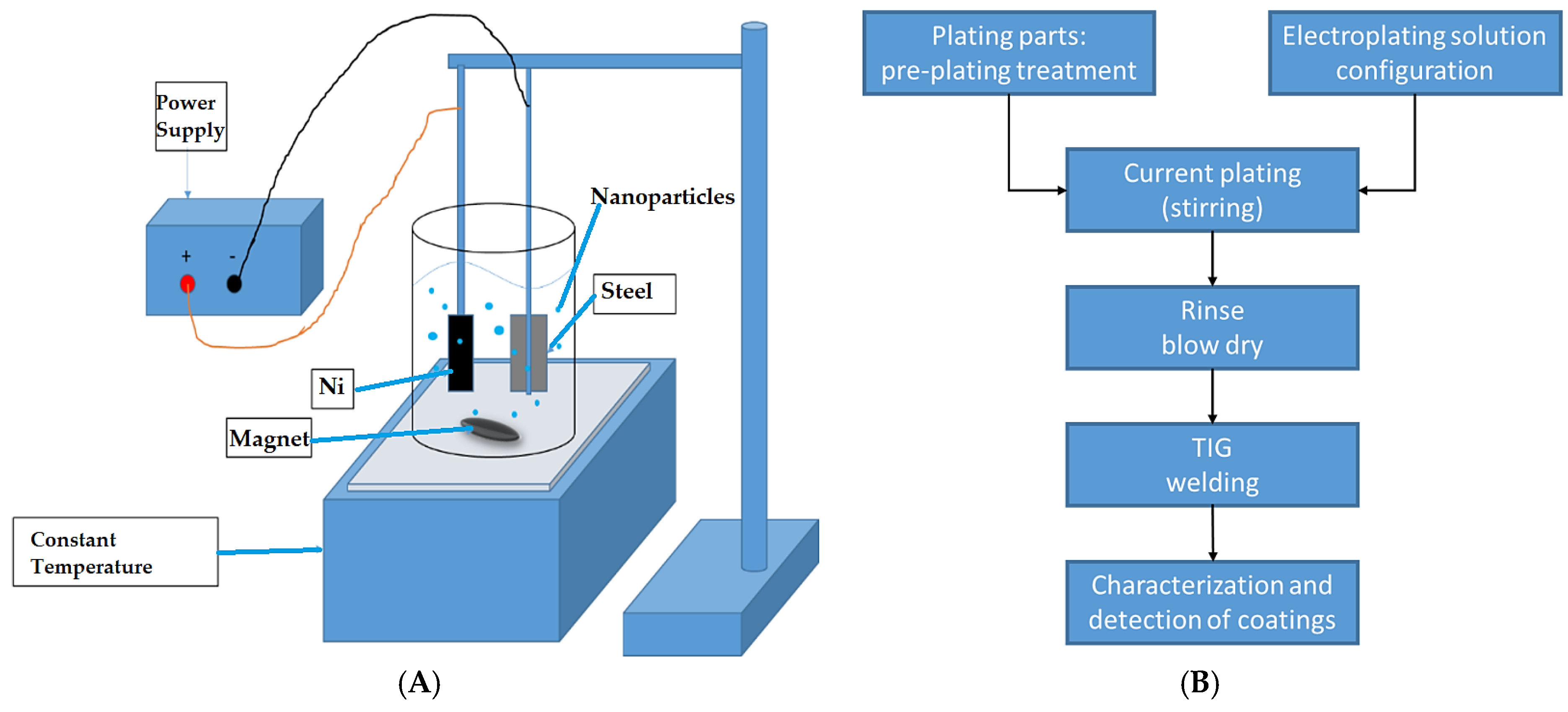
2. Materials and Methods
Modification of the Surface of the Mild Steel
3. Results and Discussion
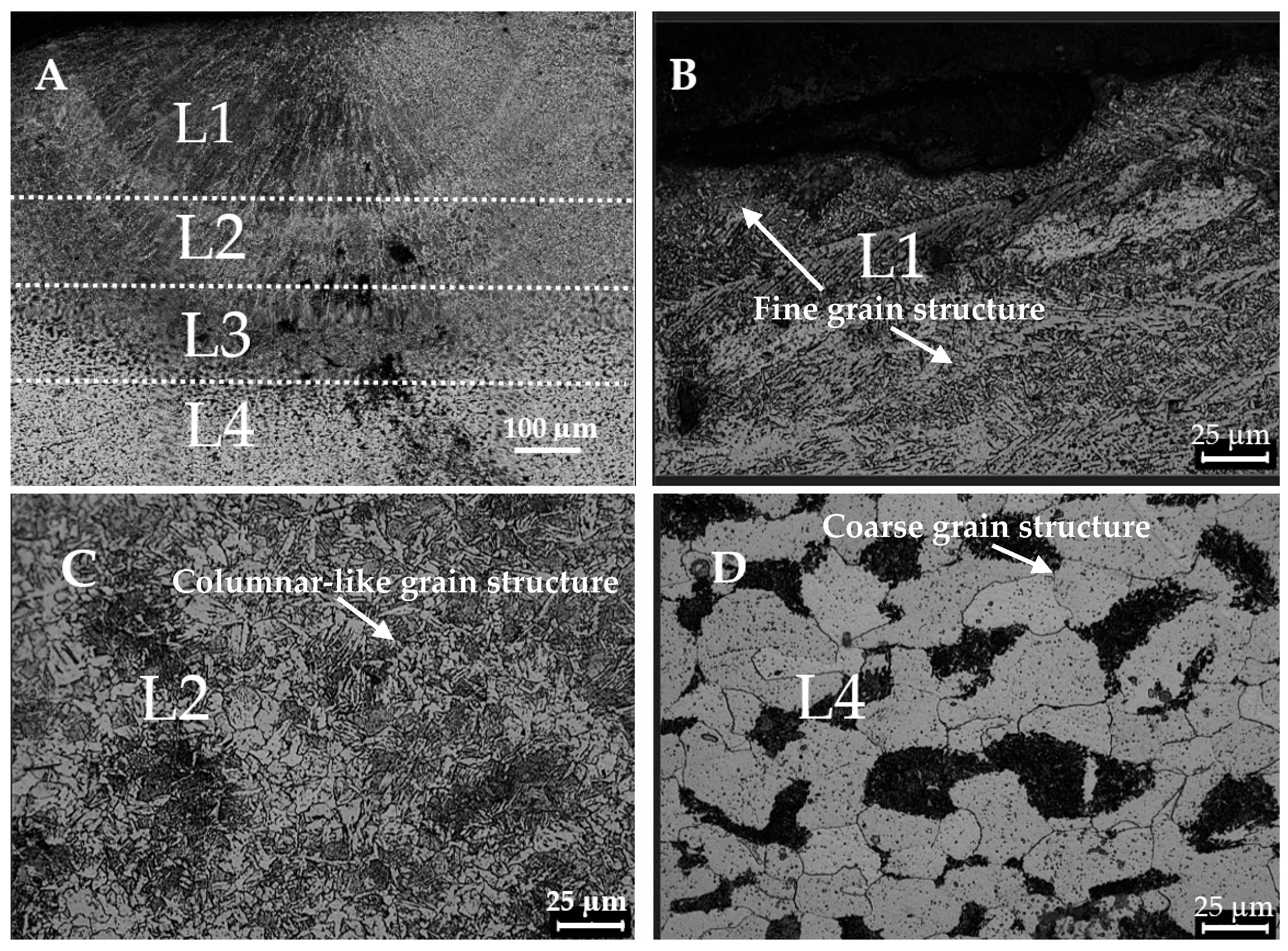
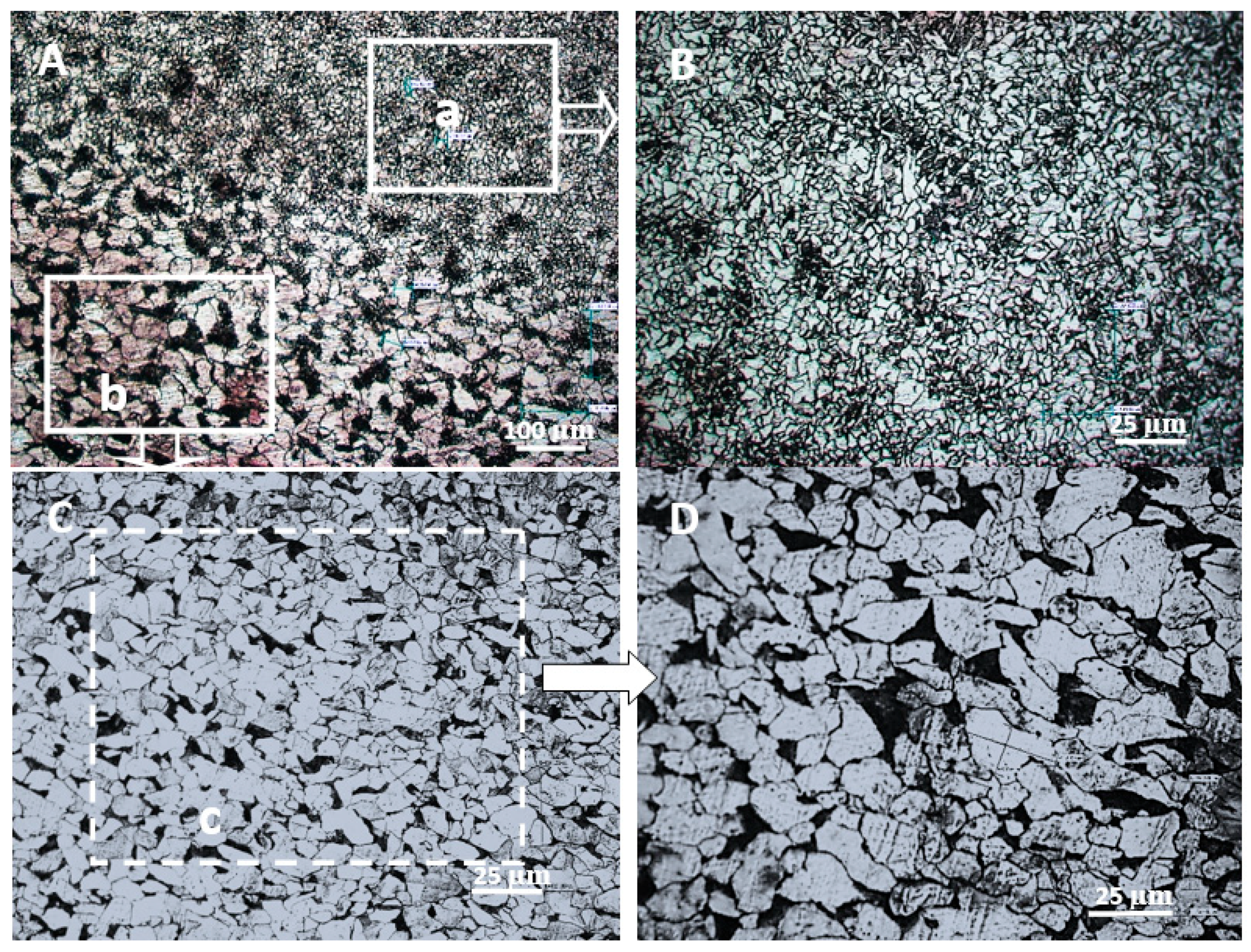
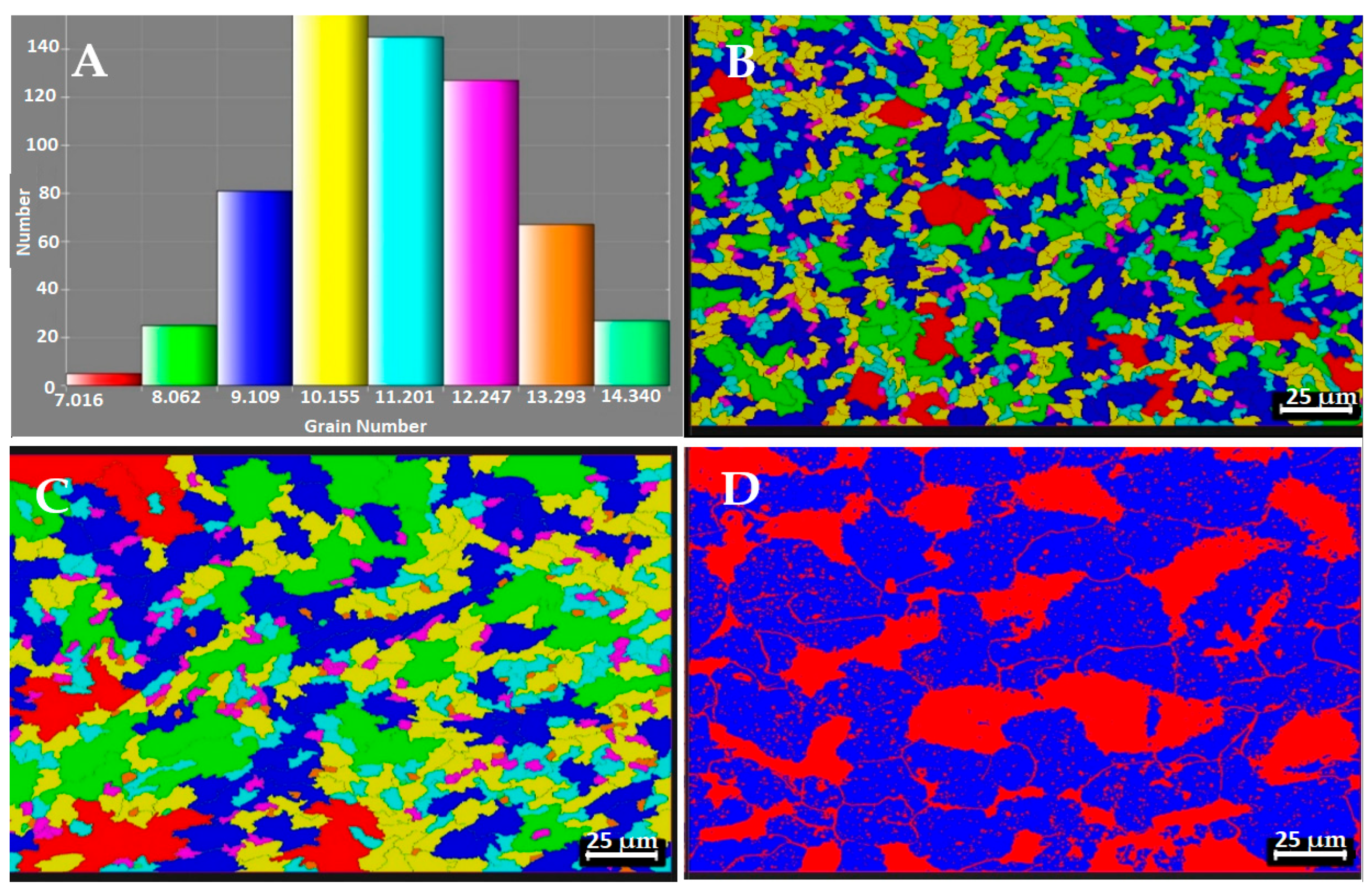
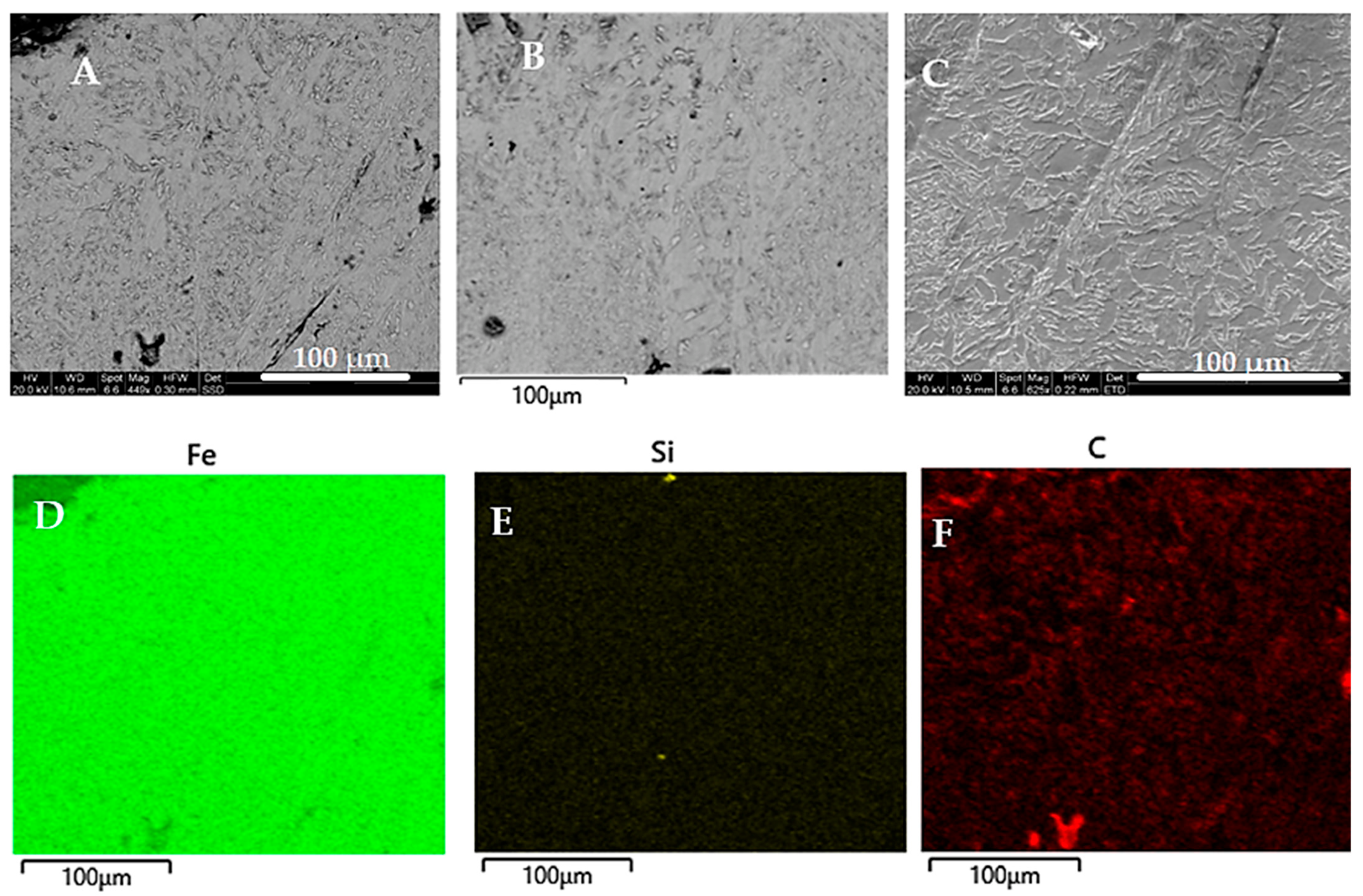
3.1. Microstructural Changes During the Hardening Process
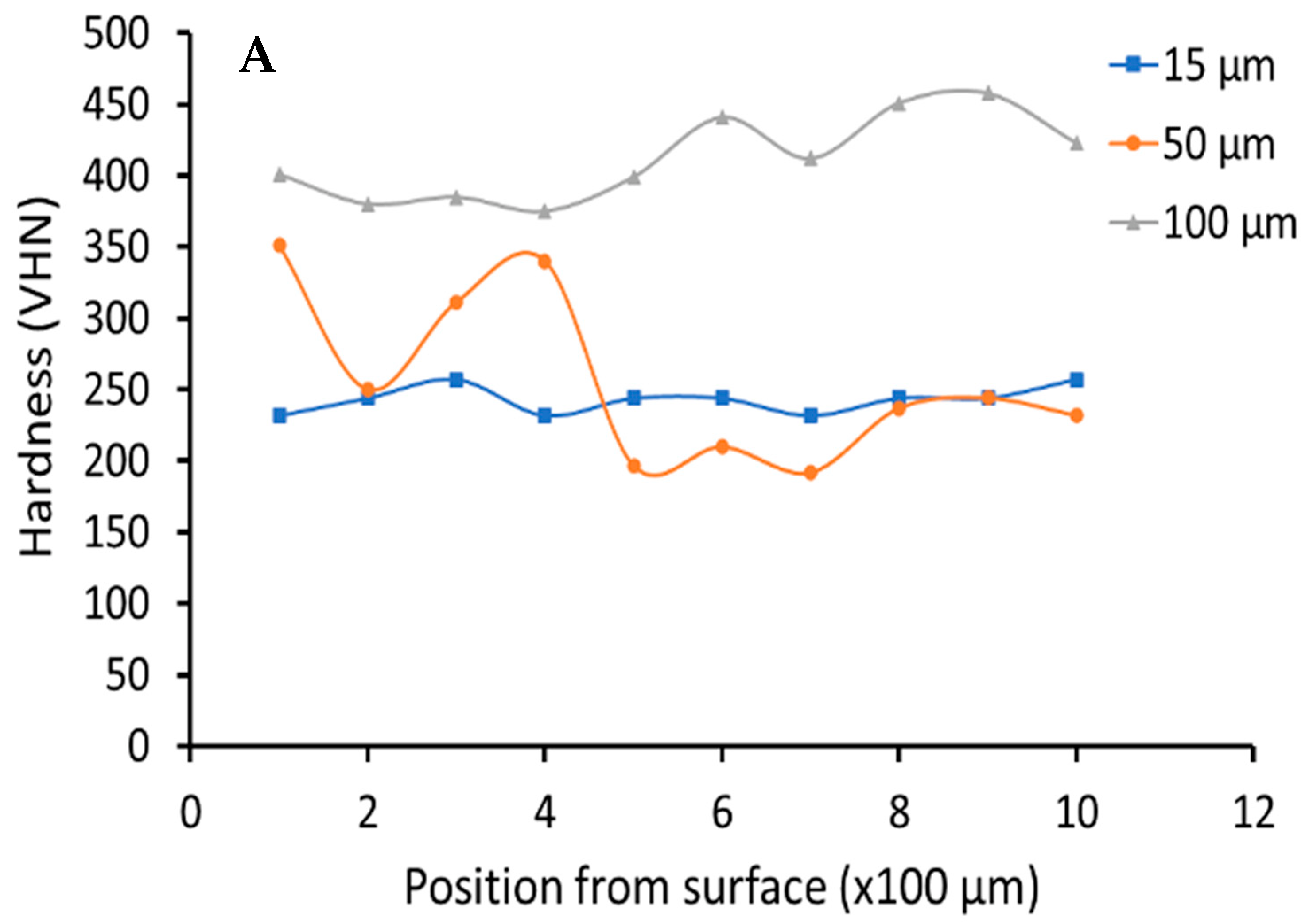
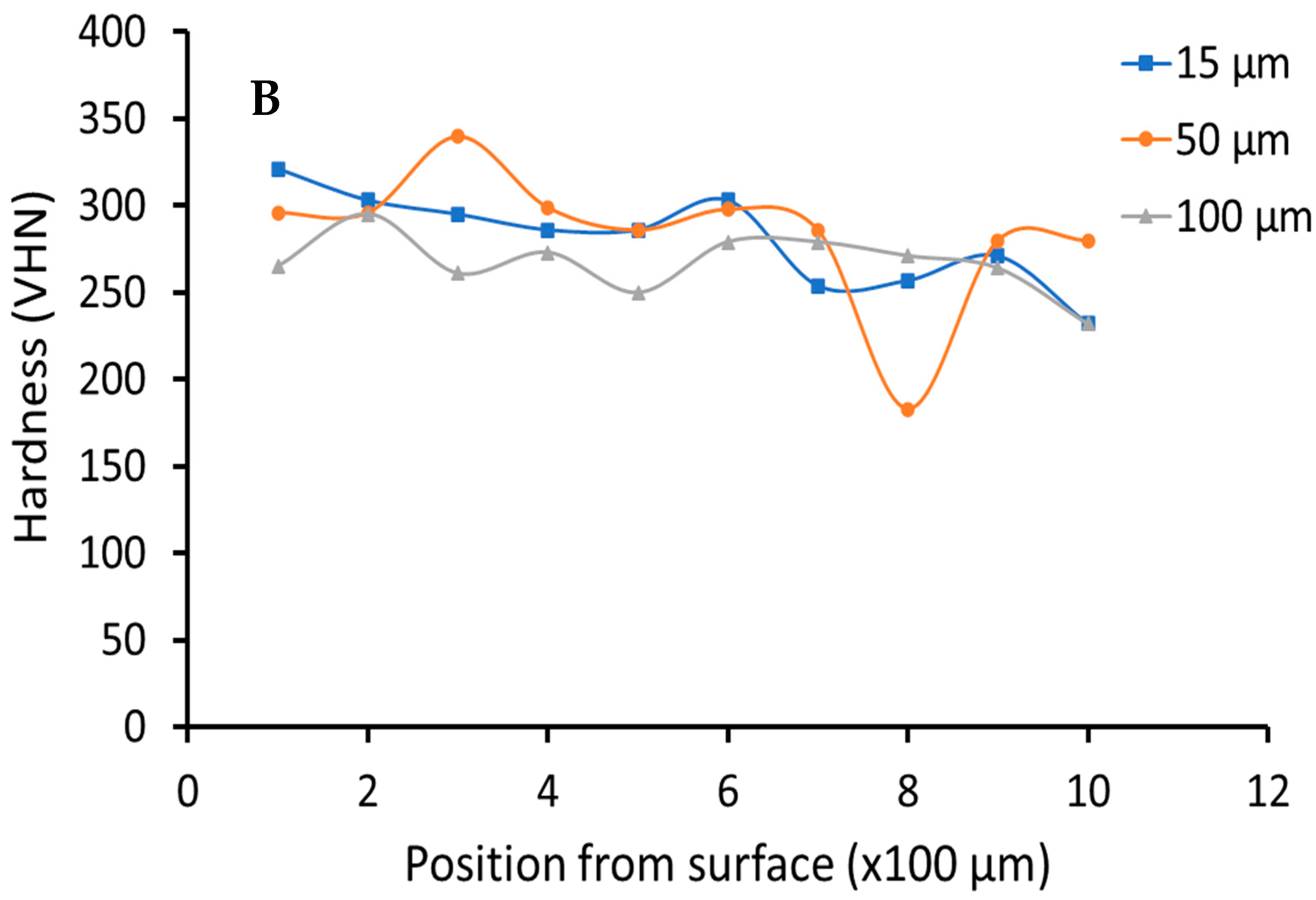
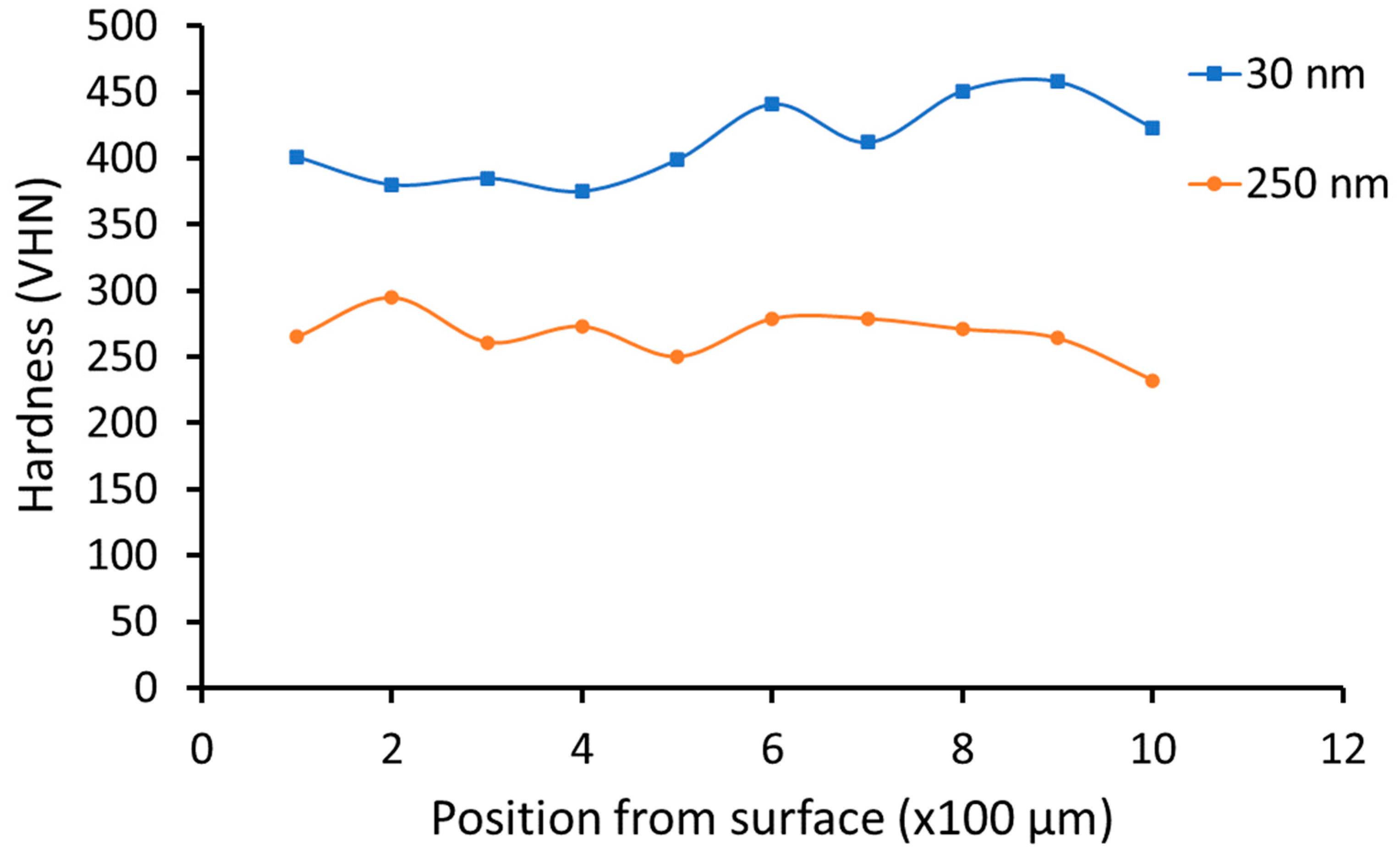
3.2. Hardness Testing
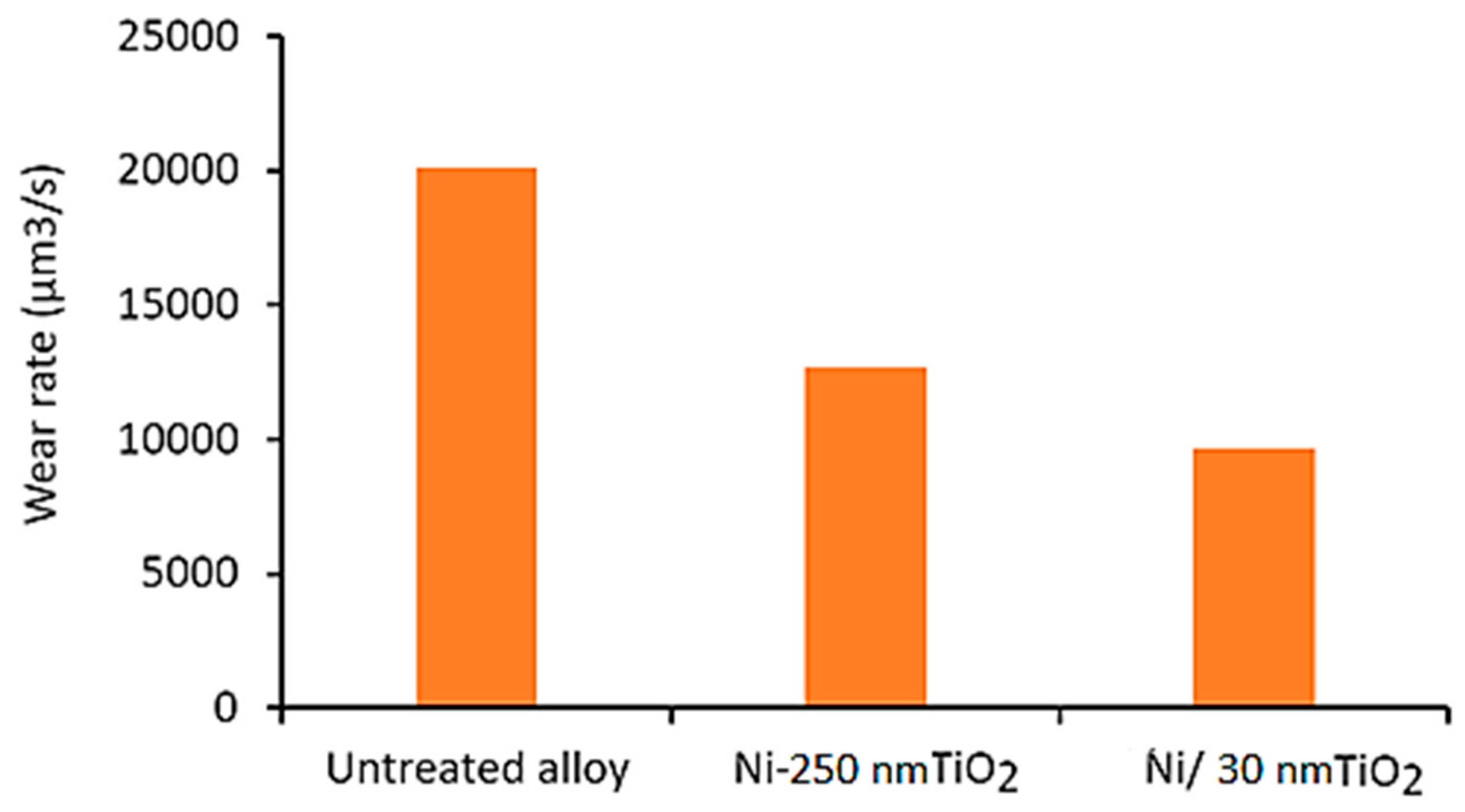
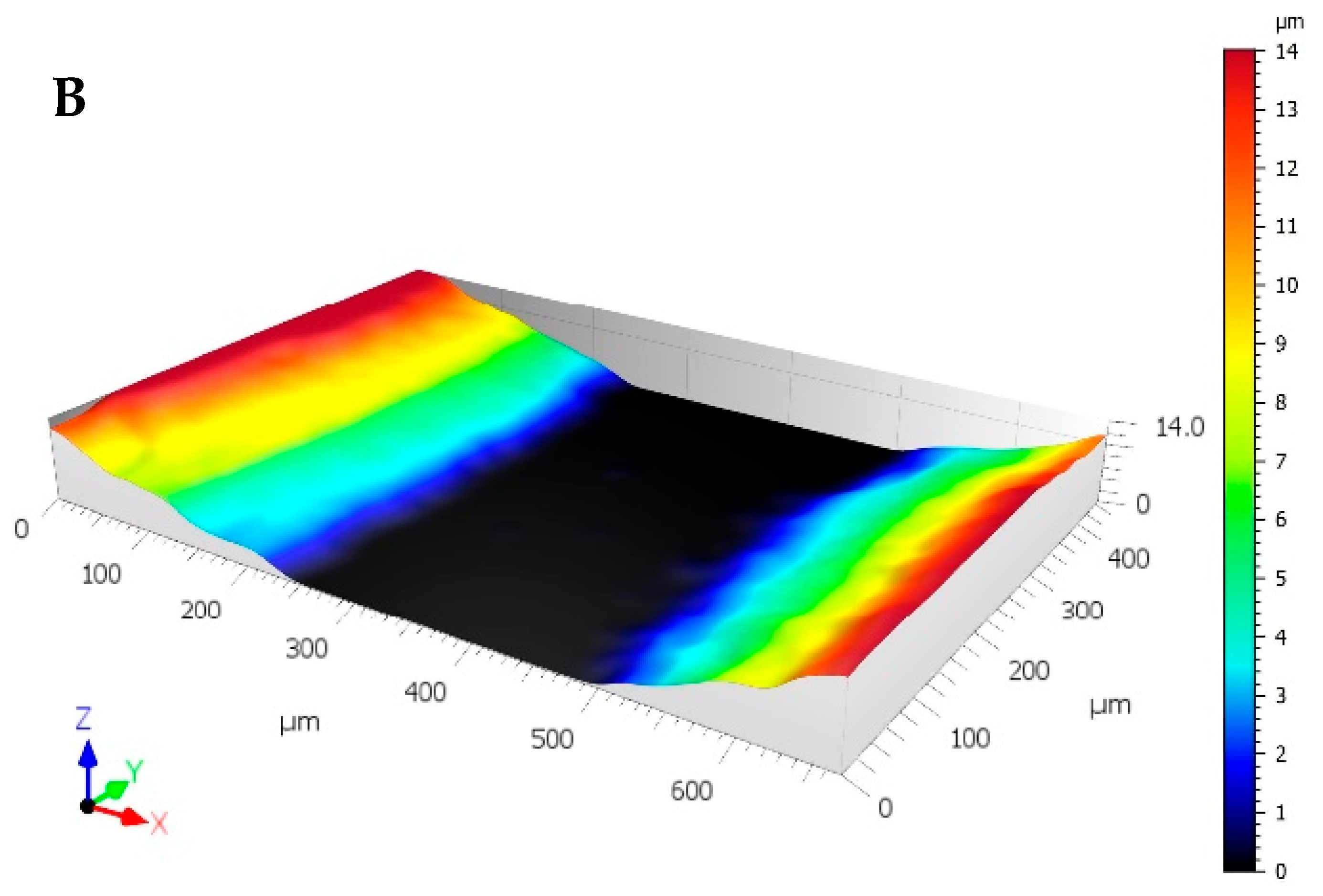
3.3. Wear Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jurči, P. History, developments and trends in the heat treatment of steel. Materials 2020, 13, 4003. [Google Scholar] [CrossRef] [PubMed]
- Lakkannavar, V.; Yogesha, K.B.; Prasad, C.D.; Mruthunjaya, M.; Suresh, R. A Review on Tribological and Corrosion Behaviour of Thermal Spray Coatings. J. Inst. Eng. India Ser. D 2024. [Google Scholar] [CrossRef]
- Cooke, K.O. Parametric Analysis of Electrodeposited Nano-composite Coatings for Abrasive Wear Resistance. In Electrodeposition of Composite Materials; InTech: Penang, Malaysia, 2016. [Google Scholar] [CrossRef]
