Abstract
The geometric and electronic structures and the bonding of U5M+ and T5M+ (U = uracil, T = thymine, M = Ag, Au) cluster cations have been investigated with density functional theory methods. They have a perfectly planar structure with C5h symmetry and significant stability, containing self-complementary N-H···O hydrogen bonds and five Au-O or Ag-O contacts. The energy gap between the LUMO and HOMO in the U5Ag+ cluster is 4.2 eV, which is twice as large as the HOMO-LUMO gap observed in the U5Au+ cluster. This notable difference clearly indicates that the U5Ag+ cluster possesses substantially greater stability compared to the U5Au+ cluster. This finding is consistent with the results from the energy decomposition analyses, which show that the total interaction energy of U5Ag+ is significantly higher than that of U5Au+. The same trend is observed in T5M+ as well. The interaction between the metal atoms, whether gold (Au) or silver (Ag), and the nucleobase is not predominantly controlled by electrostatic forces, as initially believed. Instead, it is primarily characterized by pronounced covalent bonding effects.
1. Introduction
Highly organized self-pairing systems, such as the guanine quartet, which are formed by guanine-rich DNAs and RNAs, have recently attracted considerable interest in both biological and supramolecular chemistry fields [1]. The G-quartet, which is assembled through the cation-templated process of guanosine, was initially discovered in 1962 [2]. Specifically, the alkaline earth metal ions, including Na+, K+, and Rb+, along with other cations like NH4+, Tl+, Sr2+, Ba2+, and Pb2+ cations, are known to effectively stabilize the G-quartet [3,4]. In contrast, cations such as Mg2+ and Ca2+ ions do not specifically stabilize G-quartets. Nevertheless, they generally facilitate the formation and stabilization of G-quadruplexes through more generalized electrostatic stabilization mechanisms [5]. In 1999, Marlow and colleagues discovered that the synthetic nucleoside isoguanosine (isoG) could notably induce the formation of pentameric isoG-quintets when present with the Cs+ cation in CDCl3 [6]. Subsequently, Chaput and Switzer [7] proposed that the directionality of hydrogen bond donor or acceptor groups on G and isoG may influence their behavior. They suggested that, while G tends to favor the formation of planar cyclic tetramers in the presence of K+ ion isoG is more inclined to form planar pentamers when Cs+ cations are present. Gu et al. [8,9] and Meyer et al. [10] meticulously performed theoretical calculations on iso-guanine quintet complexes, which were coordinated by monovalent cations such as Na+, K+, Rb+, and Cs+ cations. They discovered that, in contrast to smaller cations like Li+ and Na+, larger cations such as K+, Rb+, and Cs+ cations tend to predominantly induce the formation of pentamers. Additionally, their findings revealed that, in the presence of specific cations, including Rb+, NH4+, and Sr2+, isoG nucleosides can effectively form cation-assisted isoG-quartets or -quintets. Notably, the geometry of the backbone-unconstrained isoG-quintets, templated by cations such as NH4+, was observed to be planar. In contrast, the isoG-quartets exhibited significant deviations from planarity. Electrospray ionization experiments have effectively shown that uracil, thymine, and their homologs can consistently yield self-assembled quintet structures in the presence of K+, Rb+, and Cs+ cations [11]. Ding and colleagues [12], through the careful integration of high-resolution scanning tunneling microscopy imaging with precise density functional theory calculations, have convincingly demonstrated that alkali metal ions (Li+, Na+, and K+) can indeed form pentameric uracil clusters on the Au(111) surface.
Not only have researchers thoroughly examined the interactions between monovalent cations, such as Na+, K+, Rb+, and Cs+ cations, and nucleobases [13,14,15], but they have also extensively investigated the interactions between transition metal cations, particularly copper, silver, and gold, and nucleobases. This comprehensive exploration, both experimental and theoretical, is driven by the significant implications these interactions hold for fields such as biology and nanotechnology [16,17,18,19,20,21,22,23,24,25]. Rogers et al. [25] meticulously examined the kinetic energy dependencies and straightforward collision-induced dissociation processes of (adenine)M+ with xenon, where M represents various cations including Sc+, Ti+, V+, Cr+, Mn+, Fe+, Co+, Ni+, Cu+, and Zn+ cations, utilizing a guided ion beam mass spectrometer. Meanwhile, Valdespino-Saenz [16] and Martinez [18] convincingly proposed that the low-energy coordination sites for Cu+ in cytosine are primarily the N3 and O2 atoms. Similarly, in uracil and thymine, these are the O4 and O2 atoms, while, in adenine, the critical sites are the N9, N7, N6, and N3 atoms. Moreover, for guanine, the essential binding sites are identified as the N9, N7, O6, and N3 atoms. On the other hand, for Ag+ and Au+, the favored binding sites in uracil were determined to be the O4 and O2/N3 atoms. It was also observed that the Cu+ cation remarkably shows a stronger affinity for neutral cytosine in comparison to Al+ and Ag+ within the M–cytosine+ complex [17]. Furthermore, experimental investigations into metal ions, particularly transition metal ions, bound to nucleobases in the gas phase have already been extensively explored. Understanding the interactions between metals and nucleobases is crucial, as it not only provides essential insights for self-assembly nanomaterials but also for the innovative development of new pharmaceuticals and metal detection platforms [26].
