Insights into Crystallization of Neuronal Nicotinic α4β2 Receptor in Polarized Lipid Matrices
Abstract
1. Introduction
2. Materials and Methods
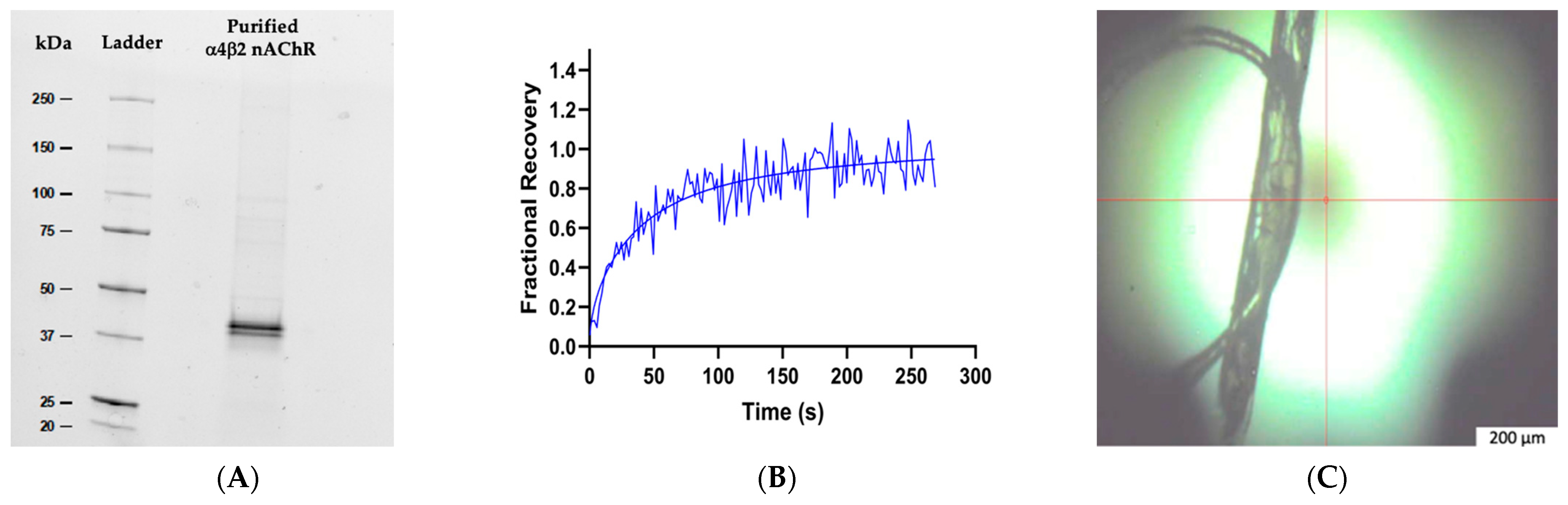
2.1. Expression and Purification of Human α4β2 nAChR
2.2. In Meso Crystallization with the RPM@LMx Device and X-ray Diffraction
2.3. Lipidic Cubic Phase Fluorescence Recovery after Photobleaching (LCP–FRAP)
2.4. Analysis of Phospholipid Molecular Species by Ultra-Performance Liquid Chromatography (UPLC) Coupled to Electrospray Ionization Tandem Mass Spectrometry (ESI-MS/MS)
3. Results
3.1. Lipid Analysis of Purified Human α4β2 nAChR-DCs
3.2. α4β2 nAChR-DC Diffusion in LCP
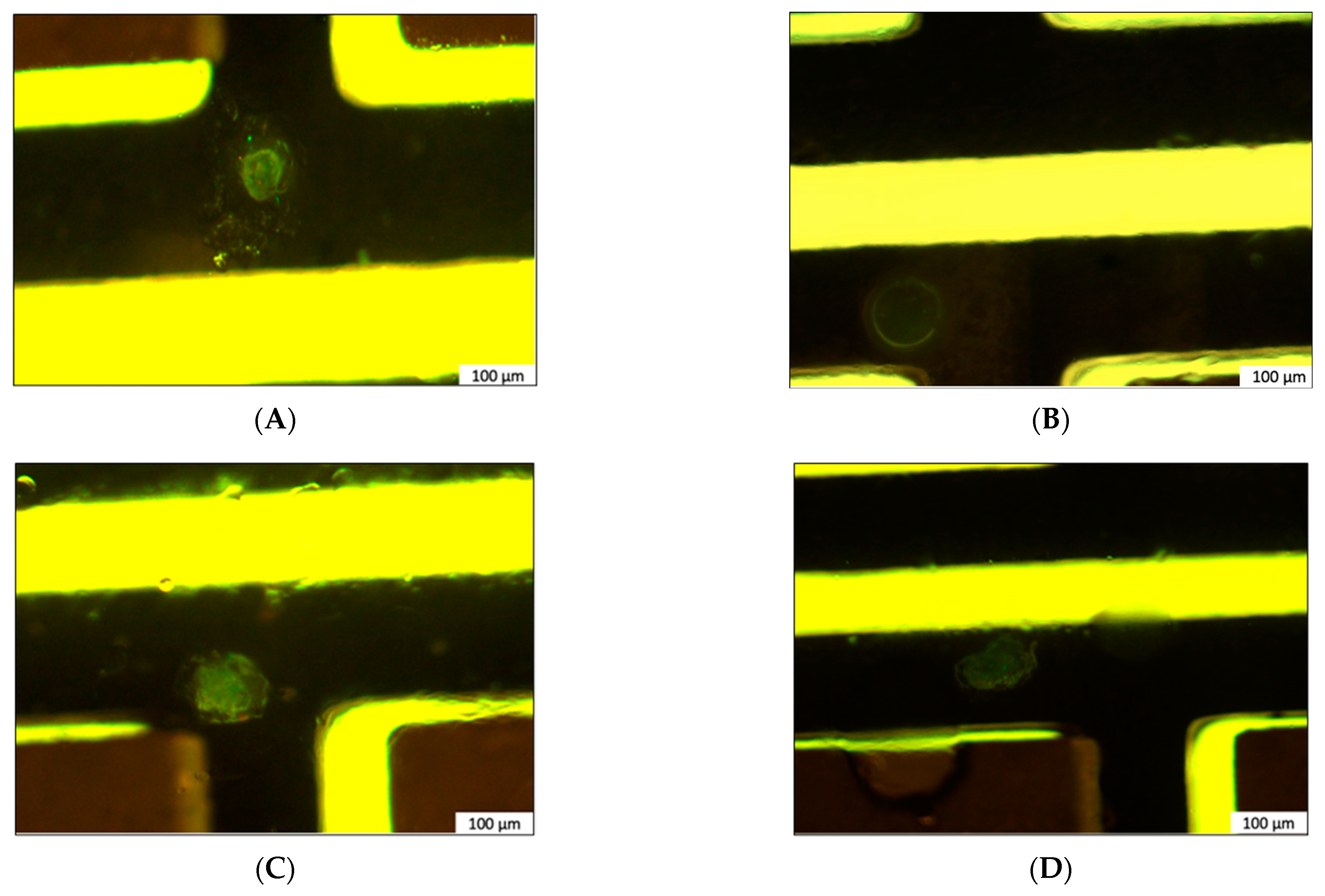
3.3. Polarized In Meso Crystallization Screening of Human α4β2 nAChR
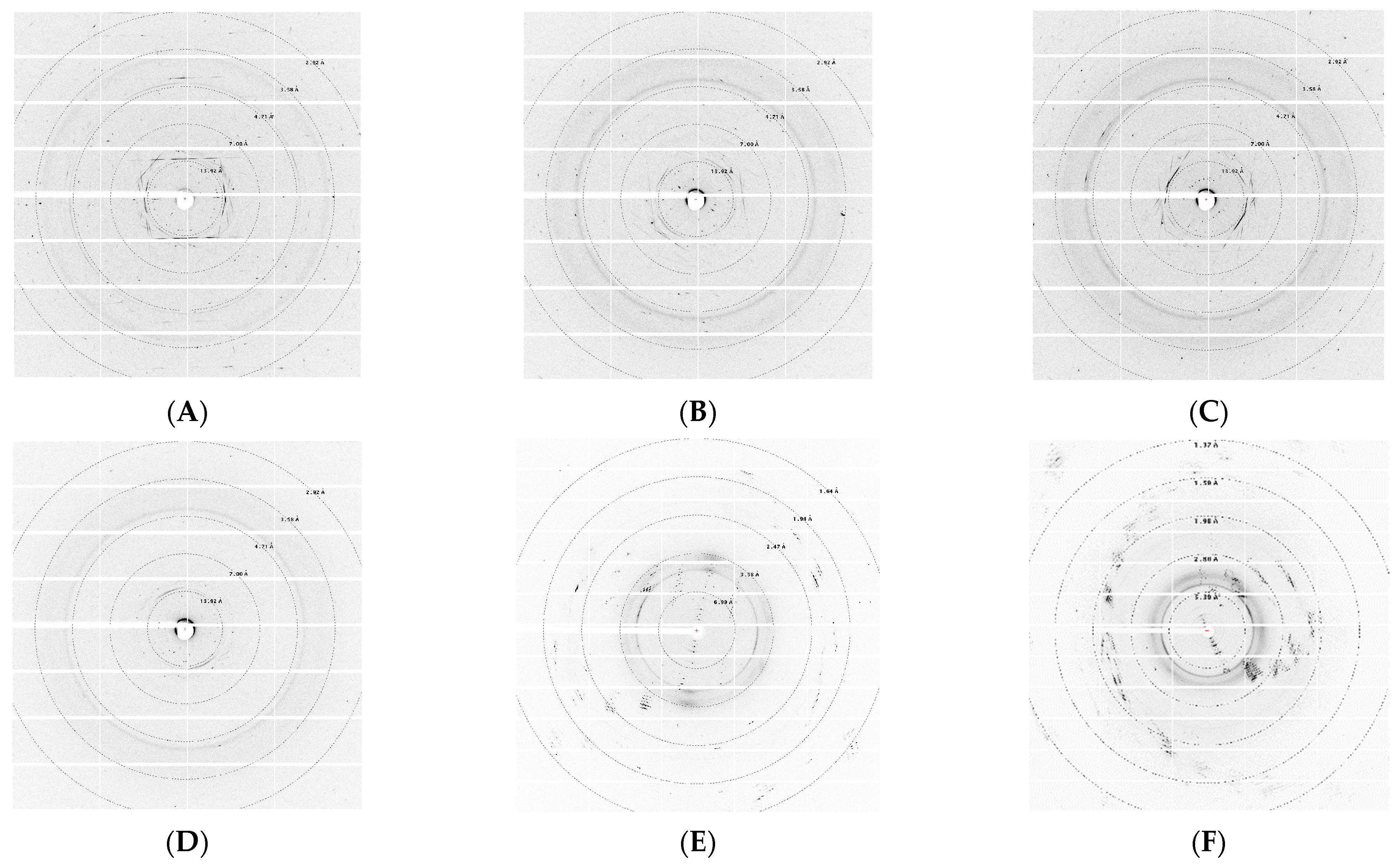
3.4. Synchrotron X-ray Diffraction
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado-Vélez, M.; Quesada, O.; Villalobos-Santos, J.C.; Maldonado-Hernández, R.; Asmar-Rovira, G.; Stevens, R.C.; Lasalde-Dominicci, J.A. Pursuing high-resolution structures of nicotinic acetylcholine receptors: Lessons learned from five decades. Molecules 2021, 26, 5753. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P. Discovery of the First Neurotransmitter Receptor: The Acetylcholine Nicotinic Receptor. Biomolecules 2020, 10, 547. [Google Scholar] [CrossRef]
- Delgado-Vélez, M.; Báez-Pagán, C.A.; Gerena, Y.; Quesada, O.; Santiago-Pérez, L.I.; Capó-Vélez, C.M.; Wojna, V.; Meléndez, L.; León-Rivera, R.; Silva, W.; et al. The α 7-nicotinic receptor is upregulated in immune cells from HIV-seropositive women: Consequences to the cholinergic anti-in flammatory response. Clin. Transl. Immunol. 2015, 4, e53. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic acetylcholine receptors: From basic science to therapeutics. Pharmacol. Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef]
- Polosa, R.B.N.L. Treatment of nicotine addiction: Present therapeutic options and pipeline developments. Trends Pharmacol Sci. 2011, 32, 281–289. [Google Scholar] [CrossRef]
