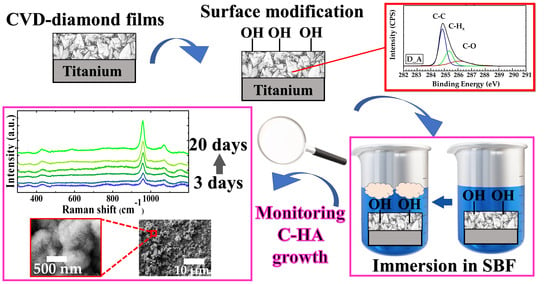
Monitoring of Carbonated Hydroxyapatite Growth on Modified Polycrystalline CVD-Diamond Coatings on Titanium Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Diamond Films Synthesis and Processing
2.3. Hydroxyapatite Precipitation
2.4. Characterization Techniques
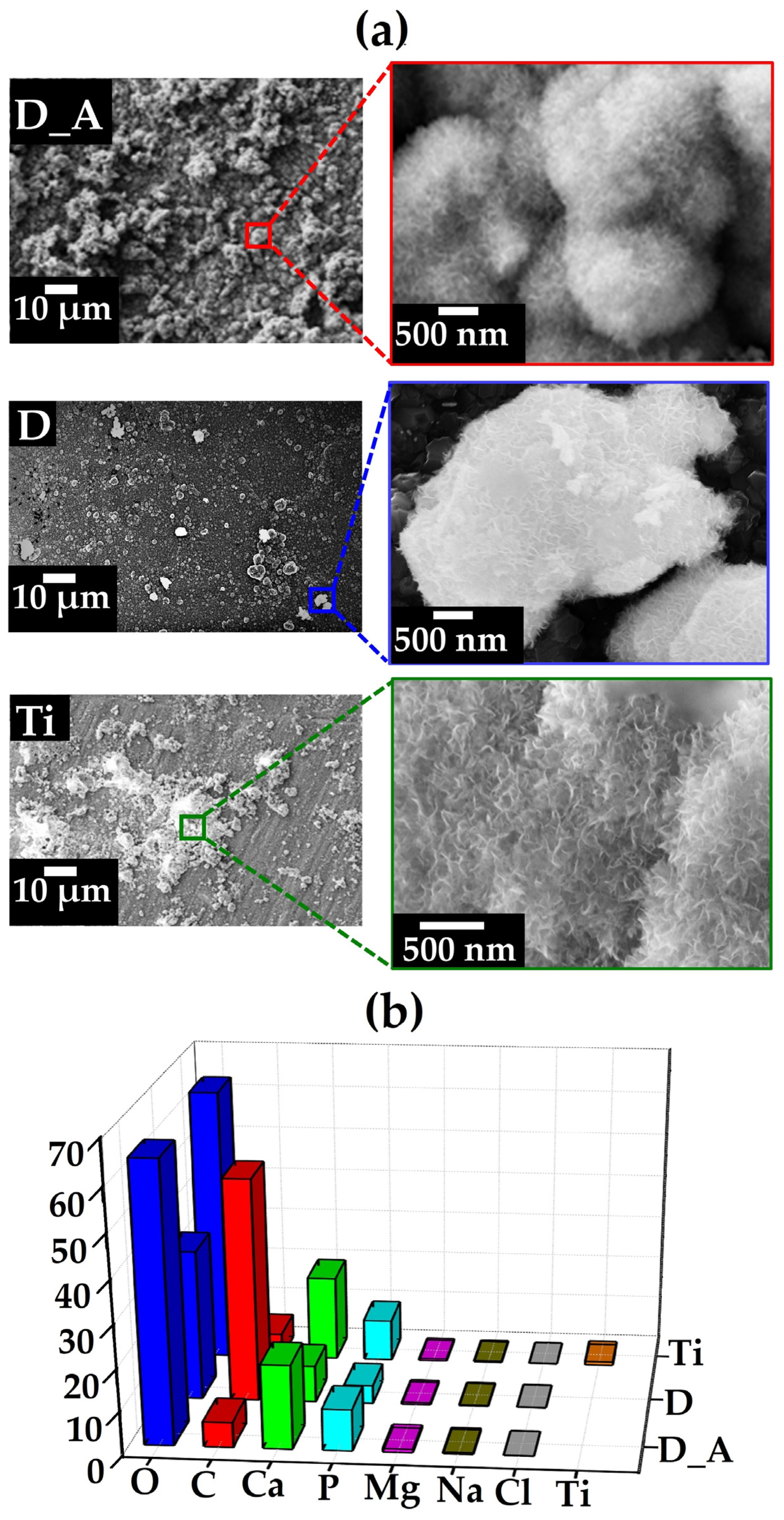
3. Results
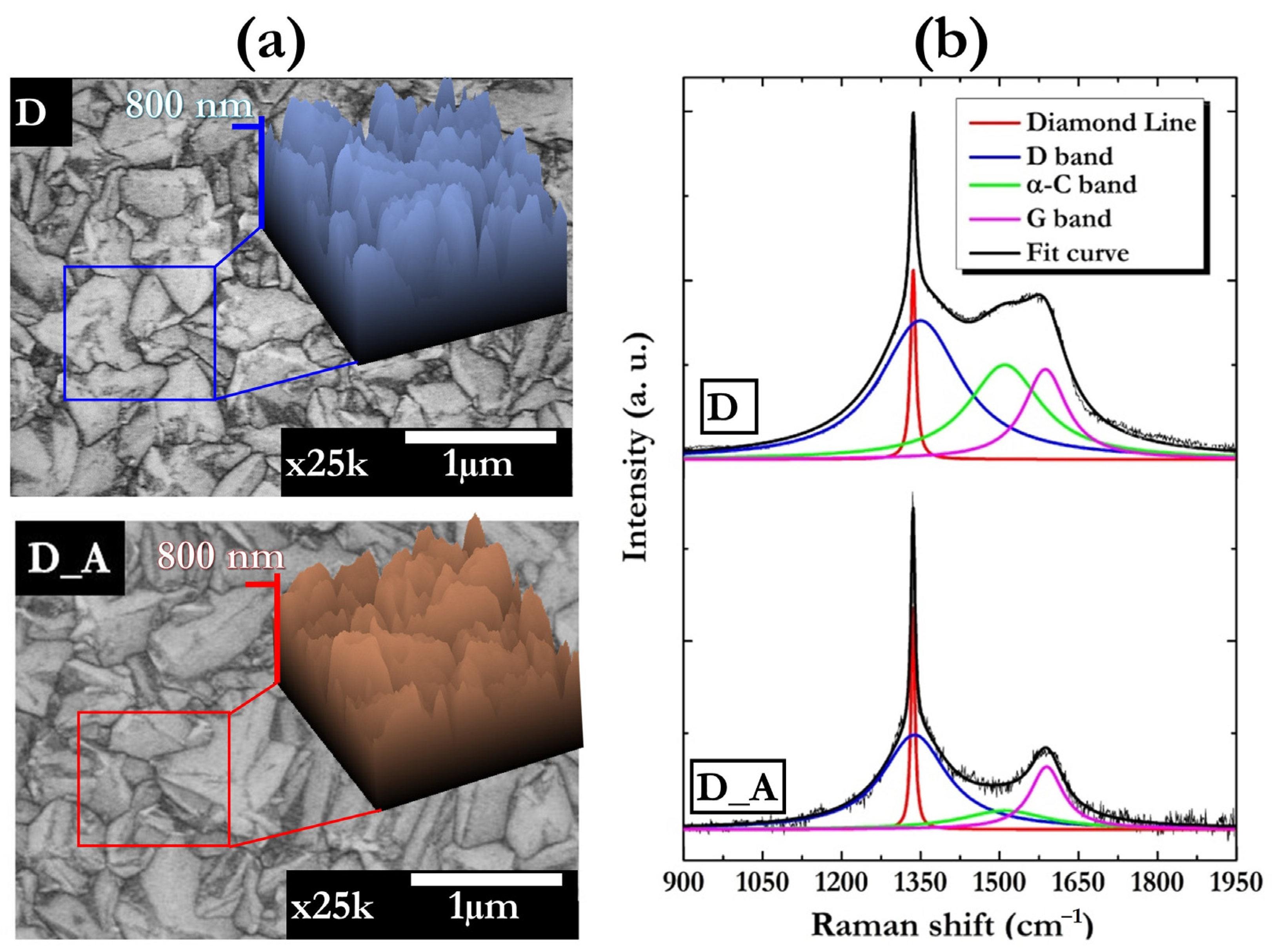
3.1. Diamond Films
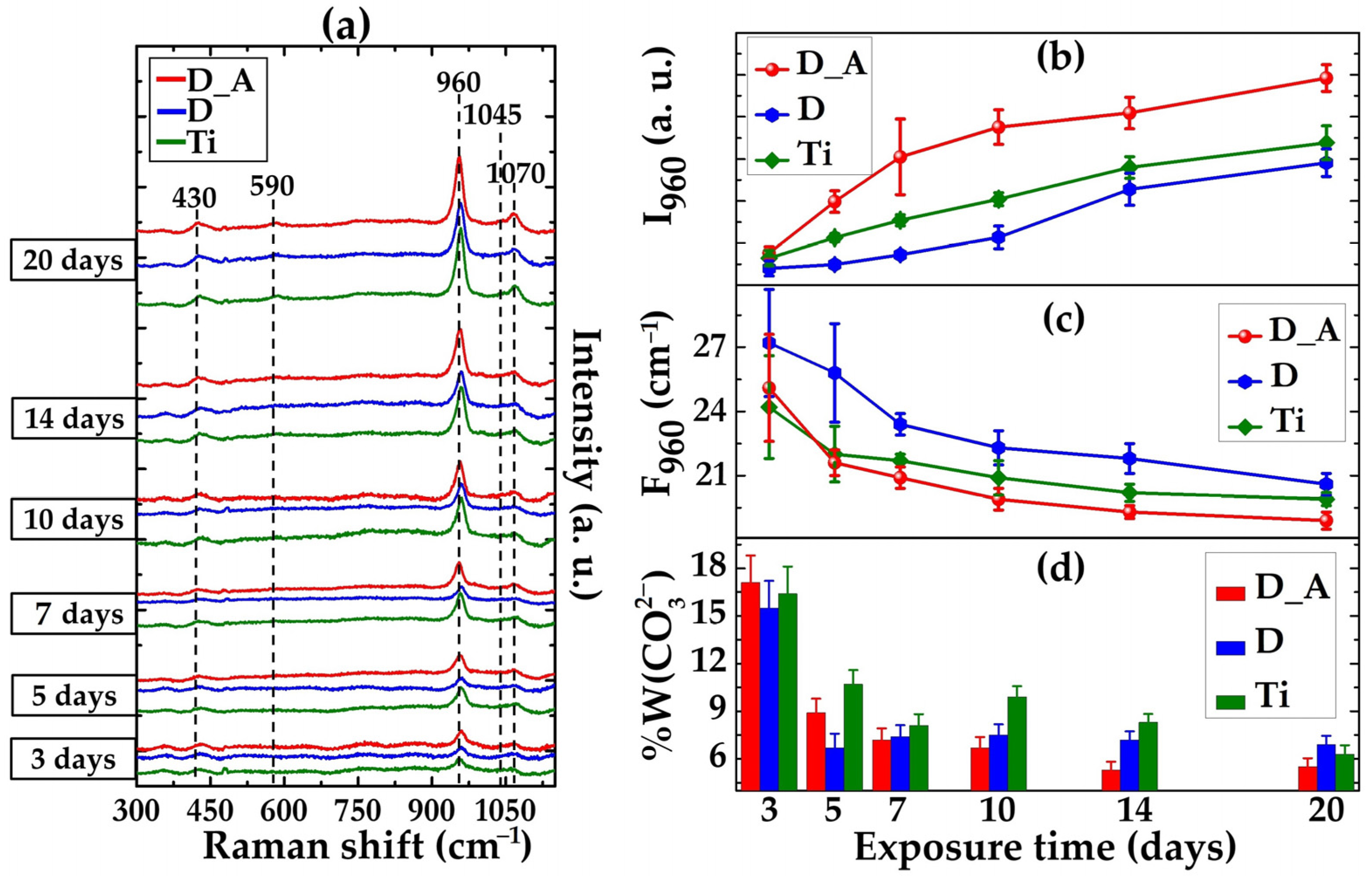
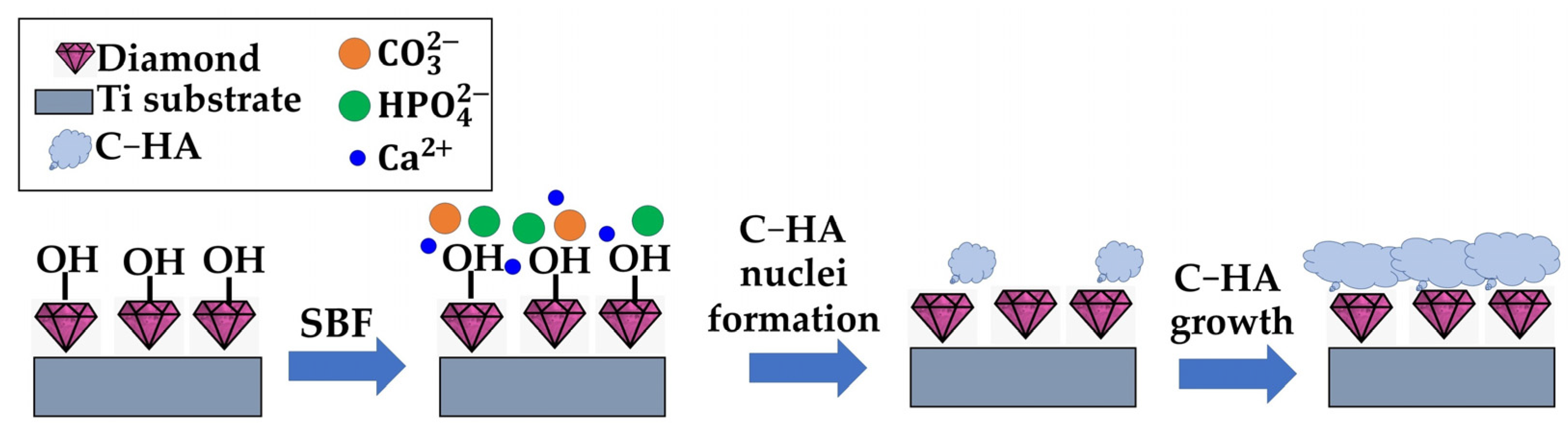
3.2. Carbonated Hydroxyapatite Growth
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raymakers, J.; Haenen, K.; Maes, W. Diamond Surface Functionalization: From Gemstone to Photoelectrochemical Applications. J. Mater. Chem. C 2019, 7, 10134–10165. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Application of Diamond Electrodes to Electrochemical Processes. Electrochim. Acta 2005, 51, 191–199. [Google Scholar] [CrossRef]
- Politi, S.; Battistoni, S.; Carcione, R.; Montaina, L.; Macis, S.; Lupi, S.; Tamburri, E. PANI-Modified Ti-Doped CVD Diamond As Promising Conductive Platform to Mimic Bioelectricity Functions. Adv. Mater. Interfaces 2021, 8, 2101401. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Jaworska, I.; Jędruchniewicz, K. Application of Unmodified Boron-Doped Diamond Electrode for Determination of Dopamine and Paracetamol. Microchem. J. 2019, 146, 664–672. [Google Scholar] [CrossRef]
- Fox, K.; Palamara, J.; Judge, R.; Greentree, A.D. Diamond as a Scaffold for Bone Growth. J. Mater. Sci. Mater. Med. 2013, 24, 849–861. [Google Scholar] [CrossRef]
- Baluchová, S.; Brycht, M.; Taylor, A.; Mortet, V.; Krůšek, J.; Dittert, I.; Sedláková, S.; Klimša, L.; Kopeček, J.; Schwarzová-Pecková, K. Enhancing Electroanalytical Performance of Porous Boron-Doped Diamond Electrodes by Increasing Thickness for Dopamine Detection. Anal. Chim. Acta 2021, 1182, 338949. [Google Scholar] [CrossRef]
- Fox, K.; Mani, N.; Rifai, A.; Reineck, P.; Jones, A.; Tran, P.A.; Ramezannejad, A.; Brandt, M.; Gibson, B.C.; Greentree, A.D.; et al. 3D-Printed Diamond-Titanium Composite: A Hybrid Material for Implant Engineering. ACS Appl. Bio Mater. 2020, 3, 29–36. [Google Scholar] [CrossRef]
