Abstract
Alzheimer’s disease (AD) is a complicated disease for which there are still no ideal one-target drugs, while multi-target drugs are closer to ideal drugs and will provide new solutions for the clinical treatment of AD. DL0410 is a promising multi-target drug candidate for AD treatment that is not only a significant inhibitor against both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) but also an antagonist of histamine H3 receptor (H3R), and its therapeutic efficacy in treating cognitive dysfunction has been validated in a series of AD-related animal models, including scopolamine-induced mice, D-galactose-induced and Aβ-induced mice, and APP/PS1 and SAMP8 mice. Although the structure of DL0410 has been analyzed using various detection techniques, such as MS and NMR, its three-dimensional crystal structure still requires further confirmation. In this study, the crystal of DL0410 was grown in aqueous solution, and its structure was detected using the X-ray diffraction method. The crystal data, atomic coordinates, bond lengths, angles, and hydrogen bonds of DL0410 were obtained. Its stability was proven by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Based on this study, the molecular docking of DL0410 with AChE, BuChE, and H3R was performed to uncover their interaction mechanisms and explain their bioactivities. This study provides important information for new multi-target drug design and the research and development of new drugs for AD treatment.
1. Introduction
Alzheimer’s disease (AD), the most common type of dementia in elderly people [1], is a progressive neurodegenerative disorder of the brain marked by the progressive loss of short-term memory [2] and impairment of language function [3], followed by spatial discrimination disorder [4], agnosia [5], and even changes in personality and behavior as the disease progresses [6,7]. Presently, this illness impacts over 30 million individuals globally. The World Alzheimer Report 2021’s figures indicate that there are currently approximately 50 million AD patients in the world, and that number is expected to rise to 152 million by 2050 [8]. Numerous pharmaceutical platforms have invested hundreds of millions of dollars in researching and developing anti-AD drugs in recent decades [9,10]; only cholinesterase inhibitors and glutamate receptor blockers are able to lessen the symptoms of AD, but they are still unable to effectively delay disease progression [11,12]. The main pathological features of AD manifest as Aβ plaques and tangled nerve fibers. There are multiple hypotheses [13] about the etiology of AD, such as Aβ hypothesis, tau protein hyperphosphorylation hypothesis, cholinergic hypothesis, neuroinflammation hypothesis, mitochondrial free radical damage hypothesis, etc., reflecting the complex pathological mechanisms of AD. For such a complex disease, scientists have proposed a multi-target treatment strategy [14], which means that drugs can simultaneously intervene in multiple stages of AD, block the disease process, and jointly improve cognitive function and even delay disease development [15].
DL0410 ((1,1′-([1,1′-biphenyl]-4,4′-diyl) bis (3-(piperidin-1-yl) propan-1-one) dihydrochloride), a structurally novel compound discovered through high-throughput screening and optimized by our laboratory (Figure 1), is a potential candidate drug against multiple targets for the treatment of AD. It is not only a significant inhibitor against both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) but also an antagonist of the histamine H3 receptor (H3R), and it also attenuates oxidative stress and neuroinflammation. The therapeutic efficacy of DL0410 on cognitive dysfunction has been validated in a series of AD-related animal models, including scopolamine-induced model mice, D-galactose-induced model mice and Aβ-induced mice, and APP/PS1 and SAMP8 mice [16]. Consequently, DL0410 was as effective as or more effective than donepezil and memantine at treating cognitive impairments. Multi-target drugs will be the focus of new drug research and development in the future [17,18]. In vitro and in vivo studies showed that DL0410 is a promising drug candidate for AD treatment (Figure 1).
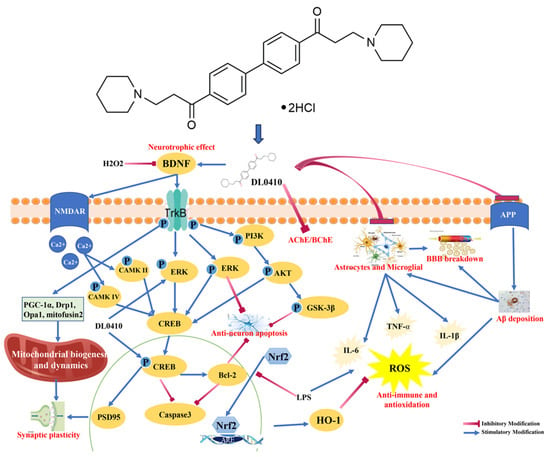
Figure 1.
