The Solid-Phase Transition of Carbapenem CS-023 Polymorphs and the Change in Helicity Observed in the Transition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Crystallization
2.3. Phase Transition on Instrumental Analysis
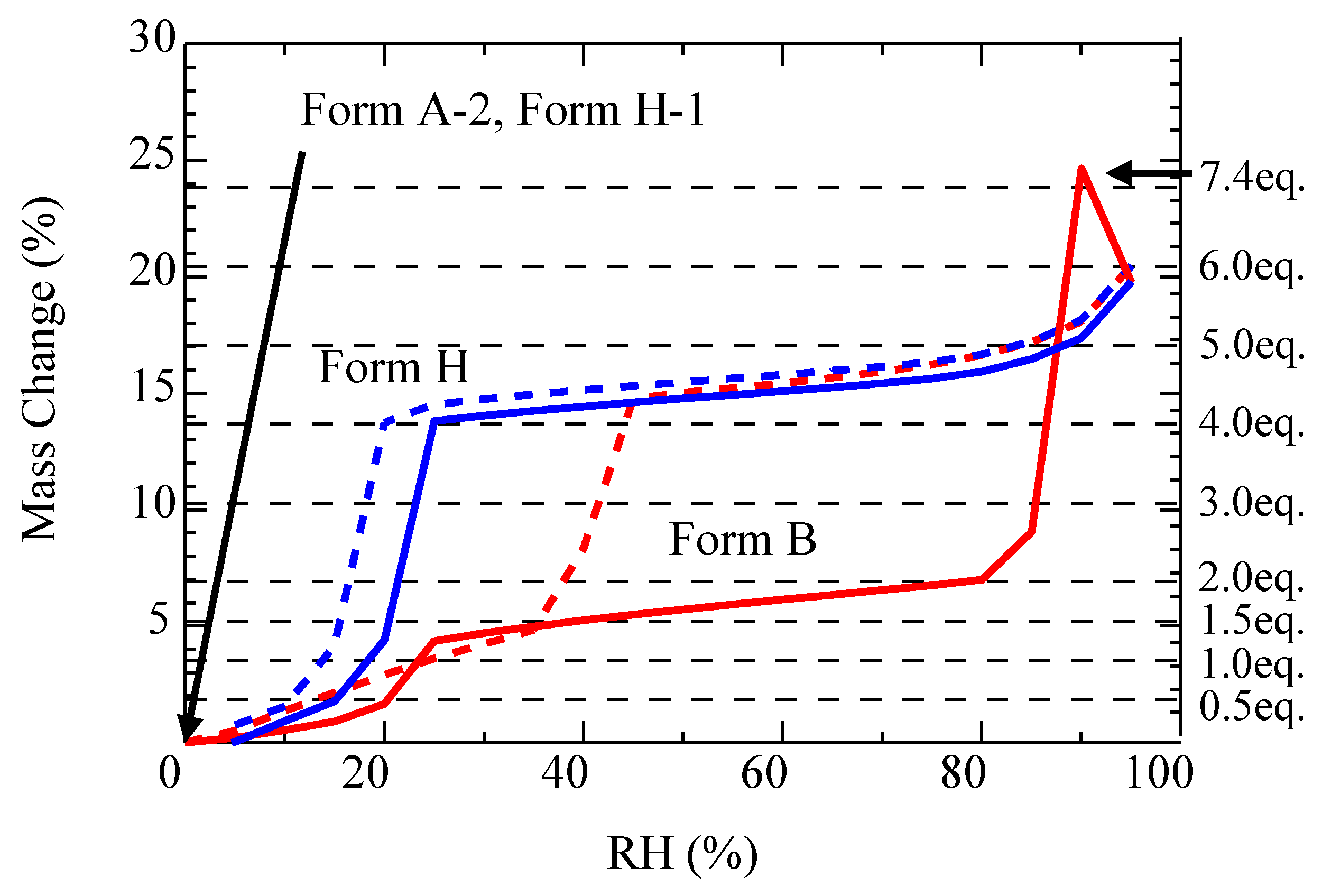
2.4. Phase Transition by Absorption and Desorption of Water and Ethanol
