Density Functional Theory Study of Lanthanide Monoxides under High Pressure: Pressure-Induced B1–B2 Transition
Abstract
1. Introduction
2. Materials and Methods
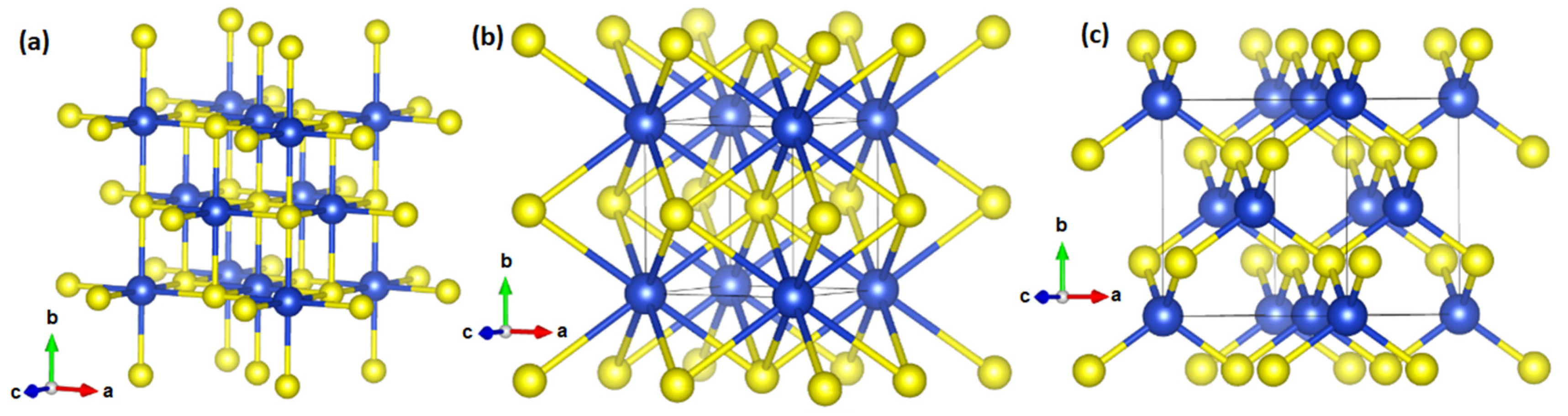
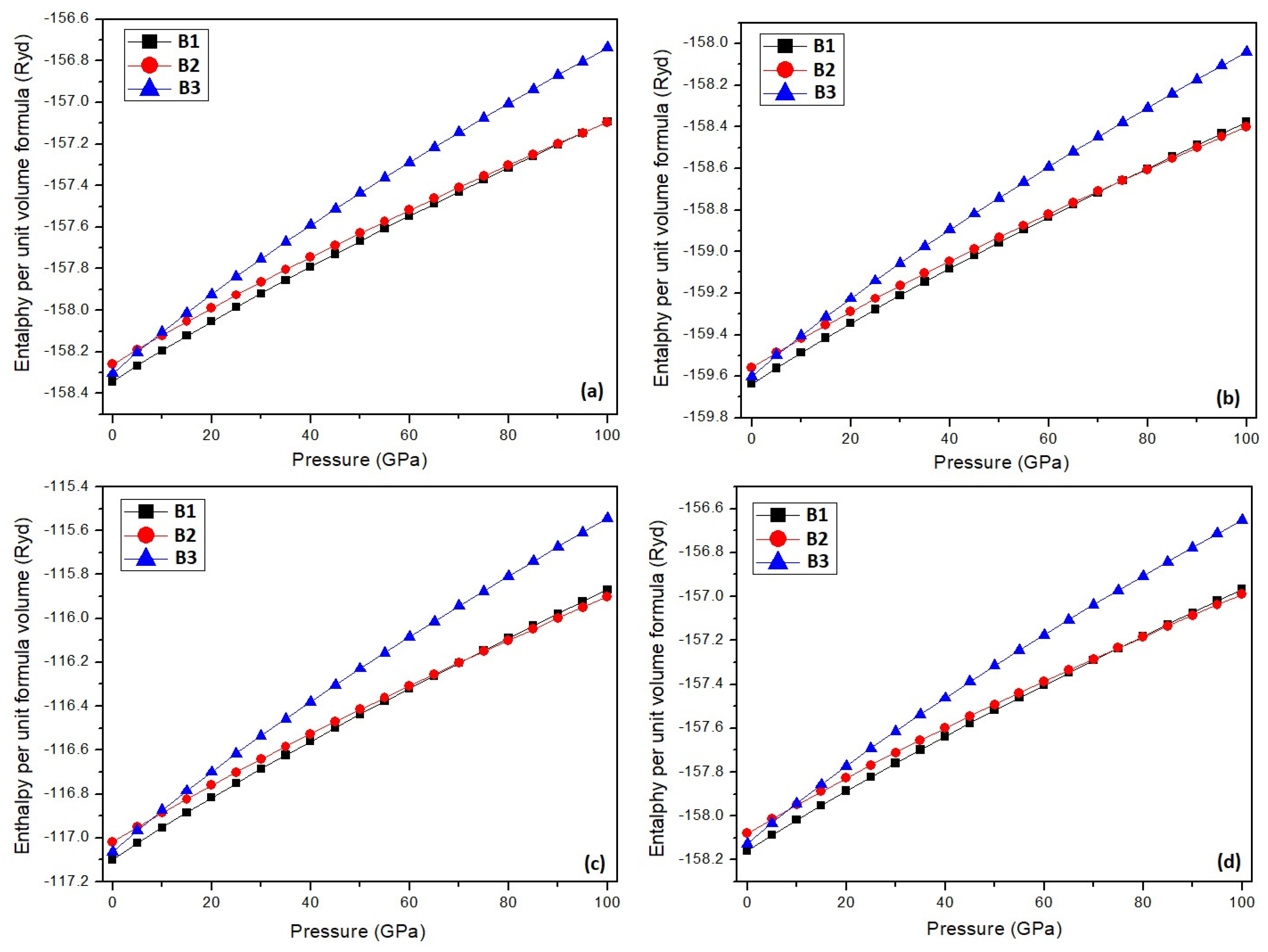
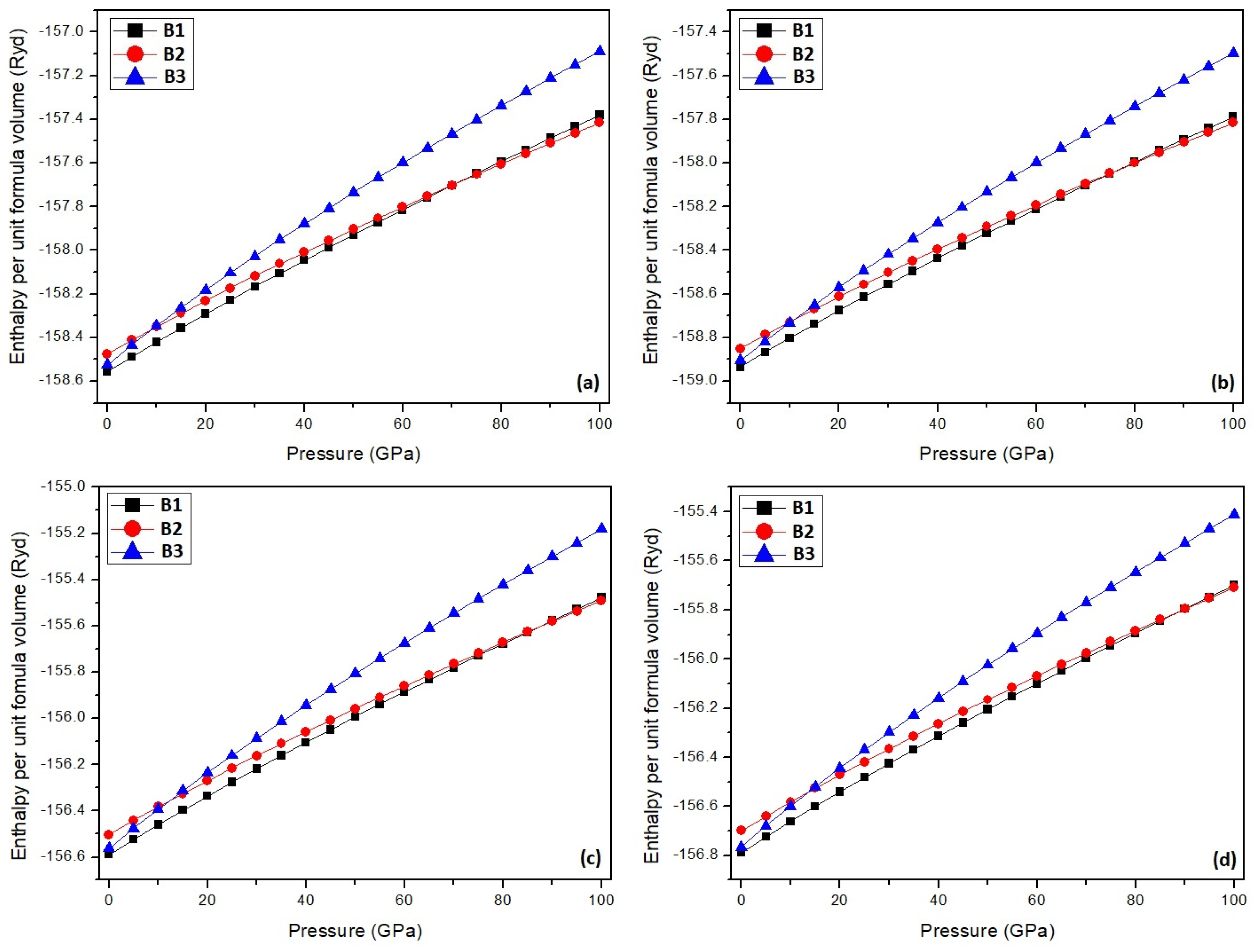
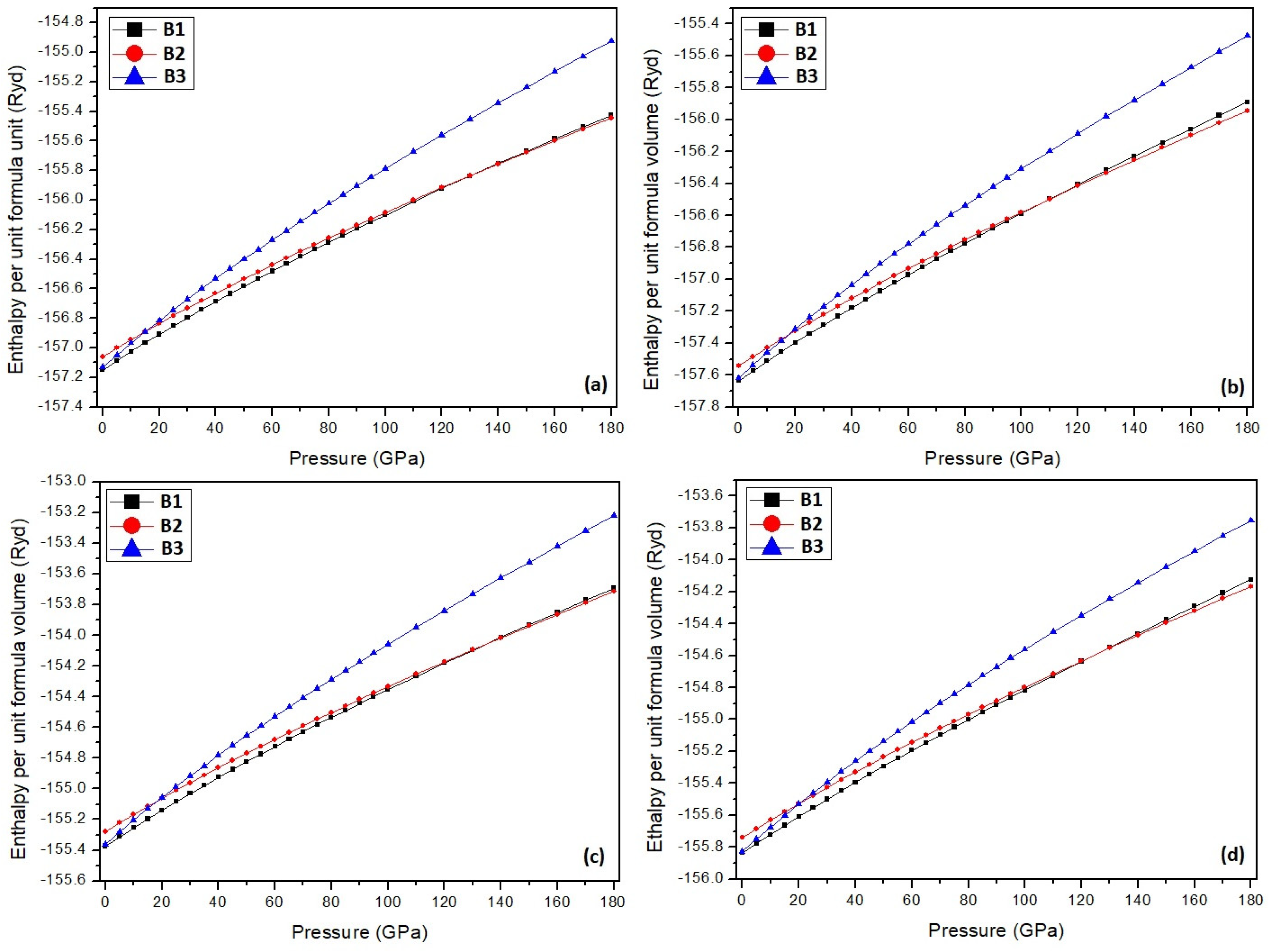
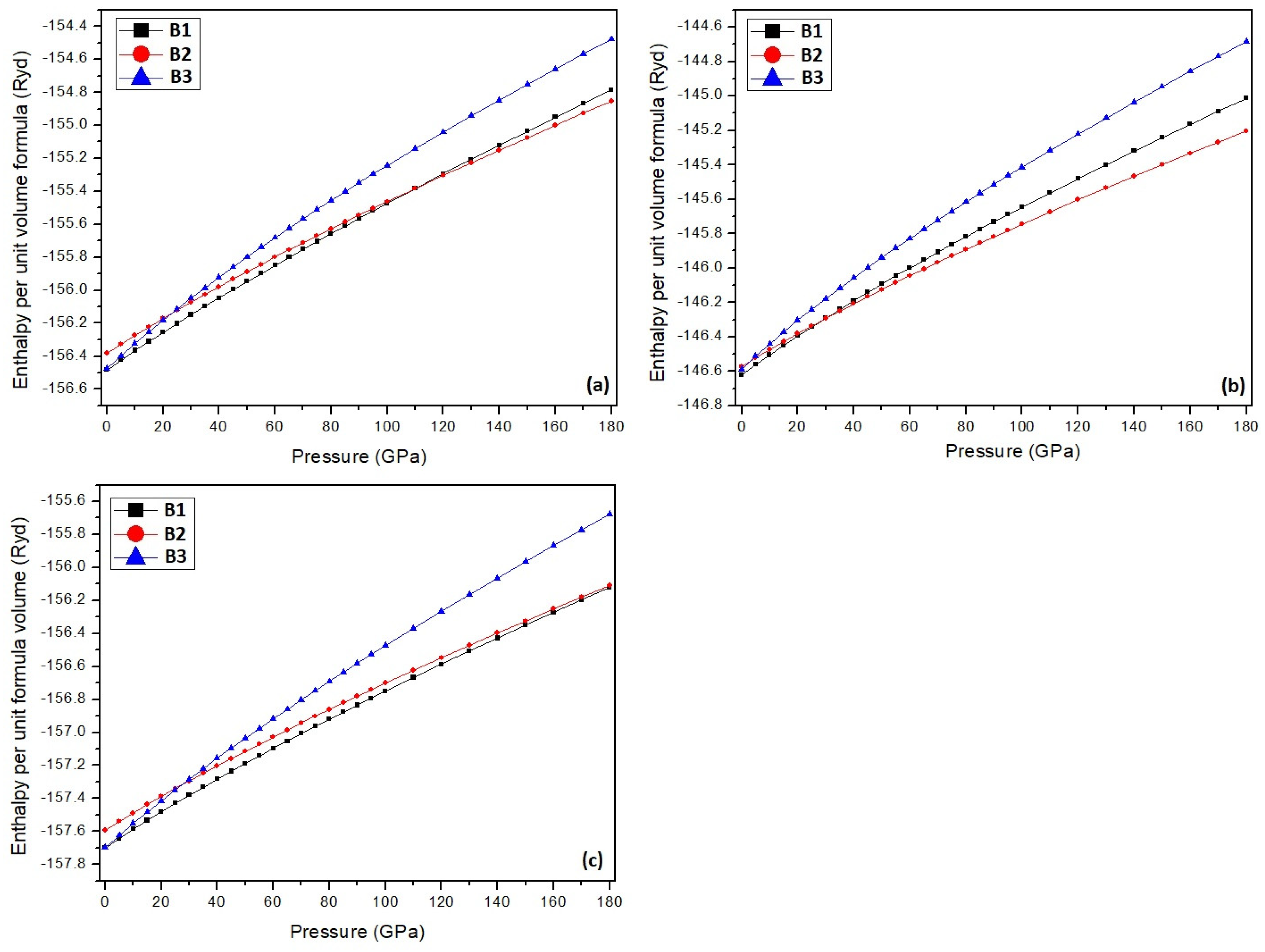
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauh, E.G.; Ackermann, R.J. The first ionization potentials of neptunium and neptunium monoxide. J. Chem. Phys. 1975, 62, 1584. [Google Scholar] [CrossRef]
- Murad, E.; Hildenbrand, D.L. Dissociation energies of GdO, HoO, ErO, TmO, and LuO; correlation of results for the lanthanide monoxide series. J. Chem. Phys. 1980, 73, 4005–4011. [Google Scholar] [CrossRef]
- Ellinger, F.H.; Zachariasen, W.H. The Crystal Structure of Samarium Metal and of Samarium Monoxide. J. Amer. Chem. Soc. 1956, 76, 5650–5653. [Google Scholar]
- Gan, H.; Zhang, C.; Du, X.Z.; Jiang, P.; Niu, C.P.; Zheng, X.H.; Yin, Y.W.; Li, X.G. Insights into superconductivity of LaO from experiments and first-principles calculations. Phys. Rev. B 2021, 104, 054515. [Google Scholar] [CrossRef]
- Shafiq, M.; Arif, S.; Ahmad, I.; Asadabadi, S.J.; Maqbool, M.; Aliabad, H.A.R. Elastic and mechanical properties of lanthanide monoxides. J. Alloys Compd. 2015, 618, 292–298. [Google Scholar] [CrossRef]
- Kimura, R.; Kaminaga, K.; Kobayashi, T.; Cho, Y.; Maruyama, S.; Maeda, A.; Matsumoto, Y. Elevating the Superconducting Temperature in Epitaxially Stabilized Rock-Salt NbO. Chem. Mater. 2024, 36, 5028–5036. [Google Scholar] [CrossRef]
- Han, Y.; Brugman, B.L.; Leinbach, L.J.; Guo, X.; Leinenweber, K.; Navrotsky, A. Thermochemical properties of high-pressure neodymium monoxide. Inorg. Chem. 2024, 63, 13468–13473. [Google Scholar] [CrossRef]