- Fong, J.S.L.; Booth, M.A.; Rifai, A.; Fox, K.; Gelmi, A. Diamond in the Rough: Toward Improved Materials for the Bone−Implant Interface. Adv. Healthc. Mater. 2021, 10, 2100007. [Google Scholar] [CrossRef]
- Booth, M.A.; Pope, L.; Sherrell, P.C.; Stacey, A.; Tran, P.A.; Fox, K.E. Polycrystalline Diamond Coating on 3D Printed Titanium Scaffolds: Surface Characterisation and Foreign Body Response. Mater. Sci. Eng. C 2021, 130, 112467. [Google Scholar] [CrossRef]
- Hupert, M.; Muck, A.; Wang, J.; Stotter, J.; Cvackova, Z.; Haymond, S.; Show, Y.; Swain, G.M. Conductive Diamond Thin-Films in Electrochemistry. Diam. Relat. Mater. 2003, 12, 1940–1949. [Google Scholar] [CrossRef]
- Dutta, G.; Siddiqui, S.; Zeng, H.; Carlisle, J.A.; Arumugam, P.U. The Effect of Electrode Size and Surface Heterogeneity on Electrochemical Properties of Ultrananocrystalline Diamond Microelectrode. J. Electroanal. Chem. 2015, 756, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, H.; Yegani, R.; Babaeipour, V. Study on the Reinforcing Effect of Nanodiamond Particles on the Mechanical, Thermal and Antibacterial Properties of Cellulose Acetate Membranes. Diam. Relat. Mater. 2016, 69, 166–176. [Google Scholar] [CrossRef]
- Tamburri, E.; Carcione, R.; Vitale, F.; Valguarnera, A.; Macis, S.; Lucci, M.; Terranova, M.L. Exploiting the Properties of Ti-Doped CVD-Grown Diamonds for the Assembling of Electrodes. Adv. Mater. Interfaces 2017, 4, 1700222. [Google Scholar] [CrossRef]
- Reina, G.; Orlanducci, S.; Cairone, C.; Tamburri, E.; Lenti, S.; Cianchetta, I.; Rossi, M.; Terranova, M.L. Rhodamine/Nanodiamond as a System Model for Drug Carrier. J. Nanosci. Nanotechnol. 2015, 15, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R. Low-Pressure Diamond: From the Unbelievable to Technical Products. ChemTexts 2021, 7, 10. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Auciello, O.; Birrell, J.; Carlisle, J.A.; Curtiss, L.A.; Goyette, A.N.; Gruen, D.M.; Krauss, A.R.; Schlueter, J.; Sumant, A.; et al. Synthesis and Characterization of Highly-Conducting Nitrogen-Doped Ultrananocrystalline Diamond Films. Appl. Phys. Lett. 2001, 79, 1441–1443. [Google Scholar] [CrossRef]