In our proceeding paper, we thoroughly conducted a combined study involving photoelectron spectroscopy and theoretical analysis to comprehensively investigate the gold–nucleobase complexes. This research has convincingly provided experimental evidence confirming the presence of non-conventional hydrogen bonds (NCHBs) within these nucleobase–gold complexes, while also revealing that gold anions can effectively stabilize specific tautomers of nucleobases [27,28]. Furthermore, we meticulously carried out photodissociation experiments on metal–base complexes, specifically involving Cu, Ag, and Au, and seamlessly integrated these findings with density functional theory (DFT) calculations to accurately identify their dissociation pathways [29]. The principal aim of our study is to extensively employ DFT calculations to examine the geometric and electronic structures, as well as the inherent stabilities, of the U5M+ and T5M+ cations (where U denotes uracil, T represents thymine, and M stands for Ag or Au).
2. Computational Methods
To thoroughly investigate the structures and energetics of these metal ion–nucleobase complexes, we optimized their full geometric structures without imposing any symmetry constraints. This detailed optimization was conducted using density functional theory (DFT) computations, rigorously performed with the GAUSSIAN 09 program, thereby ensuring both accuracy and precision in the results [30]. The optimized minimum structures were thoroughly verified by confirming the absence of imaginary frequencies during the analysis of vibrational frequencies. For uracil and thymine, the hybrid three-parameter B3LYP functional [31,32] was diligently employed in combination with the 6–31++G(d,p) basis set. Additionally, for Ag, Au, K, Rb, and Cs, the ECP28MDF, ECP60MDF, ECP10MDF, ECP28MDF, and ECP46MDF relativistic effective core potentials (RECP) [33] developed by the Stuttgart–Cologne groups were precisely selected, ensuring accurate representation of the electronic environment for each metal ion [33]. Gaussian-type one-electron basis sets, specifically ECP28MDF_VDZ and ECP60MDF_VDZ for Ag and Au, respectively, were employed [33,34]. It has been confirmed that the energetic ordering of various tautomers of nucleobases aligns well with experimental results when density functional approaches (B3LYP) and the 6–31++G(d,p) basis sets are utilized for the comprehensive geometry optimizations of nucleobases [35]. This consistent agreement highlights the reliability of these methods in accurately predicting the relative stabilities of the tautomers. The bond dissociation energy (BDE) is accurately determined by first calculating the total energy of the parent molecule in its completely optimized ground-state geometry and then carefully subtracting the total energy of all individual fragments. This approach rigorously guarantees that the BDE precisely represents the exact energy difference between the intact molecule and its separated components. Moreover, corrections for zero-point energy (ZPE) were carefully applied to the cationic metal–nucleobase complexes, ensuring greater accuracy when calculating the relative energies.
To conduct the energy decomposition analyses systematically on these complexes, the geometries were precisely optimized using generalized gradient approximation of the Becke–Perdew BP86 [36,37], as implemented in the Amsterdam Density Functional program (ADF 2013.01) [38,39,40]. This thorough optimization process ensured a detailed and accurate examination of the energy components within the complexes, achieving convergence with an energy gradient of less than 10−5 Hartree∙Å−1 and a Kohn–Sham SCF criterion of below 10−8 a.u., thereby enabling a highly precise analysis. The uncontracted Slater basis sets, featuring triple-zeta quality with two polarization functions (TZ2P) [41], were meticulously employed. The frozen core approximation was consistently applied to the [1s2–3d10] core for Ag, the [1s2–4f14] core for Au, and the [1s2] cores for C, N, and O. This method ensured both precision and efficiency in the calculations, thereby facilitating a comprehensive analysis of the complexes. The scalar relativistic (SR) was taken into account by the zero-order regular approximation (ZORA). The geometric optimization of the U5M+ and T5M+ cations was confirmed to have converged according to these stringent standards, providing a reliable foundation for further calculations. The theoretical method was thoroughly validated by precisely calculating the harmonic frequencies of the G2(H2O) cluster and then rigorously comparing these calculated results with experimental measurements. This detailed comparison revealed a strong concordance between the theoretical predictions and the observed experimental data, thereby confirming the accuracy and reliability of the theoretical approach [42,43].