2.5. Determination of Crystal Structure with Single-Crystal X-ray Diffraction
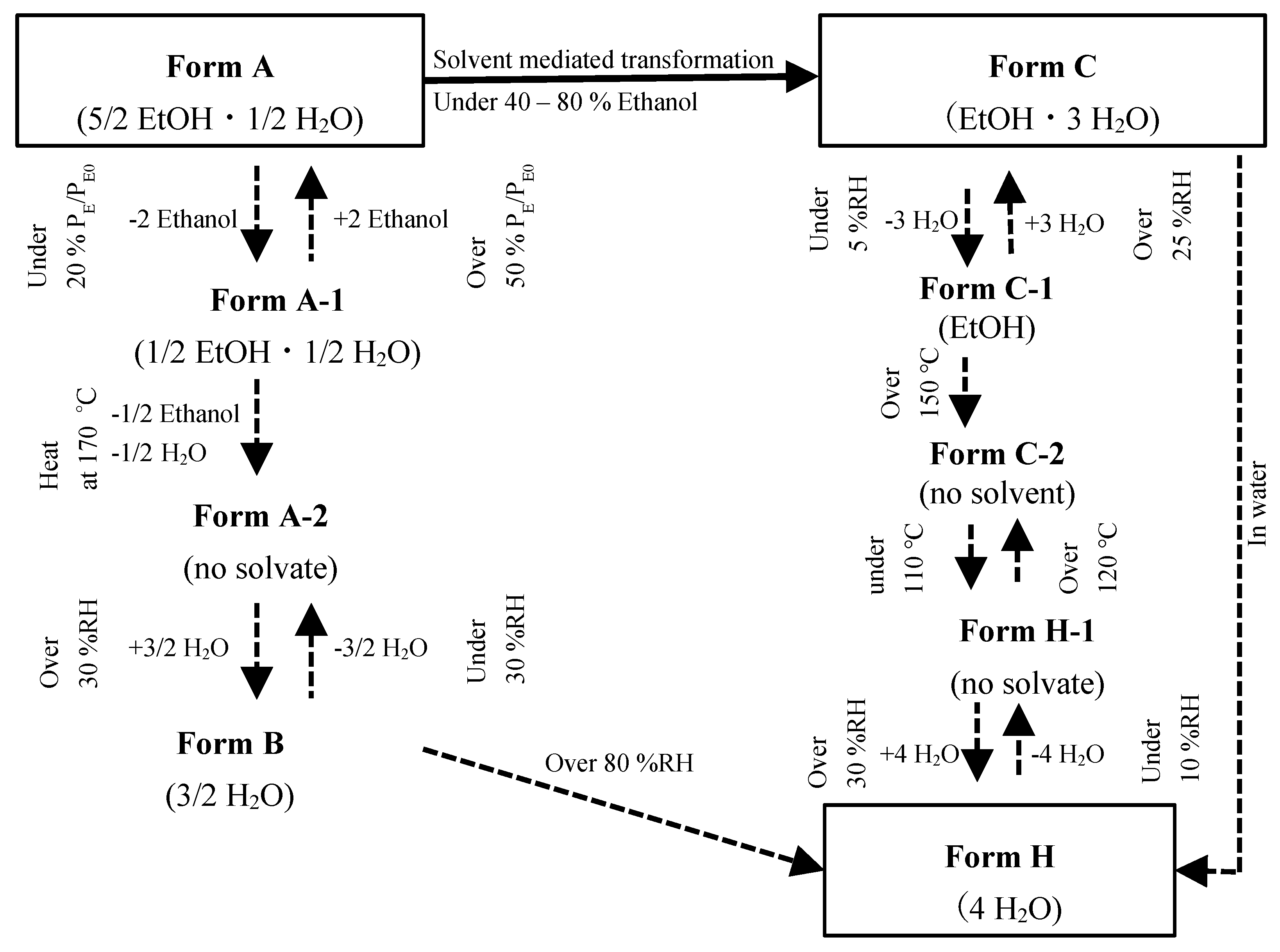
3. Results and Discussion
3.1. The Solid-Phase Transition of Form C to Form H
3.2. The Relationship of CS-023 Polymorphs, Form A, Form C, and Form H
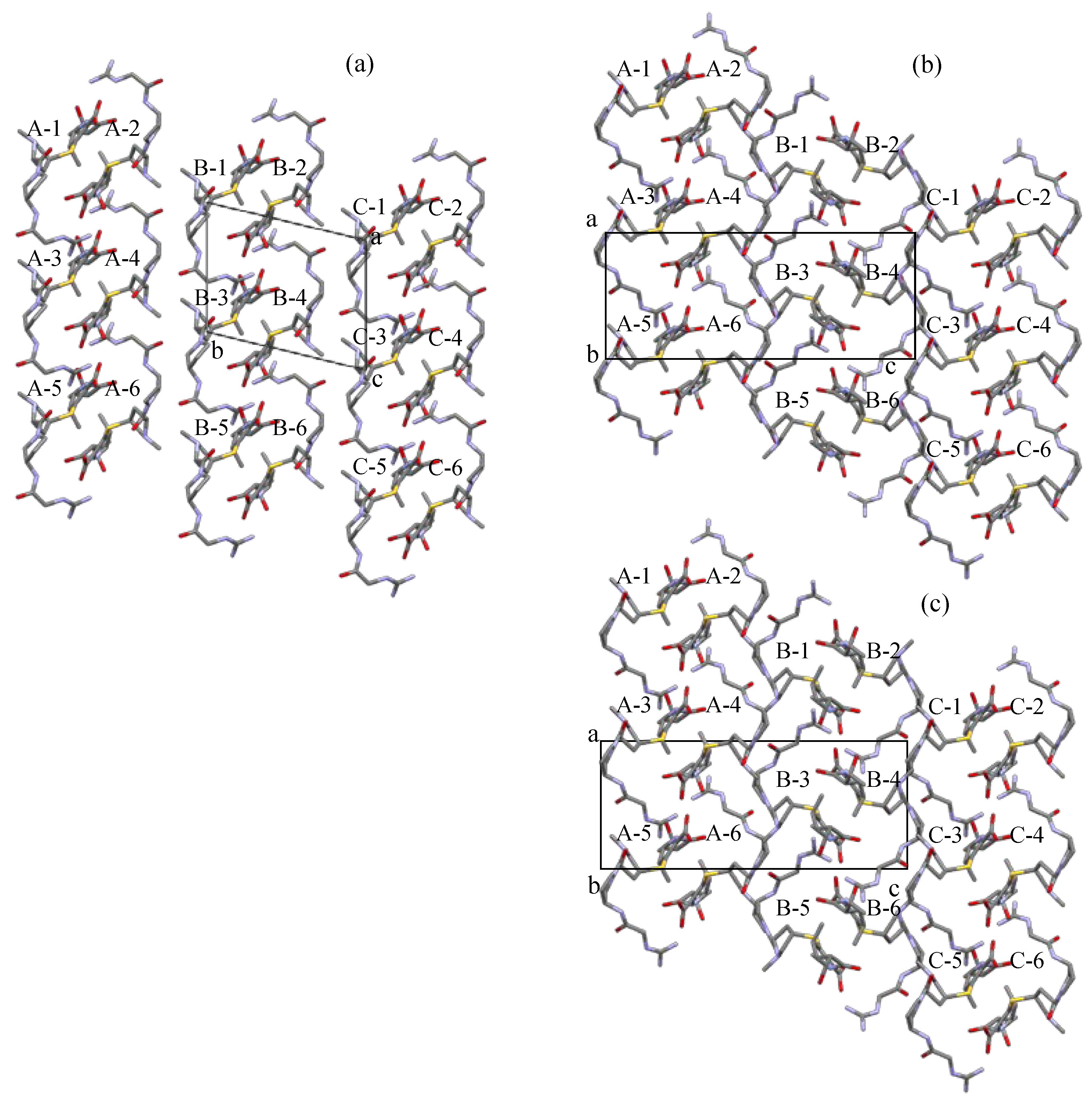