- Cooke, K.O.; Khan, T.I.; Shar, M.A. Effect of heat-treatment on the thermal and mechanical stability of Ni/Al2O3 nanocrystalline coatings. J. Manuf. Mater. Process. 2020, 4, 17. [Google Scholar] [CrossRef]
- Eo, D.R.; Chung, S.G.; Yang, J.H.; Cho, W.T.; Park, S.H.; Cho, J.W. Surface modification of high-Mn steel via laser-DED: Microstructural characterization and hot crack susceptibility of clad layer. Mater. Des. 2022, 223, 111188. [Google Scholar] [CrossRef]
- Balbande, S.; Paraye, N.K.; Das, S. Surface Modification of Steel via In Situ Formed Tantalum Carbide Through TIG Arcing. Met. Mater. Trans. A Phys. Met. Mater. Sci. 2023, 54, 1–5. [Google Scholar] [CrossRef]
- Leitner, M.; Pichler, P.; Steinwender, F.; Guster, C. Wear and fatigue resistance of mild steel components reinforced by arc welded hard layers. Surf. Coat Technol. 2017, 330, 140–148. [Google Scholar] [CrossRef]
- Ci, W.; Chen, X.; Dai, X.; Liu, C.; Ma, Y.; Zhao, D.; Pan, F. Achieving ultra-high corrosion-resistant Mg-Zn-Sc alloys by forming Sc-assisted protective corrosion product film. J. Mater. Sci. Technol. 2024, 181, 138–151. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Wu, D.; Zheng, X.; Sun, J.; Geng, P.; Ma, N. Enhanced mechanical properties of Fe-based hardfacing alloy with Al additions fabricated by laser cladding. Surf. Coat Technol. 2024, 478, 130447. [Google Scholar] [CrossRef]