The Amsterdam Density Functional (ADF 2013.01) program [38,39,40] was precisely utilized to thoroughly conduct combined energy decomposition approaches (EDAs) and natural orbitals for the chemical valence (NOCV) [44,45] studies on the reactions M+ + U5 → U5M+ or M+ + T5 → T5M+ (where M represents Ag or Au). These highly detailed EDA-NOCV calculations were rigorously completed based on the lowest-energy structures of the U5M+ and T5M+ cluster cations, employing the generalized gradient approximation (GGA) with the Becke–Perdew (BP86) functional [36,37] and the TZ2P basis set. Additionally, to systematically analyze the charge distribution, the effective atomic charges on Ag and Au, as well as on K, Rb, and Cs atoms, were attentively computed using various population analysis methods, including the Voronoi and Hirshfeld partitioning schemes, all diligently executed at the PBE/TZ2P level of theory.
3. Results and Discussion
In our thorough investigation of their low-energy configurations, we carefully examined all possible complexes formed with Ag+ or Au+ ions and the predominant tautomers of uracil or thymine [35]. Figure 1 clearly presents the optimized structures of the lowest-energy isomers of the U5M+ and T5M+ cluster cations. The geometric parameters for the U5M+ and T5M+ cluster cations, where M represents Ag, Au, K, Rb, or Cs, are detailed in Table 1. In these arrangements, the metal ion, centrally positioned within a perfectly planar cyclic pentamer, interacts with five nucleobases through five Ag-O or Au-O bonds, thus significantly stabilizing the pentameric clusters. Moreover, each nucleobase efficiently forms two self-complementary N-H···O hydrogen bonds with neighboring nucleobases, further reinforcing the overall stability of the complexes. All of these clusters exhibit C5h symmetry, whereas the U5M+ and T5M+ cluster cations (where M = Rb, Cs) display a distinctly non-planar configuration. Specifically, in the U5Au + cation, the Au-O bond distances are approximately 2.734 Å, which are slightly shorter compared to the Ag-O bond distances of around 2.737 Å found in the U5Ag+ cation. This difference reflects the trend in atomic radii, where gold (1.24 Å) is noticeably smaller than silver (1.28 Å), largely due to the significant relativistic effects associated with gold. The metal ion–oxygen distances in the U5M+ cations are subtly longer compared to those in the T5M+ cations. Specifically, the angles of the inner N-H···O hydrogen bonds in the U5M+ and T5M+ (where M = Au or Ag) cations are approximately 172°, which are distinctly smaller than the angles of about 179° observed in the U5Cs+ and T5Cs+ cations. Additionally, the angles of the inner N-H···O hydrogen bonds in the U5M+ and T5M+ (where M = Au or Ag) cations are somewhat greater than those of the outer N-H···O hydrogen bonds. In contrast, the angles of the inner N-H···O hydrogen bonds in the U5M+ and T5M+ (where M = K, Rb, or Cs) cations are larger than the angles of the outer N-H···O hydrogen bonds. This clearly demonstrates a notable discrepancy in the impact of inner versus outer hydrogen bonds between halogen and noble metal elements.
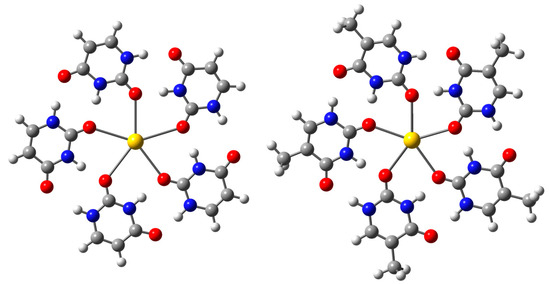
Figure 1.
Structures of the lowest-lying isomers of U5M+ and T5M+ (M = Ag, Au) cluster cations.

Table 1.
The geometric parameters of U5M+ and T5M+ (M = Ag, Au, K, Rb, Cs) cluster cations.
To thoroughly investigate the charge distribution of metal atoms in the U5M+ and T5M+ cluster cations (as presented in Table 2), we precisely calculated the effective atomic charges of K, Rb, Cs, Au, and Ag at the DFT/PBE/TZ2P level using various analytical methods. Both the Hirshfeld and Voronoi deformation schemes consistently revealed that the charge transfers between the central metal atoms and the surrounding oxygen atoms are notably significant, although the exact figures show slight variations. These charge transfers depend critically on the charge density distributions as well as the chosen density-partitioning scheme [46]. The effective atomic charges of the central metal atoms in the U5M+ cluster cations closely resemble those in the T5M+ cluster cations. The inclusion of the methyl group has a negligible effect on the overall charge distribution. The computed atomic partial charges indicate that the metal atoms in both U5M+ and T5M+ cluster cations predominantly act as electron donors rather than electron acceptors. For the lighter Ag atom centrally located, fewer electrons are transferred to the peripheral ligands (i.e., M → U5 or M → T5), which corresponds to Ag’s lower electron affinity. Consequently, the oxygen atoms, which are bonded to both the metal atoms and the uracil or thymine ligands, acquire a negative charge. The Metal-to-Ligand Charge Transfer (MLCT) involves the transfer of electron density from the metal to the ligands, which can strengthen the metal–ligand bonds and enhance the overall stability of the complex. Systematically, the Mayer bond orders [47] of the Ag–O and Au–O bonds within the U5Ag+ and U5Au+ clusters were carefully analyzed by employing the BP86/TZ2P computational method. The computational analysis clearly revealed that the Mayer bond order for the Ag-O bond stands at 0.13, while the Au-O bond demonstrates a marginally higher bond order of 0.16. This subtle difference underscores the inherently distinct bonding characteristics of silver and gold in these metal–uracil complexes.