- Quik, M.; O’Leary, K.; Tanner, C.M. Nicotine and Parkinson’s disease; implications for therapy. Physiol. Behav. 2008, 23, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.Y.; Akers, A.T.; Journigan, V.B.; Henderson, B.J. Novel putative positive modulators of α4β2 nachrs potentiate nicotine reward-related behavior. Molecules 2021, 26, 4793. [Google Scholar] [CrossRef]
- Buisson, B.; Bertrand, D. Open-channel blockers at the human α4β2 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 1998, 53, 555–563. [Google Scholar] [CrossRef]
- Vallés, A.S.; Barrantes, F.J. Nicotinic acetylcholine receptor dysfunction in addiction and in some neurodegenerative and neuropsychiatric diseases. Cells 2023, 12, 2051. [Google Scholar] [CrossRef]
- Walsh, R.M., Jr.; Roh, S.H.; Gharpure, A.; Morales-Perez, C.L.; Teng, J.; Hibbs, R.E. Structural principles of distinct assemblies of the human α 4 β 2 nicotinic receptor. Nature 2018, 557, 261–265. [Google Scholar] [CrossRef]
- Gharpure, A.; Teng, J.; Zhuang, Y.; Noviello, C.M.; Walsh, R.M., Jr.; Cabuco, R.; Howard, R.J.; Zaveri, N.T.; Lindahl, E.; Hibbs, R.E. Agonist Selectivity and Ion Permeation in the α3β4 Ganglionic Nicotinic Receptor. Neuron 2019, 104, 501–511.e6. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.B.; Hibbs, R.E. Structure of the Native Muscle-type Nicotinic Receptor and Inhibition by Snake Venom Toxins. Neuron 2020, 106, 952–962.e5. [Google Scholar] [CrossRef]
- Rahman, M.; Basta, T.; Teng, J.; Lee, M.; Worrell, B.T.; Stowell, M.H.B.; Hibbs, R.E. Structural mechanism of muscle nicotinic receptor desensitization and block by curare. Nat. Struct. Mol. Biol. 2022, 4, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Noviello, C.M.; Gharpure, A.; Mukhtasimova, N.; Cabuco, R.; Baxter, L.; Borek, D.; Sine, S.M.; Hibbs, R.E. Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 2021, 184, 2121–2134. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Zaidi, S.A.; Xu, L.; Zhan, Y.; Chen, A.; Guo, J.; Huang, X.; Roth, B.L.; Katritch, V.; et al. Structural insight into angiotensin receptor signaling modulation by balanced and biased agonists. EMBO J. 2023, 42, e112940. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Structural insight into apelin receptor-G protein stoichiometry. Nat. Struct. Mol. Biol. 2022, 29, 688–697. [Google Scholar]