- Brugman, B.L.; Han, Y.; Leinbach, L.J.; Leinenweber, K.; van de Walle, A.; Ushakov, S.V.; Hong, Q.J.; Navrotsky, A. Computationally led high-pressure synthesis and experimental thermodynamics of Rock Salt yttrium monoxide. Chem. Mater. 2024, 36, 332–339. [Google Scholar] [CrossRef]
- Sano, Y.; Kaminaga, K.; Maruyama, S.; Matsumoto, Y. Ferromagnetic semiconductor EuO thin films characterized by vacuum electrochemical process with ionic liquid. Mater. Sci. Semicond. Process. 2024, 181, 108629. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, H.; Karaca, E.; Zhou, J.; Kawaguchi, S.; Kadobayashi, H.; Yu, X.; Errandonea, D.; Yue, B.; Hong, F. Pressure-Sensitive Multiple Superconducting Phases and Their Structural Origin in Van der Waals HfS2 up to 160 GPa. Phys. Rev. Lett. 2024, 133, 066001. [Google Scholar] [CrossRef]
- Sun, P.H.; Zhang, J.F.; Liu, K.; Han, Q.; Lu, Z.Y. First-principles study of the superconductivity in LaO. Phys. Rev. B 2021, 104, 045121. [Google Scholar] [CrossRef]
- Qian, J.; Shen, Z.; Wei, X.; Li, W. ℤ2 nontrivial topology of rare-earth binary oxide superconductor LaO. Phys. Rev. B 2022, 105, L020508. [Google Scholar] [CrossRef]
- Uchida, Y.; Kaminaga, K.; Fukumura, T.; Hasegawa, T. Samarium monoxide epitaxial thin film as a possible heavy-fermion compound. Phys. Rev. B 2017, 95, 125111. [Google Scholar] [CrossRef]
- Kaminaga, K.; Oka, D.; Hasegawa, T.; Fukumura, T. Superconductivity of rock-salt structure LaO epitaxial thin film. J. Am. Chem. Soc. 2018, 140, 6754–6757. [Google Scholar] [CrossRef]
- Jurkutat, M.; Kattinger, C.; Tsankov, S.; Haase, J. How pressure enhances the critical temperature of superconductivity in YBa2Cu3O6+y. Proc. Natl. Acad. Sci. USA 2023, 120, e22154581. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.X.; Xie, H.; Cong, R.; Tang, Z.C.; Zhou, M.F. Electron Affinities of the Early Lanthanide Monoxide Molecules. Chin. J. Chem. Phys. 2011, 24, 604–610. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Hong, Q.-J.; Gilbert, D.A.; Navrotsky, A.; Walle, A.V.D. Thorium and Rare Earth Monoxides and Related Phases. Materials 2023, 16, 1350. [Google Scholar] [CrossRef]