Table 2.
Effective atomic charge of central atoms of U5M+ and T5M+ (M = Ag, Au) cluster cations.
The energy-level correlation diagrams for the Ag+ and U5Ag+ cations are illustratively presented in Figure 2, while the corresponding diagrams for the Au and U5Au+ cations are clearly depicted in Figure 3. Furthermore, the plots of the occupied molecular orbitals for both the U5Ag+ and U5Au+ cations are comprehensively shown in Figure 4 and Figure 5, respectively. These visualizations provide deeper insights into the nature of these metal–oxygen bonds within the molecular structure. Additionally, the detailed mixing character, orbital energy (measured in eV), electronic occupation number, and atomic orbital contributions in percentage for the Ag-O mixed-valence molecular orbitals of the U5Ag+ and U5Au+ cations are systematically listed in Table 3 and Table 4, respectively. The Au ion–uracil interactions occur mainly through the stabilization of 3e2′, 2e2′, 2e1′, 1e1′, 1a′, and 1e2′ MOs. The 1a′ orbital is consistently characterized by an energy level of −10.5 eV and is fully occupied by two electrons, whereas the remaining five orbitals are collectively filled by four electrons. Notably, the 2e2′ orbital, possessing an energy level of −9.3 eV, predominantly contains the highest proportion of Au 5d character, accounting for 66%. Conversely, the 3e2′ orbital, which also exhibits an energy of −8.9 eV, is distinctively marked by the lowest Au 5d character, contributing just 10%. The 1e1′ and 1a’ MOs are primarily composed of πu bonds, which are predominantly formed by the interaction of O 2p and Au 5d orbitals. The interaction of O 2p and Au 5d orbitals also effectively produces σ bonds in the 2e1′ MO and πg bonds in the 1e2′, 2e2′, and 3e2′ MOs. These four MOs predominantly contain effective π bonding within the intra-nucleobases and complementary N-H···O hydrogen bonds between the nucleobases. The LUMO, specifically designated as the 2a’ orbital, is accurately characterized by an energy level of −6.8 eV. This orbital is predominantly constituted by 82% Au 6s, with an additional 2% contribution from Au 5d, and the remainder derived from O 2p orbitals. Moreover, there exists a notably significant energy gap of 2.1 eV between the LUMO and the HOMO, highlighting a crucial aspect of the electronic structure. The interactions between the Ag ion and uracil are remarkably similar to those observed in Au ion–uracil interactions. These interactions are primarily mediated through the significant involvement of the 2e1″, 2e2′, 1e1′, 1e1″, 1a′, and 1e2′ MOs, ensuring a close correspondence between the two types of interactions. The 1e1′ and 1a′ orbitals, which exhibit energies of −10.1 eV and −10.7 eV, respectively, each occupy only two electrons. In contrast, the other four orbitals collectively accommodate four electrons. With the exception of the 2e1″ orbital, the d-orbital contributions in the remaining orbitals are notably larger compared to those in the bonding orbitals of the U5Au+ cluster. Consequently, this variation highlights a significant difference in the electronic characteristics of these orbitals. The 1e1″ orbital, energetically positioned at −10.6 eV, exhibits the highest d-orbital contribution, which reaches an impressive 94%. In contrast, the 2e1″ orbital, with an energy of −9.8 eV, shows the smallest d-orbital contribution, amounting to only 3%. The 1e1′ orbital is uniquely the only one among these six occupied orbitals that inclusively contains Ag 5s components. The LUMO orbital, specifically 2a′, has an energy of −5.6 eV and is predominantly composed of 91% Ag 5s and 1% Ag 4d, with the remaining contribution coming from O 2p orbitals. Notably, the energy gap between the LUMO and HOMO is 4.2 eV, which is twice as large as the HOMO-LUMO gap observed in the U5Au+ cluster. This significant difference indicates that the U5Ag+ cluster exhibits considerably greater stability compared to its U5Au+ counterpart.