The chemical structure of DL0410 and a summary of studies on the anti-AD effect and mechanisms of DL0410 in vitro and in vivo.
Since dosage forms are also an important part of new drug development, they can impact their bioavailability and stability [19], while the structure of compounds and their stability can provide crucial information for the formulation of drug delivery systems [20]. However, not much research about the structure and stability of DL0410 has been carried out. In this study, we examined the structure and properties of a DL0410 sample using cutting-edge analytical techniques to characterize its materials. We investigated its crystal structure using single-crystal X-ray diffraction and studied its stability with other analytical techniques such as differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA).
Molecular docking represents a structure-based drug design [21]. During the molecular docking process, small-molecule ligands and target protein active sites are matched and recognized according to their geometric space, energy, chemical environment, and other aspects, and the optimal binding mode between ligands and receptors is searched for through the continuous optimization of molecular conformation [22]. Based on the structure of DL0410, we performed molecular docking computations of DL0410 with AChE, BuChE, and H3R to explore their interaction mechanisms and in vitro bioactivities, which will provide important information for new multi-target drug designs and promote new drug research and development for AD.
2. Materials and Methods
2.1. Materials
DL0410 (99.7%, HPLC) was provided by the Institute of Materia Medica, Chinese Academy of Medical Sciences (Beijing, China).
2.2. Crystal Preparation
We weighed an amount of DL0410, which was poured into a conical bottle with 35 mL aqueous solution, making it a saturated solution. Then, the solution was heated and stirred. After the solution became clear, it was filtered. Then, we put the conical bottle into a low-temperature environment such as a refrigerator at 4 °C for 6 h, waiting for crystal growth. This work was completed in Prof. Li’s laboratory in the College of Pharmacy, Purdue University.
2.3. Single Crystal Detection
A colorless plate-shaped crystal of sample DL0410 (recorded as DL0410_0m in the crystal detection report) with formula C28H50Cl2N2O8 with approximate dimensions of 0.03 × 0.50 × 0.61 mm was mounted on a Mitegen micromesh mount in a random orientation. Triumph curved graphite crystal was used as a monochromator to collect data from a shock-cooled single crystal at 150(2) K on a Bruker AXS D8 Quest CMOS diffractometer (Billerica, MA, USA) with a precise focus-sealed tube X-ray source. The diffractometer used MoKα radiation (λ = 0.71073 Å). All data were integrated with SAINT V8.38A (Bruker AXS Inc., Madison, WI, USA), and SADABS 2016/2 was used to apply multi-scan absorption correction [23]. Direct methods using SHELXS-97 were used to solve the structure, and full-matrix least-squares methods using SHELXL-2018/3 were used to improve the solution against F2 [24,25]. All non-hydrogen atoms were refined with anisotropic displacement parameters. Carbon H atoms were refined isotropically on calculated positions using a riding model. Positions of pyramidal (sp3 hybridized) amine H atoms and water H atoms were refined isotropically. Uiso values were constrained to 1.5 times the Ueq of their pivot atoms for water molecules and 1.2 times for all other hydrogen atoms.
2.4. Thermal Analyses
DSC thermograms were acquired using a Q2000 differential scanning calorimeter equipped with an RCS90 refrigerated cooling system (TA instruments, Newcastle, DE, USA). Nitrogen was used as the purge gas at a flow rate of 50 mL/min. Temperature and enthalpy were calibrated using indium. The sample (~7 mg) was put into Tzero aluminum pans, and a single pinhole-equipped Tzero aluminum hermetic lid (TA instruments) was used to seal. The samples were heated to 300 °C at a rate of 10 °C/min. Data were processed using Universal Analysis software (TA instruments) [26].