3.3. A Comparison of the Crystal Structure between Form A, Form C, and Form H
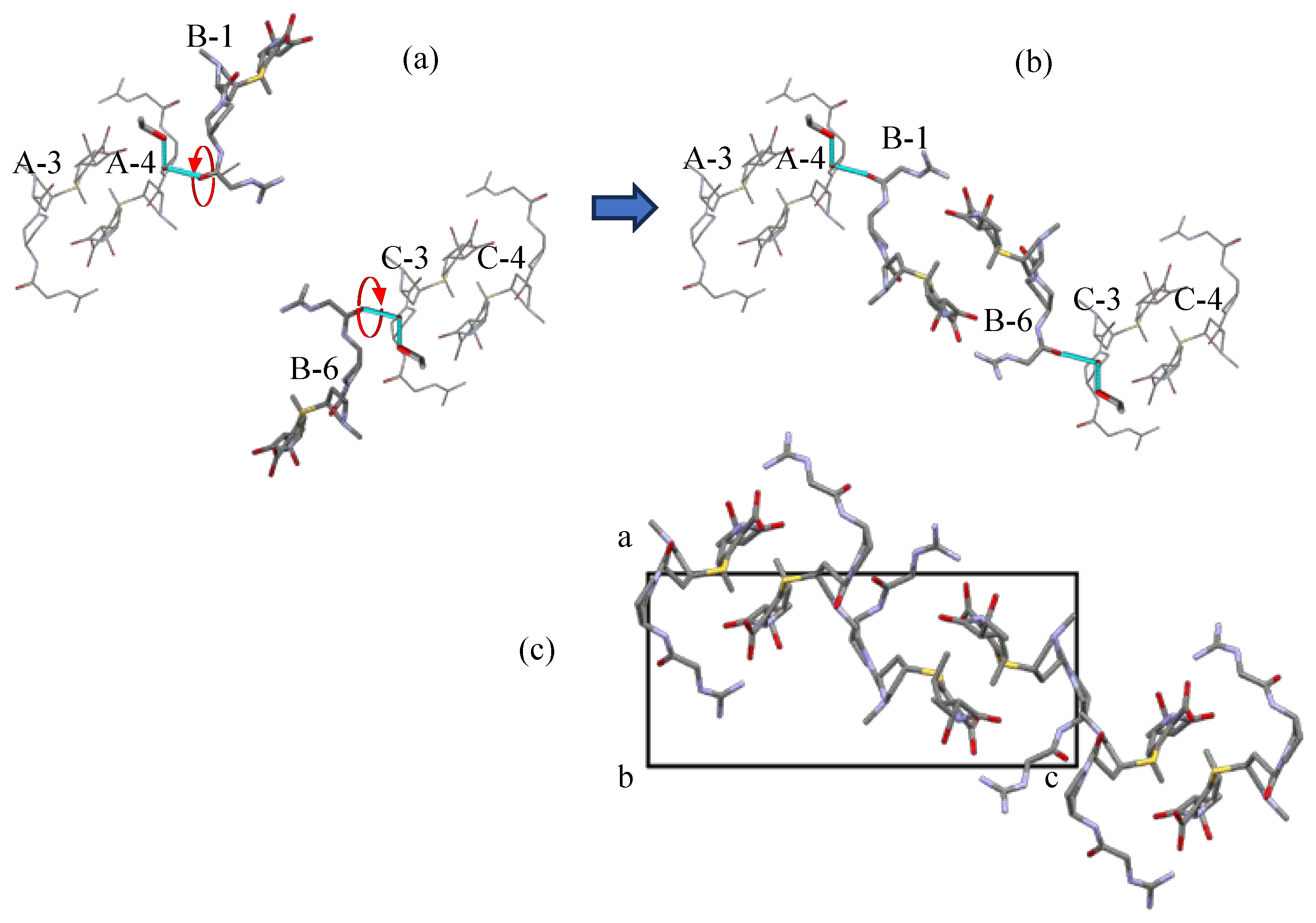
3.4. The Mechanism of the Solid-Phase Transition of Form A into Form H
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.pmda.go.jp/int-activities/int-harmony/ich/0043.html (accessed on 18 July 2021).