- Harilal, S.S.; Kautz, E.J.; Bernacki, B.E.; Phillips, M.C.; Skrodzki, P.J.; Burger, M.; Jovanovic, I. Physical conditions for UO formation in laser-produced uranium plumes. Phys. Chem. Chem. Phys. 2019, 21, 16161–16169. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Sahni, V.; Bohnen, K.P.; Harbola, M.K. Analysis of the local-density approximation of density-functional theory. Phys. Rev. A 1988, 37, 1895–1907. [Google Scholar] [CrossRef] [PubMed]
- Attfiled, J.P.; Ferey, G. Structural correlations within the lanthanum palladium oxide family. J. Sol. State Chem. 1989, 80, 286–298. [Google Scholar] [CrossRef]
- Garg, A.B.; Muñoz, A.; Anzellini, S.; Sanchez-Martín, J.; Turnbull, R.; Díaz-Anichtchenko, D.; Popescu, C.; Errandonea, D. Role of GdO addition in the structural stability of cubic Gd2O3 at high pressures: Determination of the equation of states of GdO and Gd2O3. Materialia 2024, 34, 102064. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Prandini, G.; Marrazzo, A.; Castelli, I.E.; Mounet, N.; Marzari, N. Precision and efficiency in solid-state pseudopotential calculations. npj Comput. Mater. 2018, 4, 72. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Recio, J.M.; Flórez, M.; Francisco, E.; Blanco, M.A.; Pendás, A.M. Microscopic Study of the Rock Salt-Caesium Chloride phase stability in alkali halides. High Press. Res. 2002, 22, 443–446. [Google Scholar] [CrossRef]
- Errandonea, D.; Gomis, O.; Santamaría-Perez, D.; García-Domene, B.; Muñoz, A.; Rodríguez-Hernández, P.; Achary, S.N.; Tyagi, A.K.; Popescu, C. Exploring the high-pressure behavior of the three known polymorphs of BiPO4: Discovery of a new polymorph. J. Appl. Phys. 2015, 117, 105902. [Google Scholar] [CrossRef]