The DL0410 crystal sample’s water content, that is the hydration state, was examined using a TGA 5500 system (TA Instruments) (New Castle, DE, USA). For the balance and samples, nitrogen was utilized as the purge gas at flow rates of 10 mL/min and 25 mL/min, respectively. Alumel and nickel standards were used to calibrate the temperature, and a magnetic bar was used to determine the Curie point temperature. Weight was calibrated using weight fixtures (343 mg, 443 mg, and 1240 mg). A platinum pan containing around 15 mg of the material was heated to 300 °C at a rate of 5 °C per minute from room temperature. The total weight loss from room temperature to ~94 °C was analyzed for water content [27,28].
2.5. Molecular Docking
The binding modes of DL0410 with acetylcholinesterase (AChE), butyrylcholinesterase (BuChE), and histamine 3 receptor (H3R) were revealed by CDOCKER [29] in Discovery Studio 2016 based on the crystal structure of DL0410. The crystal complex structure of AChE and its active ligand donepezil with a resolution of 2.45 Å (PDB ID:7E3H), the crystal complex structure of BuChE with N-((1-(2,3-dihydro-1H-inden-2-yl)piperidin-3-yl)methyl)-N-(2-(dimethylamino)ethyl)-2-naphthamide with a resolution of 2.10 Å (PDB ID:5NN0), and the crystal complex structure of H3R and N-ethyl-3-fluoranyl-3-[3-fluoranyl-4-(pyrrolidin-1-ylmethyl)phenyl]cyclobutane-1-carboxamide (CAS:935840-31-6) with a resolution of 2.60 Å (PDB ID:7F61) were downloaded from the Protein Data Bank (https://www.rcsb.org/ accessed on 7 December 2023) for the comparison of the molecular docking of DL0410 and the original ligands. Before molecular docking, protein crystals needed to be pretreated, including removing ligand molecules from crystal complexes. The preprocessed protein structure was defined as the receptor for docking, and the binding site of molecular docking was also defined. At the same time, the structure of DL0410 was optimized. After all the docking parameters were in appropriate positions, the original ligand molecules in the crystal structure were cut out and reconnected back to the pre-defined active sites to calculate the value of root mean square deviation (RMSD). Finally, the values of docking energy between DL0410 and proteins were calculated, and the interaction modes of DL0410 with AChE, BuChE, and H3R were explored by molecular docking.
3. Results
3.1. The Crystal Structure of DL0410
After the saturated solution of DL0410 was cooled, we obtained the colorless crystal for its crystal structure detection. The database accession number for deposited atomic coordinates for DL0410 is 2289807. The detection results showed that the crystal size is 0.030 × 0.500 × 0.610 mm3, and the main crystal cell parameters are as follows, a = 7.2995(4), b = 8.6002(5), and c = 13.0646(7). Alpha = 102.669(2), beta = 94.052(2), gamma = 100.705(2), and the molecular number in the unit cell (Z) is one. This belongs to the triclinic crystal system, space group P1, and the crystal density (Dx) is 1.305 g cm−3. Detailed information is listed in Figure 2. The chloride anion and water molecules were disordered, with the single chloride anion being disordered with all four water molecules. The positions of the disordered Cl and O atoms were freely refined, but the atomic displacement parameters (ADPs) of the overlapping atoms were restricted to be pairwise identical. In a slight simplification of the disorder model, water H atoms were assigned the same occupancy as their carrying oxygen atoms and were not positionally split. The water H atom positions were refined, and the O-H and H···H distances were restrained to 0.84(2) and 1.36(2) Å, respectively. The chloride occupancy rates were refined to 0.500(2) for Cl5, 0.183(2) for Cl2, 0.249(2) for Cl3, and 0.067(2) for Cl4 under these conditions. Water and chloride, when H-bonded, have very similar van der Waals radii and get swapped easily. The total Cl to H2O ratio is thus 1:3, and for one whole molecule (which is a detection), the formula is C28H38N2O2, 2(Cl), 6(H2O) (thus a hexahydrate). The bond lengths and angles for DL0410 are shown in Table 1, the torsion angles are shown in Table 2, and the hydrogen bonds are displayed in Table 3.