- Mazzaferro, S.; Kang, G.; Natarajan, K.; Hibbs, R.E.; Sine, S.M. Structural bases for Stoichiometry-Selective Calcium Potentiation of a Neuronal Nicotinic Receptor. Br. J. Pharmacol. 2024, 181, 1973–1992. [Google Scholar] [CrossRef]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. X-ray structure of the human α4β2 nicotinic receptor. Nature 2016, 538, 411–415. [Google Scholar] [CrossRef]
- Padilla-Morales, L.F.; Colón-Sáez, J.O.; González-Nieves, J.E.; Quesada-González, O.; Lasalde-Dominicci, J.A. Functionality and stability data of detergent purified nAChR from Torpedo using lipidic matrixes and macroscopic electrophysiology. Data Brief 2016, 6, 433–437. [Google Scholar] [CrossRef]
- Padilla-Morales, L.F.; Colón-Sáez, J.O.; González-Nieves, J.E.; Quesada-González, O.; Lasalde-Dominicci, J.A. Assessment of the Functionality and Stability of Detergent Purified nAChR from Torpedo using Lipidic Matrixes and Macroscopic Electrophysiology. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1858, 47–56. [Google Scholar] [CrossRef]
- Caffrey, M.; Cherezov, V. Crystallizing Membrane Proteins Using Lipidic Mesophases. Nat. Protoc. 2010, 4, 706–731. [Google Scholar] [CrossRef] [PubMed]
- Luecke, H.; Schobert, B.; Richter, H.T.; Cartailler, J.P.; Lanyi, J.K. Structure of bacteriorhodopsin at 1.55 Å resolution. J. Mol. Biol. 1999, 291, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallogr. Sect. FStructural Biol. Commun. 2015, 71, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-Resolution Crystal Structure of an Engineered Human β2-adrenergic G protein–coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef]
- Martynowycz, M.W.; Shiriaeva, A.; Ge, X.; Hattne, J.; Nannenga, B.L.; Cherezov, V.; Gonen, T. MicroED structure of the human adenosine receptor determined from a single nanocrystal in LCP. Proc. Natl. Acad. Sci. USA 2021, 118, e2106041118. [Google Scholar] [CrossRef]
