Abstract
The benefits of crystallization in a microgravity environment have been documented. Herein, we update the community on the results of a retrospective meta-analysis and data curation of 507 unique crystallization experiments that have been disseminated in the literature over a broad diversity of substrates. The analysis variables in the dataset that were evaluated include individual success metrics such as size, structural improvement, improved uniformity, increased resolution limit, and improved mosaicity. The overall databases were evaluated over time and by molecular complexity. Compared to ground experiments, crystals grown in a microgravity environment continue to show improvement across all metrics evaluated. The retrospective analysis demonstrates that success rates of crystallization experiments in microgravity improved over time. There also seems to be no correlation between complexity of molecule, using molecular weight as a surrogate for complexity, and successful crystallization under microgravity conditions. The microgravity environment provides improvement to crystal fabrication across metrics utilized for evaluation and molecular types, and the datasets utilized for this investigation are excellent tools for this evaluation.
1. Introduction
Single crystals of high quality are sought for a variety of applications, from semiconductors to drug discovery. Advances in crystallization strategies for a broad variety of these diverse applications continues to be of interest. These advances could be for specific types of crystals [1], for challenging classes of compounds [2], or incremental improvements to existing strategies [3]. Methods that produce crystals that are superior in key properties will provide more accurate solid-state structures for proteins and macromolecules, may provide superior properties for inorganic compounds, and offer the possibility of new polymorphic forms of crystalline compounds.
The interest in the microgravity environment for improved fabrication of materials includes the crystallization of compounds [4,5]. Crystals as simple as table salt [6] and as complex as monoclonal antibodies [7] have been formed in microgravity, and most have improved properties or improved structures compared to their terrestrial counterparts. Crystallization experiments were among the first scientific experiments in microgravity, starting in 1973 [8], and have continued to be part of the experimental portfolio in Earth orbit to the present day.
Early and recent retrospective analyses of microgravity experimentation were very discipline-specific, and focused on subsets of materials like advanced electronic materials [4], methods like TIM crystallization [9], or targets including proteins [5,10,11], retrospective analyses of one country’s protein crystallizations [12], perspectives on the outlook of protein crystallizations [13], and overall methodologies [14]. While not part of this study, an account of types of diffusion techniques for macromolecular crystallization [15] and a communication that includes common forms of semiconductor crystallization have been performed [16]. A recent preliminary report of aggregated crystal data across disciplines indicated that microgravity conditions had positive impacts on crystal formation [17]. More data have been added to the databases utilized for this study. This affords an excellent opportunity to provide an update to the data analysis.
2. Materials and Methods
A retrospective meta-analysis of publicly available crystallization experiments (scientific articles, research reports, books, etc.) was undertaken, and two databases of the relevant metrics were constructed (see links in Supplementary Materials). The literature was searched using key search engines and repositories (Google Scholar, SciFinder, NASA data repositories, the Physical Sciences Informatics database, and National Center for Biotechnology Information system). In addition, the publication indexes of academic journals with more than one microgravity crystallization report (such as npj Microgravity, Acta Cryst., J. Cryst. Growth, etc.) were searched individually. These reports were sorted into the appropriate database depending on the identity of the compound crystallized. One database contains information for macromolecular and/or organic molecules. The macromolecular compounds included in the database are mostly proteins and enzymes, both with and without substrates bound, viruses, and multi-protein complexes. The organic compounds in this database include smaller polypeptides (30 amino acids or fewer), small organic compounds (pivalic acid, triglycine sulfate, 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile—also known as ROY, and tetrathiafulvalene-7,7,8,8-tetracyanoquinodimethane—also known as TTF-TCNQ). The second database contains information for inorganic compounds such as ice, sodium chloride, hydroxyapatite, calcium carbonate, silica, and zeolites, including semiconductors like gallium arsenide, cadmium telluride, and indium antimonide.