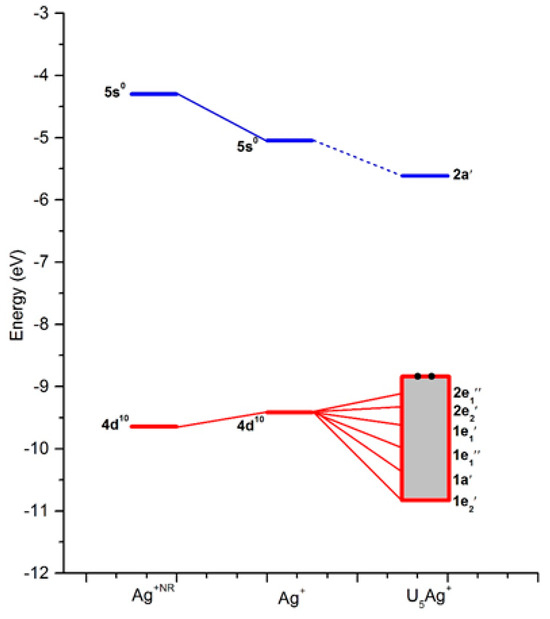
Figure 2.
The energy-level correlation diagrams of Ag+ and U5Ag+ cation. The half level between 5s0 and 4d10 of Ag has shifted to the half level between HOMO 3e2′ and LUMO 2a′ of U5Ag+ cation.
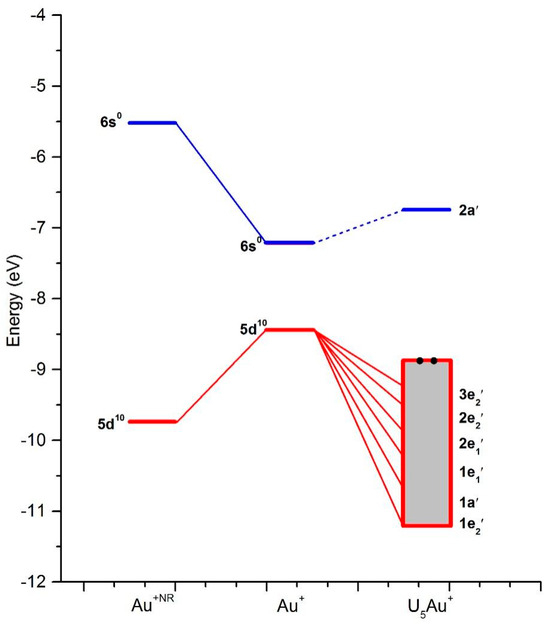
Figure 3.
The energy-level correlation diagrams of Au and U5Au+ cation. The half level between 6s1 and 5d10 of Au has shifted to the half level between HOMO 4e2′ and LUMO 2a′ of U5Au+ cation.
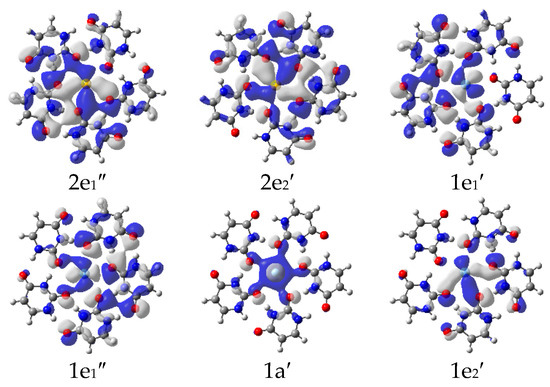
Figure 4.
Plot of occupied molecular orbitals of U5Ag+ cations (isovalve = 0.03) that are relevant for the Ag-O bonding.
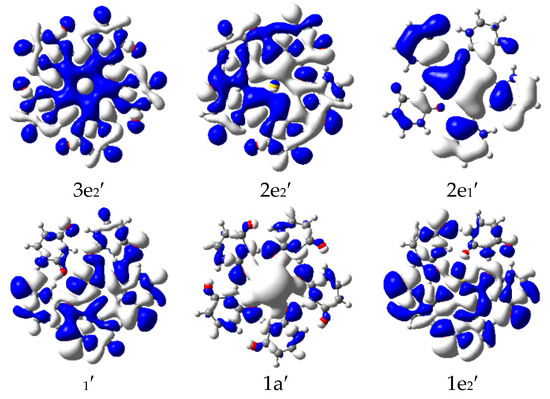
Figure 5.
Plot of occupied molecular orbitals of U5Au+ cations (isovalve = 0.03) that are relevant for the Au-O bonding.

Table 3.
Mixing character, orbital energy (in eV), electronic occupation number, and AO contributions in % of the Ag-O mixed-valence MOs of U5Ag+ cation (see Figure 2).