- Michaelian, N.; Sadybekov, A.; Besserer-Offroy, É.; Han, G.W.; Krishnamurthy, H.; Zamlynny, B.A.; Fradera, X.; Siliphaivanh, P.; Presland, J.; Spencer, K.B.; et al. Structural insights on ligand recognition at the human leukotriene B4 receptor 1. Nat. Commun. 2021, 12, 2971. [Google Scholar] [CrossRef]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. Manipulation of Subunit Stoichiometry in Heteromeric Membrane Proteins Resource Manipulation of Subunit Stoichiometry in Heteromeric Membrane Proteins. Struct. Des. 2016, 24, 797–805. [Google Scholar] [CrossRef]
- Liu, W.; Cherezov, V. Crystallization of membrane proteins in lipidic mesophases. J. Vis. Exp. 2011, 49. [Google Scholar] [CrossRef]
- Quesada, O.; González-Freire, J.E.; Colón, J.; Maldonado-Hernández, R.; González-Freire, C.; Acevedo-Cintrón, J.; Rosado-Millán, I.D.; Lasalde-Dominicci, J.A. Assessment of Purity, Functionality, Stability, and Lipid Composition of Cyclofos-nAChR-Detergent Complexes from Torpedo californica Using Lipid Matrix and Macroscopic Electrophysiology. J. Mem. Biol. 2023, 256, 271–285. [Google Scholar] [CrossRef]
- Quesada, O.; Freire, C.G.; Ferrer, M.C.; -Sáez, J.O.C.; Fernández-García, E.; Mercado, J.; Dávila, A.; Morales, R.; Lasalde-Dominicci, J.A. Uncovering the lipidic basis for the preparation of functional nicotinic acetylcholine receptor detergent complexes for structural studies. Sci. Rep. 2016, 6, 32766. [Google Scholar] [CrossRef]
- Symons, J.L.; Cho, K.J.; Chang, J.T.; Du, G.; Waxham, M.N.; Hancock, J.F.; Levental, I.; Levental, K.R. Lipidomic atlas of mammalian cell membranes reveals hierarchical variation induced by culture conditions, subcellular membranes, and cell lineages. Soft Matter 2021, 17, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Palsdottir, H.; Hunte, C. Lipids in membrane protein structures. Biochim. Biophys. Acta 2004, 1666, 2–18. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.; Gavira, J.A. Introduction to protein crystallization. Acta Cryst. 2013, F70, 2–20. [Google Scholar]
- Shoemaker, S.C.; Ando, N. X-rays in the Cryo-EM era: Structural Biology’s dynamic future. Biochemistry 2019, 57, 277–285. [Google Scholar] [CrossRef]