Each database includes source material information (material crystallized, authors/PIs, year flown, mission flown, article title, journal and/or book title, DOI—if available, sponsoring country), experimental information (crystal shape, number of crystals, whether pictures are included in the report, molecular weight, crystal size, unit cell parameters, space group, methods, conditions, data reported, flights complications, materials, temperature, B factor, I/sigma, etc.), and an analysis of improvement over ground-based experiments (larger crystal size, increased uniformity, structural improvement, superior mosaicity, improved resolution limit, and superior cycle time, throughput, yield, optical, or thermoelectric performance in the case of semiconductors). Combining the information from both databases provided 573 unique entries. All the experiments have been included in this report, including duplicate experiments (where crystals were fabricated in multiple runs of a single PI or author’s work), where experiments failed due to equipment failure and/or complications from launch or landing, where crystallization chambers failed, where crystals were unstable, and/or syringe leaks were documented. This worst-case scenario provided an accurate representation of the overall probability for success for crystallization experiments under microgravity conditions including all factors.
Given the wide variety of disciplines of the experimentalists, experimental objectives, crystallization targets, and other compounding factors, not all studies reported on the same metrics. The data provided in the literature for each metric were classified as “shows improvement”, “same as ground studies”, or “worse than ground studies”. Studies without a particular metric were excluded from the data reported. Crystal studies that report metrics where the first reported crystal was the result of a microgravity experiment were classified as improved for that metric.
For the metric of size, crystals were larger compared to ground experiments up to five times the volume [18]. If the authors in the reference discussed improved uniformity specifically in their text, often providing statements such as “improved morphology” [19] and “high isotropy” [20], it was reported as improved in our database. Due to structural enhancement, microgravity-grown crystals were described as having “fewer flaws” [18] and being “better developed” [21]. Improvements in the numerical measure of mosaicity, as in [20], and resolution limit, as in [22], where lower numbers were better, were used to determine whether comparative data were provided.
Removing the experiments where there were documented complications, listed above, provided 536 unique entries. An analysis of these data provided insight into realistic expectations for microgravity research in the present day given the technological expertise and experience gathered from previous failures. These data were evaluated on the same metrics as above and reported herein. The data reported here are as of 31 October 2023.
3. Results
3.1. Updated Results
In the preliminary report in 2022 [17], 228 (159 macromolecules/organic compounds and 69 inorganic compounds) were included in the database. Since that time, an additional 279 crystal studies with reported data for the metrics evaluated have been added for a total of 507 (316 macromolecules/organic compounds and 191 inorganic compounds). Experiments that did not report on a particular metric were excluded from the data. The updated data from these analyses can be found in Table 1. Mosaicity and resolution limit are metrics that are pertinent to macromolecules, not inorganic compounds or small organic molecules. This updated analysis is consistent with the metrics previously reported, demonstrating that the database continues to be an excellent tool for the evaluation of microgravity experimentation.

Table 1.
Overall evaluation of crystals based on individual metrics; all data, n = 507.
The number of data points in each category of the information pool have doubled or more than doubled since the preliminary report. For example, the number of inorganic molecules reported as larger in size was 39 in the preliminary report, whereas the database now contains 90 studies that report larger crystal sizes. The microgravity crystals measured larger, displayed structural improvements, and had superior uniformity compared to their terrestrial counterparts across all crystal types. The macromolecules grown in microgravity also have improved resolution limit and mosaicity, i.e., the measured values were lower, compared to crystals grown on Earth.
The improvement in at least one metric is a key indicator of the impact of microgravity on crystallization experiments (Table 2). Out of the 507 compounds, 451 (89%) showed improvement in at least one of the evaluation metrics. This is also consistent with the previous report. More than one third of the crystals grown in microgravity were reported to be improved in three or more metrics. This is especially remarkable given the broad diversity of disciplinary areas represented in this dataset (physics, material science, engineering, chemistry, medicinal chemistry, etc.), the wide range of applications for the crystals grown (crystallography, electronics, basic science, medicine, etc.), and the lack of consistency in reporting the metrics selected in Table 1 in the literature.

Table 2.
Overall improvement in one, two, three, or more metrics (n = 507); all data.
Excluding literature accounts of crystallization experiments with reported complications resulted in a smaller pool of data: 477 individual compounds. Evaluating for the same metrics in Table 1, the percentages in each of the categories either remained the same or were bettered (Table 3). The one metric that improved the most when experiments with complications were removed was crystal uniformity. More uniform crystals have more consistent performance properties [23]; physical properties like solubility, stability, dissolution rate, bioavailability, and tabletability [24]; and predictable electronic structures, bonding, surface energies, and chemical reactivity [25]. The improved uniformity across all crystal types (organic, macromolecular, inorganic) grown in microgravity is especially striking.