- Grausova, L.; Kromka, A.; Burdikova, Z.; Eckhardt, A.; Rezek, B.; Vacik, J.; Haenen, K.; Lisa, V.; Bacakova, L. Enhanced Growth and Osteogenic Differentiation of Human Osteoblast-like Cells on Boron-Doped Nanocrystalline Diamond Thin Films. PLoS ONE 2011, 6, e20943. [Google Scholar] [CrossRef]
- Terranova, M.L.; Rossi, M.; Vitali, G. Structural Investigation of the Titanium/Diamond Film Interface. J. Appl. Phys. 1998, 80, 3552–3560. [Google Scholar] [CrossRef]
- Mandal, S. Nucleation of Diamond Films on Heterogeneous Substrates: A Review. RSC Adv. 2021, 11, 10159–10182. [Google Scholar] [CrossRef]
- Carcione, R.; Tamburri, E.; Bartali, R.; Speranza, G.; Micheli, V.; Pepponi, G.; Bellutti, P.; Terranova, M.L. On the route to produce conductive Ni-related color centers in CVD-grown diamond. Multifunct. Mater. 2019, 2, 035001. [Google Scholar] [CrossRef]
- Terranova, M.L.; Piccirillo, S.; Sessa, V.; Compagnone, D.; Sbornicchia, P.; Rossi, M. Electrochemical Behaviour of Electrodes Assembled with Ti-Containing Diamond Films. Diam. Relat. Mater. 2001, 10, 627–630. [Google Scholar] [CrossRef]
- Rifai, A.; Tran, N.; Reineck, P.; Elbourne, A.; Mayes, E.; Sarker, A.; Dekiwadia, C.; Ivanova, E.P.; Crawford, R.J.; Ohshima, T.; et al. Engineering the Interface: Nanodiamond Coating on 3D-Printed Titanium Promotes Mammalian Cell Growth and Inhibits Staphylococcus Aureus Colonization. ACS Appl. Mater. Interfaces 2019, 11, 24588–24597. [Google Scholar] [CrossRef] [PubMed]
- Sur, D.; Tirado, P.; Alcantar, J.; Auciello, O.; Basim, G.B. Integration of Ultrananocrystalline Diamond (UNCD)-Coatings on Chemical-Mechanical Surface Nano-Structured (CMNS) Titanium-Based Dental Implants. MRS Adv. 2020, 5, 2261–2271. [Google Scholar] [CrossRef]
- Shen, S.; Shen, W.; Liu, S.; Li, H.; Chen, Y.; Qi, H. First-Principles Calculations of Co-Doping Impurities in Diamond. Mater. Today Commun. 2020, 23, 100847. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Hu, M.; Li, M.; Liu, X.; Su, T.; Yu, K.; Han, F. The First Principle Study and Experimental of Boron Synergistic Sulfur Doping in Diamond. Mater. Today Commun. 2020, 24, 101021. [Google Scholar] [CrossRef]
- Goss, J.P.; Briddon, P.R.; Jones, R.; Sque, S. Donor and Acceptor States in Diamond. Diam. Relat. Mater. 2004, 13, 684–690. [Google Scholar] [CrossRef]