- Barrantes, F.J. Structure and function meet at the nicotinic acetylcholine receptor-lipid interface. Pharmacol. Res. 2023, 190, 106729. [Google Scholar] [CrossRef] [PubMed]
- Barrantes, F.J. Modulation of a rapid neurotransmitter receptor-ion channel by membrane lipids. Front. Cell Dev. Biol. 2024, 11, 1328875. [Google Scholar] [CrossRef]
- Dawaliby, R.; Trubbia, C.; Delporte, C.; Noyon, C.; Ruysschaert, J.M.; Antwerpen, P.V.; Govaerts, C. Phosphatidylethanolamine is a key regulator of membrane fluidity in eukaryotic cells. J. Biol. Chem. 2016, 291, 3658–3668. [Google Scholar] [CrossRef]
- Hejazi, S.; Pahlavandanzadeh, H.; Elliott, J.A.W. Thermodynamic investigation of the effect of electric field on solid-liquid equilibrium. J. Phys. Chem. B 2021, 125, 1271–1281. [Google Scholar] [CrossRef]
- Nanev, C.N. Recent Insights into the Crystallization Process; Protein Crystal Nucleation and Growth Peculiarities; Processes in the Presence of Electric Fields. Crystals 2017, 7, 310. [Google Scholar] [CrossRef]
- Van’t Hag, L.; Knoblich, K.; Seabrook, S.A.; Kirby, N.M.; Mudie, S.T.; Lau, D.; Li, X.; Gras, S.L.; Mulet, X.; Call, M.E.; et al. Exploring the in meso crystallization mechanism by characterizing the lipid mesophase microenvironment during the growth of single transmembrane alpha-helical peptide crystals. Phil. Trans. R. Soc. A 2016, 374, 20150125. [Google Scholar] [CrossRef]
- Al-Haq, M.I.; Lebrasseur, E.; Tsuchiya, H.; Torri, T. Protein crystallization under an electric field. Cryst. Rev. 2007, 13, 29–64. [Google Scholar] [CrossRef]
- Ogata, M.; Aoki, D.; Suzuki, M.; Tanaka, D.; Wakamatsu, T. The role of an applied electric field in protein crystallization at low temperature. Jpn. J. Appl. Phys. 2019, 58, 110903. [Google Scholar] [CrossRef]
- Kan, K. The effect of fatty acids, ionic strength, and electric fields on the microscopic dynamics of BSA aggregates. Front. Phys. 2023, 11, 1282099. [Google Scholar]
- Yager, P.; Price, R.R.; Schnur, J.M.; Schoen, P.E.; Singh, A.; Rhodes, D.G. The mechanism of formation of lipid tubules from liposomes. Chem. Phys. Lipids 1988, 46, 171–179. [Google Scholar] [CrossRef]
