Chemical Composition and Spectral Variation in Gem-Quality Blue Iron-Bearing Tourmaline from Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. General Gemological Analysis
2.2.2. Electron Microprobe Analysis (EPMA)
2.2.3. Infrared Spectroscopy (IR)
2.2.4. Laser Raman Spectroscopy (RS)
2.2.5. Ultraviolet-Visible Absorption Spectroscopy (UV-VIS)
3. Results
3.1. Gemological Characteristics
3.2. Chemical Composition
3.3. Spectroscopic Characteristics
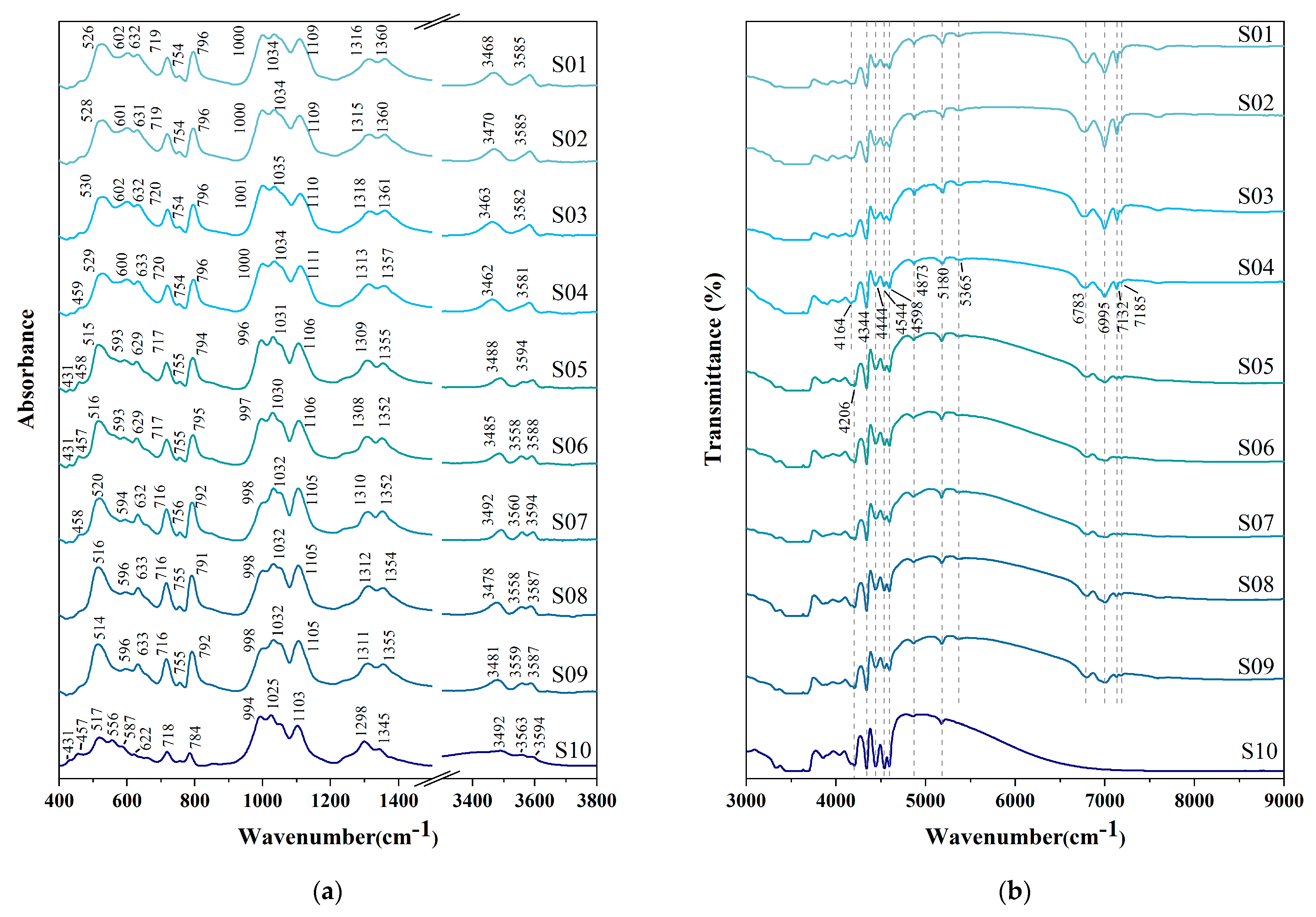
3.3.1. Infrared Spectroscopy (IR)
3.3.2. Laser Raman Spectroscopy (RS)
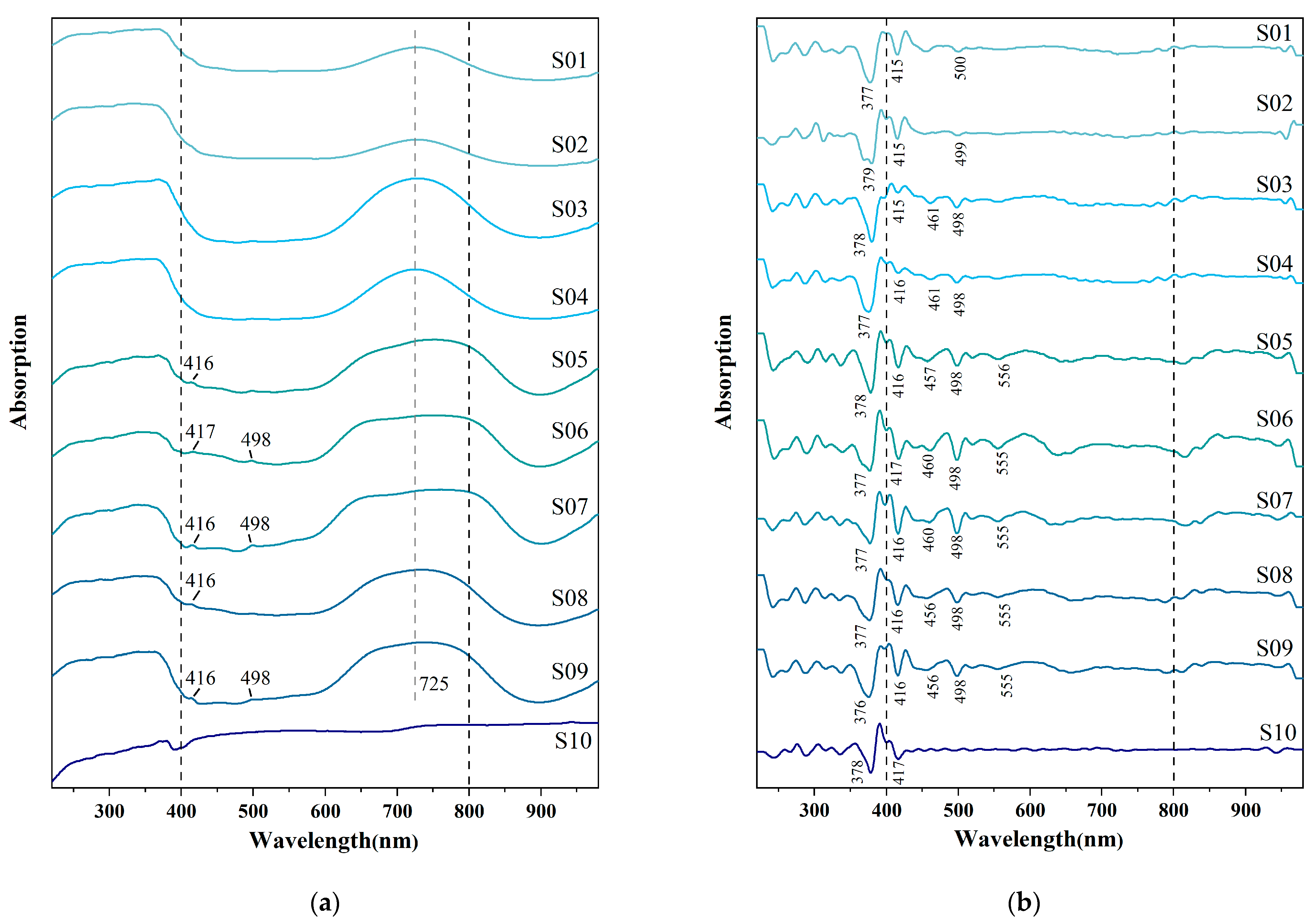
3.3.3. Ultraviolet–Visible Absorption Spectroscopy (UV-VIS)
4. Discussion
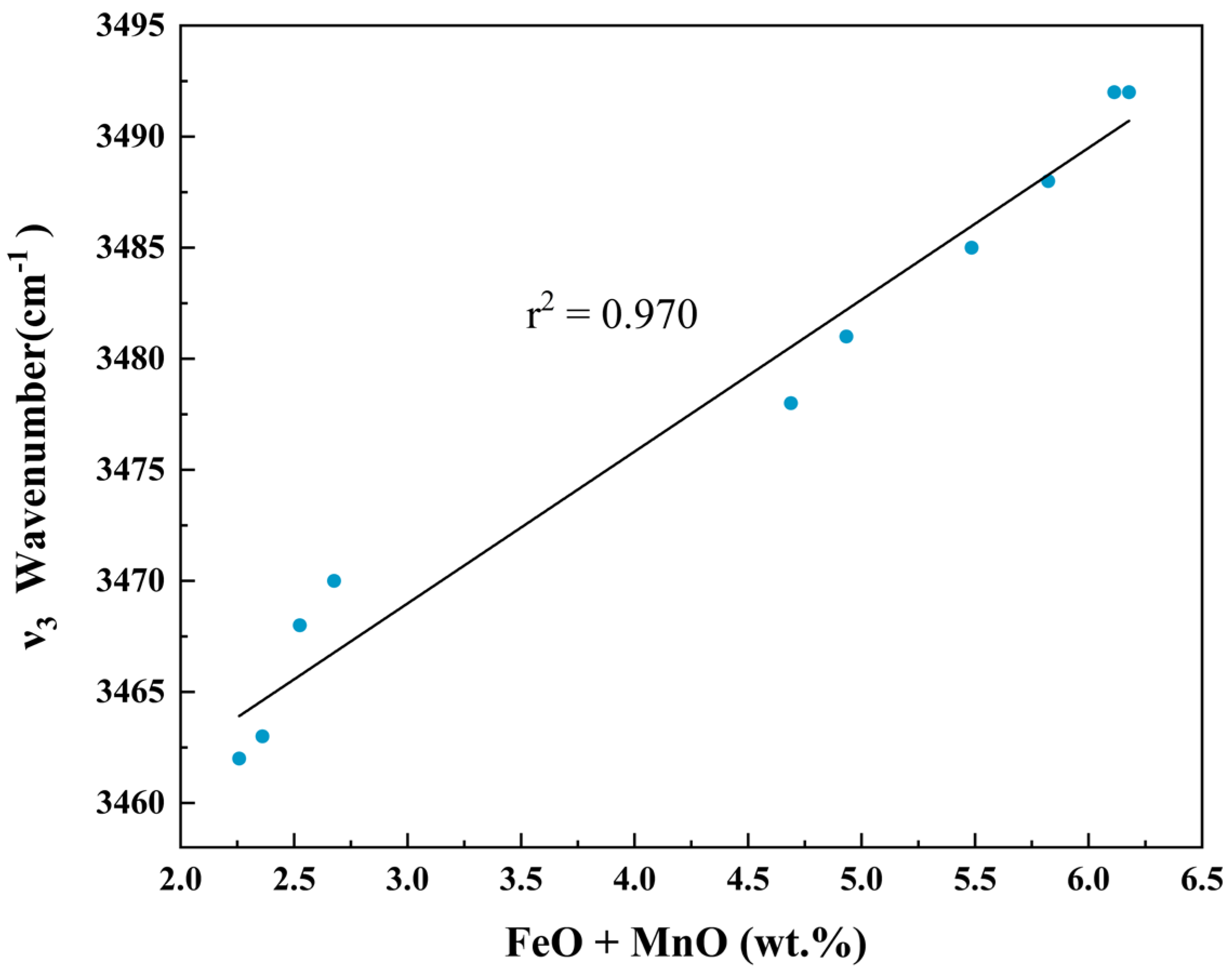
4.1. Crystal Structure and Spectral Variation
4.2. Causes of Color
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, D.J.; Novák, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.L.; Uher, P.; Pezzotta, F. Nomenclature of the Tourmaline-Supergroup Minerals. Am. Mineral. 2011, 96, 895–913. [Google Scholar] [CrossRef]
- Bosi, F. Tourmaline Crystal Chemistry. Am. Mineral. 2018, 103, 298–306. [Google Scholar] [CrossRef]
- Bosi, F. Crystal Chemistry of the Elbaite-Schorl Series. Am. Mineral. 2005, 90, 1784–1792. [Google Scholar] [CrossRef]
- Pasetti, L.; Borromeo, L.; Bersani, D.; Andò, S.; Schnellrath, J.; Hennebois, U.; Karampelas, S. Identification of Some Gem Quality Blue to Green Li-Tourmalines. Minerals 2023, 14, 44. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist Crystal Structure Database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Sun, Z.; Palke, A.C.; Breeding, C.M.; Dutrow, B. A New Method for Determining Gem Tourmaline Species by LA-ICP-MS. Gems Gemol. 2019, 55, 2–17. [Google Scholar] [CrossRef]
- Katsurada, Y.; Sun, Z. Cuprian Liddicoatite Tourmaline. Gems Gemol. 2017, 53, 34–41. [Google Scholar] [CrossRef]
- Nasdala, L.; Wildner, M.; Giester, G.; Chanmuang, N.C.; Scicchitano, M.R.; Hauzenberger, C. Blue Dravite (‘Indicolite’) from the Elahera Gem Field, Sri Lanka. J. Gemmol. 2021, 37, 618–630. [Google Scholar] [CrossRef]