- Ouahrani, T.; Muñoz, A.; Franco, R.; Boufatah, R.M.; Bedrane, Z.; Errandonea, D. High-pressure properties of thallium orthovanadate from density-functional theory calculations. J. Alloys Compd. 2024, 978, 73483. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Leger, J.M.; Yacoubi, N.; Loriers, J. Synthesis of rare earth monoxides. J. Sol. State Chem. 1981, 36, 261–270. [Google Scholar] [CrossRef]
- Griesemer, S.D.; Ward, L.; Wolverton, C. High-throughput crystal structure solution using prototypes. Phys. Rev. Mater. 2021, 5, 105003. [Google Scholar] [CrossRef]
- Krill, G.; Ravet, M.F.; Kappler, J.P.; Abadli, L.; Leger, J.M.; Yacoubi, N.; Loriers, C. Magnetic properties of some rare earth monoxides LnO (Ln = Pr, Nd, Sm) mixed valence state of SmO. Sol. State Commun. 1980, 33, 351–353. [Google Scholar] [CrossRef]
- Kobayashi, S.; Martín-Cid, A.; Toyoki, K.; Okazaki, H.; Hirosawa, S.; Nakamura, T. Influence of magnetostriction on the lattice constants of the secondary phases in Nd-Fe-B sintered magnets studied by synchrotron X-ray diffraction. AIP Adv. 2019, 9, 25154. [Google Scholar] [CrossRef]
- Eick, H.A.; Baezinger, N.C.; Eyring, L. Lower oxides of samarium and europium. The preparation and crystal structure of SmO0.4-0.6, SmO and EuO. J. Am. Chem. Soc. 1956, 78, 5147–5149. [Google Scholar] [CrossRef]
- Leger, J.M.; Aimonino, P.; Loriers, J.; Dordor, P.; Coqblin, B. Transport properties of SmO. Phys. Lett. A 1980, 80, 325–327. [Google Scholar] [CrossRef]
- Matthias, B.T.; Bozorth, R.M.; Van Vleck, J.H. Ferromagnetic Interaction in EuO. Phys. Rev. Lett. 1961, 7, 160–161. [Google Scholar] [CrossRef]