- Campeta, A.M.; Chekal, B.P.; Abramov, Y.A.; Meenan, P.A.; Henson, M.J.; Shi, B.; Singer, R.A.; Horspool, K.R. Development of a targeted polymorph screening approach for a complex polymorphic and highly solvating API. J. Pharm. Sci. 2010, 99, 3874–3886. [Google Scholar] [CrossRef] [PubMed]
- Nere, N.K.; Allen, K.C.; Marek, J.C.; Bordawekar, S.V. Drying process optimization for an API solvate using heat transfer model of an agitated filter dryer. J. Pharm. Sci. 2012, 101, 3886–3895. [Google Scholar] [CrossRef] [PubMed]
- Valentina, C.; Norberto, M.; Giovanni, P. Crystal Chemistry of the Antibiotic Doripenem. J. Pharm. Sci. 2014, 103, 3641–3647. [Google Scholar]
- Hickey, M.B.; Peterson, M.L.; Manas, E.S.; Alvarez, J.; Haeffner, F.; Almarsson, Ö. Hydrates and Solid-State Reactivity, A Survey of β-Lactam Antibiotics. J. Pharm. Sci. 2007, 96, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Young Jr, V.G.; Su, Y.; Suryanarayanan, R. Partial dehydration of levothyroxine sodium pentahydrate in a drug product environment: Structural insights into stability. Mol. Pharm. 2020, 17, 3915–3929. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bhatt, P.M.; Nangia, A.; Kruger, G.J. Stable polymorph of venlafaxine hydrochloride by solid-to-solid phase transition at high temperature. Cryst. Growth Des. 2007, 7, 476–480. [Google Scholar] [CrossRef]