- Li, M. Characterization of Blue Tourmaline from Madagascar for Exploring Its Color Origin. Adv. Condens. Matter Phys. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Okrusch, M.; Ertl, A.; Schüssler, U.; Tillmanns, E.; Brätz, H.; Bank, H. Major- and Trace-Element Composition of Paraíba-Type Tourmaline from Brazil, Mozambique and Nigeria. J. Gemmol. 2016, 35, 120–139. [Google Scholar] [CrossRef]
- Katsurada, Y.; Sun, Z.; Breeding, C.M.; Dutrow, B.L. Geographic Origin Determination of Paraiba Tourmaline. Gems Gemol. 2019, 55, 648–659. [Google Scholar] [CrossRef]
- Laurs, B.M.; Zwaan, J.C.; Breeding, C.M.; Simmons, W.B.; Beaton, D.; Rijsdijk, K.F.; Befi, R.; Falster, A.U. Copper-Bearing (Paraíba-Type) Tourmaline from Mozambique. Gems Gemol. 2008, 44, 4–30. [Google Scholar] [CrossRef]
- Shigley, J.E.; Cook, B.C.; Laurs, B.M.; Bernardes, M.O. An Update on “Paraíba” Tourmaline from Brazil. Gems Gemol. 2001, 37, 260–276. [Google Scholar] [CrossRef]
- Abduriyim, A.; Kitawaki, H.; Furuya, M.; Schwarz, D. “Paraíba”-Type Copper-Bearing Tourmaline from Brazil, Nigeria, and Mozambique: Chemical Fingerprinting by LA-ICP-MS. Gems Gemol. 2006, 42, 4–21. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Rozhdestvenskaya, I.V.; Frank-Kamenetskaya, O.V.; Zolotarev, A.A.; Mashkovtsev, R.I. Crystal Chemistry of Cu-Bearing Tourmalines. Am. Mineral. 2013, 98, 1610–1616. [Google Scholar] [CrossRef]
- Watenphul, A.; Schlüter, J.; Bosi, F.; Skogby, H.; Malcherek, T.; Mihailova, B. Influence of the Octahedral Cationic-Site Occupancies on the Framework Vibrations of Li-Free Tourmalines, with Implications for Estimating Temperature and Oxygen Fugacity in Host Rocks. Am. Mineral. 2016, 101, 2554–2563. [Google Scholar] [CrossRef]
- Watenphul, A.; Burgdorf, M.; Schlüter, J.; Horn, I.; Malcherek, T.; Mihailova, B. Exploring the Potential of Raman Spectroscopy for Crystallochemical Analyses of Complex Hydrous Silicates: II. Tourmalines. Am. Mineral. 2016, 101, 970–985. [Google Scholar] [CrossRef]
- Bronzova, Y.; Babushkina, M.; Frank-Kamenetskaya, O.; Vereshchagin, O.; Rozhdestvenskaya, I.; Zolotarev, A. Short-Range Order in Li–Al Tourmalines: IR Spectroscopy, X-ray Single Crystal Diffraction Analysis and a Bond Valence Theory Approach. Phys. Chem. Miner. 2019, 46, 815–825. [Google Scholar] [CrossRef]
