Comparative Analysis of Graphitization Characteristics in Bamboo and Oak Charcoals for Secondary Battery Anodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
3. Results and Discussion
3.1. Surface Area Analysis Based on Particle Size
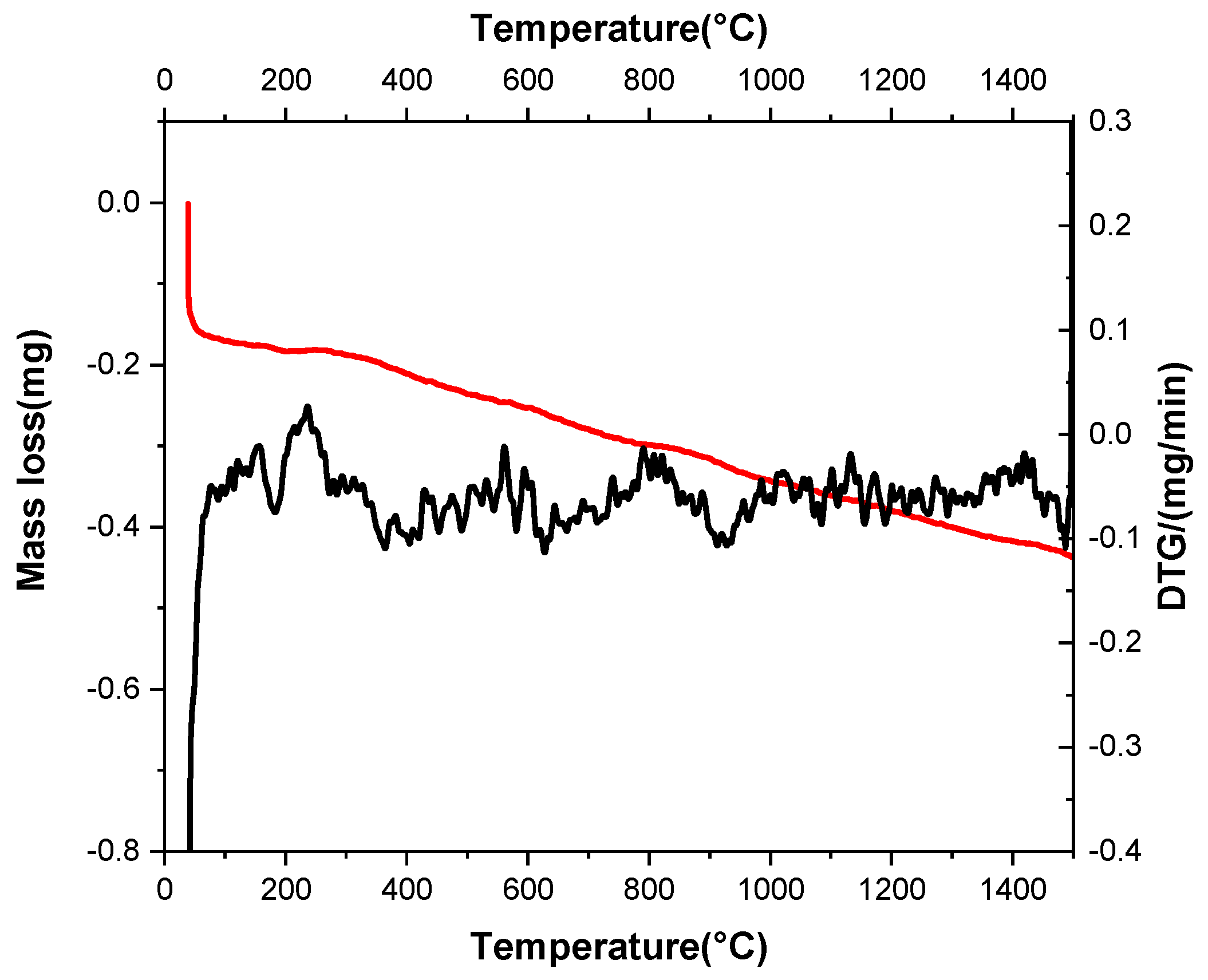
3.2. Thermogravimetric Analysis
3.3. Elemental Analysis
3.4. Surface Morphology
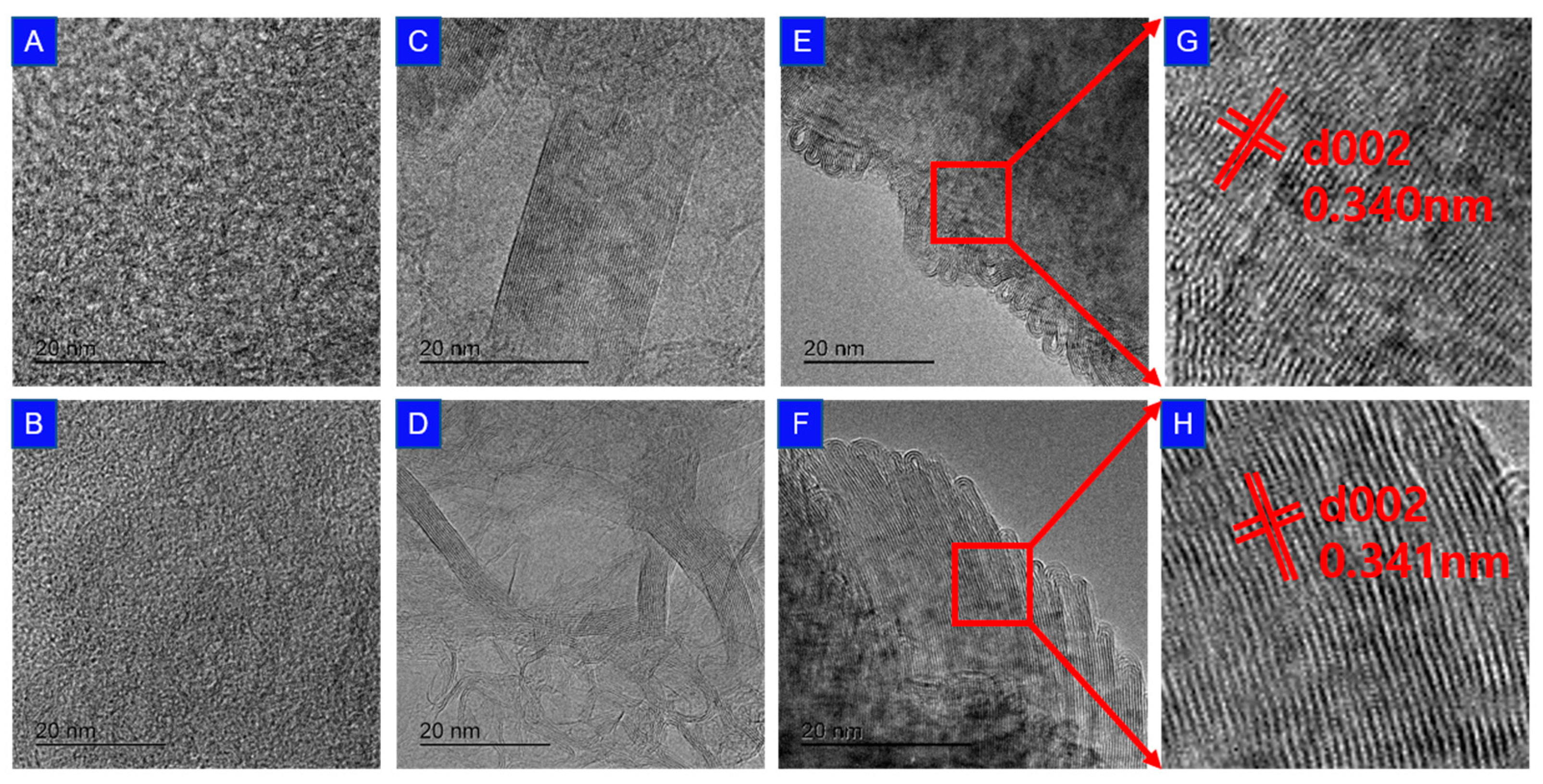
3.5. TEM Observation of Internal Structure
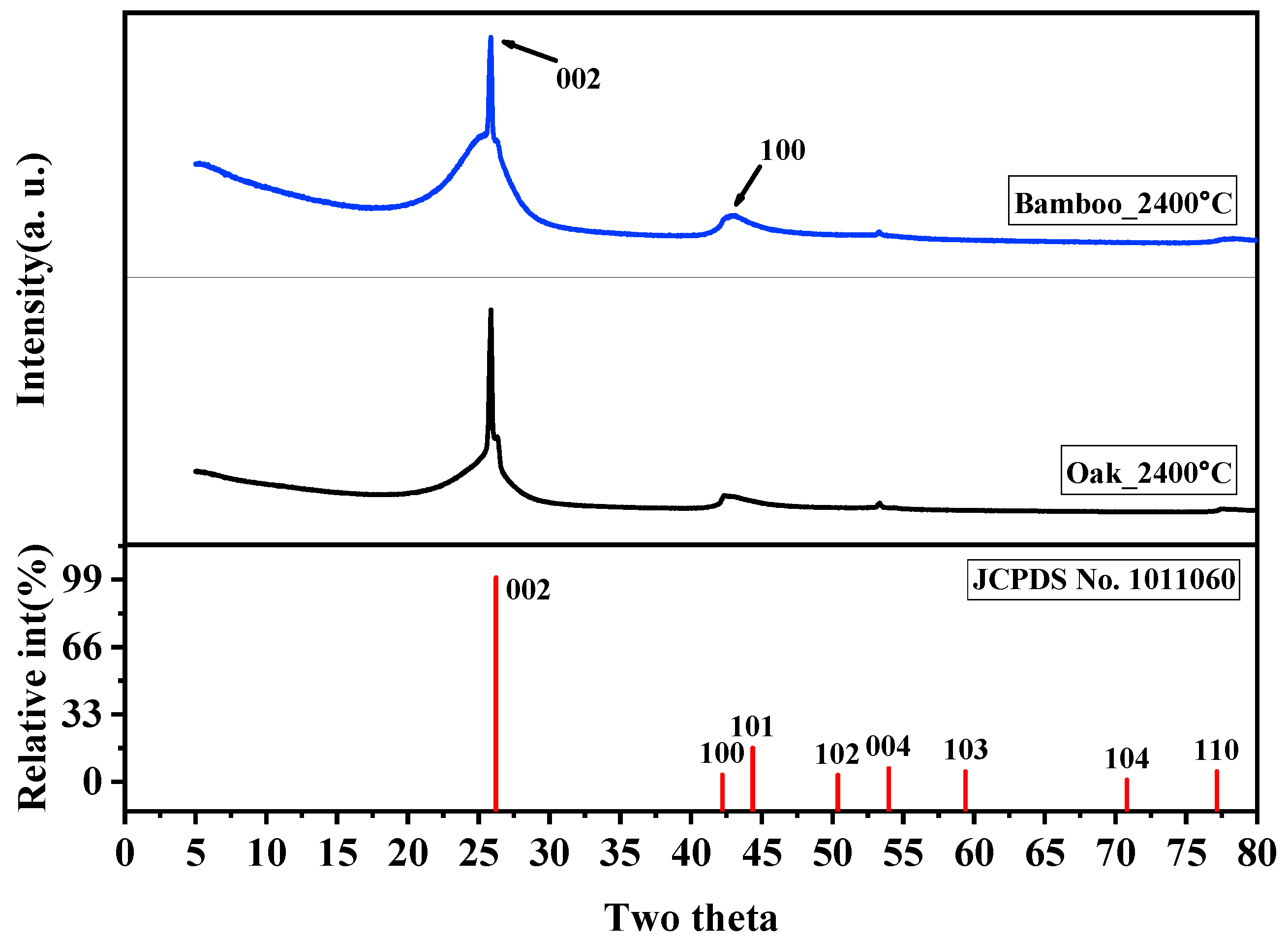
3.6. Crystal Growth by XRD
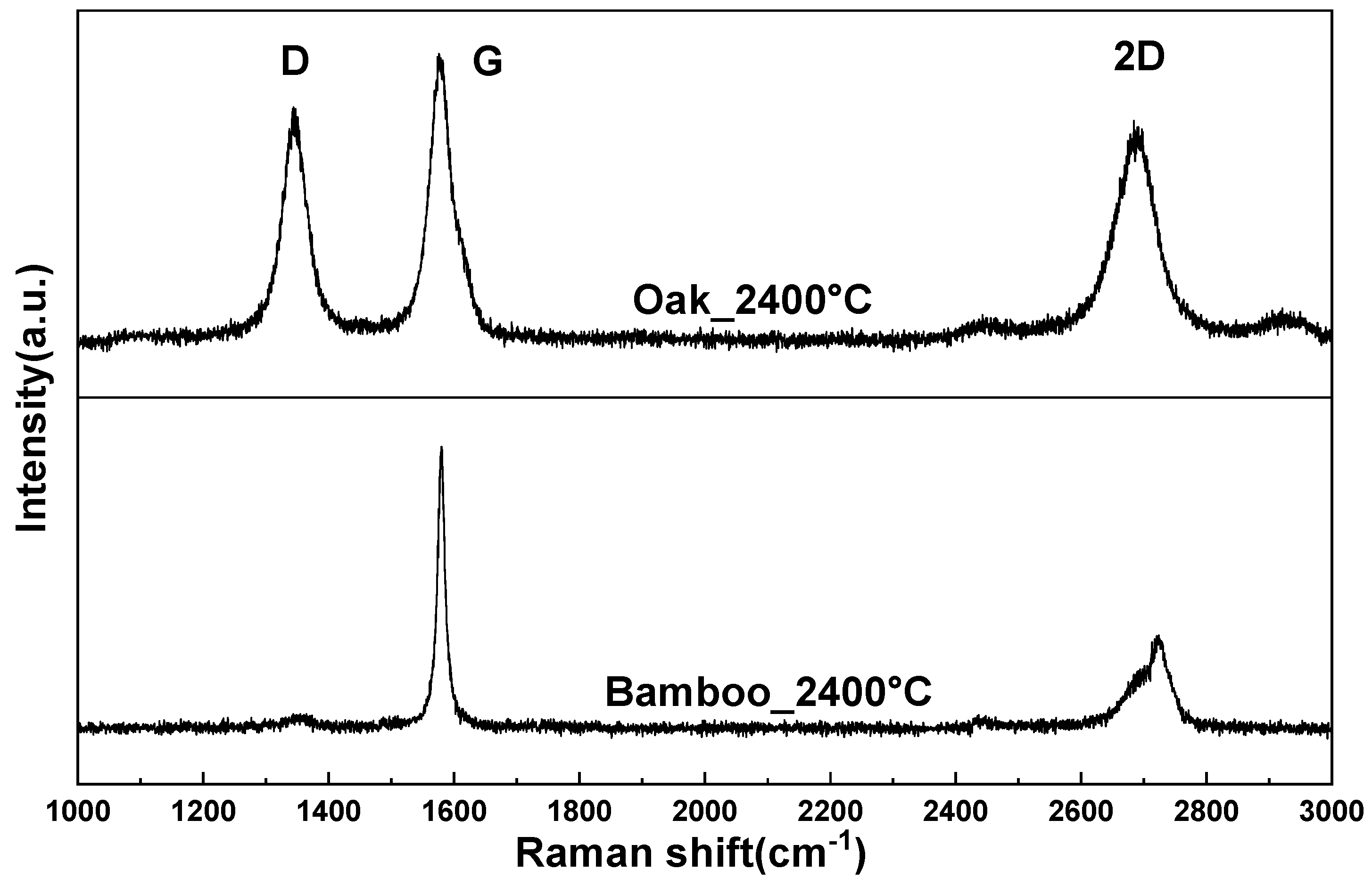
3.7. Analysis of Crystallinity by Raman Spectroscopy
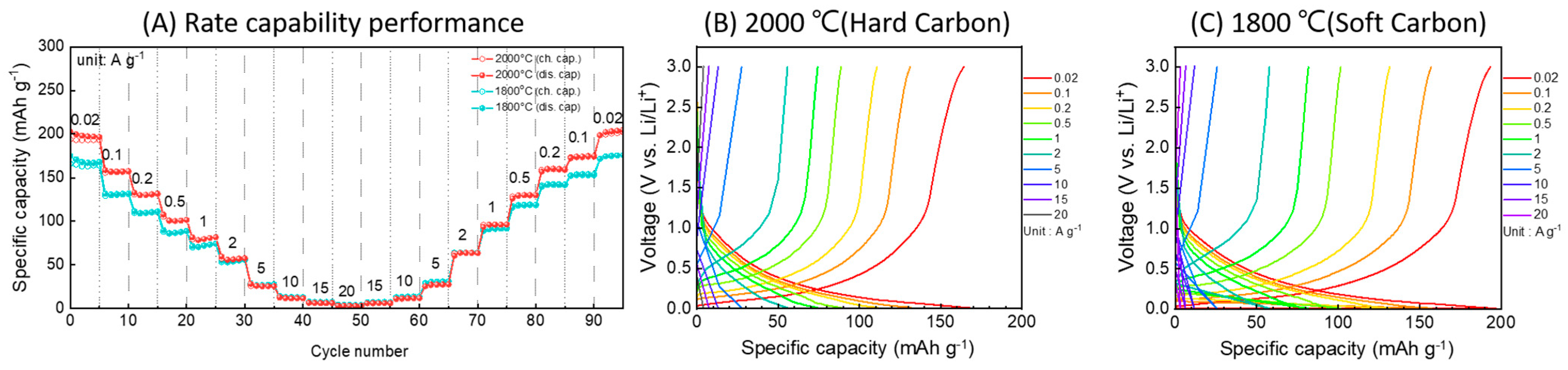
3.8. Utilization of Anode Materials for Lithium-Ion Batteries
4. Conclusions
- When charcoal is crushed to reduce particle size, the specific surface area increases significantly, and the mesopores and macropores break down and disappear, reducing the average pore size. As the particle size decreases through the crushing process, the surface-area-to-volume ratio increases, providing more surface area for the same volume. However, when the particle size falls below a certain threshold, the surface area no longer increases but decreases.