- Ödman, J.; Lekholm, U.; Jemt, T.; Brånemark, P.-I.; Thilander, B. Osseointegrated Titanium Implants—A New Approach in Orthodontic Treatment. Eur. J. Orthod. 1988, 10, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; He, G. Enhancement of the Porous Titanium with Entangled Wire Structure for Load-Bearing Biomedical Applications. Mater. Des. 2014, 56, 241–244. [Google Scholar] [CrossRef]
- Janson, O.; Gururaj, S.; Pujari-Palmer, S.; Karlsson Ott, M.; Strømme, M.; Engqvist, H.; Welch, K. Titanium Surface Modification to Enhance Antibacterial and Bioactive Properties While Retaining Biocompatibility. Mater. Sci. Eng. C 2019, 96, 272–279. [Google Scholar] [CrossRef]
- Dallago, M.; Fontanari, V.; Torresani, E.; Leoni, M.; Pederzolli, C.; Potrich, C.; Benedetti, M. Fatigue and Biological Properties of Ti-6Al-4V ELI Cellular Structures with Variously Arranged Cubic Cells Made by Selective Laser Melting. J. Mech. Behav. Biomed. Mater. 2018, 78, 381–394. [Google Scholar] [CrossRef]
- Carcione, R.; Battistoni, S.; Palmieri, E.; Orlanducci, S.; Tamburri, E. Pretreatment Strategies of Titanium Substrates to Modulate the Electrochemical Properties of CVD-Grown Ti-Doped Diamond Electrodes for Dopamine Detection. Surf. Coatings Technol. 2023, 467, 129662. [Google Scholar] [CrossRef]
- Battistoni, S.; Carcione, R.; Tamburri, E.; Erokhin, V.; Terranova, M.L.; Iannotta, S. A Ti-Doped Chemical Vapor Deposition Diamond Device as Artificial Synapse for Neuromorphic Applications. Adv. Mater. Technol. 2023, 8, 2201555. [Google Scholar] [CrossRef]
- Jozwik, K.; Karczemska, A. The New Generation Ti6Al4V Artificial Heart Valve with Nanocrystalline Diamond Coating on the Ring and with Derlin Disc after Long-Term Mechanical Fatigue Examination. Diam. Relat. Mater. 2007, 16, 1004–1009. [Google Scholar] [CrossRef]
- Papo, M.J.; Catledge, S.A.; Vohra, Y.K.; Machado, C. Mechanical Wear Behavior of Nanocrystalline and Multilayer Diamond Coatings on Temporomandibular Joint Implants. J. Mater. Sci. Mater. Med. 2004, 15, 773–777. [Google Scholar] [CrossRef]