Table 4.
Mixing character, orbital energy (in eV), electronic occupation number, and AO contributions in % of the Au-O mixed-valence MOs of U5Au+ cation (see Figure 3).
Table 5 provides a detailed presentation of the energy decomposition analysis results specifically for the reactions M+ + U5 → U5M+ and M+ + T5 → T5M+, thoroughly calculated using the BP86/TZ2P method. The total quantum effect ∆EQuant represents a comprehensive measure of the interactions within a molecular system. It is derived by meticulously summing the Pauli repulsion term (∆EPauli), which accounts for the repulsive forces between electrons due to the Pauli exclusion principle, and the orbital interaction term (∆EOrb), which reflects the stabilizing interactions arising from the overlap of molecular orbitals. The proportion of orbital interaction energy (∆EOrb) is consistently and strikingly greater than that of electrostatic interaction energy (∆Eelstat). Specifically, within the U5Ag+ complex, the contribution of ∆EOrb reaches an exceptionally high 74.3%. This observation strongly suggests that the interaction between the metal atoms (whether Au or Ag) and the nucleobase is not primarily governed by electrostatic forces, as previously assumed [48], but is instead predominantly characterized by covalent interactions. Furthermore, the overall order of instantaneous interaction energy (∆Eint) follows the sequence U5Ag+ > T5Ag+ > U5Au+ > T5Au+. This ranking aligns precisely with the order of orbital interaction energy (∆EOrb) as well as the total quantum effect (∆EQuant). Consequently, this finding clearly demonstrates that, within this particular series of clusters, U5Ag+ exhibits the highest degree of stability, whereas T5Au+ is the least stable among them.

Table 5.
Energy decomposition analyses for M+ + U5 → U5M+ or M+ + T5 → T5M+ from BP86/TZ2P calculations in eV.
4. Conclusions
The geometric and electronic structures of the U5M+ and T5M+ cluster cations (where M denotes either Ag or Au) have been meticulously investigated using both the DFT/PBE and DFT/B3LYP methods. These stable structures are distinguished by the presence of self-complementary N-H···O hydrogen bonds and five notable Au-O or Ag-O interactions. Each nucleobase forms two self-complementary N-H···O hydrogen bonds with adjacent nucleobases, which significantly enhances the overall stability of the complexes. Specifically, the Au-O bond distances are approximately 2.730 ± 0.04 Å, which are slightly shorter compared to the Ag-O bond distances, which measure around 2.734 ± 0.03 Å. Using the BP86/TZ2P computational method, it was clearly determined that the Ag-O bond has a Mayer bond order of 0.13, whereas the Au-O bond shows a slightly higher bond order of 0.16. The metal ion–oxygen distances observed within the U5M+ cations are marginally and consistently longer when compared to those within the T5M+ cations. Importantly, in the U5M+ and T5M+ complexes, where M stands for either Ag or Au, it has been comprehensively noted that neither Ag nor Au actively contributes to the bonding of the HOMO. Furthermore, the energy gap between the LUMO and HOMO in the U5Ag+ cluster has been meticulously calculated to be approximately 4.2 eV, which is, remarkably, double the size of the HOMO-LUMO gap identified in the U5Au+ cluster. This significant difference strongly implies that the U5Ag+ cluster demonstrates a considerably higher degree of stability compared to its U5Au+ counterpart. This inference is further substantiated by energy decomposition analyses, which clearly show that the total interaction energy of U5Ag+ is appreciably greater than that of U5Au+. The presence of the methyl group results in a narrowing of the HOMO-LUMO gap. The interaction between the metal atoms, whether they are gold (Au) or silver (Ag), and the nucleobase is not predominantly dictated by electrostatic forces, as was earlier presumed. Instead, this interaction is primarily characterized by substantial covalent bonding effects. This shift in understanding highlights that the strength and nature of these interactions are significantly influenced by covalent interactions rather than by electrostatic forces alone. These findings shed important light on the energetic contributions and interactions that influence the formation of U5M+ and T5M+ clusters. A similar trend is consistently observed in the T5M+ complexes as well.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst14100865/s1.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further enquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Davis, J.T. G-quartets 40 years later: From 5′-GMP to molecular biology and supramolecular chemistry. Angew. Chem. Int. Ed. 2004, 43, 668–698. [Google Scholar] [CrossRef] [PubMed]
