Assessment and Improvement of Melt Quality of Recycled Secondary A357 Alloy by Application of the High Shear Melt Conditioning (HSMC) Technology
Abstract
1. Introduction
2. Experimental
2.1. Materials Preparation and Experimental Setup
2.2. Characterization of Non-Metallic Inclusions
2.3. Material Properties and Melt Quality Characterization
3. Results
3.1. Identification of the Predominant Inclusions in the Primary A357 Alloys
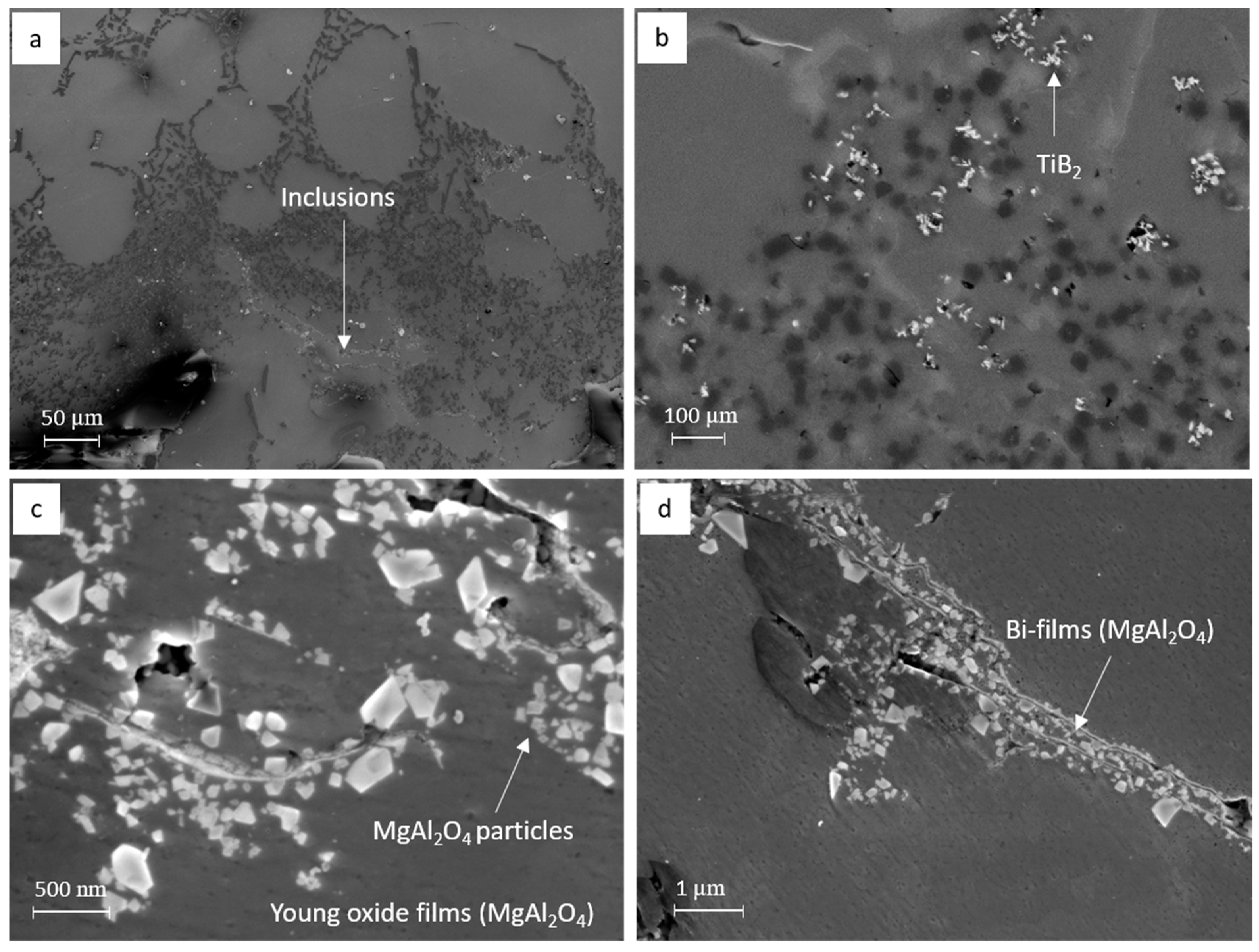
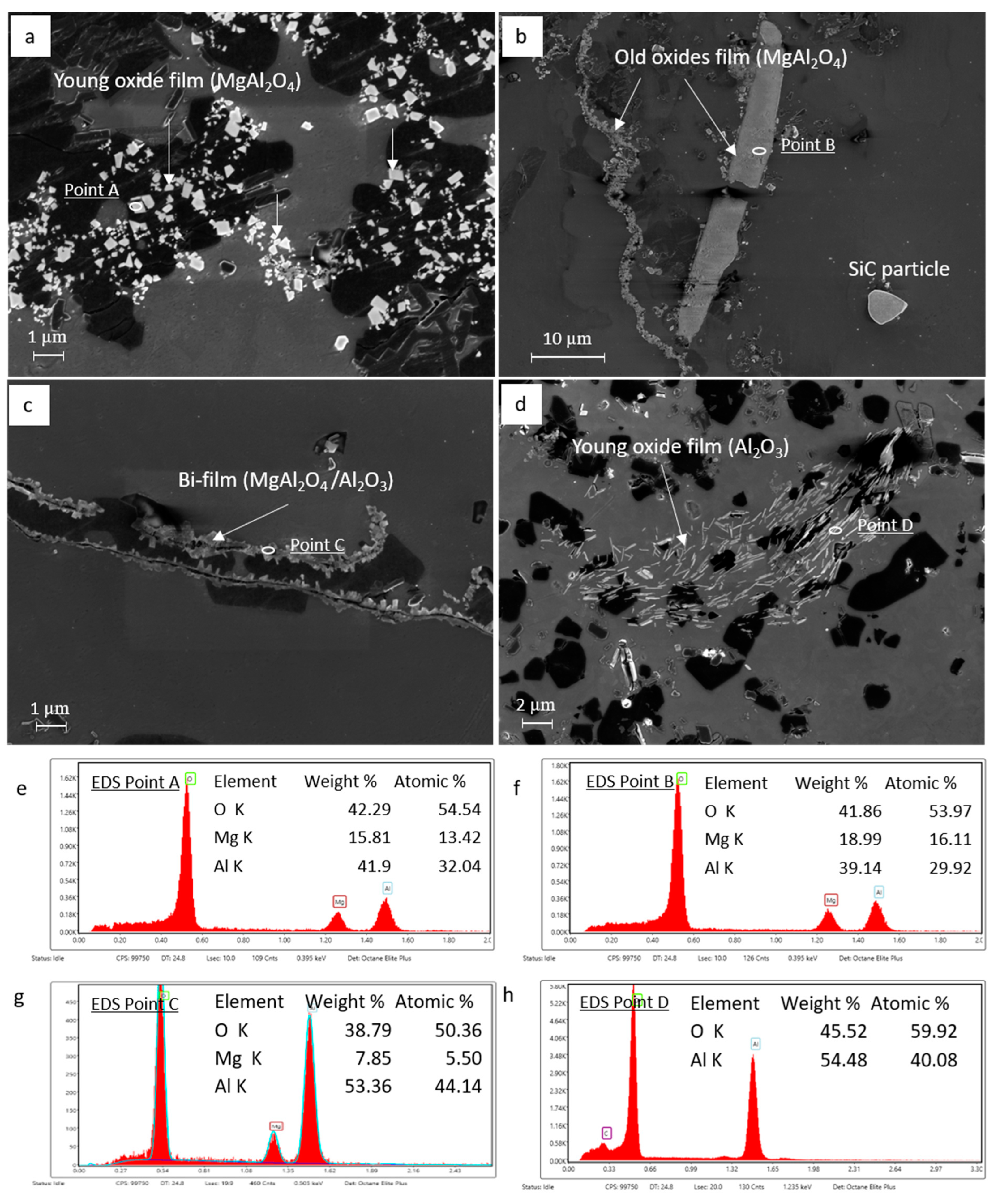
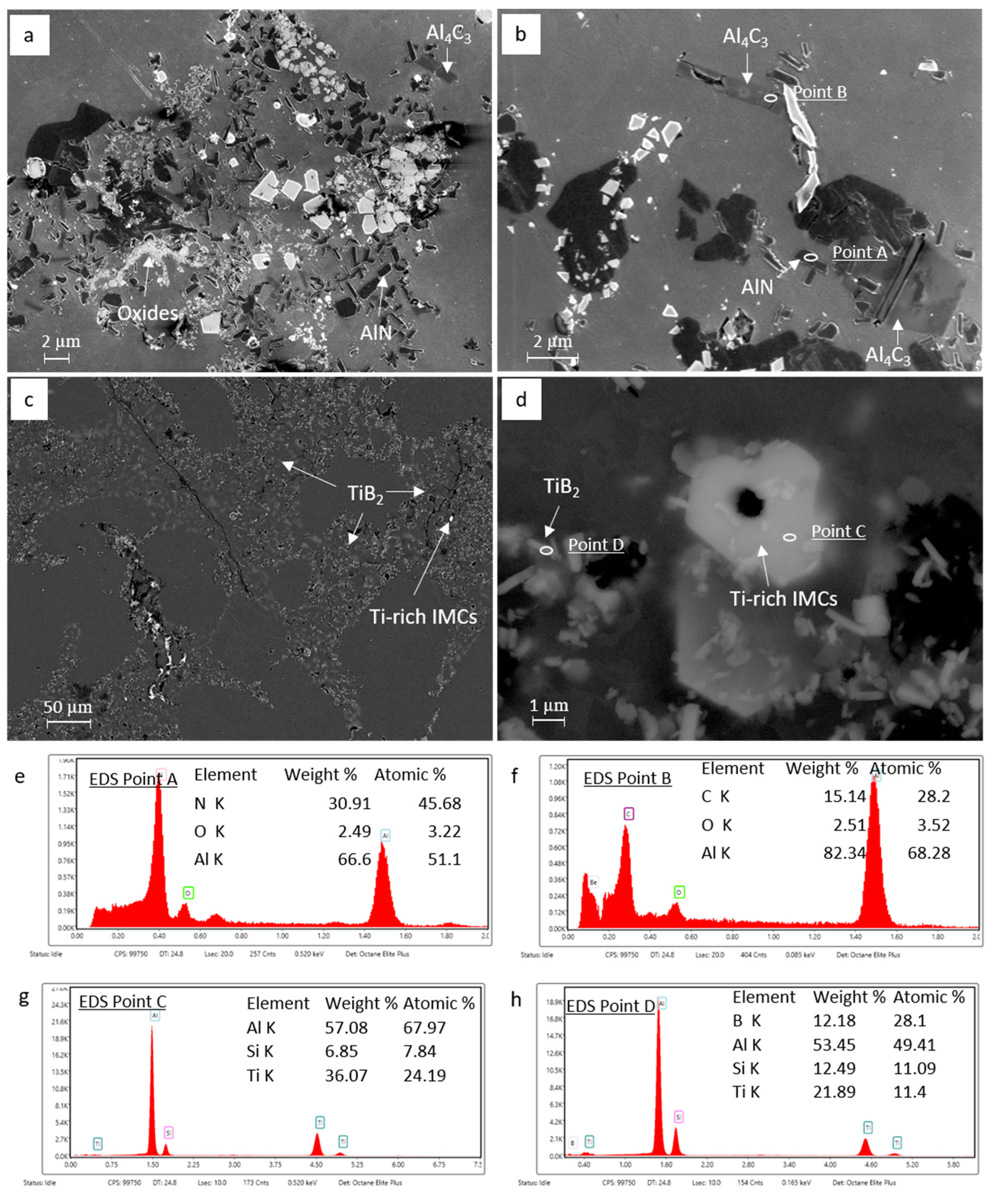