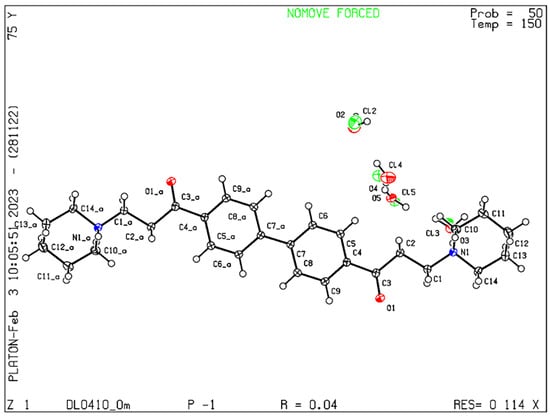
Figure 2.
X-Ray structure of DL0410.

Table 1.
Bond lengths and angles for DL0410.

Table 2.
Torsion angles for DL0410.

Table 3.
Hydrogen bonds for DL0410.
3.2. Thermal Analyses
3.2.1. DSC Analysis
By using a thermoanalytical technique called differential scanning calorimetry or DSC, the difference in the amount of heat required to increase the temperature of a sample is measured as a function of temperature. The DSC results are shown in Figure 3. The heat flow of the crystal sample changed rapidly between approximately 75 and 110 °C since there are water molecules in the crystal. The lowest peak was at about 238 °C since it was the melting point of the compound DL0410.
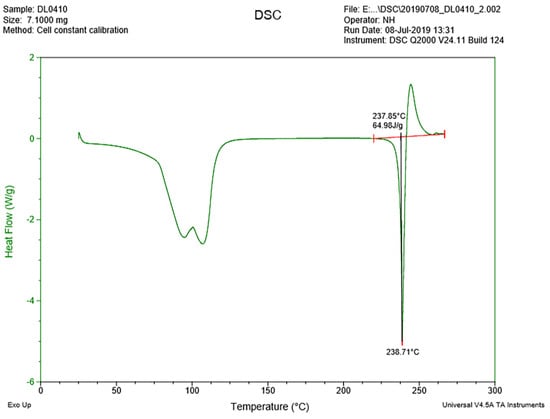
Figure 3.
The DSC thermogram for DL0410 crystal sample.
3.2.2. TGA Result
Thermal gravimetric analysis (TGA) is a method of thermal analysis in which the water content can be investigated. From Figure 4, we can see that the weight loss of the crystal sample started when the system was heated, and this ended at about 100 °C, and the lost weight is 17.338%, which is consistent with the percentage of water in the crystal structure.
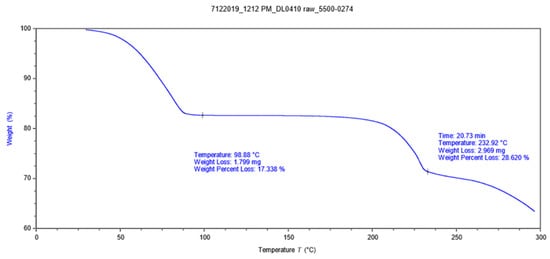
Figure 4.
TGA plots for DL0410 crystal sample.
3.3. Molecular Docking of DL0410 with AChE, BuChE, and H3R
DL0410 is a potent AChE inhibitor with IC50 = 0.29 µM, and its ability to inhibit BuChE was further discovered by machine learning with an IC50 of 3.96 µM. In addition, DL0410 also displayed significant antagonism on H3R with IC50 = 0.31 µM, while donepezil does not affect it [30,31]. As we all know, AChE, BuChE, and H3R are very important drug targets against AD [32,33], so the interaction between DL0410 and these drug targets should provide important information for promoting its further study and for new multi-target drug design. Therefore, based on the crystal structure of DL0410, we performed molecular docking of DL0410 with three targets: AChE, BuChE, and H3R.