- Fries, M.D.; Vohra, Y.K. Nanostructured Diamond Film Deposition on Curved Surfaces of Metallic Temporomandibular Joint Implant. J. Phys. D Appl. Phys. 2002, 35, L105. [Google Scholar] [CrossRef]
- Aspenberg, P.; Anttila, A.; Konttinen, Y.T.; Lappalainen, R.; Goodman, S.B.; Nordsletten, L.; Santavirta, S. Benign Response to Particles of Diamond and SiC: Bone Chamber Studies of New Joint Replacement Coating Materials in Rabbits. Biomaterials 1996, 17, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Kopova, I.; Stankova, L.; Liskova, J.; Vacik, J.; Lavrentiev, V.; Kromka, A.; Potocky, S.; Stranska, D. Bone Cells in Cultures on Nanocarbonbased Materials for Potential Bone Tissue Engineering: A Review. Phys. Status Solidi (A) 2014, 211, 2688–2702. [Google Scholar] [CrossRef]
- Carcione, R.; Politi, S.; Iacob, E.; Potrich, C.; Lunelli, L.; Vanzetti, L.E.; Bartali, R.; Micheli, V.; Pepponi, G.; Terranova, M.L.; et al. Exploring a New Approach for Regenerative Medicine: Ti-Doped Polycrystalline Diamond Layers as Bioactive Platforms for Osteoblast-like Cells Growth. Appl. Surf. Sci. 2021, 540, 148334. [Google Scholar] [CrossRef]
- Ali, M.; Ali, F.; Yang, B.; Abbas, A. A Comprehensive Account of Biomedical Applications of CVD Diamond Coatings. J. Phys. D Appl. Phys. 2021, 54, 443001. [Google Scholar] [CrossRef]
- Strąkowska, P.; Beutner, R.; Gnyba, M.; Zielinski, A.; Scharnweber, D. Electrochemically Assisted Deposition of Hydroxyapatite on Ti6Al4V Substrates Covered by CVD Diamond Films—Coating Characterization and First Cell Biological Results. Mater. Sci. Eng. C 2016, 59, 624–635. [Google Scholar] [CrossRef]
- Rifai, A.; Tran, N.; Lau, D.W.; Elbourne, A.; Zhan, H.; Stacey, A.D.; Mayes, E.L.H.; Sarker, A.; Ivanova, E.P.; Crawford, R.J.; et al. Polycrystalline Diamond Coating of Additively Manufactured Titanium for Biomedical Applications. ACS Appl. Mater. Interfaces 2018, 10, 8474–8484. [Google Scholar] [CrossRef] [PubMed]
- El-wassefy, N.A.; Hammouda, I.M.; Habib, A.N.E.-D.A.; El-awady, G.Y.; Marzook, H.A. Assessment of Anodized Titanium Implants Bioactivity. Clin. Oral Implants Res. 2014, 25, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nishiguchi, S.; Nakamura, T. Graded Surface Structure of Bioactive Titanium Prepared by Chemical Treatment. J. Biomed. Mater. Res. 1999, 45, 100–107. [Google Scholar] [CrossRef]