- Namba, M.; Takatsu, H.; Mikita, R.; Sijia, Y.; Murayama, K.; Li, H.-B.; Terada, R.; Tassel, C.; Ubukata, H.; Ochi, M.; et al. Large perpendicular magnetic anisotropy induced by an intersite charge transfer in strained EuVO2H films. J. Am. Chem. Soc. 2023, 145, 21807–21816. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kaminaga, K.; Saito, D.; Oka, D.; Fukumura, T. Rock salt structure GdO epitaxial thin film with a high ferromagnetic Curie temperature. Appl. Phys. Lett. 2020, 117, 052402. [Google Scholar] [CrossRef]
- Sasaki, S.; Oka, D.; Kaminaga, K.; Saito, D.; Yamamoto, T.; Abe, N.; Shimizu, H.; Fukumura, T. A high-Tc heavy rare earth monoxide semiconductor TbO with a more than half-filled 4f orbital. Dalton Trans. 2022, 51, 16648–16652. [Google Scholar] [CrossRef]
- Amrillah, T.; Oka, D.; Shimizu, H.; Sasaki, S.; Saito, D.; Kaminaga, K.; Fukumura, T. Rock salt-type HoO epitaxial thin film as a heavy rare-earth monoxide ferromagnetic semiconductor with a Curie temperature above 130 K. Appl. Phys. Lett. 2022, 120, 082403. [Google Scholar] [CrossRef]
- Leger, J.M.; Maugrion, J.; Albert, L.; Achard, J.C.; Lories, C. High pressure synthesis of ytterbium oxides (YbO and Yb3O4). C. R. Acad. Sci. Ser. C Sci. Chim. 1978, 286, 201. [Google Scholar]
- Fishel, N.A.; Haschke, J.M.; Eick, H.A. Preparation of ytterbium and europium oxides in liquid ammonia. Inor. Chem. 1970, 9, 413–414. [Google Scholar] [CrossRef]
- Leger, J.M.; Yacoubi, N.; Loriers, J.; McCarthy, G.J.; Rhyne, J.J.; Silber, H.B. (Eds.) The Rare Earths in Modern Science and Technology; Plenum Press: New York, NY, USA; London, UK, 1979; Volume 2, p. 203. [Google Scholar]
- Zhang, G.X.; Reilly, A.M.; Tkatchenko, A.; Scheffler, M. Performance of various density-functional approximations for cohesive properties of 64 bulk solids. New J. Phys. 2018, 20, 063020. [Google Scholar] [CrossRef]