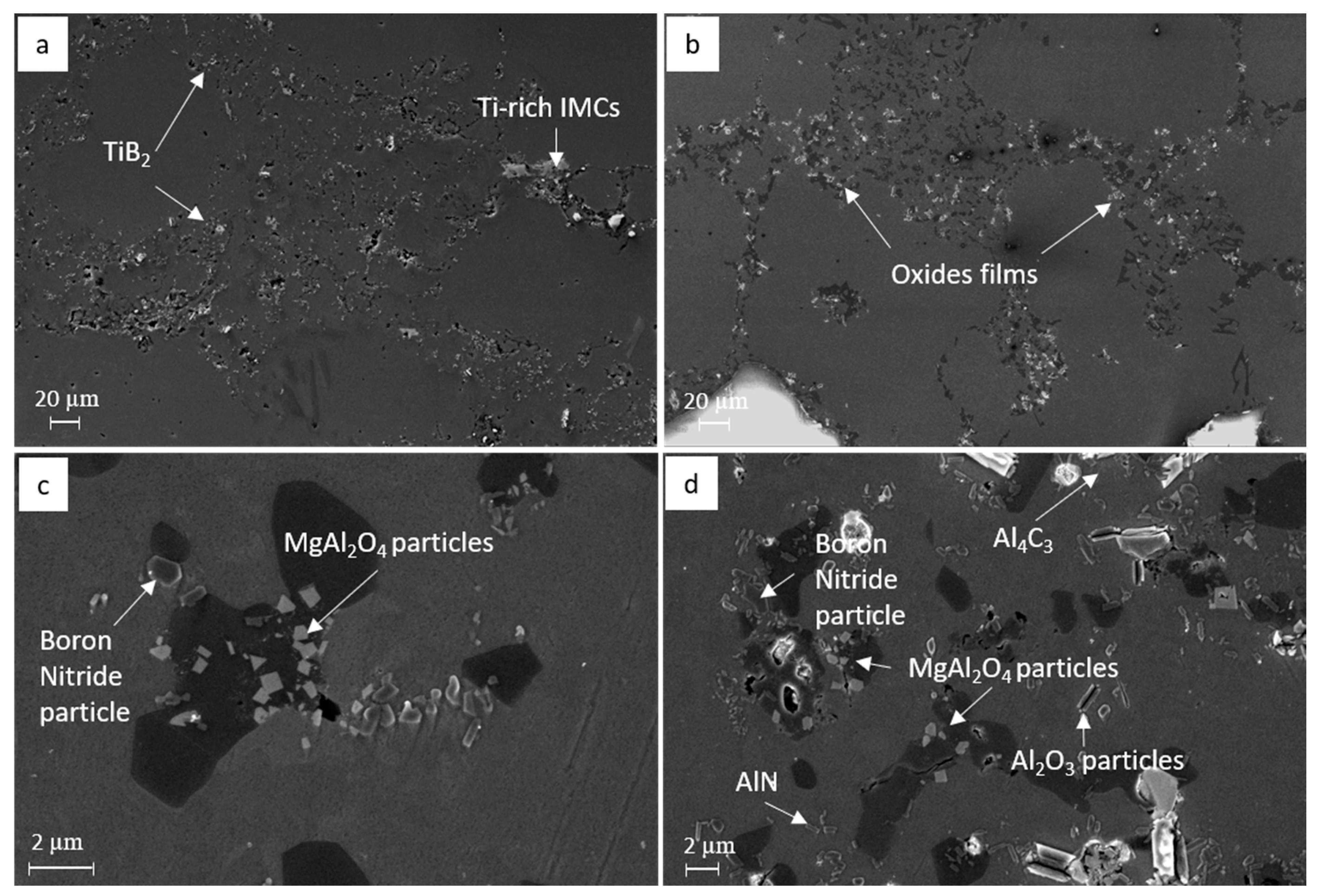
3.2. Identification of the Predominant Inclusions in the Recycled A357 Alloys
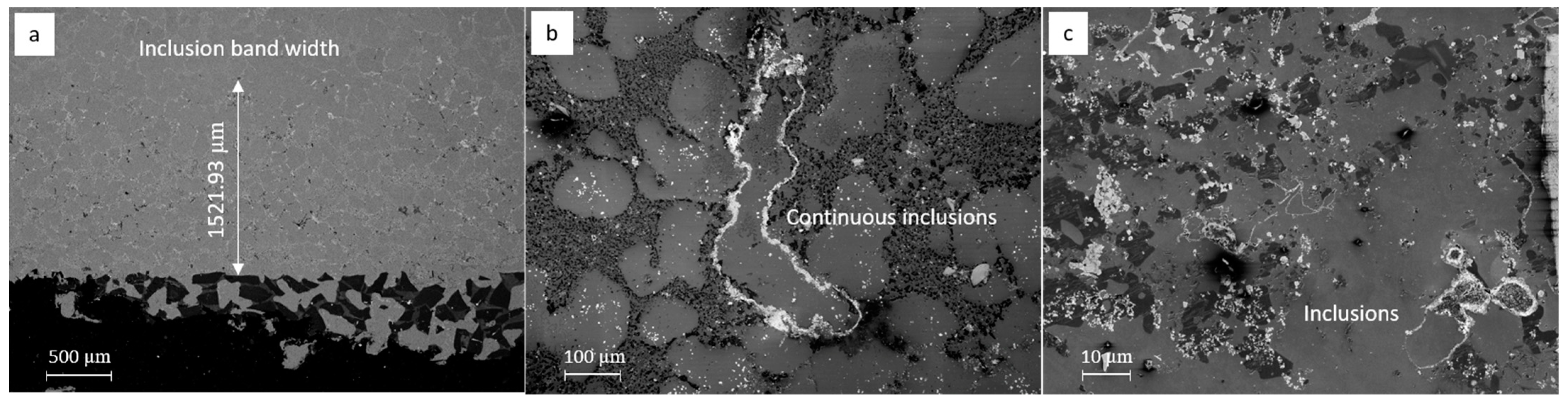
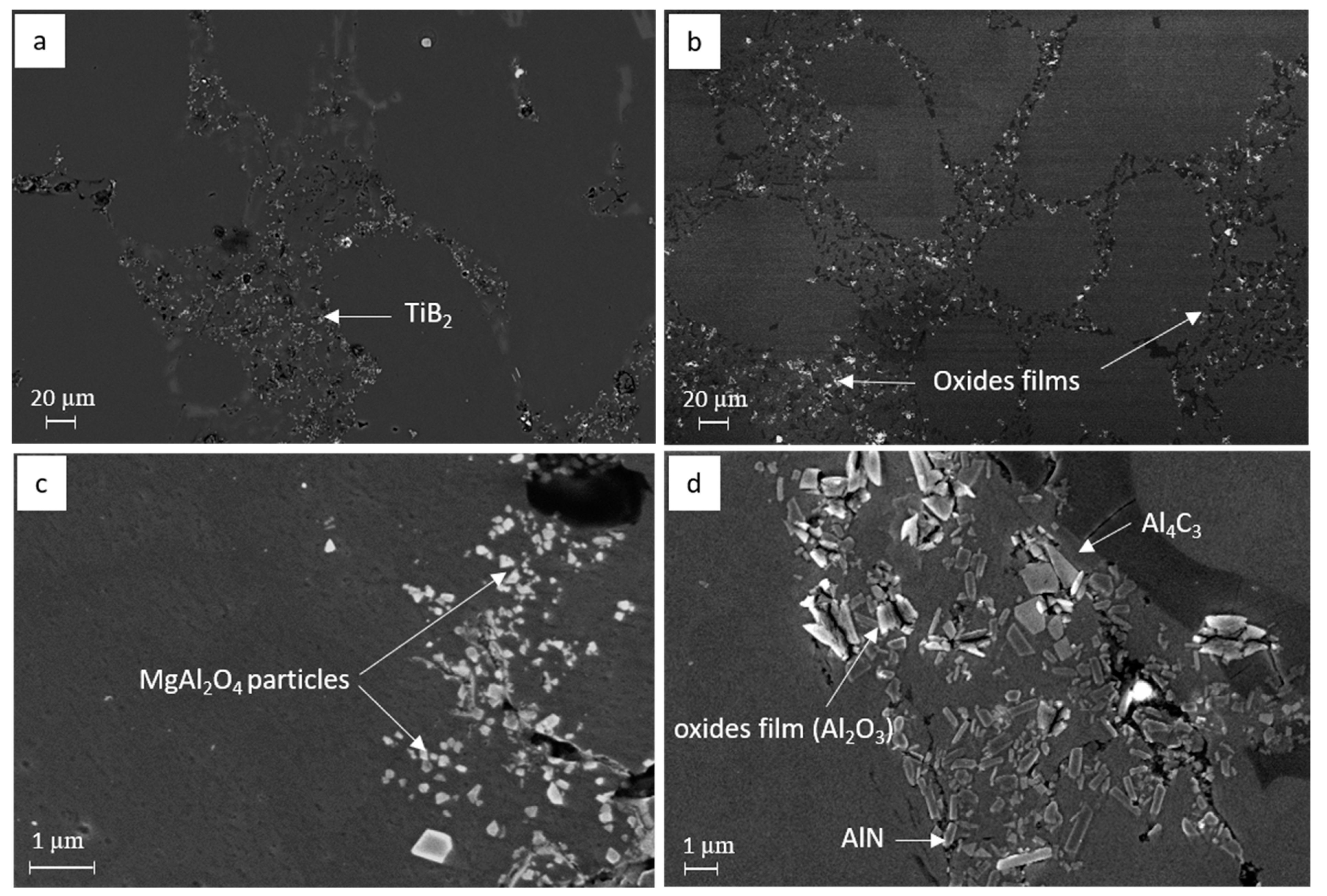
3.3. Characterization of Inclusions in the Recycled A357 Alloys under Different Melt Conditions