Table 3.
Overall evaluation of crystals based on individual metrics; complications removed.
When compounds with descriptions of experimental complications in the account were excluded, the overall improvement in at least one metric ticked up slightly to 90% (Table 4). Seven of the experiments with complications gave results that were the same or showed no improvement in the metrics when compared to their Earth-grown counterparts. This was 13% of the “no improvement or the same” pool, which is a significant percentage of the poorer results. Notably, there were crystallization experiments with known complications (5 or 3%) that showed improvement in three or more metrics that have also been removed from the pool for this analysis.

Table 4.
Overall improvement in one, two, three, or more metrics (n = 477); complications removed.
Updated information will be added to the databases as research from microgravity crystallizations are added to the literature. Researchers in the field are encouraged to report on as many of the key metrics as possible. Microgravity research implementation partners are also encouraged to share key pieces of data on crystallization success, as this can be conducted without revealing sensitive information about experiments or experimental design.
3.2. Analysis by Year
An analysis of the combined dataset by the year the experiment was completed in microgravity was undertaken. Our hypothesis was that scientific experience and improved techniques would lead to improved crystal growth in at least one metric over time. Preliminary work indicated that, for proteins, this did not hold true [15].
An overall analysis of the data in ten-year increments (1970s, 1980s, 1990s, 2000s, and 2010s) demonstrates that the percentage of crystals that were superior in at least one metric (size, uniformity, structure, mosaicity, resolution limit, and/or performance) and did improve over time. From an initial starting point in the mid-60% range, the enhancement by microgravity seemed to have leveled out to the low 80% range across all crystal types (Figure 1). The number of experiments in each decade ranged from 29 in the 1970s, to a high of 187 in the 1990s.
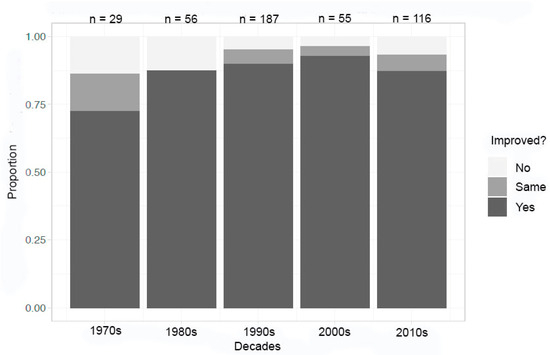
Figure 1.
Decadal summary of improvement in at least one metric.
The analysis of organic/macromolecules in the same time ranges is consistent with Wilkinson’s work [19], demonstrating that macromolecules/organic compounds do not seem to benefit from improvements made over time and from scientific experience (Figure 2). It should be noted that several examples in the dataset (seven) were successfully crystallized for the first time ever in microgravity, which were classified as “improved” for all metrics reported. In addition, macromolecule/organic compound crystallization in microgravity did not begin until the mid-1980s, mirroring advancements in terrestrial crystallization of proteins [26] and crystallographic visualization techniques [27]. It could also be that more challenging problems, like the proteins that were first crystallized in microgravity, were chosen for experimentation. The number of crystallization experiments was greatest in the 1990s, fell in the 2000s, and has seen a recent uptick in the 2010s.
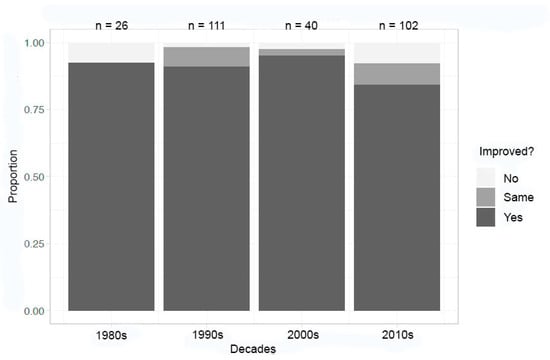
Figure 2.
Decadal summary of improvement in at least one metric—organic/macromolecules.
The analysis of inorganic compounds in the same time ranges shows significant improvement in success in at least one metric by the 2010s (Figure 3). The rate of improvement in at least one metric began at about 67% in the 1970s and improved to near 100% in the 2010s. This improvement was commensurate with additional crystallization methods becoming available in a low gravity environment. Notably, the number of crystallization reports has fallen significantly since the high reported in the 1990s.