- Söderlind, P.; Turchi, P.E.A.; Landa, A.; Lordi, V. Ground-state properties of rare-earth metals: An evaluation of density-functional theory. J. Phys. Cond. Matter 2014, 26, 416001. [Google Scholar] [CrossRef] [PubMed]
- Gschneidner, K.A., Jr. Systematics in lanthanide and actinide solids. J. Alloys Compd. 1995, 223, 165–169. [Google Scholar] [CrossRef]
- Horne, M.; Strange, P.; Temmerman, W.M.; Szotek, Z.; Svane, A.; Winter, H. The electronic structure of europium chalcogenides and pnictides. J. Phys. Cond. Matter 2004, 16, 5061. [Google Scholar] [CrossRef]
- Johansson, B. Nature of the electrons in the actinide series. Phys. Rev. B 1975, 11, 2740. [Google Scholar] [CrossRef]
- Kalpana, G.; Palanivel, B.; Rajagopalan, M. Electronic and structural properties of alkaline-earth oxides under high pressure. Phys. Rev. B 1995, 52, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mao, H.K.; Somayazulu, M.; Ding, Y.; Meng, Y.; Häusermann, D. B1-to-B2 phase transition of transition-metal monoxide under strong compression. Phys. Rev. B 2004, 70, 094114. [Google Scholar] [CrossRef]
- Sims, C.E.; Barrera, G.D.; Allan, N.L.; Mackrodt, W.C. Thermodynamics and mechanism of the B1-B2 phase transition in group-I halides and group-II oxides. Phys. Rev. B 1998, 57, 11164. [Google Scholar] [CrossRef]
- Richet, P.; Mao, H.K.; Bell, P.M. Static compression and equation of state of CaO to 1.35 Mbar. J. Geophys. Res. 1988, 93, 15279–15288. [Google Scholar] [CrossRef]
- Mao, H.K.; Bell, P.M. Equations of state of MgO and ε Fe under static pressure conditions. J. Geophys. Res. 1979, 84, 4533–4536. [Google Scholar] [CrossRef]
| Experiments | Average of Experimental Values | QE Calculations GGA This Work | QE Calculations LDA This Work | WIEN2k Calculations GGA, Ref. [5] | |
|---|---|---|---|---|---|
| LaO | 5.144 [32] | 5.156 (4) | 5.1643 | 5.0583 | 5.1102 |
| 5.125 [33] | |||||
| 5.198 [14] | |||||
| CeO | 5.089 [32] | 5.089 (1) | 5.1312 | 5.0244 | 4.9931 |