- Kong, H.; Liu, Y.; Ren, H.; Li, F.; Kang, K.; Tao, Y.; Sun, Q. Surface modification of mild steel via heterogeneous double-wire arc directed energy deposition: Microstructure and performance of cladding layer. Surf. Coat Technol. 2024, 482, 130751. [Google Scholar] [CrossRef]
- Dowling, M.; Al-Hamaoy, A.R.; Obeidi, M.A. Laser surface cladding of metal parts. Results Surf. Interfaces 2023, 12, 100142. [Google Scholar] [CrossRef]
- Qiu, F.; Kujanpää, V. Transformation hardening of medium-carbon steel with a fiber laser: The influence of laser power and laser power density. Mechanika 2011, 17, 318–323. [Google Scholar] [CrossRef]
- Sarkar, S.; Gopinath, M.; Chakraborty, S.S.; Syed, B.; Nath, A.K. Analysis of temperature and surface hardening of low carbon thin steel sheets using Yb-fiber laser. Surf. Coat Technol. 2016, 302, 344–358. [Google Scholar] [CrossRef]
- Yu, H.; Sun, F.; Zhang, J. Laser and plasma nitriding of titanium using CW-CO2 laser in the atmosphere. Curr. Appl. Phys. 2009, 9, 227–233. [Google Scholar] [CrossRef]
- Obeidi, M.A.; McCarthy, E.; Brabazon, D. Laser surface processing with controlled nitrogen-argon concentration levels for regulated surface life time. Opt. Lasers Eng. 2018, 102, 154–160. [Google Scholar] [CrossRef]