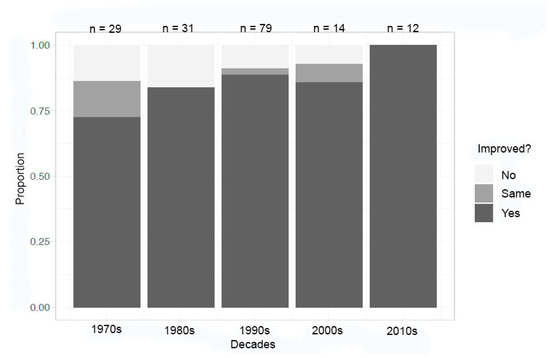
Figure 3.
Decadal summary of improvement in at least one metric—inorganic compounds.
Performing the same analyses removing the experiments with known complications provided similar improvements in crystallization experiments in microgravity over the decades (Figure 4). However, by excluding the experiments with known complications reported in the literature, the large leap in improvement in at least one metric to the mid-80% range occurred between the 1970s and 1980s and remained until present. The complication percentage (the number of experiments in each decade with reported complications) ranges between 0 and 14%. The 1980s had the largest percentage of experimental complications at 14%, and the 2000s had no technical or experimental challenges reported.
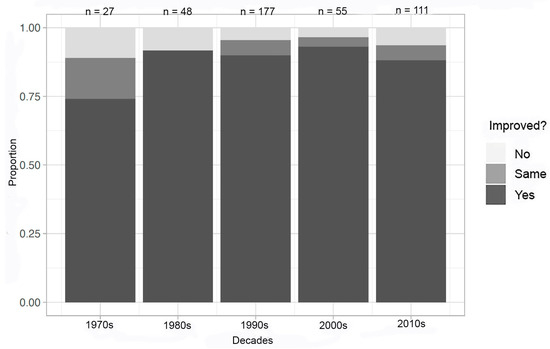
Figure 4.
Decadal summary of improvement in at least one metric—all compounds; complications removed.
An evaluation of organic/macromolecular compounds through the decades also exhibited no overall improvement over the decades (Figure 5). However, the highest percentage of experiments with known complications took place in the 1980s (17% of experiments reported in the literature in that decade). This percentage dropped to 5% in the 1990s, 0% in the 2000s, and 3% in the 2010s. For both inorganic and organic/macromolecular compounds, the highest percentage of technical or experimental challenges occurred in the 1980s.
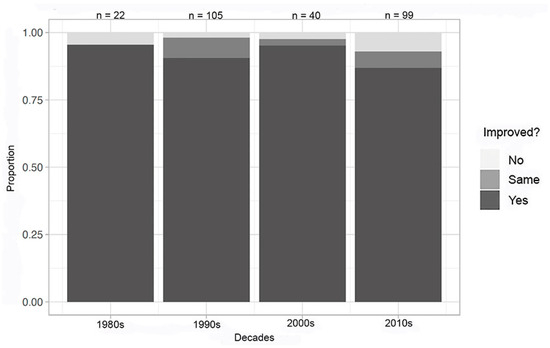
Figure 5.
Decadal summary of improvement in at least one metric—organic/macromolecular compounds; complications removed.
The analysis of the inorganic compounds crystallized through the decades with the experimental problems removed demonstrates the overall improvement leap between the 1970s and 1980s as well (Figure 6). Notably, there were 7% reported complications in the 1970s, 16% reported complications in the 1980s, 3% complications in the 1990s, and no reported complications in the 2000s or 2010s for inorganic compounds (although the number of experiments that were performed are comparatively fewer for the 2000s and 2010s).
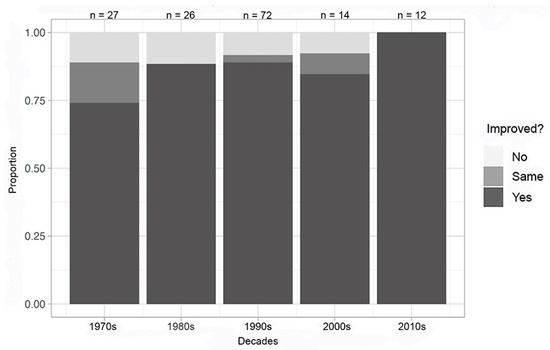
Figure 6.