| 5.089 [32] | |||||
| PrO | 5.031 [32] | 5.0312 (3) | 5.0677 | 4.9600 | 4.9295 |
| 5.031 [32] | |||||
| 5.0316 [34] | |||||
| NdO | 4.994 [32] | 5.000 (8) | 5.0144 | 4.9051 | 4.9510 |
| 4.9960 [32] | |||||
| 5.0101 [35] | |||||
| SmO | 4.943 [32] | 4.97 (4) | 4.9256 | 4.8141 | 4.9592 |
| 4.9414 [34] | |||||
| 4.9414 [35] | |||||
| 5.015–5.050 [36] | |||||
| 4.9883 [14] | |||||
| 4.94 [37] | |||||
| EuO | 5.142 [36] | 5.1416 (6) | 4.8867 | 4.6103 | 4.9954 |
| 5.1439 [14] | |||||
| 5.141 [38] | |||||
| 5.1419 [39] | |||||
| GdO | 4.99 [40] | 4.99 | 4.8542 | 4.73634 | |
| TbO | 4.92 [41] | 4.92 | 4.8243 | 4.58015 | 4.8009 |
| HoO | 4.904 [42] | 4.904 | 4.7629 | 4.6367 | 4.7661 |
| YbO | 4.877 [43] | 4.876 (5) | 4.7205 | 4.5767 | 4.6566 |
| 4.87 [44] | |||||
| 4.88 [45] |
| Lanthanide Monoxide | a (Å) | B0 (GPa) | B0′ | B1–B2 Transition Pressure (GPa) | % of ΔV at the B1–B2 Transition | Bulk Modulus Ref. [5] (GPa) |
|---|---|---|---|---|---|---|
| LaO | 5.1643 | 125.1 | 4.51 | 96 | −0.082 | 102.663 |
| CeO | 5.1312 | 128.0 | 4.68 | 75 | −0.076 | 162.659 |
| PrO | 5.0677 | 133.6 | 5.17 | 71 | −0.078 | 146.852 |
| NdO | 5.0144 | 136.9 | 4.95 | 77 | −0.079 | 162.557 |
| PmO | 4.9673 | 140.5 | 6.06 | 71 | −0.078 | - |
| SmO | 4.9256 | 138.5 | 6.14 | 77 | −0.078 | 108.327 |
| EuO | 4.8867 | 139.1 | 5.5 | 86 | −0.077 | 94.287 |
| GdO | 4.8542 | 137.5 | 5.63 | 90 | −0.077 | - |
| TbO | 4.8243 | 142.4 | 3.02 | 133 | −0.077 | 129.013 |
| DyO | 4.7916 | 148.7 | 4.84 | 110 | −0.076 | - |
| HoO | 4.7629 | 151.6 | 3.38 | 135 | −0.075 | 125.880 |
| ErO | 4.7364 | 149.4 | 5.64 | 124 | −0.073 | 106.973 |
| TmO | 4.7070 | 146.6 | 6.35 | 111 | −0.072 | - |
| YbO | 4.7205 | 124.6 | 4.44 | 29 | −0.159 | 226.067 |
| LuO | 4.6566 | 131.1 | 3.01 | 209 | −0.069 | - |