- Harnett, A.; Obeidi, M.A.; Ahad, I.U.; Al-Hamaoy, A.R. Comparing the surface hardness of mild steel processed with CO₂ and fibre lasers. Results Mater. 2023, 18, 100400. [Google Scholar] [CrossRef]
- Psyllaki, P.P.; Griniari, A.; Pantelis, D.I. Parametric study on laser nitriding of 1.5919 steel. J. Mater Process. Technol. 2008, 195, 299–304. [Google Scholar] [CrossRef]
- Kong, H.; Liu, Y.; Ren, H.; Li, F.; Kang, K.; Tao, Y.; Sun, Q. Corrosion and abrasion behavior of high-temperature carburized 20MnCr5 gear steel with Nb and B microalloying. J. Mater. Res. Technol. 2023, 25, 5845–5854. [Google Scholar] [CrossRef]
- Valente, E.H.; Jellesen, M.S.; Somers, M.A.J.; Christiansen, T.L. Gaseous surface hardening of Ti-6Al-4V fabricated by selective laser melting. Surf. Coat Technol. 2020, 383, 125278. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Liu, G.; Qian, Y.; Xu, Y.; Xiang, D. Surface Modification of 42CrMo Steels: A Review from Wear and Corrosion Resistance. Coatings 2024, 14, 337. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, S.; Liu, S.; Guo, Y.; Liang, S.; Zhang, S.; Zhang, X.; Zhang, J.; Liu, R. Effect of annealing treatment on microstructure and mechanical properties of lightweight steels micro-alloyed with vanadium and niobium. Mater. Sci. Eng. A 2023, 886, 145700. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Vikram, R.; Sambath, S.; Sowrirajan, M.; Arunachalashiva, M.; Abhijith, P.V.; Deepak, D. Optimization of flux cored arc welding parameters to minimize the dilution percentage of AISI 316L stainless steel cladding on mild steel. Int. J. Interact. Des. Manuf. 2023. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Y.; Yang, Y.; Li, C. Toughening and strengthening mechanism of plasma sprayed nanostructured Al2O3-13 wt.%TiO2 coatings. Surf. Coat Technol. 2009, 204, 642–649. [Google Scholar] [CrossRef]
- Varis, T.; Lagerbom, J.; Suhonen, T.; Terho, S.; Laurila, J.; Vuoristo, P. On the Applicability of Iron-Based Coatings Against Abrasion and Cavitation Erosion Wear. J. Therm. Spray Technol. 2023, 32, 473–487. [Google Scholar] [CrossRef]
- Li, Y.; Meng, X.; Li, R.; Zeng, F.; Gu, Y. Effect of Ni Content on Microstructure and Performance of Ni/Ceramic Composite Coating. J. Mater. Eng. Perform. 2020, 29, 2853–2864. [Google Scholar] [CrossRef]
- Dudás, A.; Laki, G.; Nagy, A.L.; Zsoldos, I.; Hanula, B.; Bartel, D. Wear behaviour of ceramic particle reinforced atmospheric plasma spray coatings on the cylinder running surface of internal combustion engines. Wear 2022, 502–503, 204373. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, R.; Gao, Y.; Li, Y.; Gong, W.; Li, X.; Lü, W. Microstructure and wear-resistant behaviors of Al2O3-TiO2 reinforced Ni-based composite coating plasma-sprayed on 6061 aluminum alloy. Surf. Coat Technol. 2024, 487, 131032. [Google Scholar] [CrossRef]
- Kumar, N.; Choubey, V.K. Comparative Evaluation of Oxidation Resistance of Detonation Gun-Sprayed Al2O3–40%TiO2 Coating on Nickel-Based Superalloys at 800 °C and 900 °C. High Temp. Corros. Mater. 2023, 99, 359–373. [Google Scholar] [CrossRef]
- Cooke, K.; Alhubaida, A. Microstructural response and wear behaviour of Ti-6Al-4V impregnated with Ni/Al2O3 + TiO2 nanostructured coating using an electric arc. Sci. Rep. 2022, 12, 21978. [Google Scholar] [CrossRef]
- Bhansali, K.; Keche, A.J.; Gogte, C.L.; Chopra, S. Effect of grain size on Hall-Petch relationship during rolling process of reinforcement bar. Mater. Today Proc 2020, 26, 3173–3178. [Google Scholar] [CrossRef]
- Kawasaki, M.; Figueiredo, R.B.; Langdon, T.G. The Role of Grain Size in the Mechanical Properties of Metals. Solid State Phenom. 2023, 353, 149–156. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Kawasaki, M.; Langdon, T.G. The role of grain size in achieving excellent properties in structural materials. J. Mater. Res. Technol. 2024, 30, 3448–3462. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.; Yang, Z.; Zhang, X.; Dong, Y.; Jiang, J.; Wang, J.; Ren, Z. Surface melt nitriding and strengthening mechanism of plasma torched M50 bearing steel. J. Manuf. Process. 2024, 124, 1214–1226. [Google Scholar] [CrossRef]
- Cooke, K.O.; Shar, M.A.; Hussain, S. Micro-alloying and surface texturing of Ti-6Al-4V alloy by embedding nanoparticles using gas tungsten arcwelding. J. Manuf. Mater. Process. 2020, 4, 29. [Google Scholar] [CrossRef]
- Cardona, D.M.M.; Wongsa-Ngam, J.; Jimenez, H.; Langdon, T.G. Effects on hardness and microstructure of AISI 1020 low-carbon steel processed by high-pressure torsion. J. Mater. Res. Technol. 2017, 6, 355–360. [Google Scholar] [CrossRef]







| Elements | Fe | Mn | C | Cu | S | P |
|---|---|---|---|---|---|---|
| Composition (wt%) | Bal | 0.75 | 0.26 | 0.2 | 0.05 | 0.04 |
| Number of Baths | Bath Composition | Particle Size | Volume of Particles Added | Coating Thickness (µm) |
|---|---|---|---|---|
| Bath 1 | 250 g/L NiSO4·6H2O, 45 g/LNiCl2·6H2O, 35 g/L H3BO3, 1 g/L Saccharin, 1 L H2O | 15, 50 and 120 | ||
| Bath 2 | 250 g/L NiSO4·6H2O, 45 g/LNiCl2·6H2O, 35 g/L H3BO3, 1 g/L Saccharin, 1 L H2O | 250 nm TiO2 particles | 20 g/L—TiO2 | 15, 50 and 120 |
| Bath 3 | 250 g/L NiSO4·6H2O, 45 g/LNiCl2·6H2O, 35 g/L H3BO3, 1 g/L Saccharin, 1 L H2O | 30 nm TiO2 particles | 20 g/L—TiO2 | 15, 50 and 120 |