- Long, S.; Zhang, M.; Zhou, P.; Yu, F.; Parkin, S.; Li, T. Tautomeric polymorphism of 4-hydroxy nicotinic acid. Cryst. Growth Des. 2016, 16, 2573–2580. [Google Scholar] [CrossRef]
- Shi, G.; Li, S.; Shi, P.; Gong, J.; Zhang, M.; Tang, W. Distinct pathways of solid-to-solid phase transitions induced by defects: The case of dl-methionine. IUCrJ 2021, 8, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Suzuki, M.; Yang, Y.; Yoshikawa, I.; Yin, Q.; Houjou, H. Seed-triggered solid-to-solid transformation between color polymorphs: Striking differences between quasi-isomorphous crystals of dichloro-substituted salicylideneaniline regioisomers. CrystEngComm 2020, 22, 4903–4913. [Google Scholar] [CrossRef]
- Li, M.; Yue, Z.; Chen, Y.; Tong, H.; Tanaka, H.; Tan, P. Revealing thermally-activated nucleation pathways of diffusionless solid-to-solid transition. Nat. Commun. 2021, 12, 4042. [Google Scholar] [CrossRef] [PubMed]
- Patyk-Kaźmierczak, E.; Kaźmierczak, M. Hydrate vs. anhydrate under a pressure-(De) stabilizing effect of the presence of water in solid forms of sulfamethoxazole. Cryst. Growth Des. 2021, 21, 6879–6888. [Google Scholar] [CrossRef]
- Kitagawa, D.; Kawasaki, K.; Tanaka, R.; Kobatake, S. Mechanical behavior of molecular crystals induced by a combination of photochromic reaction and reversible single-crystal-to-single-crystal phase transition. Chem. Mater. 2017, 29, 7524–7532. [Google Scholar] [CrossRef]