Decadal summary of improvement in at least one metric—inorganic compounds; complications removed.
As microgravity crystallization experiments began in the 1970s, it is no surprise that there were more challenges both from a technical standpoint (complications) and from an experimental standpoint (fewer “successful” experiments). In the 1980s, significant improvements in the experimental conditions were realized to produce favorable results, but there were still significant technical complications. By the 1990s, moving forward, both technical and experimental conditions for crystallization in microgravity had improved to very high numbers.
3.3. Analysis by Complexity
The molecules represented in this study range from simple ionic compounds like table salt, with a molecular weight of 58 g/mol, to a multi-subunit protein complex, photosystem 1, which has a molecular weight of 1,200,000 g/mol (1200 kDa). Using molecular weight as a surrogate for complexity, the compounds were separated into molecular weight ranges. It was our hypothesis that molecular weight of the compound crystallized has no impact on the improvement in at least one metric (size, uniformity, structure, mosaicity, resolution limit, and/or performance) in microgravity. An analysis using a series of molecular weight ranges was undertaken.
The entire dataset was sorted using molecular weight ranges of under 200, 200–500, 500–25,000, 25,000–75,000, 75,000–250,000, and 250,000–1,200,000 g/mol (Figure 7). Success in at least one metric did not seem to be dependent on molecular weight as the percentage was at its lowest (76%) in the 200–500 g/mol range and near 100% in the 250,000–1,200,000 g/mol range. All the other molecular weight ranges fell in between these values with no pattern to the proportion percentage.
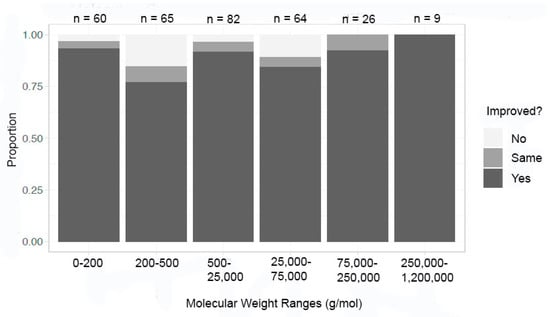
Figure 7.
Improvement in at least one metric by molecular weight—all crystals.
Recognizing that the overall dataset was skewed towards inorganic compounds in the lower molecular weights and towards macromolecules in the higher molecular weights, each of those datasets was evaluated independently. Utilizing the molecular weight ranges of under 100, 100–200, 200–300, 300–400, 400–500, and 500–3000 g/mol for inorganic compounds, an additional grouping was performed (Figure 8). Molecular weight, again, does not seem to have an impact on improvement in at least one metric, with a 74% success rate for 200–500 g/mol and a 91% success rate in both 100–200 g/mol and 300–400 g/mol. The other molecular weight ranges fell in between these proportions of success.
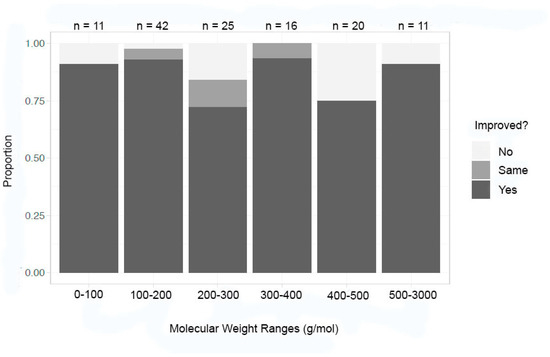
Figure 8.
Improvement in at least one metric by molecular weight—inorganic compounds.
When the data for exclusively organic/macromolecular compounds were sorted, the molecular weight ranges that were selected were 0–1000; 1000–15,000; 15,000–50,000; 50,000–100,000; and 100,000–1,200,000 g/mol (Figure 9). The ranges for success in at least one metric in these categories were 88% (15,000–50,000 g/mol) to near 100% (0–1000 g/mol). The other molecular weight ranges were between these two proportional values. There seemed to be no impact on molecular weight and success for improving in at least one metric when organic or macromolecular compounds were crystallized in microgravity.