- When oak and bamboo charcoal powders were heated to about 1500 °C at 10 K/min in a nitrogen atmosphere, thermal analysis results showed that 86.87 wt% of oak charcoal and 88.33 wt% of bamboo charcoal remained. This indicates that a yield of over 85% can be obtained when charcoal is graphitized.
- SEM examination of the surface shape revealed that 10~20 µm of the average pore size breaks down or becomes blocked and disappears as graphitization progresses. TEM observation shows that graphitization occurs in the form of a turbostratic structure.
- XRD analysis indicates that isotropic peaks disappear and carbon atoms are converted to graphite crystals when heat treated at 2400 °C. Raman spectroscopy analysis shows that bamboo charcoal has superior crystallinity compared to other wood-based charcoals such as oak charcoal.
- Bamboo charcoal was used as an anode material for lithium-ion batteries. It was confirmed that hard carbon achieved 168 mAh/g at a current density of 0.02 A/g, and soft carbon achieved 196 mAh/g.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.S.; Hanif, M.A.; Song, H.; Kim, S.; Cho, Y.; Ryu, S.K.; Kim, H.G. Wood-Derived Graphite: A Sustainable and Cost-Effective Material for the Wide Range of Industrial Applications. Crystals 2024, 14, 309. [Google Scholar] [CrossRef]
- Chidumayo, E.N.; Gumbo, D.J. The environmental impacts of charcoal production in tropical ecosystems of the world: A synthesis. Energy Sustain. Dev. 2013, 17, 86–94. [Google Scholar] [CrossRef]
- Hunt, J.; DuPonte, M.; Sato, D.; Kawabata, A. The basics of biochar: A natural soil amendment. Soil Crop Manag. 2010, 30, 1–6. [Google Scholar]
- Zuo, Y.; Feng, J.; Soyol-Erdene, T.O.; Wei, Z.; Hu, T.; Zhang, Y.; Tang, W. Recent advances in wood-derived monolithic carbon materials: Synthesis approaches, modification methods and environmental applications. Chem. Eng. J. 2023, 463, 142332. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhang, C.H. Study on structural evolution of synthetic graphite derived from lignite prepared by high temperature–high pressure method. Crystals 2022, 12, 464. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, D.S.; Roh, J.S. Bulk graphite: Materials and manufacturing process. Carbon Lett. 2015, 16, 135–146. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, C.; Wu, H.; Li, L.; Zhang, C. Progress, challenge and perspective of graphite-based anode materials for lithium batteries: A review. J. Energy Storage 2024, 81, 110409. [Google Scholar] [CrossRef]
- Fan, C.L.; Chen, H. Preparation, structure, and electrochemical performance of anodes from artificial graphite scrap for lithium ion batteries. J. Mater. Sci. 2011, 46, 2140–2147. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, J.; He, X.; Li, J.; Jiang, C.; Wan, C. Modification of natural graphite for lithium ion batteries. Solid State Sci. 2008, 10, 612–617. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Komarov, F.F.; Lipkin, M.S.; Milchanin, O.V.; Parfimovich, I.D.; Shchegolkov, A.V.; Semenkova, A.V.; Velichko, A.V.; Chebotov, A.V.; Nokhaeva, V.A. Synthesis and study of cathode materials based on carbon nanotubes for lithium-ion batteries. Inorg. Mater. Appl. Res. 2021, 12, 1281–1287. [Google Scholar] [CrossRef]
- Kamal, A.S.; Othman, R.; Jabarullah, N.H. Preparation and synthesis of synthetic graphite from biomass waste: A review. Syst. Rev. Pharm. 2020, 11, 881–894. [Google Scholar]
- Lu, L.L.; Lu, Y.Y.; Xiao, Z.J.; Zhang, T.W.; Zhou, F.; Ma, T.; Ni, Y.; Yao, H.B.; Yu, S.H.; Cui, Y. Wood-inspired high-performance ultrathick bulk battery electrodes. Adv. Mater. 2018, 30, 1706745. [Google Scholar] [CrossRef]
- Song, P.; Chen, C.; Shen, X.; Zeng, S.; Premlatha, S.; Ji, Z.; Zhai, L.; Yuan, A.; Liu, Q. Metal-organic frameworks-derived carbon modified wood carbon monoliths as three-dimensional self-supported electrodes with boosted electrochemical energy storage performance. J. Colloid Interface Sci. 2022, 620, 376–387. [Google Scholar] [CrossRef]
- Min, S.; Deng, W.; Li, Y.; Wang, F.; Zhang, Z. Self-Supported CoP Nanoparticle-Embedded Wood-Derived Porous Carbon Membrane for Efficient H2 Evolution in Both Acidic and Basic Solutions. ChemCatChem 2020, 12, 3929–3936. [Google Scholar] [CrossRef]
- Ao, K.; Zhang, X.; Nazmutdinov, R.R.; Wang, D.; Shi, J.; Yue, X.; Sun, J.; Schmickler, W.; Daoud, W.A. Hierarchical CoFe@ N-Doped Carbon Decorated Wood Carbon as Bifunctional Cathode in Wearable Zn-Air Battery. Energy Environ. Mater. 2024, 7, e12499. [Google Scholar] [CrossRef]
- Wood, A.R.; Garg, R.; Justus, K.; Cohen-Karni, T.; LeDuc, P.; Russell, A.J. Intact mangrove root electrodes for desalination. RSC Adv. 2019, 9, 4735–4743. [Google Scholar] [CrossRef]
- Liese, W.; Kohl, M. Bamboo: The Plant and Its Uses; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Buziquia, S.T.; Lopes, P.V.F.; Almeida, A.K.; de Almeida, I.K. Impacts of bamboo spreading: A review. Biodivers. Conserv. 2019, 28, 3695–3711. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Ray, S.S. Bamboos: From Bioresource to Sustainable Materials and Chemicals. Sustainability 2021, 13, 12200. [Google Scholar] [CrossRef]
- Park, Y.N.; Lee, J.J.; Kwac, L.K.; Ryu, S.K.; Kim, H.G. Graphitization of Oak-Tree-Based White Charcoals by High Temperature Heat Treatment. Korean J. Chem. Eng. 2024, 41, 1841–1849. [Google Scholar] [CrossRef]
- ISO 3310-1; Test Sieves—Technical Requirements and Testing—Part 1: Test Sieves of Metal Wire Cloth. International Organization for Standardization: Geneva, Switzerland, 2016.