3.3.1. Molecular Docking of DL0410 with AChE
It is generally believed that the docking system is more stable when the value of root mean square deviation (RMSD), a parameter used to evaluate the stability of the docking system, is less than two. As shown in Figure 5A, the RMSD values of the ten conformations of donepezil generated by docking were all less than two, indicating that the docking system was relatively stable. The maximum value of −CDOCKER ENERGY generated by DL0410 docking with AChE was 23.227, which is greater than that of donepezil docking with AChE (Figure 5B). As shown in Figure 5C–H, the original ligand donepezil can form Pi-Alkyl interactions with AChE amino acid residues Tyr341 and Tyr337; a conventional hydrogen bond with AChE amino acid residues Phe295; Pi-Pi stacked interactions with AChE amino acid residues Tyr341, Trp286, and Trp86; and carbon hydrogen bonds with AChE amino acid residues Ser293 and Tyr341. Furthermore, the interactions of donepezil also include Pi-Sigma interaction with AChE amino acid residues Tyr341. DL0410 can form four similar interactions to donepezil, including Pi-Alkyl interactions with AChE amino acid residues Trp86 and His447; a conventional hydrogen bond with AChE amino acid residues Ser293; Pi-Pi stacked interactions with AChE amino acid residues Tyr341 and Trp286; and a carbon hydrogen bond with AChE amino acid residues Ser293 and Tyr341.
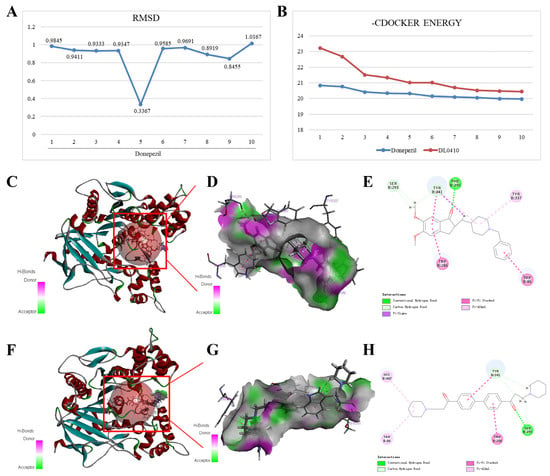
Figure 5.
Interactions of AchE (PDB ID: 7E3H) with the original ligand donepezil and DL0410. (A) The RMSD values of the ten conformations of donepezil generated by docking. (B) The −CDOCKER ENERGY values of ten conformations generated by donepezil and DL0410 docking with AchE, respectively. Interaction diagrams of donepezil (C–E) and DL0410 (F–H) docking with AchE, respectively.
3.3.2. Molecular Docking of DL0410 with BuChE
As shown in Figure 6A, the RMSD values of the six conformations generated by the original ligand docking with BuChE were less than 2.5, and the minimum RMSD value was 0.8238, indicating that the system was relatively stable and used for subsequent docking. The maximum values of −CDOCKER ENERGY generated by the original ligand and DL0410 docking with BuChE were 6.189 and 20.331, respectively (Figure 6B). As shown in Figure 6C–H, the original ligand can form Pi-Alkyl and Alkyl interactions with BuChE amino acid residues Ala328, Phe329, and Leu286; carbon hydrogen bonds with BuChE amino acid residues Gly116, Glu197, and Ser198; and Pi-Pi T-shaped and amide-Pi stacked with BuChE amino acid residues Phe329, Trp231, and Gly116. Furthermore, the potential interaction also included Pi-Sigma interaction with BuChE amino acid residues Tyr332. Dl0410 can form similar interactions to the original ligand, including Pi-Alkyl and Alkyl interactions with BuChE amino acid residues Ala328 and Tyr332; a carbon hydrogen bond with BuChE amino acid residues Ser287; and amide-Pi stacked with BuChE amino acid residues Gly116. In addition, DL0410 can form conventional hydrogen bonds with BuChE amino acid residues Glu197 and His438.
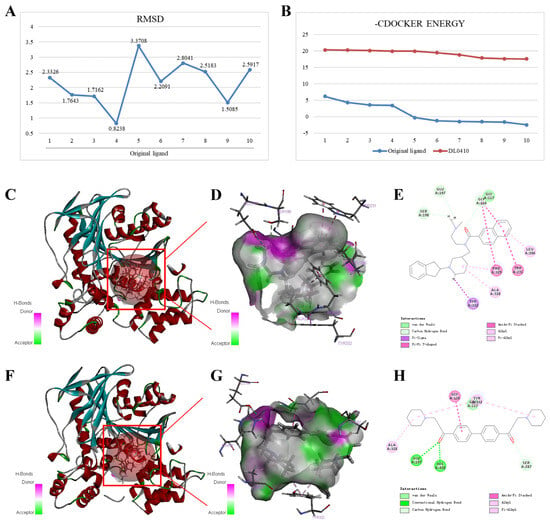
Figure 6.