- Gellert, M.; Lipsett, M.N.; Davies, D.R. Helix formation by guanylic acid. Proc. Natl. Acad. Sci. USA 1962, 48, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Gilbert, W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature 1990, 344, 410–414. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, D.; van Dongen, J.L.; Lutz, M.; Spek, A.L.; Schenning, A.P.; Meijer, E. G-quadruplex self-assembly regulated by Coulombic interactions. Nat. Chem. 2009, 1, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Sigel, H.; Sigel, R.K. Interplay between Metal Ions and Nucleic Acids. Metal Ions in Life Sciences; Springer: Berlin/Heidelberg, Germany, 2012; Volume 10, pp. 119–134. [Google Scholar]
- Marlow, A.L.; Davis, J.T. Self-assembled ionophores as phase transfer catalysts. Tetrahedron Lett. 1999, 40, 3539–3542. [Google Scholar] [CrossRef]
- Chaput, J.C.; Switzer, C. A DNA pentaplex incorporating nucleobase quintets. Proc. Natl. Acad. Sci. USA 1999, 96, 10614–10619. [Google Scholar] [CrossRef]
- Gu, J.; Leszczynski, J. Isoguanine complexes: Quintet versus tetrad. J. Phys. Chem. B 2003, 107, 6609–6613. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Leszczynski, J. Iso-guanine quintet complexes coordinated by mono valent cations (Na+, K+, Rb+, and Cs+). J. Comput. Chem. 2007, 28, 1790–1795. [Google Scholar] [CrossRef]
- Meyer, M.; Sühnel, J. Self-association of isoguanine nucleobases and molecular recognition of alkaline ions: Tetrad vs pentad structures. J. Phys. Chem. A 2003, 107, 1025–1031. [Google Scholar] [CrossRef]
- Qiu, B.; Liu, J.; Qin, Z.; Wang, G.; Luo, H. Quintets of uracil and thymine: A novel structure of nucleobase self-assembly studied by electrospray ionization mass spectrometry. Chem. Commun. 2009, 2009, 2863–2865. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Li, D.; Xu, W. Real-Space Evidence of Trimeric, Tetrameric, and Pentameric Uracil Clusters Induced by Alkali Metals. J. Phys. Chem. C 2020, 124, 5257–5262. [Google Scholar] [CrossRef]
- Rajabi, K.; Gillis, E.A.L.; Fridgen, T.D. Structures of Alkali Metal Ion-Adenine Complexes and Hydrated Complexes by IRMPD Spectroscopy and Electronic Structure Calculations. J. Phys. Chem. A 2010, 114, 3449–3456. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.; Toscano, M.; Grand, A. Bond Energies and Attachments Sites of Sodium and Potassium Cations to DNA and RNA Nucleic Acid Bases in the Gas Phase. J. Am. Chem. Soc. 2001, 123, 10272–10279. [Google Scholar] [CrossRef] [PubMed]
- Krasnokutski, S.A.; Lee, J.S.; Yang, D.-S. High-resolution electron spectroscopy and structures of lithium-nucleobase (adenine, uracil, and thymine) complexes. J. Chem. Phys. 2010, 132, 044304–044308. [Google Scholar] [CrossRef] [PubMed]
- Valdespino-Saenz, J.; Martinez, A. Theoretical Study of Neutral, Anionic, and Cationic Uracil−Ag and Uracil−Au Systems: Nonconventional Hydrogen Bonds. J. Phys. Chem. A 2008, 112, 2408–2414. [Google Scholar] [CrossRef]
- Vazquez, M.-V.; Martinez, A. Theoretical Study of Cytosine−Al, Cytosine−Cu and Cytosine−Ag (Neutral, Anionic and Cationic). J. Phys. Chem. A 2008, 112, 1033–1039. [Google Scholar] [CrossRef]
- Martinez, A. Theoretical study of guanine—Cu and uracil—Cu (neutral, anionic, and cationic). Is it possible to carry out a photoelectron spectroscopy experiment? J. Chem. Phys. 2005, 123, 024311–024319. [Google Scholar] [CrossRef]
- Soto-Verdugo, V.; Metiu, H.; Gwinn, E. The properties of small Ag clusters bound to DNA bases. J. Chem. Phys. 2010, 132, 195102–195110. [Google Scholar] [CrossRef]
- Kryachko, E.S.; Remacle, F. Complexes of DNA Bases and Gold Clusters Au3 and Au4 Involving Nonconventional N−H⋯Au Hydrogen Bonding. Nano Lett. 2005, 5, 735–739. [Google Scholar] [CrossRef]
- Martinez, A. Do Anionic Gold Clusters Modify Conventional Hydrogen Bonds? The Interaction of Anionic Aun (n = 2 − 4) with the Adenine-Uracil Base Pair. J. Phys. Chem. A 2009, 113, 1134–1140. [Google Scholar] [CrossRef]