- Hariharan, P.S.; Pan, C.; Karthikeyan, S.; Xie, D.; Shinohara, A.; Yang, C.; Wang, L.; Anthony, S.P. Solvent vapor induced rare single-crystal-to-single-crystal transformation of stimuli-responsive fluorophore: Solid state fluorescence tuning, switching and role of molecular conformation and substituents. Dye. Pigment. 2020, 174, 108067. [Google Scholar] [CrossRef]
- Matsuura, S.; Igarashi, K.; Azuma, M.; Ooshima, H. Polymorphic Crystallization Design to Prevent the Degradation of the b-Lactam Structure of a Carbapenem. Crystals 2021, 11, 931. [Google Scholar] [CrossRef]
- Matsuura, S.; Igarashi, K.; Azuma, M.; Ooshima, H. The Identification and Characterization of a New Carbapenem CS-023 Solvate Polymorph Achieved by the Appropriate Crystal Washing and Drying. J. Chem. Eng. Jpn. 2023, 51, 2215225. [Google Scholar] [CrossRef]
- Kawamoto, I.; Shimoji, Y.; Kanno, O.; Kojima, K.; Ishikawa, K.; Matsuyama, E.; Ashida, Y.; Shibayama, T.; Fukuoka, T.; Ohya, S. Synthesis and structure-activity relationships of novel parenteral carbapenems, CS-023 (R-115685) and related compounds containing an amidine moiety. J. Antibiot. 2003, 56, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, T.; Sugiyama, D.; Kamiyama, E.; Tokui, T.; Hirota, T.; Ikeda, T. Characterization of CS-023 (RO4908463), a Novel Parenteral Carbapenem Antibiotic, and Meropenem as Substrates. Drug Metab. Pharmacokinet. 