- Kizuki, T.; Matsushita, T.; Kokubo, T. Antibacterial and Bioactive Calcium Titanate Layers Formed on Ti Metal and Its Alloys. J. Mater. Sci. Mater. Med. 2014, 25, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Wopenka, B.; Pasteris, J.D. A Mineralogical Perspective on the Apatite in Bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Sotiropoulou, P.; Fountos, G.; Martini, N.; Koukou, V.; Michail, C.; Kandarakis, I.; Nikiforidis, G. Bone Calcium/Phosphorus Ratio Determination Using Dual Energy X-ray Method. Phys. Medica 2015, 31, 307–313. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of Hydroxyapatite from Natural Sources. Heliyon 2017, 5, e01588. [Google Scholar] [CrossRef]
- Haberko, K.; Bućko, M.M.; Brzezińska-Miecznik, J.; Haberko, M.; Mozgawa, W.; Panz, T.; Pyda, A.; Zarebski, J. Natural Hydroxyapatite—Its Behaviour during Heat Treatment. J. Eur. Ceram. Soc. 2006, 26, 537–542. [Google Scholar] [CrossRef]
- Atemni, I.; Ouafi, R.; Hjouji, K.; Mehdaoui, I.; Ainane, A.; Ainane, T.; Taleb, M.; Rais, Z. Extraction and Characterization of Natural Hydroxyapatite Derived from Animal Bones Using the Thermal Treatment Process. Emergent Mater. 2023, 6, 551–560. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce In Vivo Surface-structure Changes in Bioactive Glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman Spectroscopy of Disordered, Amorphous, and Diamondlike Carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman Spectroscopy of Amorphous, Nanostructured, Diamondlike Carbon, and Nanodiamond. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Vorlíček, V.; Rosa, J.; Vaněček, M.; Nesládek, M.; Stals, L.M. Quantitative Study of Raman Scattering and Defect Optical Absorption in CVD Diamond Films. Diam. Relat. Mater. 1997, 6, 704–707. [Google Scholar] [CrossRef]
- Liu, X.; Lu, P.; Wang, H.; Ren, Y.; Tan, X.; Sun, S.; Jia, H. Morphology and Structure of Ti-Doped Diamond Films Prepared by Microwave Plasma Chemical Vapor Deposition. Appl. Surf. Sci. 2018, 442, 529–536. [Google Scholar] [CrossRef]