3.4. Variations in the Size and Number Density of Inclusions in the Recycled A357 Alloys
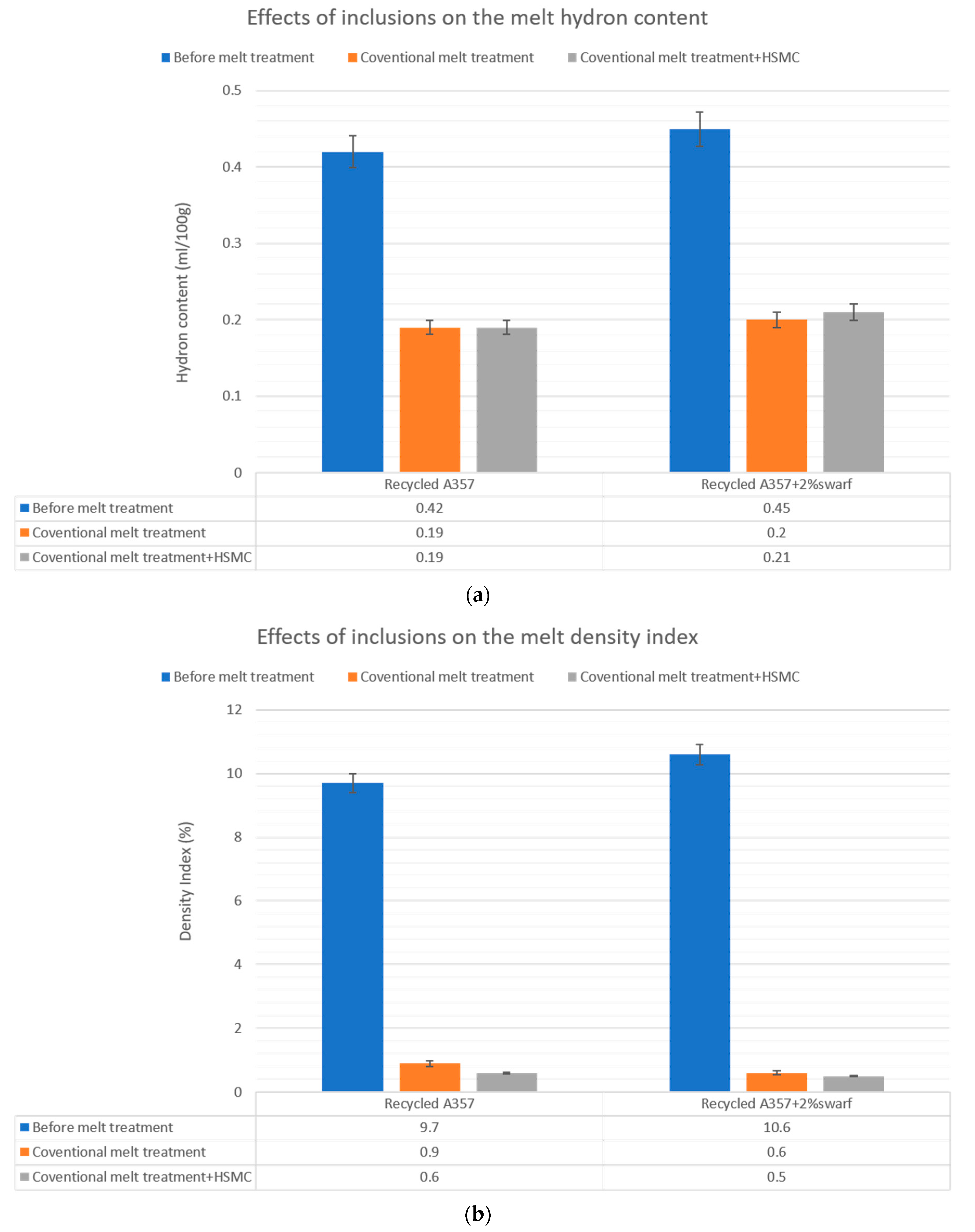
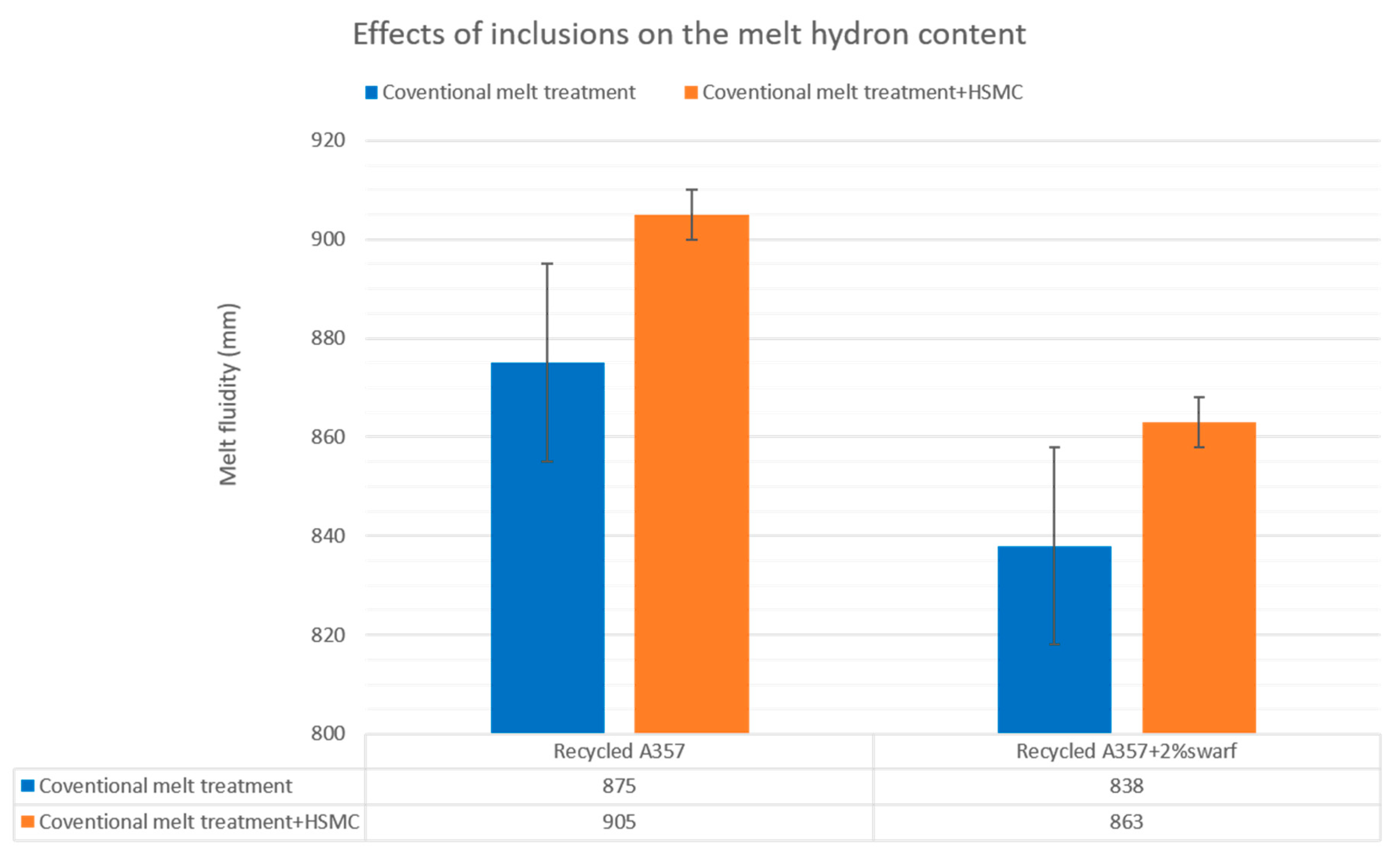
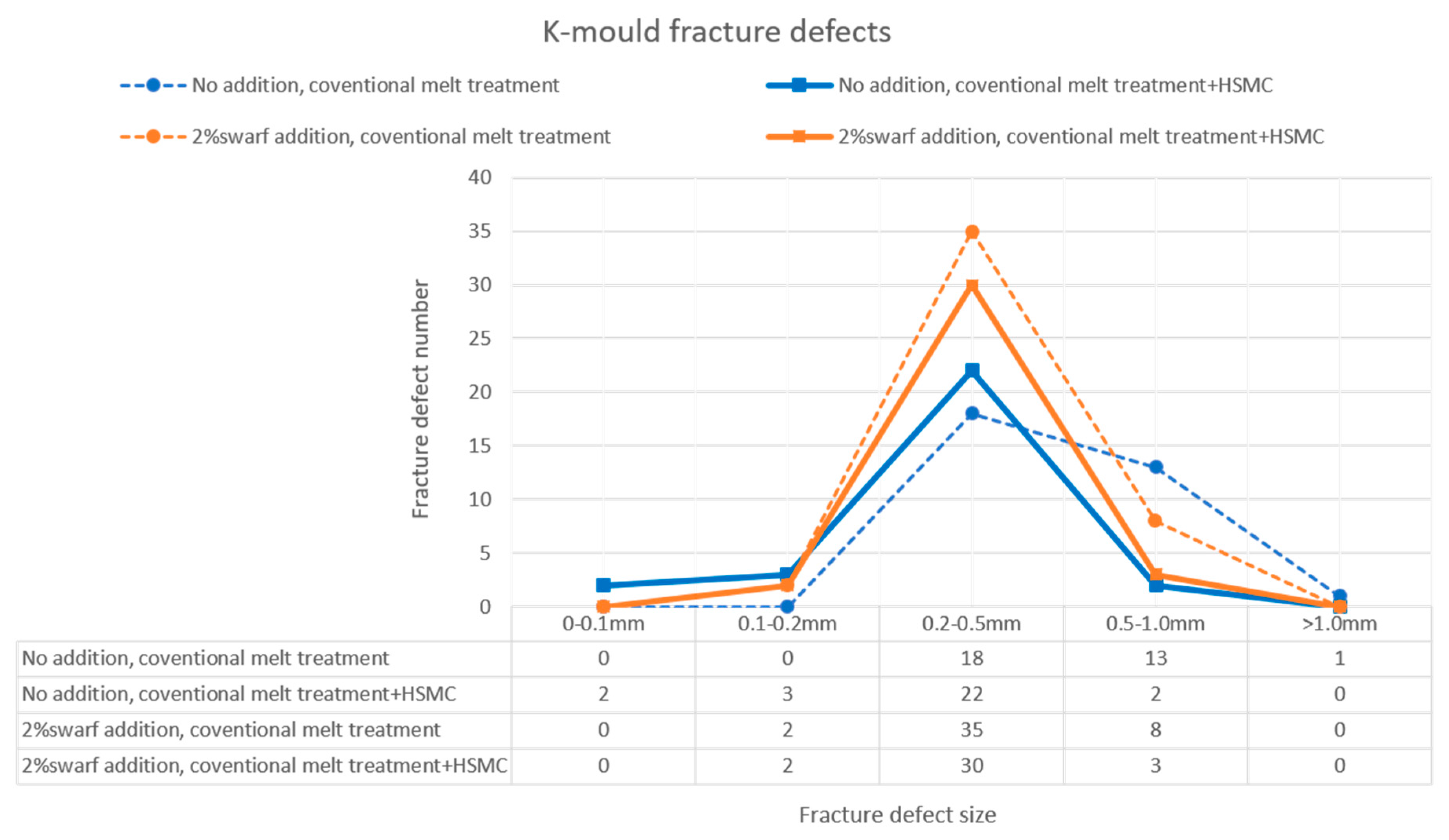
3.5. Effects of Inclusions on the Melt Quality and Material Properties of the Recycled A357 Alloys
4. Discussion
4.1. Formation Mechanism of the Inclusions in the Recycled A357 Alloy
4.2. Benefits of HSMC in Improving the Melt Quality of High Contaminated Recycled A357 Alloy
5. Conclusions
- The predominant inclusions in the recycled A357 alloys consisted primarily of a significant amount of non-metallic inclusions, particularly TiB2 particles and oxide films composed of discrete MgAl2O4 particles. Al4C3, AlN, Ti-rich, and Fe-rich intermetallic compounds (IMCs) were also present as inclusions, although their number densities were relatively small.