- Rincon, E.; Yanez, M.; Toro-Labbe, A.; Mo, O. Effect of Ni (II), Cu (II) and Zn (II) association on the keto-enol tautomerism of thymine in the gas phasewz. Phys. Chem. Chem. Phys. 2007, 9, 2531–2537. [Google Scholar] [CrossRef]
- Šponer, J.; Sabat, M.; Burda, J.V.; Leszczynski, J.; Hobza, P.; Lippert, B. Metal ions in non-complementary DNA base pairs: An ab initio study of Cu(I), Ag(I), and Au(I) complexes with the cytosine-adenine base pair. J. Biol. Inorg. Chem. 1999, 4, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Šponer, J.; Šponer, J.E.; Gorb, L.; Leszczynski, J.; Lippert, B. Metal-Stabilized Rare Tautomers and Mispairs of DNA Bases: N6-Metalated Adenine and N4-Metalated Cytosine, Theoretical and Experimental Views. J. Phys. Chem. A 1999, 103, 11406–11413. [Google Scholar] [CrossRef]
- Rodgers, M.T.; Armentrout, P.B. Influence of d Orbital Occupation on the Binding of Metal Ions to Adenine. J. Am. Chem. Soc. 2002, 124, 2678–2691. [Google Scholar] [CrossRef] [PubMed]
- Boychuk, B.T.A.; Wetmore, S.D. Assessment of Density Functional Theory Methods for the Structural Prediction of Transition and Post-Transition Metal–Nucleic Acid Complexes. J. Chem. Theory Comput. 2023, 19, 5273–5288. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-J.; Xu, H.-G.; Zheng, W.-J.; Li, J. Theoretical and experimental studies of the interactions between Au2− and nucleobases. Phys. Chem. Chem. Phys. 2014, 16, 2928–2935. [Google Scholar] [CrossRef]
- Cao, G.-J.; Xu, H.-G.; Li, R.-Z.; Zheng, W.-J. Hydrogen bonds in the nucleobase-gold complexes: Photoelectron spectroscopy and density functional calculations. J. Chem. Phys. 2012, 136, 014305. [Google Scholar] [CrossRef]
- Cao, G.-J.; Xu, H.-G.; Xu, X.-L.; Wang, P.; Zheng, W.-J. Photodissociation and density functional calculations of A2M+ and G2M+ (A = adenine, G = guanine, M = Cu, Ag, and Au) cluster ions. Int. J. Mass Spectrom. 2016, 407, 118–125. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.G.A.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Figgen, D.; Rauhut, G.; Dolg, M.; Stoll, H. Energy-consistent pseudopotentials for group 11 and 12 atoms: Adjustment to multi-configuration Dirac–Hartree–Fock data. Chem. Phys. 2005, 311, 227–244. [Google Scholar] [CrossRef]
- Peterson, K.A.; Puzzarini, C. Systematically convergent basis sets for transition metals. II. Pseudopotential-based correlation consistent basis sets for the group 11 (Cu, Ag, Au) and 12 (Zn, Cd, Hg) elements. Theor. Chem. Acc. 2005, 114, 283–296. [Google Scholar] [CrossRef]
- Cao, G.-J.; Zheng, W.-J. Structures, Stabilities, and Physicochemical Properties of Nucleobase Tautomers. Acta Phys.-Chim. Sin. 2013, 29, 2135–2147. [Google Scholar]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Guerra, C.F.; Snijders, J.; Te Velde, G.; Baerends, E. Towards an order-N DFT method. Theor. Chem. Acc. 1998, 99, 391–403. [Google Scholar]
- Baerends, E.J.; Ziegler, T.; Autschbach, J.; Bashford, D.; Bérces, A.; Bickelhaupt, F.M.; Bo, C.; Boerrigter, P.M.; Cavallo, L.; Chong, D.P.; et al. ADF, SCM, Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2013; Available online: http://www.scm.com (accessed on 1 January 2024).
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Cao, G.-J. A dinuclear Cu(i)-mediated complex: Theoretical studies of the G2Cu24+ cluster ion. J. Chem. Phys. 2018, 149, 144308. [Google Scholar] [CrossRef]
- Nir, E.; Plützer, C.; Kleinermanns, K.; de Vries, M. Properties of isolated DNA bases, base pairs and nucleosides examined by laser spectroscopy. Eur. Phys. J. D 2002, 20, 317–329. [Google Scholar] [CrossRef]
- Hopffgarten, M.V.; Frenking, G. Energy decomposition analysis. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 43–62. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-J.; Schwarz, W.H.E.; Li, J. An 18-Electron System Containing a Superheavy Element: Theoretical Studies of Sg@Au12. Inorg. Chem. 2015, 54, 3695–3701. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I. Charge, bond order and valence in the AB initio SCF theory. Chem. Phys. Lett. 1983, 97, 270–274. [Google Scholar] [CrossRef]
- Cao, G.-J.; Hou, H.-L. Dinuclear Metal-Mediated Guanine–Uracil Base Pairs: Theoretical Studies of GUM22+(M = Cu, Ag, and Au) Ions. J. Clust. Sci. 2019, 30, 439–448. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).