Interactions of BuChE (PDB ID: 5NN0) with the original ligand and DL0410. (A) The RMSD values of the ten conformations of original ligand generated by docking. (B) The −CDOCKER ENERGY values of ten conformations generated by original ligand and DL0410 docking with BuChE, respectively. Interaction diagrams of original ligand (C–E) and DL0410 (F–H) docking with BuChE, respectively.
3.3.3. Molecular Docking of DL0410 with H3R
As shown in Figure 7A, the RMSD values of the ten conformations of the original ligand generated by docking were all less than two, indicating that the docking system was relatively stable. The maximum values of −CDOCKER ENERGY generated by the original ligand and DL0410 docking with H3R were 4.771 and 13.053, respectively (Figure 7B). As shown in Figure 7C–H, the original ligand can form Pi-Alkyl and Alkyl interactions with H3R amino acid residues Cys118, Leu401, Trp402, Phe398, Tyr374, Tyr189, and Phe193; conventional hydrogen bonds with H3R amino acid residues Asp114, Tyr91, and Tyr115; Pi-Pi T-shaped and Pi-Pi stacked with H3R amino acid residues Phe398 and Tyr115; and carbon hydrogen bonds with H3R amino acid residues Tyr374 and Tyr189. Furthermore, the potential interactions also included Halogen (Fluorine) bond with H3R amino acid residues Cys188 and unfavorable donor–donor interaction with H3R amino acid residues Tyr94. Dl0410 can form similar interactions to the original ligand, including Pi-Alkyl and Alkyl interactions with H3R amino acid residues Cys118, Leu401, Trp402, Phe398, Tyr374, His187, and Arg27; conventional hydrogen bonds with H3R amino acid residues Tyr115 and Arg27; Pi-Pi T-shaped with H3R amino acid residues Phe193 and Phe398; and carbon hydrogen bonds with H3R amino acid residues Tyr374, Tyr189, and Gly98. In addition, DL0410 can form a Pi-Donor hydrogen bond with H3R amino acid residue Tyr91.
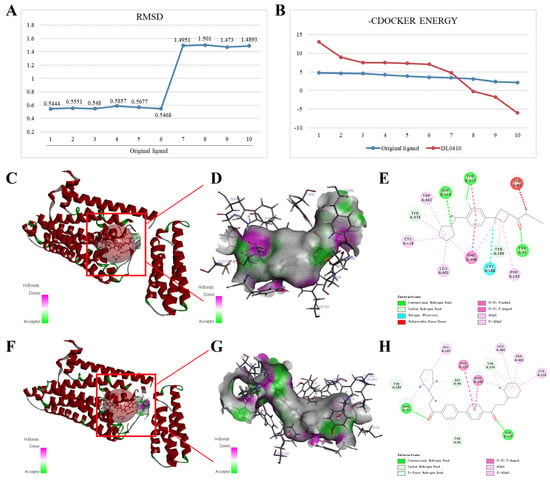
Figure 7.
Interactions of H3R (PDB ID: 7F61) with the original ligand and DL0410. (A) The RMSD values of the ten conformations of original ligand generated by docking. (B) The −CDOCKER ENERGY values of ten conformations generated by original ligand and DL0410 docking with H3R, respectively. Interaction diagrams of original ligand (C–E) and DL0410 (F–H) docking with H3R, respectively.