- Pesquera, A.; Gil-Crespo, P.P.; Torres-Ruiz, F.; Torres-Ruiz, J.; Roda-Robles, E. A Multiple Regression Method for Estimating Li in Tourmaline from Electron Microprobe Analyses. Mineral. Mag. 2016, 80, 1129–1133. [Google Scholar] [CrossRef]
- Yavuz, F.; Karakaya, N.; Yıldırım, D.K.; Karakaya, M.Ç.; Kumral, M. A Windows Program for Calculation and Classification of Tourmaline-Supergroup (IMA-2011). Comput. Geosci. 2014, 63, 70–87. [Google Scholar] [CrossRef]
- Šontevska, V.; Jovanovski, G.; Makreski, P. Minerals from Macedonia. Part XIX. Vibrational Spectroscopy as Identificational Tool for Some Sheet Silicate Minerals. J. Mol. Struct. 2007, 834–836, 318–327. [Google Scholar] [CrossRef]
- Li, W.; Wu, R.; Dong, Y. Study on Infrared Spectra and Infrared Radiation Characteristics of Tourmaline. Geol. J. China Univ. 2008, 14, 426–432. [Google Scholar]
- Li, M. Spectroscopic Characteristics and Color Origin of Red Tourmaline from Brazil. J. Spectrosc. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G. Minerals from Macedonia. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 460–467. [Google Scholar] [CrossRef]
- Fuchs, Y.; Lagache, M.; Linares, J. Oxydation expérimentale de Fe-tourmalines et corrélation avec une déprotonation des groupes hydroxyle. Comptes Rendus Geosci. 2002, 334, 245–249. [Google Scholar] [CrossRef]
- Prasad, P.S.R. Study of Structural Disorder in Natural Tourmalines by Infrared Spectroscopy. Gondwana Res. 2005, 8, 265–270. [Google Scholar] [CrossRef]
- Ertl, A.; Rossman, G.R.; Hughes, J.M.; London, D.; Wang, Y.; O’Leary, J.A.; Dyar, M.D.; Prowatke, S.; Ludwig, T.; Tillmanns, E. Tourmaline of the Elbaite-Schorl Series from the Himalaya Mine, Mesa Grande, California: A Detailed Investigation. Am. Mineral. 2010, 95, 24–40. [Google Scholar] [CrossRef]
- Maneewong, A.; Seong, B.S.; Shin, E.J.; Kim, J.S.; Kajornrith, V. Color Change of Tourmaline by Heat Treatment and Electron Beam Irradiation: UV-Visible, EPR, and Mid-IR Spectroscopic Analyses. J. Korean Phys. Soc. 2016, 68, 83–92. [Google Scholar] [CrossRef]
- Castañeda, C.; Oliveira, E.F.; Gomes, N.; Soares, A.C.P. Infrared Study of OH Sites in Tourmaline from the Elbaite-Schorl Series. Am. Mineral. 2000, 85, 1503–1507. [Google Scholar] [CrossRef]
- Reddy, B.J.; Frost, R.L.; Martens, W.N.; Wain, D.L.; Kloprogge, J.T. Spectroscopic Characterization of Mn-Rich Tourmalines. Vib. Spectrosc. 2007, 44, 42–49. [Google Scholar] [CrossRef]