- Alba, G.; Eon, D.; Villar, M.P.; Alcántara, R.; Chicot, G.; Cañas, J.; Letellier, J.; Pernot, J.; Araujo, D. H-Terminated Diamond Surface Band Bending Characterization by Angle-Resolved XPS. Surfaces 2020, 3, 61–71. [Google Scholar] [CrossRef]
- Merel, P.; Tabbal, M.; Chaker, M.; Moisa, S.; Margot, J. Direct Evaluation of the Sp 3 Content in Diamond-like-Carbon Films by XPS. Appl. Surf. Sci. 1998, 136, 105–110. [Google Scholar] [CrossRef]
- Park, C.K.; Chang, S.M.; Uhm, H.S.; Seo, S.H.; Park, J.S. XPS and XRR Studies on Microstructures and Interfaces of DLC Films Deposited by FCVA Method. Thin Solid Films 2002, 420, 235–240. [Google Scholar] [CrossRef]
- Ferro, S.; Dal Colle, M.; De Battisti, A. Chemical Surface Characterization of Electrochemically and Thermally Oxidized Boron-Doped Diamond Film Electrodes. Carbon 2005, 43, 1191–1203. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR Studies of Synthetic and Natural Apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Awonusi, A.; Morris, M.D.; Tecklenburg, M.M.J. Carbonate Assignment and Calibration in the Raman Spectrum of Apatite. Calcif. Tissue Int. 2007, 81, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Okagbare, P.I.; Begun, D.; Tecklenburg, M.; Awonusi, A.; Goldstein, S.A.; Morris, M.D. Noninvasive Raman Spectroscopy of Rat Tibiae: Approach to In Vivo Assessment of Bone Quality. J. Biomed. Opt. 2012, 17, 090502. [Google Scholar] [CrossRef] [PubMed]
- Yasar, O.F.; Liao, W.C.; Mathew, R.; Yu, Y.; Stevensson, B.; Liu, Y.; Shen, Z.; Edén, M. The Carbonate and Sodium Environments in Precipitated and Biomimetic Calcium Hydroxy-Carbonate Apatite Contrasted with Bone Mineral: Structural Insights from Solid-State NMR. J. Phys. Chem. C 2021, 125, 10572–10592. [Google Scholar] [CrossRef]
- Nosenko, V.V.; Yaremko, A.M.; Dzhagan, V.M.; Vorona, I.P.; Romanyuk, Y.A.; Zatovsky, I.V. Nature of Some Features in Raman Spectra of Hydroxyapatite-Containing Materials. J. Raman Spectrosc. 2016, 47, 726–730. [Google Scholar] [CrossRef]
- Taylor, E.A.; Mileti, C.J.; Ganesan, S.; Kim, J.H.; Donnelly, E. Measures of Bone Mineral Carbonate Content and Mineral Maturity/Crystallinity for FT-IR and Raman Spectroscopic Imaging Differentially Relate to Physical–Chemical Properties of Carbonate-Substituted Hydroxyapatite. Calcif. Tissue Int. 2021, 109, 77–91. [Google Scholar] [CrossRef]