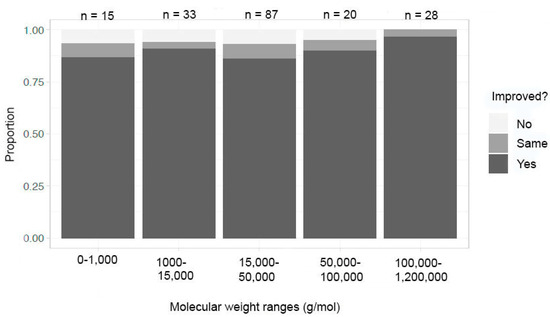
Figure 9.
Improvement in at least one metric by molecular weight—organic/macromolecules.
Removing experiments with known complications and performing the analysis by molecular weight, our surrogate for molecular complexity, the tabulation from combined datasets continued to indicate that molecular weight does not appear to have an impact on success, with all ranges reporting improvement in at least one metric at a rate of 80% or higher (Figure 10). The 0–200 g/mol range had the least complications (2%), and the 200–500 g/mol and 75,000–25,000 g/mol ranges had the most complications (12%). The other molecular weight ranges had complication rates between these two percentages. While the highest molecular weight range had a relatively high percentage of complications (11%), this was only a single experimental report.
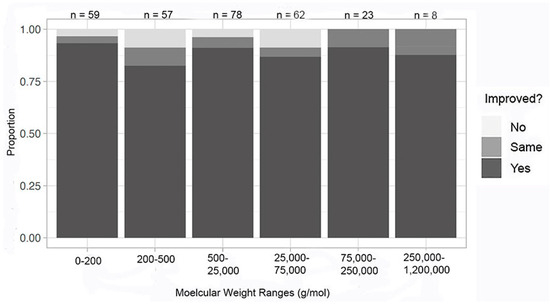
Figure 10.
Improvement in at least one metric by molecular weight—all crystals; complications removed.
The improvement in at least one metric for inorganic compounds once complications had been removed continued to show no impact of molecular weight (Figure 11). Both the low molecular weight (under 100 g/mol) and high molecular weight (above 300 g/mol) groups had no complications. The range of complications that were seen in the rest of the molecular weights ranged from 5–19%, but improvement in at least one metric observed were above 75% in at least one of the key metrics.
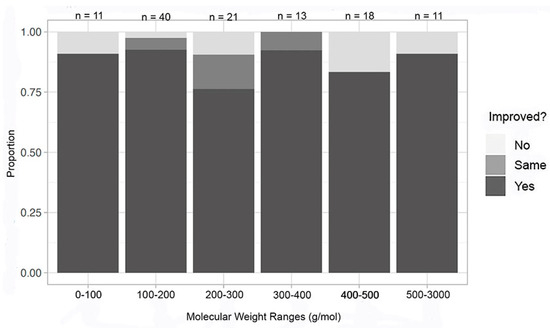
Figure 11.
Improvement in at least one metric by molecular weight—inorganic compounds; complications removed.
The analysis of the organic/macromolecular experiments with problematic conditions removed demonstrates no difference by molecular weight in success in at least one metric (Figure 12). Almost half of the macromolecules had a molecular weight in the 15,000–50,000 g/mol range. This is consistent with the average molecular weight of all proteins, having a median of approximately 25,000 g/mol (human) [28].
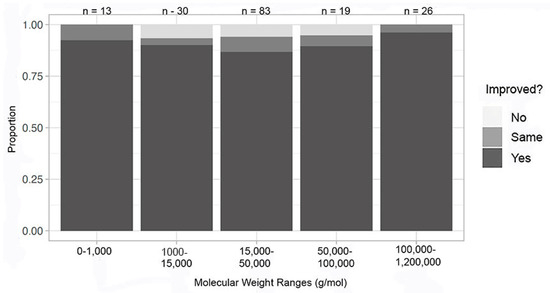
Figure 12.
Improvement in at least one metric by molecular weight—organic/macromolecular compounds; complications removed.
Even taking into consideration that lower-molecular-weight compounds tended to be inorganic and higher-molecular-weight compounds were macromolecules, there was no impact of molecular weight on the success in at least one metric. The analysis of the experimental conditions in our databases indicates that improved crystallization can be expected in microgravity regardless of the size (complexity) of a molecule.