- Li, X.; Zu, E. Near-Infrared Spectrum Analysis of Cyclosilicates Gem Minerals. Bull. Chin. Ceram. Soc. 2016, 35, 1318–1321. [Google Scholar] [CrossRef]
- Peng, M.; Wang, H. A Study on the Vibrational Spectra of Water in Tourmaline. Acta Mineral. Sin. 1995, 15, 372–377. [Google Scholar] [CrossRef]
- Hoang, L.H.; Hien, N.T.M.; Chen, X.B.; Minh, N.V.; Yang, I. Raman Spectroscopic Study of Various Types of Tourmalines. J. Raman Spectrosc. 2011, 42, 1442–1446. [Google Scholar] [CrossRef]
- Hoang, L.H.; Hien, N.T.M.; Chen, X.-B.; Yang, I.S. Annealing Effect in Raman Scattering of Various Types of Tourmalines. J. Appl. Spectrosc. 2013, 79, 881–887. [Google Scholar] [CrossRef]
- Fantini, C.; Tavares, M.C.; Krambrock, K.; Moreira, R.L.; Righi, A. Raman and Infrared Study of Hydroxyl Sites in Natural Uvite, Fluor-Uvite, Magnesio-Foitite, Dravite and Elbaite Tourmalines. Phys. Chem. Miner. 2013, 41, 247–254. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Natkaniec-Nowak, L. A Raman Microspectroscopic Study of Organic Inclusions in “Watermelon” Tourmaline from the Paprok Mine (Nuristan, Afghanistan). Vib. Spectrosc. 2011, 57, 248–253. [Google Scholar] [CrossRef]
- Chenguang, Y.; Ruifeng, K.; Zhenyu, X.; Guangle, Z.; Jianguo, L. Second derivative of Voigt function. Acta Phys. Sin. 2014, 63, 142–147. [Google Scholar] [CrossRef]
- Mattson, S.M.; Rossman, G.R. Fe2+-Ti4+ Charge Transfer in Stoichiometric Fe2+,Ti4+-Minerals. Phys. Chem. Miner. 1988, 16, 78–82. [Google Scholar] [CrossRef]
- Bosi, F.; Skogby, H.; Agrosi, G.; Scandale, E. Tsilaisite, NaMn3Al6(Si6O18)(BO3)3(OH)3OH, a New Mineral Species of the Tourmaline Supergroup from Grotta d’Oggi, San Pietro in Campo, Island of Elba, Italy. Am. Mineral. 2012, 97, 989–994. [Google Scholar] [CrossRef]
- Rossman, G.R. Optical Spectroscopy. Rev. Mineral. Geochem. 2014, 78, 371–398. [Google Scholar] [CrossRef]
- Pezzotta, F.; Laurs, B.M. Tourmaline: The Kaleidoscopic Gemstone. Elements 2011, 7, 333–338. [Google Scholar] [CrossRef]
- Schwarzinger, C.; Wildner, M.; Ulatowski, S.; Sawyer, M. Vanadium-Bearing Tourmaline from the Commander Mine, Nadonjukin, Tanzania. J. Gemmol. 2019, 36, 534–543. [Google Scholar] [CrossRef]