| Lanthanide Monoxide | a B2 Structure (Å) | B0 B2 Structure (GPa) | B0′ B2 Structure | a B3 Structure (Å) | B0 B3 Structure (GPa) | B0′ B2 Structure |
|---|---|---|---|---|---|---|
| LaO | 3.1614 | 121.0 | 4.60 | 5.6698 | 81.6 | 3.95 |
| CeO | 3.1482 | 123.3 | 4.65 | 5.6300 | 86.2 | 4.24 |
| PrO | 3.1072 | 128.0 | 4.64 | 5.5624 | 89.5 | 4.13 |
| NdO | 3.0737 | 131.7 | 4.69 | 5.5053 | 93.0 | 4.20 |
| PmO | 3.0451 | 134.3 | 4.56 | 5.4553 | 95.7 | 4.09 |
| SmO | 3.0204 | 135.7 | 4.62 | 5.4103 | 98.4 | 4.08 |
| EuO | 2.9969 | 137.4 | 4.57 | 5.3667 | 101.3 | 4.14 |
| GdO | 2.9778 | 139.5 | 4.39 | 5.3290 | 103.7 | 4.02 |
| TbO | 2.9589 | 141.9 | 4.40 | 5.2920 | 106.4 | 4.43 |
| DyO | 2.9403 | 144.7 | 4.61 | 5.2565 | 108.4 | 4.13 |
| HoO | 2.9230 | 147.6 | 5.01 | 5.2229 | 109.5 | 4.60 |
| ErO | 2.9089 | 148.1 | 5.23 | 5.1948 | 110.5 | 4.08 |
| TmO | 2.8924 | 143.2 | 4.85 | 5.1623 | 111.8 | 3.59 |
| YbO | 2.8071 | 133.0 | 4.44 | 5.1267 | 85.9 | 4.10 |
| LuO | 2.8641 | 142.6 | 4.89 | 5.0996 | 120.1 | 4.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, S.; Errandonea, D. Density Functional Theory Study of Lanthanide Monoxides under High Pressure: Pressure-Induced B1–B2 Transition. Crystals 2024, 14, 831. https://doi.org/10.3390/cryst14100831
Ferrari S, Errandonea D. Density Functional Theory Study of Lanthanide Monoxides under High Pressure: Pressure-Induced B1–B2 Transition. Crystals. 2024; 14(10):831. https://doi.org/10.3390/cryst14100831
Chicago/Turabian StyleFerrari, Sergio, and Daniel Errandonea. 2024. "Density Functional Theory Study of Lanthanide Monoxides under High Pressure: Pressure-Induced B1–B2 Transition" Crystals 14, no. 10: 831. https://doi.org/10.3390/cryst14100831
APA StyleFerrari, S., & Errandonea, D. (2024). Density Functional Theory Study of Lanthanide Monoxides under High Pressure: Pressure-Induced B1–B2 Transition. Crystals, 14(10), 831. https://doi.org/10.3390/cryst14100831