- Compared to the primary alloy, the inclusions identified in the recycled A357 alloy exhibited an extremely high number density and poor agglomeration, making them difficult to remove effectively from the melt using conventional degassing techniques. The addition of swarf further exacerbated the size variation and increased the number density of the inclusions by introducing more fresh continuous oxides, carbide particles, and nitride films.
- High shear melt conditioning (HSMC) technology was found to be an effective method for breaking up the agglomeration of entrapped inclusions, rendering them discontinuous and well-dispersed in the melt, thereby facilitating the removal of most large inclusions using conventional melt cleanliness techniques and converting the rest of the large inclusions into a highly dispersed state to minimize their detrimental effects.
- The melt fluidity was restored to a higher value, and the size and number of the defects significantly decreased after the HSMC. The combination of HSMC technology with conventional melt treatment has the potential to enhance the melt condition before casting, resulting in noticeable improvements in the melt quality and material castability.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taub, A.I.; Krajewski, P.E.; Luo, A.A.; Owens, J.N. The evolution of technology for materials processing over the last 50 years: The automotive example. JOM 2007, 59, 48–57. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Wang, C.F.; Yin, S.; Teng, D.; Guan, R.G. Development of low-carbon energy storage material: Electrochemical behavior and discharge properties of iron-bearing Al-Li-based alloys as Al-air battery anodes. J. Power Sources 2023, 585, 233654. [Google Scholar] [CrossRef]
- Mukhopadhyay, J.; Ramana, Y.V.; Singh, U. Extraction of Value Added Products from Aluminum Dross Material to Achieve Zero Waste. In Proceedings of the Light Metals 2005: Proceedings of the Technical Sessions Presented by the TMS Aluminum Committee at the 134th TMS Annual Meeting, San Francisco, CA, USA, 13–17 February 2005; pp. 1209–1212.
- Das, S.K.; Green, J.A.S. The development of recycle-friendly automotive aluminum alloys. JOM 2007, 59, 47–51. [Google Scholar] [CrossRef]
- Zhang, L.; Damoah, L.; Li, S.; Abebe, W. Mechanisms of Inclusion Removal from Aluminum through Filtration. In Proceedings of the Light Metals 2008, Technical Session on Light Metals 2008 Held at the 137th TMS Annual Meeting, New Orleans, LA, USA, 9–13 March 2008; pp. 649–655. [Google Scholar]
- Gallo, R. “I Have Inclusions! Get Me the Cheapest and Best Flux for Cleaning My Melt!”–Is This the Best Driven, Cost-Saving Approach by a Foundry? In Proceedings of the 121st Metal casting Congress of the American Foundry Society, Milwaukee, WI, USA, 25-27 April 2017; pp. 17–105. [Google Scholar]
- Gesing, A. Assuring the continued recycling of light metals in end-of-life vehicles: A global perspective. J. Mater. 2004, 56, 18–27. [Google Scholar] [CrossRef]
- Gaustad, G.; Olivetti, E.; Kirchain, R. Improving aluminum recycling: A survey of sorting and impurity removal technologies. Resour. Conserv. Recycl. 2012, 58, 79–87. [Google Scholar] [CrossRef]
- Ashtari, P.; Gerard, T.K.; Sadayappan, K. Removal of iron from recycled aluminum alloys. Can. Metall. Q. 2012, 51, 75–80. [Google Scholar] [CrossRef]
- Campbell, J. Castings, 2nd ed.; Elsevier: Oxford, UK, 2003. [Google Scholar]
- Alimi, S.A. Recycling aluminum for sustainable development: A review of different processing technologies in green manufacturing. Results Eng. 2024, 23, 102566. [Google Scholar] [CrossRef]
- Cabrera, N.; Mott, N.F.; Wills, H.H. Theory of the Oxidation of Metals. Rep. Prog. Phys. 1949, 12, 163–184. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M. Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. A critical review of techniques for the removal of oxide films (including the heat treatment of liquid metal). In Proceedings of the 2nd International Aluminum Casting Technology Symposium, Columbus, OH, USA, 7–9 October 2002; pp. 135–146. [Google Scholar]
- Fan, Z.; Zuo, Y.B.; Jiang, B. Apparatus and Method for Liquid Metals Treatment. WO Patent 2012035357 A1, 22 March 2012. [Google Scholar]