- Cui, L.; Guo, Y.; Tang, J.; Yang, Y. Spectroscopy Characteristics and Color-Influencing Factors of Green Iron-Bearing Elbaite. Crystals 2023, 13, 1461. [Google Scholar] [CrossRef]
- Laurs, B.M.; Simmons, W.B.; Rossman, G.R.; Fritz, E.A.; Koivula, J.I.; Anckar, B.; Falster, A.U. Yellow Mn-Rich Tourmaline From The Canary Mining Area, Zambia. Gems Gemol. 2007, 43, 314–331. [Google Scholar] [CrossRef][Green Version]
- Thongnopkun, P.; Naowabut, P. Effect of Heat Treatment on Madagascar Dravite Tourmaline: UV-Visible and Diffuse Reflectance Infrared Spectroscopic Characterization. J. Appl. Spectrosc. 2018, 85, 616–623. [Google Scholar] [CrossRef]
- Kurtz, D.; Rossman, G.; Hunter, B. The Nature of the Mn(III) Color Centers in Elbaite Tourmalines. Inorg. Chem. 2020, 59, 9618–9626. [Google Scholar] [CrossRef]
- Bosi, F.; Celata, B.; Skogby, H.; Hålenius, U.; Tempesta, G.; Ciriotti, M.E.; Bittarello, E.; Marengo, A. Mn-Bearing Purplish-Red Tourmaline from the Anjanabonoina Pegmatite, Madagascar. Mineral. Mag. 2021, 85, 242–253. [Google Scholar] [CrossRef]
- Ertl, A.; Hughes, J.M.; Prowatke, S.; Ludwig, T.; Lengauer, C.L.; Meyer, H.-P.; Giester, G.; Kolitsch, U.; Prayer, A. Alumino-Oxy-Rossmanite from Pegmatites in Variscan Metamorphic Rocks from Eibenstein an Der Thaya, Lower Austria, Austria: A New Tourmaline That Represents the Most Al-Rich End-Member Composition. Am. Mineral. 2022, 107, 157–166. [Google Scholar] [CrossRef]
- Suwanmanee, W.; Sutthirat, C.; Wanthanachaisaeng, B.; Utapong, T. Colour Enhancement of Pink Tourmaline from Nigeria by Electron-Beam and Gamma Irradiation. J. Gemmol. 2021, 37, 514–526. [Google Scholar] [CrossRef]
- Mattson, S.M.; Rossman, G.R. Fe2+-Fe3+ Interactions in Tourmaline. Phys. Chem. Miner. 1987, 14, 163–171. [Google Scholar] [CrossRef]

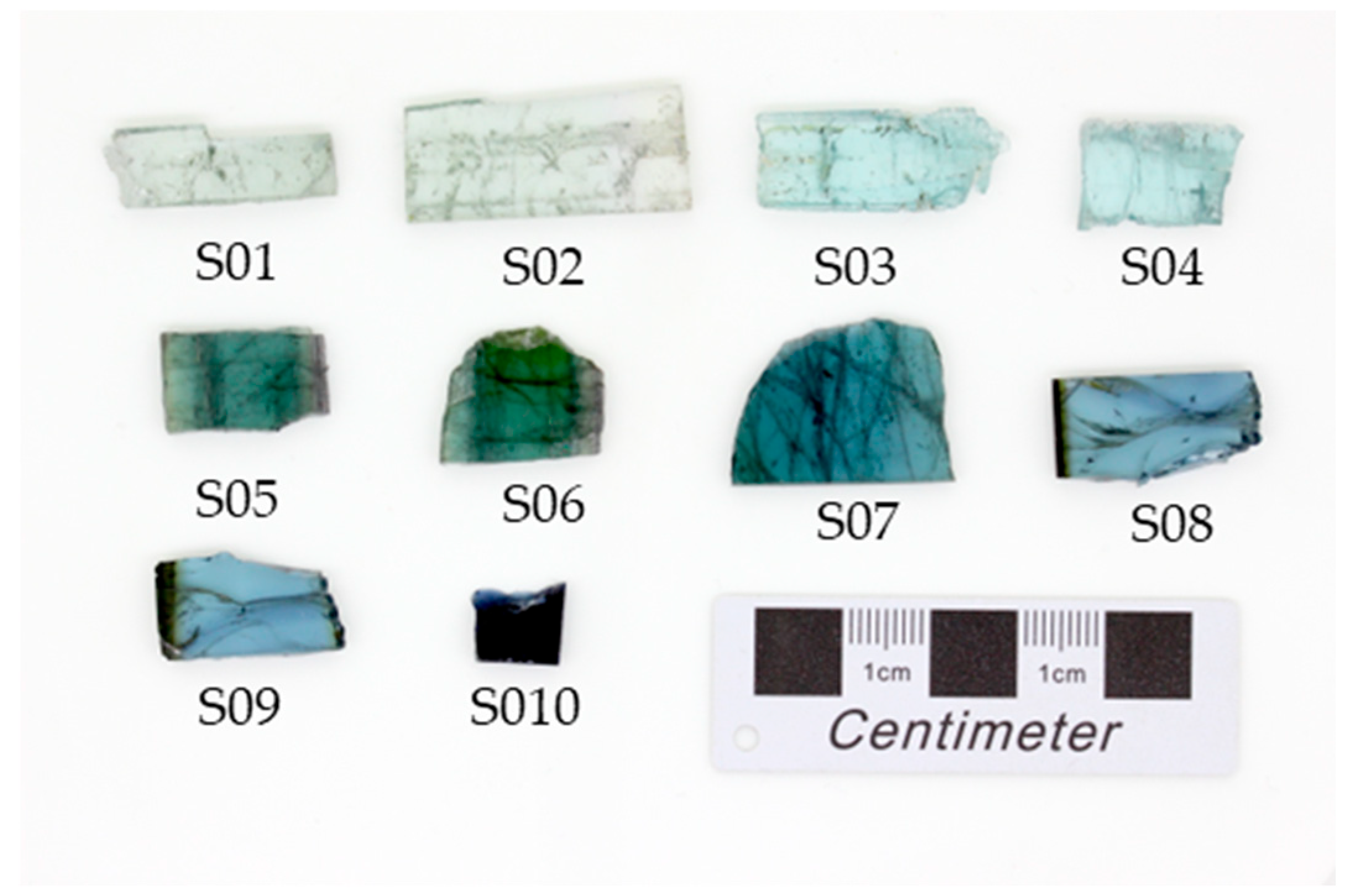



| Sample | Color | Size (cm3) | Weight (ct) | Source |
|---|---|---|---|---|
| S01 | Greyish blue | 2.22 × 0.93 × 0.30 | 8.44 | Cruzeiro Mine—Vein 1, Governador Valadares |
| S02 | Greyish blue | 3.25 × 1.46 × 0.24 | 15.46 | Cruzeiro Mine—Vein 1, Governador Valadares |
| S03 | Light blue | 2.90 × 1.20 × 0.26 | 9.12 | Golconda Mine, Governador Valadares |
| S04 | Light blue | 1.88 × 1.20 × 0.24 | 6.37 | Golconda Mine, Governador Valadares |