- Kim, D.W.; An, K.S.; Kwak, L.K.; Kim, H.G.; Ryu, S.K.; Lee, Y.S. A Study on Moisture Adsorption Capacity by Charcoals. Korean Chem. Eng. Res. 2022, 60, 377–385. [Google Scholar]
- An, K.S.; Kwak, L.K.; Kim, H.G.; Ryu, S.K. Characterization of Charcoals prepared by Korean Traditional Kiln. Korean Chem. Eng. Res. 2022, 60, 208–216. [Google Scholar]
- Farivar, F.; Yap, P.L.; Karunagaran, R.U.; Losic, D. Thermogravimetric analysis (TGA) of graphene materials: Effect of particle size of graphene, graphene oxide and graphite on thermal parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Park, S.H.; Jang, J.H.; Wistara, N.J.; Febrianto, F.; Lee, M. Fuel properties of Indonesian bamboo carbonized at different temperatures. BioResources 2019, 14, 4224–4235. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Yang, J.; Gao, Q.; Ni, L.; Hou, Y.; He, Y.; Liu, Z. Combustion behaviors of molded bamboo charcoal: Influence of pyrolysis temperatures. Energy 2021, 226, 120253. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithium ion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Su, R.; Dong, X. Preparation and electrochemical properties of bamboo-based carbon for lithium-ion-battery anode material. Int. J. Electrochem. Sci. 2019, 14, 2452–2461. [Google Scholar] [CrossRef]
- Hata, T.; Yamane, K.; Kobayashi, E.; Imamura, Y.; Ishihara, S. Microstructural investigation of wood charcoal made by spark plasma sintering. J. Wood Sci. 1998, 44, 332–334. [Google Scholar] [CrossRef]
- Yamane, T.; Ishihara, S.; Hata, T. Effects of sintering temperature on structural changes of wood charcoal. Tanso 1999, 1999, 2–6. [Google Scholar] [CrossRef]
- Kawamura, K.; Amari, M.; Aruga, A.; Ozawa, S. Changes of Bending Strength and Microstructure of Charcoals with High Temperature Treatment. Tanso 2000, 2000, 32–36. [Google Scholar] [CrossRef]
- Hata, T.; Vystavel, T.; Bronsveld, P.; DeHosson, J.; Kikuchi, H.; Nishimiya, K.; Imamura, Y. Catalytic carbonization of wood charcoal: Graphite or diamond? Carbon 2004, 42, 961–964. [Google Scholar] [CrossRef]
- Fogg, J.L.; Putman, K.J.; Zhang, T.; Lei, Y.; Terrones, M.; Harris, P.J.; Harris, P.J.F.; Marks, N.A.; Suarez-Martinez, I. Catalysis-free transformation of non-graphitising carbons into highly crystalline graphite. Commun. Mater. 2020, 1, 47. [Google Scholar] [CrossRef]
- Inagaki, M.; Naka, S. Crystallite orientation in polycrystalline graphites made from glass-like carbons under high pressure. J. Mater. Sci. 1975, 10, 814–818. [Google Scholar] [CrossRef]
- Nishimiya, K.; Hata, T.; Kikuchi, H.; Imamura, Y. Effect of aluminum compound addition on graphitization of wood charcoal by direct electric pulse heating method. J. Wood Sci. 2004, 50, 177–181. [Google Scholar] [CrossRef]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 1989, 4, 385–393. [Google Scholar] [CrossRef]
- Ramirez-Rico, J.; Gutierrez-Pardo, A.; Martinez-Fernandez, J.; Popov, V.V.; Orlova, T.S. Thermal conductivity of Fe graphitized wood derived carbon. Mater. Des. 2016, 99, 528–534. [Google Scholar] [CrossRef]
- Yoshida, A.; Kaburagi, Y.; Hishiyama, Y. Full width at half maximum intensity of G band in first order Raman spectrum of carbon material as a parameter for graphitization-a study with pyrolytic carbons. Tanso 2006, 2006, 2–7. [Google Scholar] [CrossRef][Green Version]