4. Discussion and Conclusions
Due to the complex pathological mechanisms of AD, there are currently no effective therapeutic drugs available, making the development of multi-target anti-AD drugs particularly important [34,35]. DL0410 is not only a potent inhibitor against both AChE and BuChE but also an antagonist of histamine H3R, and its therapeutic efficacy on cognitive dysfunction has been validated in a series of AD-related animal models, including scopolamine-induced mice, D-galactose-induced and Aβ-induced mice, APP/PS1 transgene mice, and SAMP8 mice [16]. DL0410 has multiple effects which can improve AD, including protecting mitochondrial structure and function, inhibiting oxidative stress damage and neuroinflammation, promoting neurotrophic effects, inhibiting neuronal apoptosis, improving cholinergic transmission, and synaptic plasticity. Therefore, DL0410 was shown to have a beneficial effect on cognitive defects. Although we have analyzed the structure of DL0410 using various detection techniques such as MS and NMR, its three-dimensional structure still requires further confirmation of the crystal structure. In this study, the crystals of DL0410 were tried to prepare and detect. Fortunately, we successfully obtained the crystals and detected these with advanced crystal detection equipment.
DL0410 is a novel symmetrical small molecule. In the crystal structure of DL0410 (Figure 2), it can be seen that the part of piperidine from C(12a) to the other part of piperidine C(12) is fully extended, the biphenyl ring C(4a) to C(4) is a conjugated system, and the two benzene rings are coplanar. Two linker propionyl chains (CH2CH2CO−) connecting biphenyl and two piperidine moieties from both C(1a)H2C(2a)H2C(3a)O and C(3)OC(2)H2C(1)H2 are in staggered conformation. This structure can be identified using DSC and TGA. Future studies should consider studying other possible crystal forms of DL0410 and their impact on their bioavailability, as well as their activities in vivo, in order to study the formulation of preparation and the optimal crystal form.
The structure of DL0410 (Figure 1) contains biphenyl and piperidine scaffolds. Biphenyl is robust, which is the basis for the stability of this molecular structure, while the carbonyl groups and nitrogen atoms in the structure are very important for its bioactivities, as the molecular docking results described. The maximum values of the −CDOCKER ENERGY generated by the docking of DL0410 with AChE, BuChE, and H3R are all greater than those generated by the docking of the original ligand with the three proteins, respectively. Four similar interactions are formed between DL0410 and AChE, as well as between donepezil and AChE. In addition, DL0410 can form conventional hydrogen bonds with BuChE amino acid residues Glu197 and His438. Similarly, the interaction between DL0410 and H3R has four similarities, with the interaction between the original ligand and H3R; additionally, DL0410 can form a Pi-Donor hydrogen bond with H3R amino acid residue Tyr91. The molecular docking results showed that DL0410 possess high interaction with AChE, BuChE and H3R.
The bioactivities of DL0410 on these three targets of AChE, BuChE, and H3R were detected in our laboratory and published. Further in vivo studies on AD animal models will be able to confirm that DL0410 is a promising drug candidate for AD treatment. Therefore, the crystal structure studies of DL4010 will produce very important information for further drug research and development.
Author Contributions
Methodology, A.L., J.Z., N.J.H., M.Z., L.W. and T.L.; software, A.L. and J.Z.; validation, A.L., J.Z., M.Z. and L.W.; formal analysis, A.L., J.Z., N.J.H., M.Z. and T.L.; investigation, A.L., N.J.H. and L.W.; resources, A.L.; data curation, A.L. and T.L.; writing—original draft, A.L. and J.Z.; writing—review and editing, A.L. and T.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Great Science Technology Projects (2014ZX09507003-002, 2018ZX09711001-003-002), the Beijing National Science Foundation (7192134), and CAMS Innovation Fund for Medical Sciences (CIFMS no. 2021-I2M-1-028, 2021-I2M-1-057).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Afrashteh, N.; Kolb, B.E.; Mohajerani, M.H. Hearing loss and impaired short-term memory in an Alzheimer’s disease mouse model of amyloid-beta pathology. Exp. Neurol. 2023, 365, 114413. [Google Scholar] [CrossRef] [PubMed]