| S05 | Bluish green | 1.92 × 1.21 × 0.28 | 8.47 | Cruzeiro Mine—Vein 2, Governador Valadares |
| S06 | Bluish green | 1.84 × 1.56 × 0.26 | 8.25 | Cruzeiro Mine—Vein 2, Governador Valadares |
| S07 | Dark greyish blue | 2.56 × 1.91 × 0.32 | 13.51 | Cruzeiro Mine—Vein 2, Governador Valadares |
| S08 | Dark greyish blue | 2.36 × 1.28 × 0.35 | 10.9 | Rubelita mining District, Aracuaí |
| S09 | Dark greyish blue | 2.11 × 1.20 × 0.32 | 8.77 | Rubelita mining District, Aracuaí |
| S10 | Dark blue | 0.98 × 0.94 × 0.28 | 3.12 | Rubelita mining District, Aracuaí |
| Sample | Color | Pleochroism | Specific Gravity | Refractive Index | Double Refraction | UV Fluorescence | |
|---|---|---|---|---|---|---|---|
| S01 | Greyish blue | Weak | 3.07 | 1.620 | 1.640 | 0.020 | inert |
| S02 | Greyish blue | Weak | 3.06 | 1.620 | 1.640 | 0.020 | inert |
| S03 | Light blue | Strong—light blue/grey | 2.94 | 1.620 | 1.640 | 0.020 | inert |
| S04 | Light blue | Strong—light blue/grey | 2.93 | 1.620 | 1.639 | 0.019 | inert |
| S05 | Bluish green | Strong—light blue/greenish blue | 3.06 | 1.622 | 1.642 | 0.020 | inert |
| S06 | Bluish green | Strong—light blue/greenish blue | 3.07 | 1.622 | 1.642 | 0.020 | inert |
| S07 | Dark greyish blue | Strong—light blue/blue | 3.10 | 1.620 | 1.640 | 0.020 | inert |
| S08 | Dark greyish blue | Strong—light green/blue | 3.07 | 1.622 | 1.642 | 0.020 | inert |
| S09 | Dark greyish blue | Strong—light green/blue | 3.07 | 1.620 | 1.640 | 0.020 | inert |
| S10 | Dark blue | Strong—blue/dark blue | 3.09 | 1.622 | 1.642 | 0.020 | inert |
| S01 | S02 | S03 | S04 | S05 | S06 | S07 | S08 | S09 | S10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Major oxide (wt.%) analyses | ||||||||||
| SiO2 | 37.462 | 37.537 | 37.666 | 36.652 | 37.119 | 37.345 | 36.467 | 36.669 | 37.692 | 36.083 |
| TiO2 | 0.046 | 0.000 | 0.000 | 0.006 | 0.000 | 0.008 | 0.000 | 0.000 | 0.000 | 0.016 |
| Al2O3 | 39.336 | 39.668 | 38.611 | 38.246 | 36.577 | 37.448 | 35.742 | 37.138 | 38.008 | 36.169 |
| V2O3 | 0.022 | 0.000 | 0.013 | 0.015 | 0.012 | 0.025 | 0.017 | 0.012 | 0.025 | 0.000 |
| Cr2O3 | 0.023 | 0.054 | 0.000 | 0.034 | 0.015 | 0.000 | 0.000 | 0.036 | 0.000 | 0.009 |