- Stachowiak, J.C.; Hayden, C.C.; Sasaki, D.Y. Steric confinement of proteins on lipid membranes can drive curvature and tubulation. Proc. Natl. Acad. Sci. USA 2010, 107, 7781–7786. [Google Scholar] [CrossRef]
- Pebay-Peyroula, E.; Rummel, G.; Rosenbusch, J.P.; Landau, E.M. X-ray structure of bacteriorhodopsin at 2.5 angstroms from microcrystals grown in lipidic cubic phases. Science 1997, 277, 1676–1681. [Google Scholar] [CrossRef]
| Molecular Species | m/z (M + H)+ | Mean Peak Area |
|---|---|---|
| LPC 16:0 | 496.3398 | 17,941,873.71 |
| Cer m22:0 | 338.3417 | 12,200,000.00 |
| TG P-23:6/21:1 | 721.5766 | 4,509,407.999 |
| LPC 17:0 | 554.3463 | 3,323,174.888 |
| LPMt 16:0 | 423.2517 | 1,824,723.222 |
| LPC (PC 16:0) | 518.3217 | 1,595,348.877 |
| DAP O-11:0/O-5:0 | 391.2843 | 922,029.400 |
| DAP O-7:0/O-7:0 | 363.2529 | 769,424.966 |
| SM d34:1 | 703.5749 | 480,092.577 |
| LPC 18:0 | 524.3711 | 266,859.633 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-Santos, J.C.; Carrasquillo-Rivera, M.; Rodríguez-Cordero, J.A.; Quesada, O.; Lasalde-Dominicci, J.A. Insights into Crystallization of Neuronal Nicotinic α4β2 Receptor in Polarized Lipid Matrices. Crystals 2024, 14, 889. https://doi.org/10.3390/cryst14100889
Villalobos-Santos JC, Carrasquillo-Rivera M, Rodríguez-Cordero JA, Quesada O, Lasalde-Dominicci JA. Insights into Crystallization of Neuronal Nicotinic α4β2 Receptor in Polarized Lipid Matrices. Crystals. 2024; 14(10):889. https://doi.org/10.3390/cryst14100889
Chicago/Turabian StyleVillalobos-Santos, Juan C., Mallerie Carrasquillo-Rivera, Josué A. Rodríguez-Cordero, Orestes Quesada, and José Antonio Lasalde-Dominicci. 2024. "Insights into Crystallization of Neuronal Nicotinic α4β2 Receptor in Polarized Lipid Matrices" Crystals 14, no. 10: 889. https://doi.org/10.3390/cryst14100889
APA StyleVillalobos-Santos, J. C., Carrasquillo-Rivera, M., Rodríguez-Cordero, J. A., Quesada, O., & Lasalde-Dominicci, J. A. (2024). Insights into Crystallization of Neuronal Nicotinic α4β2 Receptor in Polarized Lipid Matrices. Crystals, 14(10), 889. https://doi.org/10.3390/cryst14100889