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, Y.; Tian, X.; Lu, W.; Wang, Z.; Zeng, X.; Wang, L. Multi-resolution 3D-HOG feature learning method for Alzheimer’s Disease diagnosis. Comput. Methods Programs Biomed. 2022, 214, 106574. [Google Scholar] [CrossRef] [PubMed]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 107. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf. (accessed on 7 December 2023). [CrossRef]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frolich, L.; Jack, C.R., Jr.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimers Res. Ther. 2016, 8, 39. [Google Scholar] [CrossRef]
- Goldman, D.P.; Fillit, H.; Neumann, P. Accelerating Alzheimer’s disease drug innovations from the research pipeline to patients. Alzheimers Dement. 2018, 14, 833–836. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Sorbi, S. The complexity of Alzheimer’s disease: An evolving puzzle. Physiol. Rev. 2021, 101, 1047–1081. [Google Scholar] [CrossRef] [PubMed]
- Benek, O.; Korabecny, J.; Soukup, O. A Perspective on Multi-target Drugs for Alzheimer’s Disease. Trends Pharmacol. Sci. 2020, 41, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar] [PubMed]
- Zhang, B.; Lian, W.; Zhao, J.; Wang, Z.; Liu, A.; Du, G. DL0410 Alleviates Memory Impairment in D-Galactose-Induced Aging Rats by Suppressing Neuroinflammation via the TLR4/MyD88/NF-κB Pathway. Oxid. Med. Cell Longev. 2021, 2021, 6521146. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Barbarossa, A.; Leuci, R.; Carrieri, A.; Brunetti, L.; Laghezza, A.; Catto, M.; Limongelli, F.; Chaves, S.; Tortorella, P.; et al. Novel Phenothiazine/Donepezil-like Hybrids Endowed with Antioxidant Activity for a Multi-Target Approach to the Therapy of Alzheimer’s Disease. Antioxidants 2022, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Dai, C.L.; Liu, F.; Iqbal, K. Multi-Targets: An Unconventional Drug Development Strategy for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 837649. [Google Scholar] [CrossRef]
- Rocha, B.; de Morais, L.A.; Viana, M.C.; Carneiro, G. Promising strategies for improving oral bioavailability of poor water-soluble drugs. Expert Opin. Drug Discov. 2023, 18, 615–627. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Crampon, K.; Giorkallos, A.; Deldossi, M.; Baud, S.; Steffenel, L.A. Machine-learning methods for ligand-protein molecular docking. Drug Discov. Today 2022, 27, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Moon, H.K. Gravimetric analysis and differential scanning calorimetric studies on glycerin-induced skin hydration. Arch. Pharm. Res. 2007, 30, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Delaney, S.P.; Nethercott, M.J.; Mays, C.J.; Winquist, N.T.; Arthur, D.; Calahan, J.L.; Sethi, M.; Pardue, D.S.; Kim, J.; Amidon, G.; et al. Characterization of Synthesized and Commercial Forms of Magnesium Stearate Using Differential Scanning Calorimetry, Thermogravimetric Analysis, Powder X-ray Diffraction, and Solid-State NMR Spectroscopy. J. Pharm. Sci. 2017, 106, 338–347. [Google Scholar] [CrossRef]
- Kumar, M.; Sabbarwal, S.; Mishra, P.K.; Upadhyay, S.N. Thermal degradation kinetics of sugarcane leaves (Saccharum officinarum L.) using thermo-gravimetric and differential scanning calorimetric studies. Bioresour. Technol. 2019, 279, 262–270. [Google Scholar] [CrossRef]
- Wu, G.; Robertson, D.H.; Brooks, C.L., 3rd; Vieth, M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER-A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef]
- Fang, J.; Li, Y.; Liu, R.; Pang, X.; Li, C.; Yang, R.; He, Y.; Lian, W.; Liu, A.L.; Du, G.H. Discovery of multitarget-directed ligands against Alzheimer’s disease through systematic prediction of chemical-protein interactions. J. Chem. Inf. Model. 2015, 55, 149–164. [Google Scholar] [CrossRef]
- Fang, J.; Yang, R.; Gao, L.; Zhou, D.; Yang, S.; Liu, A.L.; Du, G.H. Predictions of BuChE inhibitors using support vector machine and naive Bayesian classification techniques in drug discovery. J. Chem. Inf. Model. 2013, 53, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bolanos, J.G.; Lopez, O. Butyrylcholinesterase inhibitors as potential anti-Alzheimer’s agents: An updated patent review (2018-present). Expert Opin. Ther. Pat. 2022, 32, 913–932. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Kishi, T.; Matsunaga, S.; Iwata, N. Histamine H3 Receptor Antagonists for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. J. Alzheimers Dis. 2015, 48, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).