- Patel, J.B.; Yang, X.; Mendis, C.L.; Fan, Z. Melt Conditioning of Light Metals by Application of High Shear for Improved Microstructure and Defect Control. JOM 2017, 69, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Jaime, L.N.; Patel, J.B.; Fan, Z. Improved degassing efficiency and mechanical properties of A356 aluminum alloy castings by high shear melt conditioning (HSMC) technology. J. Mater. Process. Technol. 2021, 294, 117146. [Google Scholar]
- Niu, Z.; Wang, S.; Gao, F.; Fan, Z. Nature of Oxides in Al–Mg Alloys. Trans. Indian. Inst. Met. 2024, 77, 2929–2933. [Google Scholar] [CrossRef]
- Simensen, C.J.; Berg, C. A Survey of Inclusions in Aluminum. Alum. Dusseld. 1980, 56, 335–340. [Google Scholar]
- Nyahumwa, C.; Green, N.R.; Campbell, J. Effect of Mold-Filling Turbulence on the Fatigue Properties of Cast Aluminum Alloys. AFS Trans. 1998, 106, 215–223. [Google Scholar]
- Raiszadeh, R.; Griffiths, W.D. A method to study the history of a double oxide film defect in liquid aluminum alloys. Metall. Mater. Trans. B 2006, 37, 865–871. [Google Scholar] [CrossRef]
- Lloyed, D.J.; Lagace, H.; McLeod, A.; Morris, P.L. Microstructural aspects of aluminum-silicon carbide particulate composites produced by a casting method. Mater. Sci. Eng. A 1989, 107, 73–80. [Google Scholar] [CrossRef]
- Limmaneevichitr, C.; Eidhed, W. Fading mechanism of grain refinement of aluminum–silicon alloy with Al–Ti–B grain refiners. Mater. Sci. Eng. A 2003, 349, 197–206. [Google Scholar] [CrossRef]
- Limmaneevichitr, C.; Eidhed, W.; Eisuke, N. Effects of Residual TiB2 and TiAl3 Nucleant on Grain-Refinement of Recycled Aluminum-Silicon Alloy Castings. In Proceedings of the 9th International Conference on Aluminum Alloys, Brisbane, Australian, 2 August 2004; pp. 576–581. [Google Scholar]
- Dong, X.X.; He, L.J.; Huang, X.S.; Li, P.J. Effect of electromagnetic transport process on the improvement of hydrogen porosity defect in A380 aluminum alloy. Int. J. Hydrogen Energy 2015, 40, 9287–9297. [Google Scholar] [CrossRef]





| Alloys | Cu | Mg | Si | Fe | Mn | Ni | Zn | Pb | Sn | Ti | Sr | Be | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A(ref) | 0.004 | 0.67 | 6.54 | 0.02 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.102 | 0.020 | 0.000 | Bal. |
| A0 | 0.008 | 0.68 | 6.59 | 0.05 | 0.005 | 0.002 | 0.004 | 0.000 | 0.003 | 0.148 | 0.012 | 0.000 | Bal. |
| A1 | 0.007 | 0.64 | 6.60 | 0.05 | 0.004 | 0.003 | 0.004 | 0.000 | 0.003 | 0.138 | 0.014 | 0.000 | Bal. |
| A2 | 0.007 | 0.67 | 6.62 | 0.05 | 0.004 | 0.003 | 0.004 | 0.000 | 0.003 | 0.138 | 0.017 | 0.000 | Bal. |
| A3 | 0.007 | 0.63 | 6.56 | 0.05 | 0.004 | 0.002 | 0.004 | 0.000 | 0.003 | 0.140 | 0.022 | 0.000 | Bal. |
| A4 | 0.007 | 0.65 | 6.63 | 0.05 | 0.004 | 0.002 | 0.004 | 0.000 | 0.003 | 0.138 | 0.019 | 0.000 | Bal. |
| TiB2 (nm) | AlN (nm) | Al4C3 (nm) | Oxide (nm) | Oxide Film (µm) | |
|---|---|---|---|---|---|
| A1 | 986 ± 188 | 867 ± 223 | 2009 ± 398 | 580 ± 163 | 39 ± 11 |
| A2 | 949 ± 158 | 771 ± 202 | 1522 ± 471 | 481 ± 134 | 60 ± 22 |
| A3 | 987 ± 176 | 879 ± 213 | 1927 ± 411 | 337 ± 121 | 17 ± 3 |
| A4 | 949 ± 158 | 765 ± 177 | 1579 ± 429 | 317 ± 101 | 20 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Z.; Que, Z.; Patel, J.B.; Fan, Z. Assessment and Improvement of Melt Quality of Recycled Secondary A357 Alloy by Application of the High Shear Melt Conditioning (HSMC) Technology. Crystals 2024, 14, 1044. https://doi.org/10.3390/cryst14121044
Niu Z, Que Z, Patel JB, Fan Z. Assessment and Improvement of Melt Quality of Recycled Secondary A357 Alloy by Application of the High Shear Melt Conditioning (HSMC) Technology. Crystals. 2024; 14(12):1044. https://doi.org/10.3390/cryst14121044
Chicago/Turabian StyleNiu, Zhichao, Zhongping Que, Jayesh B. Patel, and Zhongyun Fan. 2024. "Assessment and Improvement of Melt Quality of Recycled Secondary A357 Alloy by Application of the High Shear Melt Conditioning (HSMC) Technology" Crystals 14, no. 12: 1044. https://doi.org/10.3390/cryst14121044
APA StyleNiu, Z., Que, Z., Patel, J. B., & Fan, Z. (2024). Assessment and Improvement of Melt Quality of Recycled Secondary A357 Alloy by Application of the High Shear Melt Conditioning (HSMC) Technology. Crystals, 14(12), 1044. https://doi.org/10.3390/cryst14121044