| FeO | 0.441 | 0.547 | 1.510 | 1.431 | 3.896 | 3.314 | 4.047 | 2.256 | 2.370 | 4.906 |
| MnO | 2.084 | 2.129 | 0.850 | 0.827 | 1.926 | 2.171 | 2.131 | 2.433 | 2.563 | 1.207 |
| ZnO | 0.000 | 0.053 | 0.172 | 0.150 | 0.058 | 0.013 | 0.067 | 0.000 | 0.039 | 0.064 |
| CuO | 0.000 | 0.000 | 0.021 | 0.000 | 0.000 | 0.024 | 0.000 | 0.006 | 0.060 | 0.006 |
| MgO | 0.016 | 0.000 | 0.000 | 0.006 | 0.042 | 0.038 | 0.023 | 0.000 | 0.000 | 0.033 |
| CaO | 0.105 | 0.117 | 0.363 | 0.259 | 0.309 | 0.175 | 0.200 | 0.449 | 0.437 | 0.259 |
| PbO | 0.000 | 0.115 | 0.013 | 0.000 | 0.000 | 0.000 | 0.076 | 0.000 | 0.000 | 0.000 |
| Na2O | 1.980 | 1.956 | 2.063 | 2.050 | 2.494 | 2.588 | 2.359 | 2.118 | 2.333 | 2.408 |
| K2O | 0.019 | 0.054 | 0.004 | 0.013 | 0.047 | 0.031 | 0.023 | 0.017 | 0.016 | 0.060 |
| Li2O * | 1.826 | 1.768 | 1.948 | 1.884 | 1.527 | 1.514 | 1.488 | 1.614 | 1.594 | 1.385 |
| F | 0.852 | 0.791 | 0.716 | 0.782 | 0.682 | 0.882 | 0.691 | 0.654 | 0.846 | 0.684 |
| H2O ** | 2.998 | 3.031 | 3.118 | 3.020 | 3.161 | 3.086 | 3.078 | 3.085 | 3.095 | 3.071 |
| B2O3 ** | 10.815 | 10.868 | 10.786 | 10.574 | 10.625 | 10.739 | 10.424 | 10.555 | 10.838 | 10.414 |
| O = F | 0.359 | 0.333 | 0.301 | 0.329 | 0.287 | 0.371 | 0.291 | 0.275 | 0.356 | 0.288 |
| Total | 97.666 | 98.356 | 97.554 | 95.622 | 98.205 | 99.032 | 96.543 | 96.767 | 99.560 | 96.486 |
| Normalization: Cations (apfu) based on 31 anions | ||||||||||
| Si | 6.020 | 6.003 | 6.069 | 6.024 | 6.072 | 6.044 | 6.080 | 6.038 | 6.044 | 6.022 |
| Ti | 0.006 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.002 |
| Al | 7.450 | 7.476 | 7.333 | 7.409 | 7.051 | 7.143 | 7.024 | 7.207 | 7.183 | 7.114 |
| V | 0.003 | 0.000 | 0.002 | 0.002 | 0.002 | 0.003 | 0.002 | 0.002 | 0.003 | 0.000 |
| Cr | 0.003 | 0.007 | 0.000 | 0.004 | 0.002 | 0.000 | 0.000 | 0.005 | 0.000 | 0.001 |
| Fe2+ | 0.059 | 0.073 | 0.203 | 0.197 | 0.533 | 0.449 | 0.564 | 0.311 | 0.318 | 0.685 |
| Mn2+ | 0.284 | 0.288 | 0.116 | 0.115 | 0.267 | 0.298 | 0.301 | 0.339 | 0.348 | 0.171 |
| Zn | 0.000 | 0.006 | 0.020 | 0.018 | 0.007 | 0.002 | 0.008 | 0.000 | 0.005 | 0.008 |
| Cu | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.003 | 0.000 | 0.001 | 0.007 | 0.001 |
| Mg | 0.004 | 0.000 | 0.000 | 0.001 | 0.010 | 0.009 | 0.006 | 0.000 | 0.000 | 0.008 |