2007, 22, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. A new tool for crystal structure determination and refinement. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M.; Schneider, T.R. [16] SHELXL: High-resolution refinement. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1997; Volume 277, pp. 319–343. [Google Scholar]
- Fujimoto, D.; Tamura, R.; Lepp, Z.; Takahashi, H.; Ushio, T. Mechanism of a New Type of Solvent-Assisted Solid-to-Solid Polymorphic Transition Causing Preferential Enrichment: Prominent Influence of C (sp2) H—O Interaction on the Control of a Crystal Structure. Cryst. Growth Des. 2003, 3, 973–979. [Google Scholar] [CrossRef]
- Horiguchi, M.; Okuhara, S.; Shimano, E.; Fujimoto, D.; Takahashi, H.; Tsue, H.; Tamura, R. Mechanistic flexibility of solvent-assisted solid-to-solid polymorphic transition causing preferential enrichment: Significant contribution of π/π and CH/π interactions as well as hydrogen bonds. Cryst. Growth Des. 2007, 7, 1643–1652. [Google Scholar] [CrossRef]
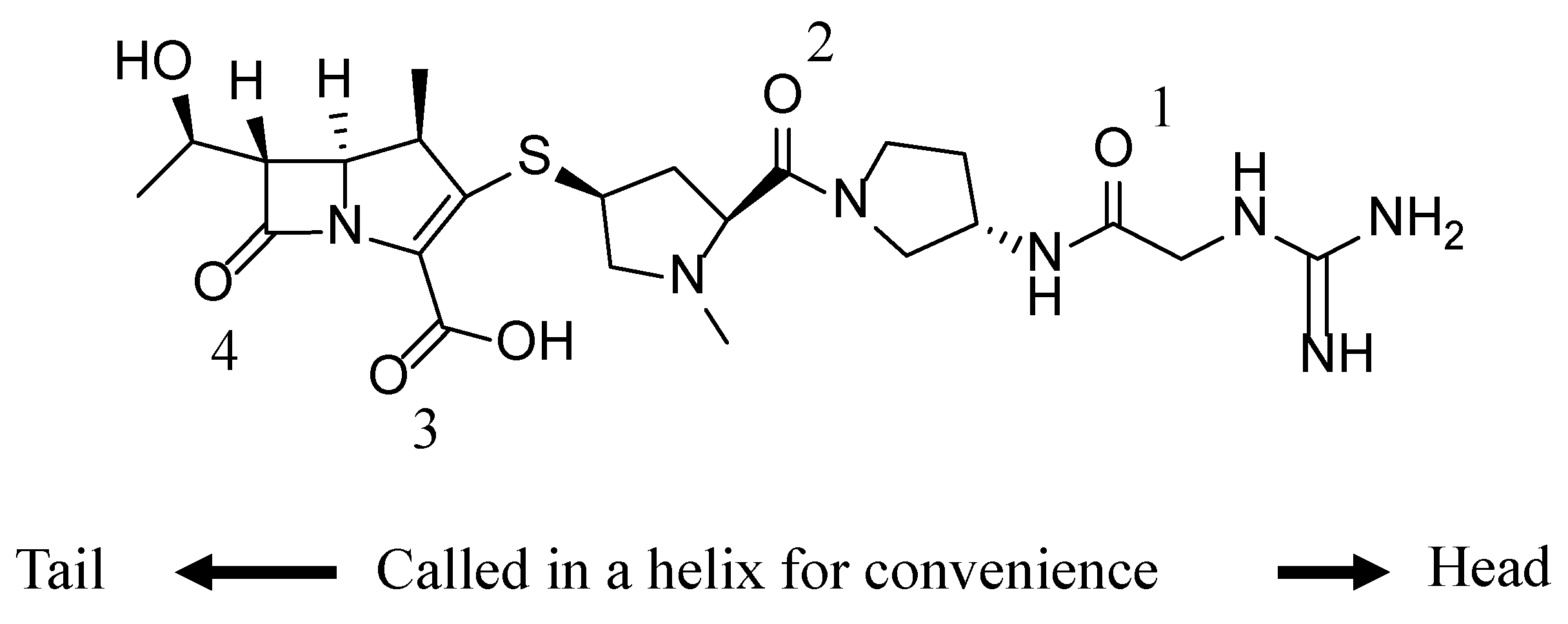
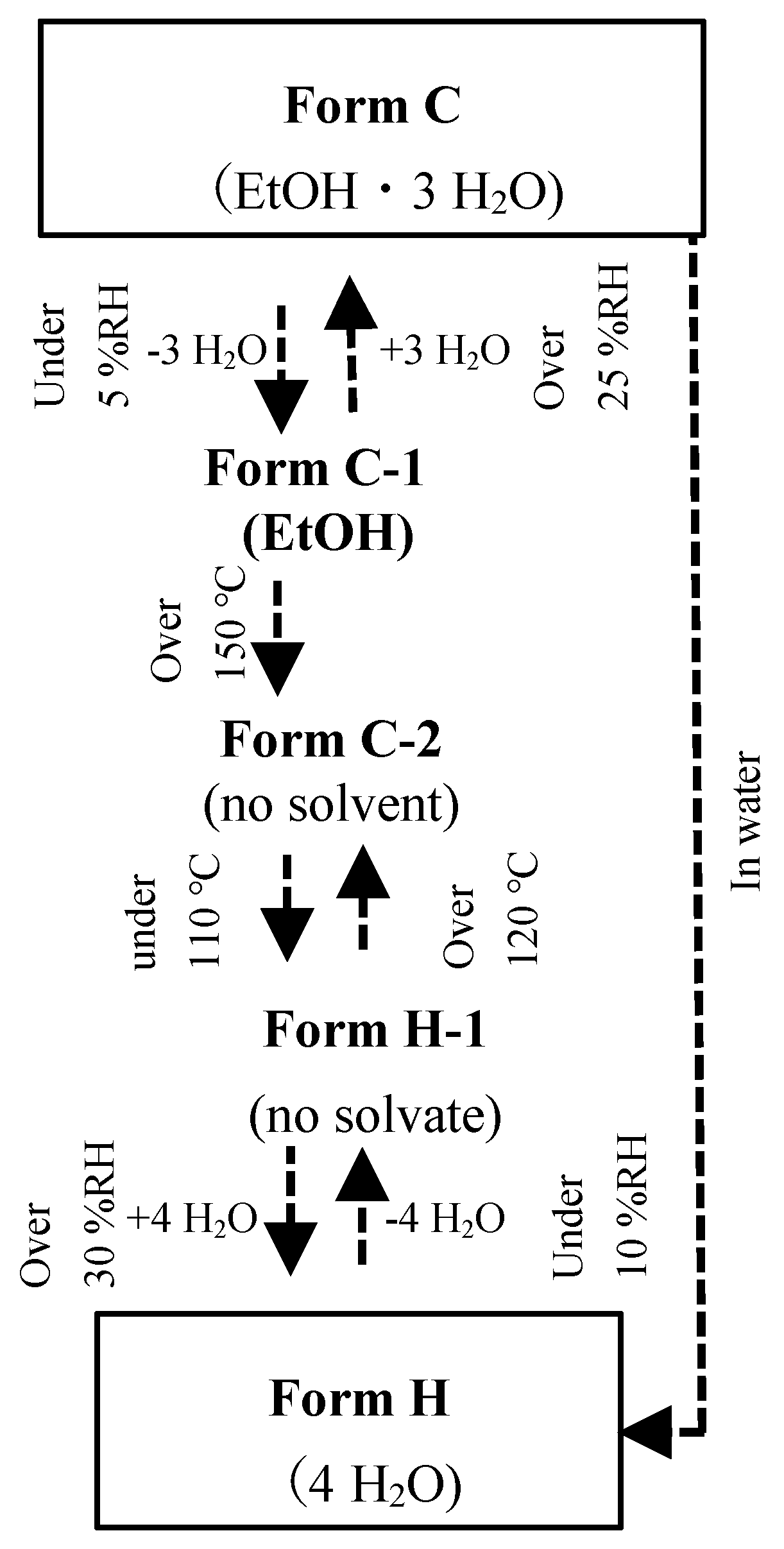
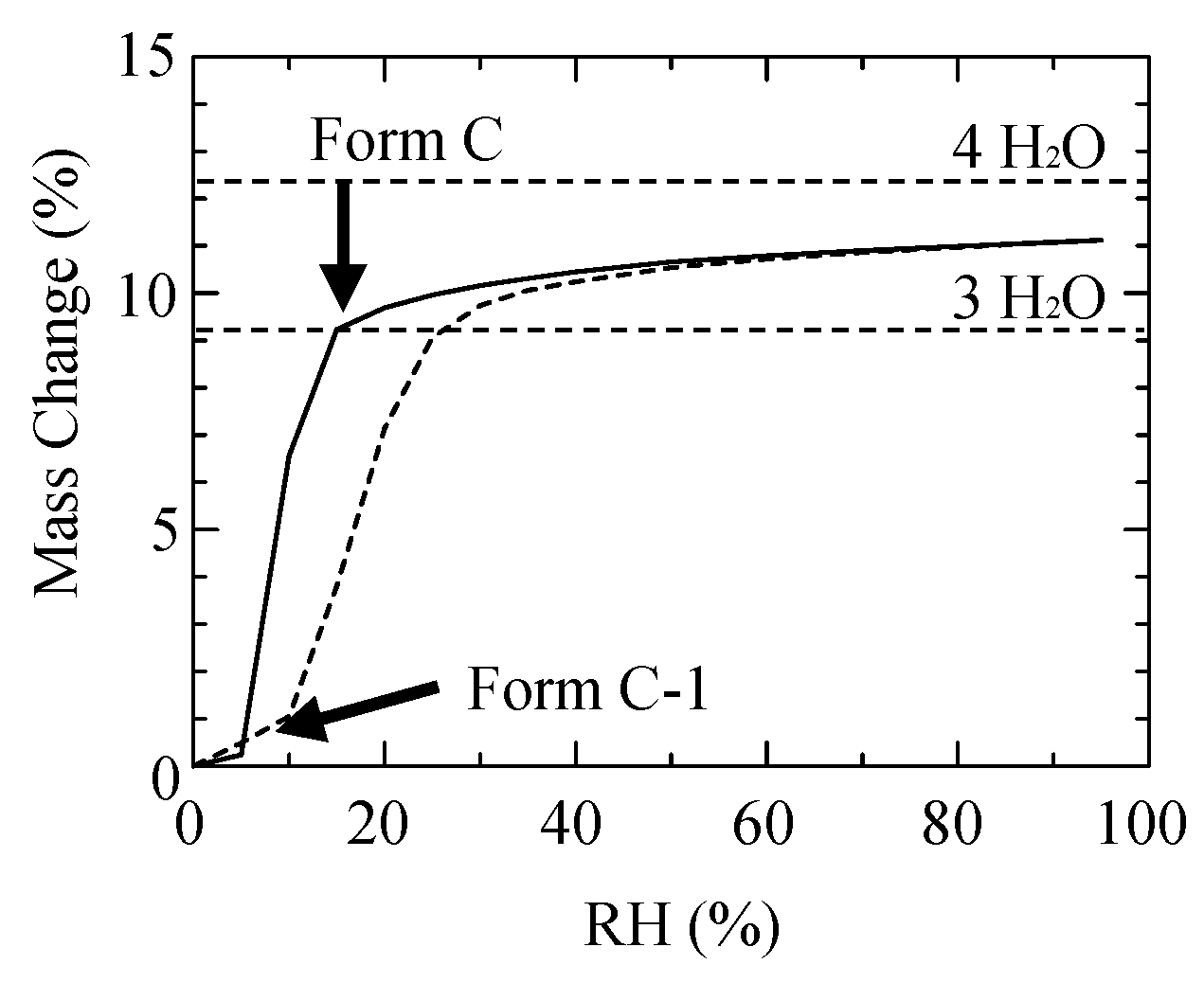
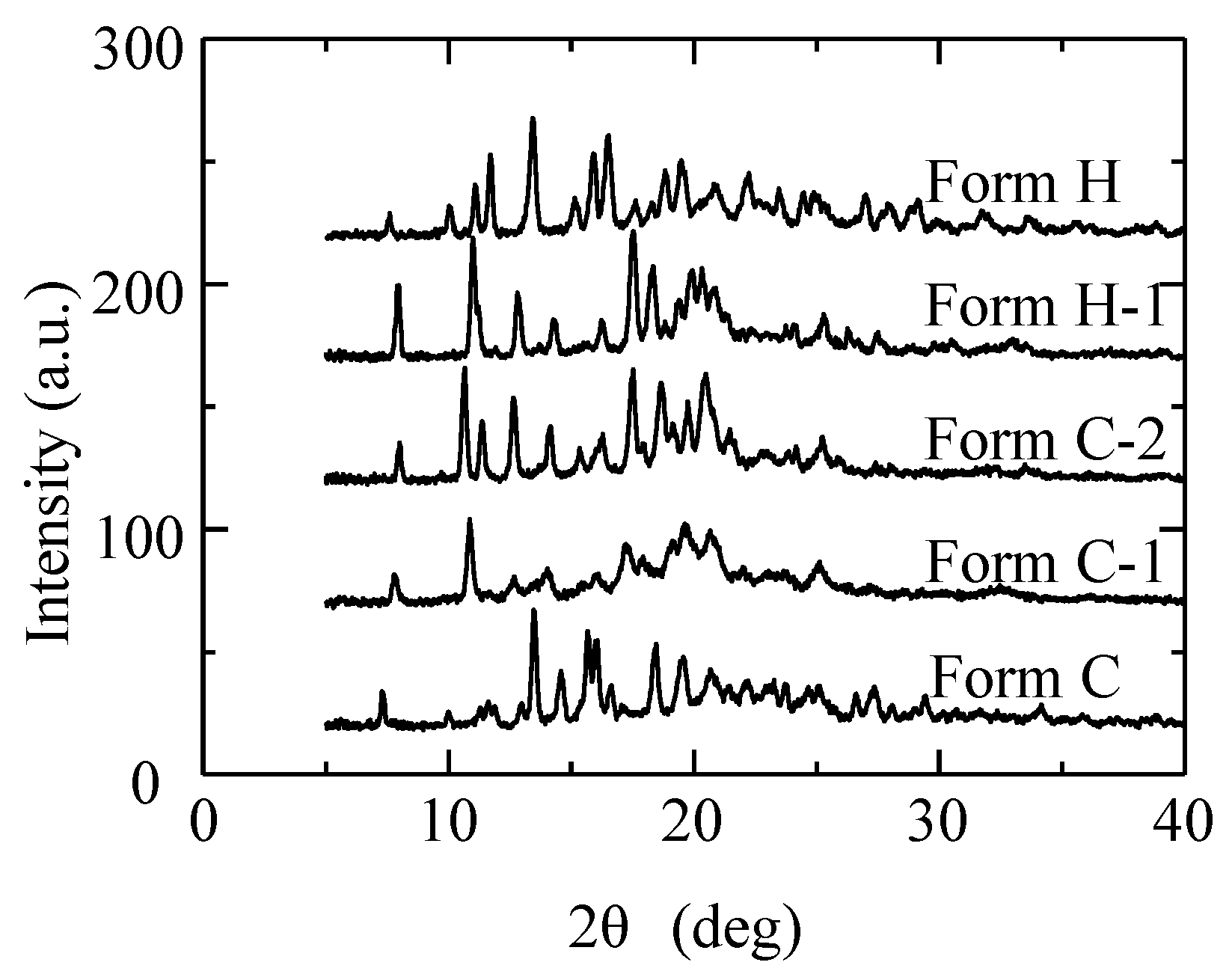
| Polymorph | Form A | Form C | Form H |
|---|---|---|---|
| Solvate | 5/2 Ethanol 1/2 H2O | 1 Ethanol 3 H2O | 4 H2O |
| Crystal system | triclinic | orthorhombic | orthorhombic |