- Yu, M.; Saunders, T.; Su, T.; Gucci, F.; Reece, M.J. Effect of heat treatment on the properties of wood-derived biocarbon structures. Materials 2018, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Togonon, J.J.H.; Chiang, P.-C.; Lin, H.-J.; Tsai, W.-C.; Yen, H.-J. Pure carbon-based electrodes for metal-ion batteries. CarbonTrends 2021, 3, 100035. [Google Scholar] [CrossRef]
- Vook, T.; Dey, S.C.; Yang, J.; Nimlos, M.; Park, S.; Han, S.D.; Sagues, W.J. Sustainable Li-ion anode material from Fe-catalyzed graphitization of paper waste. J. Energy Storage 2023, 73, 109242. [Google Scholar] [CrossRef]



| No. | Charcoal Size (mm) | ABET (m2/g) | Vt (cm3/g) | Vmi (cm3/g) (Vmi/Vt)% | Dm (nm) |
|---|---|---|---|---|---|
| 1 | >4.75 | 13.8 | 0.01 | 0.0071 (71.00) | 2.89 |
| 2 | 4.75–3.35 (4.05) | 152.56 | 0.099 | 0.0824 (83.23) | 2.8 |
| 3 | 3.35–2.36 (2.85) | 220.67 | 0.1353 | 0.1080 (79.82) | 2.45 |
| 4 | 2.36–2.00 (2.18) | 206.79 | 0.118 | 0.0882 (74.75) | 2.28 |
| 5 | 2.00–1.70 (1.85) | 319.45 | 0.1547 | 0.1304 (84.29) | 1.94 |
| 6 | <1.70 | 352.01 | 0.1453 | 0.1381 (95.04) | 1.65 |
| 7 | Powder A (10 μm) | 816.2 | 0.3287 | 0.3134 (95.35) | 1.61 |
| 8 | Powder B (4 μm) | 412.49 | 0.2124 | 0.1676 (78.91) | 2.06 |
| Charcoal (°C/min.) | Carbon (C, %) | Nitrogen (N, %) | Oxygen (O, %) | |||
|---|---|---|---|---|---|---|
| Bamboo | Oak | Bamboo | Oak | Bamboo | Oak | |
| 1000 °C, 60 min. | 92.12 | 79.36 | 0.71 | 0.88 | 7.17 | 19.36 |
| 1100 °C, 60 min. | 93.34 | 91.88 | 0.24 | 0.78 | 6.42 | 7.01 |
| 1300 °C, 60 min. | 93.64 | 92.35 | 0.16 | 0.65 | 6.20 | 6.84 |
| 1500 °C, 60 min. | 96.50 | 97.25 | 0.07 | 0.47 | 3.43 | 2.28 |
| 1800 °C, 60 min. | 98.48 | 99.88 | 0.00 | 0.00 | 1.52 | 0.04 |
| 2000 °C, 30 min. | 100.00 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2400 °C, 10 min. | 100.00 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Samples | 1500 °C | 1800 °C | 2400 °C | |||
|---|---|---|---|---|---|---|
| Oak | Bamboo | Oak | Bamboo | Oak | Bamboo | |
| Intensity (G/(G + D)) | 0.351145 | 0.4565217 | 0.495549 | 0.4680365 | 0.5517241 | 0.9175258 |
| Raman Shift (D) | 1349 | 1353 | 1347 | 1354 | 1344 | 1344 |
| Intensity (D) | 170 | 75 | 170 | 233 | 65 | 8 |
| Raman Shift (G) | 1592 | 1599 | 1583 | 1583 | 1575 | 1580 |
| Intensity (G) | 92 | 63 | 167 | 205 | 80 | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Ryu, S.-K.; Kim, H.-G.; Kwac, L.-K.; Kim, Y.-S. Comparative Analysis of Graphitization Characteristics in Bamboo and Oak Charcoals for Secondary Battery Anodes. Crystals 2024, 14, 914. https://doi.org/10.3390/cryst14110914
Lee K, Ryu S-K, Kim H-G, Kwac L-K, Kim Y-S. Comparative Analysis of Graphitization Characteristics in Bamboo and Oak Charcoals for Secondary Battery Anodes. Crystals. 2024; 14(11):914. https://doi.org/10.3390/cryst14110914
Chicago/Turabian StyleLee, Kiseon, Seung-Kon Ryu, Hong-Gun Kim, Lee-Ku Kwac, and Young-Soon Kim. 2024. "Comparative Analysis of Graphitization Characteristics in Bamboo and Oak Charcoals for Secondary Battery Anodes" Crystals 14, no. 11: 914. https://doi.org/10.3390/cryst14110914
APA StyleLee, K., Ryu, S.-K., Kim, H.-G., Kwac, L.-K., & Kim, Y.-S. (2024). Comparative Analysis of Graphitization Characteristics in Bamboo and Oak Charcoals for Secondary Battery Anodes. Crystals, 14(11), 914. https://doi.org/10.3390/cryst14110914