| Ca | 0.018 | 0.020 | 0.063 | 0.046 | 0.054 | 0.030 | 0.036 | 0.079 | 0.075 | 0.046 |
| Pb | 0.000 | 0.005 | 0.001 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 |
| Na | 0.617 | 0.606 | 0.645 | 0.653 | 0.791 | 0.812 | 0.763 | 0.676 | 0.725 | 0.779 |
| K | 0.004 | 0.011 | 0.001 | 0.003 | 0.010 | 0.006 | 0.005 | 0.004 | 0.003 | 0.013 |
| Li | 1.180 | 1.137 | 1.262 | 1.246 | 1.005 | 0.985 | 0.997 | 1.068 | 1.028 | 0.930 |
| F | 0.433 | 0.400 | 0.365 | 0.406 | 0.353 | 0.451 | 0.364 | 0.341 | 0.429 | 0.361 |
| B | 2.940 | 2.942 | 2.936 | 2.937 | 2.949 | 2.950 | 2.949 | 2.946 | 2.948 | 2.953 |
| Sample | OH(3)-ν3 | OH(3)-ν1 | OH(1)-ν2 | |
|---|---|---|---|---|
| S01 | 3486 (Al,Ti) YAlZAlZ | 3589 MgYAlZAlZ | 3653 □X | |
| S02 | 3483 AlYAlZAlZ | 3588 MgYAlZAlZ | 3653 □X | |
| S03 | 3476 AlYAlZAlZ | 3588 MgYAlZAlZ | 3652 □X | |
| S04 | 3480 AlYAlZAlZ | 3588 MgYAlZAlZ | 3655 □X | |
| S05 | 3493 FeYAlZAlZ | 3563 NaX | 3595 MgYAlZAlZ | |
| S06 | 3495 FeYAlZAlZ | 3563 NaX | 3596 MgYAlZAlZ | |
| S07 | 3495 FeYAlZAlZ | 3564 NaX | 3595 MgYAlZAlZ | |
| S08 | 3510 (Fe,Mg)YAlZAlZ | 3583 MgYAlZAlZ | 3612 (Fe,Mg)YAlZAlZ | |
| S09 | 3503 FeYAlZAlZ | 3578 MgYAlZAlZ | 3605 (Fe,Mg)YAlZAlZ | |
| S10 | 3498 FeYAlZAlZ | 3566 NaX | 3599 MgYAlZAlZ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xu, D.; Zhou, Z.; Schwarz, D.; Zheng, J.; Zhang, L. Chemical Composition and Spectral Variation in Gem-Quality Blue Iron-Bearing Tourmaline from Brazil. Crystals 2024, 14, 877. https://doi.org/10.3390/cryst14100877
Chen Y, Xu D, Zhou Z, Schwarz D, Zheng J, Zhang L. Chemical Composition and Spectral Variation in Gem-Quality Blue Iron-Bearing Tourmaline from Brazil. Crystals. 2024; 14(10):877. https://doi.org/10.3390/cryst14100877
Chicago/Turabian StyleChen, Yifang, Duo Xu, Zhengyu Zhou, Dietmar Schwarz, Junhao Zheng, and Lingmin Zhang. 2024. "Chemical Composition and Spectral Variation in Gem-Quality Blue Iron-Bearing Tourmaline from Brazil" Crystals 14, no. 10: 877. https://doi.org/10.3390/cryst14100877
APA StyleChen, Y., Xu, D., Zhou, Z., Schwarz, D., Zheng, J., & Zhang, L. (2024). Chemical Composition and Spectral Variation in Gem-Quality Blue Iron-Bearing Tourmaline from Brazil. Crystals, 14(10), 877. https://doi.org/10.3390/cryst14100877