| Space group | P1 | P212121 | P212121 |
| Unit cell dimensions | |||
| a (Å) | 10.23 | 9.857 | 9.879 |
| b (Å) | 13.21 | 13.1136 | 13.322 |
| c (Å) | 14.04 | 24.1859 | 23.45 |
| α (°) | 78.07 | 90 | 90 |
| β (°) | 89.02 | 90 | 90 |
| γ (°) | 76.63 | 90 | 90 |
| Volume (Å3) | 1805.1 | 3113.2 | 3086 |
| Z | 2 | 4 | 4 |
| Density (calculated) | 1.125 g/cm3 | 1.361 g/cm3 | 1.351 g/cm3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, S.; Igarashi, K.; Azuma, M.; Ooshima, H. The Solid-Phase Transition of Carbapenem CS-023 Polymorphs and the Change in Helicity Observed in the Transition. Crystals 2024, 14, 71. https://doi.org/10.3390/cryst14010071
Matsuura S, Igarashi K, Azuma M, Ooshima H. The Solid-Phase Transition of Carbapenem CS-023 Polymorphs and the Change in Helicity Observed in the Transition. Crystals. 2024; 14(1):71. https://doi.org/10.3390/cryst14010071
Chicago/Turabian StyleMatsuura, Shinji, Koichi Igarashi, Masayuki Azuma, and Hiroshi Ooshima. 2024. "The Solid-Phase Transition of Carbapenem CS-023 Polymorphs and the Change in Helicity Observed in the Transition" Crystals 14, no. 1: 71. https://doi.org/10.3390/cryst14010071
APA StyleMatsuura, S., Igarashi, K., Azuma, M., & Ooshima, H. (2024). The Solid-Phase Transition of Carbapenem CS-023 Polymorphs and the Change in Helicity Observed in the Transition. Crystals, 14(1), 71. https://doi.org/10.3390/cryst14010071