- Spizzirri, P.G.; Cochrane, N.J.; Prawer, S.; Reynolds, E.C. A Comparative Study of Carbonate Determination in Human Teeth Using Raman Spectroscopy. Caries Res. 2012, 46, 353–360. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hayashi, K. Carbonate Apatite Artificial Bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef]
- Nagai, H.; Kobayashi-Fujioka, M.; Fujisawa, K.; Ohe, G.; Takamaru, N.; Hara, K.; Uchida, D.; Tamatani, T.; Ishikawa, K.; Miyamoto, Y. Effects of Low Crystalline Carbonate Apatite on Proliferation and Osteoblastic Differentiation of Human Bone Marrow Cells. J. Mater. Sci. Mater. Med. 2015, 26, 1–8. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Wei, F.; Guo, J.; Guillem-Marti, J.; Ginebra, M.P.; Xiao, Y. Effect of Nano-Structural Properties of Biomimetic Hydroxyapatite on Osteoimmunomodulation. Biomaterials 2018, 181, 318–332. [Google Scholar] [CrossRef]
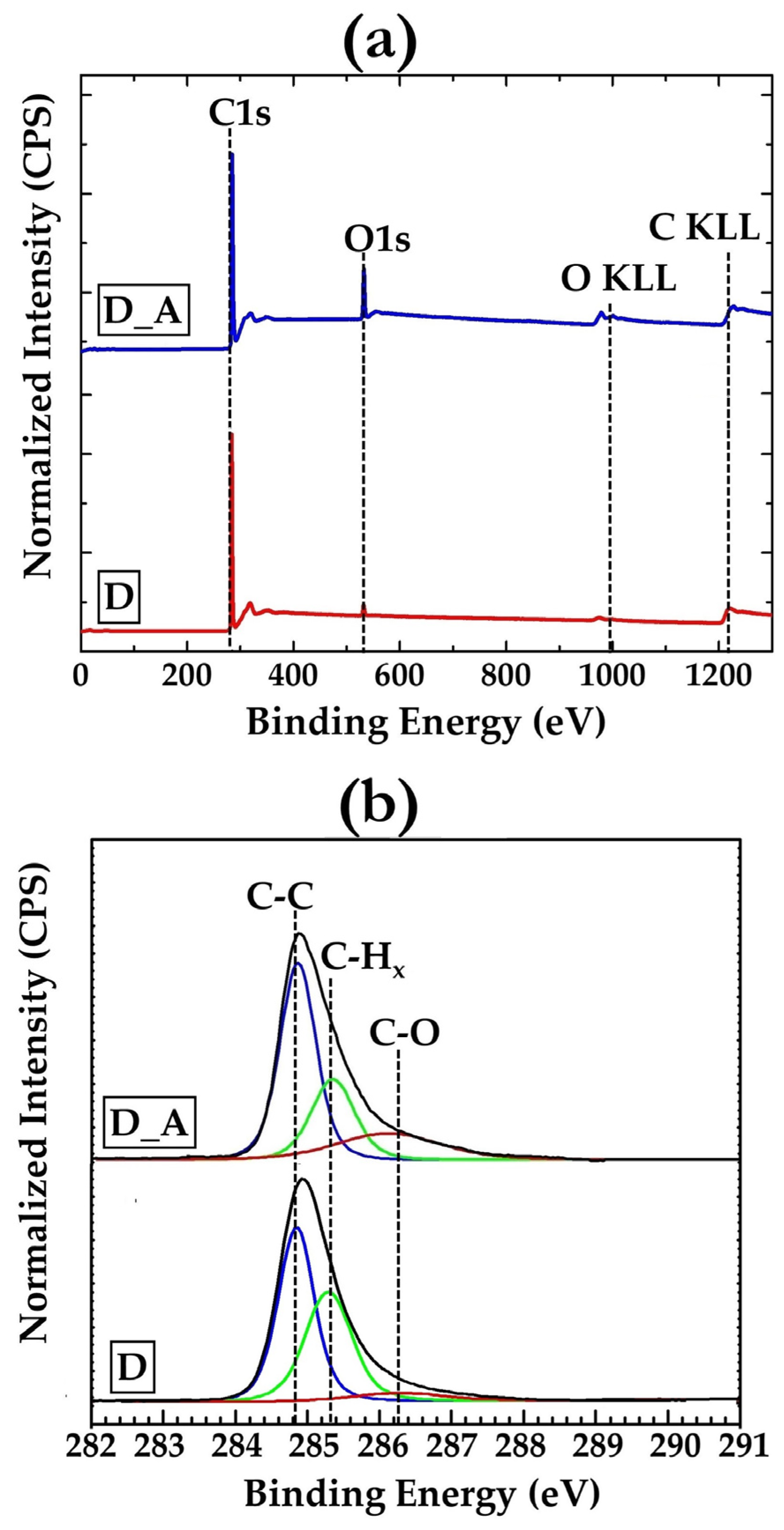
| Sample | C-C sp3 (%) | C-Hx (%) | C-O (%) |
|---|---|---|---|
| D | 56.4 | 42.1 | 1.5 |
| D_A | 54.2 | 36.3 | 9.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carcione, R.; Guglielmotti, V.; Mura, F.; Orlanducci, S.; Tamburri, E. Monitoring of Carbonated Hydroxyapatite Growth on Modified Polycrystalline CVD-Diamond Coatings on Titanium Substrates. Crystals 2024, 14, 66. https://doi.org/10.3390/cryst14010066
Carcione R, Guglielmotti V, Mura F, Orlanducci S, Tamburri E. Monitoring of Carbonated Hydroxyapatite Growth on Modified Polycrystalline CVD-Diamond Coatings on Titanium Substrates. Crystals. 2024; 14(1):66. https://doi.org/10.3390/cryst14010066
Chicago/Turabian StyleCarcione, Rocco, Valeria Guglielmotti, Francesco Mura, Silvia Orlanducci, and Emanuela Tamburri. 2024. "Monitoring of Carbonated Hydroxyapatite Growth on Modified Polycrystalline CVD-Diamond Coatings on Titanium Substrates" Crystals 14, no. 1: 66. https://doi.org/10.3390/cryst14010066
APA StyleCarcione, R., Guglielmotti, V., Mura, F., Orlanducci, S., & Tamburri, E. (2024). Monitoring of Carbonated Hydroxyapatite Growth on Modified Polycrystalline CVD-Diamond Coatings on Titanium Substrates. Crystals, 14(1), 66. https://doi.org/10.3390/cryst14010066