| TIG Welding Parameters | ||||
| Welding Type | Current | Torch Angle | Distance Between Platform andTorch | Platform Moving Speed |
| TIG parameters | 75 Amp | 45 degrees | 3 mm | 2 mm/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooke, K.O.; Mirza, A.; Chen, J.; Al Hausone, A. Surface Cladding of Mild Steel Coated with Ni Containing TiO2 Nanoparticles Using a High-Temperature Arc from TIG Welding. Crystals 2024, 14, 1048. https://doi.org/10.3390/cryst14121048
Cooke KO, Mirza A, Chen J, Al Hausone A. Surface Cladding of Mild Steel Coated with Ni Containing TiO2 Nanoparticles Using a High-Temperature Arc from TIG Welding. Crystals. 2024; 14(12):1048. https://doi.org/10.3390/cryst14121048
Chicago/Turabian StyleCooke, Kavian O., Ayesha Mirza, Junlin Chen, and Alaa Al Hausone. 2024. "Surface Cladding of Mild Steel Coated with Ni Containing TiO2 Nanoparticles Using a High-Temperature Arc from TIG Welding" Crystals 14, no. 12: 1048. https://doi.org/10.3390/cryst14121048
APA StyleCooke, K. O., Mirza, A., Chen, J., & Al Hausone, A. (2024). Surface Cladding of Mild Steel Coated with Ni Containing TiO2 Nanoparticles Using a High-Temperature Arc from TIG Welding. Crystals, 14(12), 1048. https://doi.org/10.3390/cryst14121048