4. Discussion
Updates to the data of microgravity crystallization have upheld the conclusions of the preliminary report: microgravity was a beneficial environment for crystallization regardless of crystal type. The entries in the datasets were doubled, and improvements in the overall key metrics (size, uniformity, structure, mosaicity, and resolution limit) range from 76 to 87%. When accounting for experiments where there were known complications, the ranges improve to 77–89% in the overall key metrics. These numbers could be even higher if there was a call to report as many of these key metrics as possible in future crystallization studies. For macromolecules, a 75% improvement in key metrics may provide key insights into protein structure [29]. For inorganic materials, small improvements to the structure can results in large increases in performance [30].
In the evaluation of the datasets with respect to improvement in at least one metric (size, uniformity, structure, mosaicity, resolution limit, and/or performance), the overall improvement was nearly 90%, with more than one third of the compounds crystallized improved in three or more metrics. When experiments with reported experimental problems were removed from the dataset, the improvement was 90% with respect to at least one metric. Almost 40% of the experiments reported improvement in three or more metrics. Of the five experiments that were removed due to complications that reported improvement in three or more metrics, the complications revolved around stability [31], delays in takeoff that resulted in excessive precipitation [32], premature mixing of protein solution and precipitant prior to takeoff [33], the crystals being too large to mount for analysis [34], and power loss during the experiment [35]. Even with these adverse conditions, positive experimental metrics were seen for these five experiments, perhaps indicating that the microgravity environment may be able to overcome technical challenges.
In general, the production of enhanced crystals in microgravity has improved over the decades. This is likely due to concurrent technology advances, techniques for crystallization in microgravity being refined with experience, and better understanding of the advantages of a microgravity environment, particularly capitalizing on the power of diffusion. Inorganic compounds were the large driver of the improvement in the data.
While macromolecules did not show this marked improvement, several factors can explain this result. Crystallization experiments of macromolecules were not undertaken in a microgravity environment until the 1980s. Upon initial successes, more challenging crystallization problems were chosen for microgravity experiments, including compounds that did not crystallize under terrestrial conditions. Lastly, significant advancements in Earth-grown macromolecules (particularly proteins) were taking place during the same time frame, and some of these advancements utilized lessons learned from microgravity experiments. It was hard to divorce the advancements in macromolecular crystallization in general from the advancements gained in a microgravity environment.
Fewer experiments have been reported with complications from system failures (leaks, breaches, power failures), implementation challenges (take off delays, vibration on landing), or instability (crystal fragility). It is possible that there are additional successes (and failures) from crystallization in microgravity that have not been reported in the literature due to intellectual property concerns. More recent data from the 2000s and 2010s have far fewer complications reported in the literature (between 0 and 6%). Along with the improvement in at least one evaluation metric, the opportunities for successful crystallization in the microgravity environment are very high.
Molecular weight does not have an impact on the outcomes of microgravity crystallization experiments. Molecular weight was used as a surrogate for molecular complexity, and the implication was that complex systems show the same benefits of crystallization as simple systems. In fact, several proteins had their first reports of successful crystallization in a microgravity environment: carboxypeptidase T [36]; carboxypeptidate B complex with N-sulfamoyl-L-arginine [37]; T3Ri insulin hexamer [38]; phosphoribosyl pyrophosphate synthetase from E. coli [39]; E. coli purine nucleoside phosphorylase in complex with 7-deazahypoxanthin [40]; and recombinant phosphoribosylpyrophosphate synthetase-2 from Thermus thermophilus HB27 complexed with ADP and sulfate ions [41].
There was no particular molecular weight range that was impacted by complications. Mechanical complications (power supply issues, rapid cooling, vibrations, damage in transport, etc.) comprised the bulk of the complications/failures. However, several of the crystallization experimental reports had failures due to the properties of the crystals themselves. Several of the protein crystals were sensitive to X-ray radiation and decomposed before analysis was complete [42,43]. There was one example of crystals that were too large to load for analysis [34]. In another example, crystallization occurred between the valve opening of the supply and central chambers, and these crystals were sufficiently large to block the valve open [44]. Lastly, high-molecular-weight polyethylene glycol solvents, required for some high-molecular-weight proteins, were too viscous to allow diffusion-based mixing to occur in microgravity [45].
Communication of data from proprietary information was, and continues to be, a challenge in this field. The authors encourage the addition of data to the databases from confidential experiments where the data (identity of compound, key pieces of information on size, uniformity, structure, mosaicity, resolution limit, and perhaps performance) can be released without details about the crystallization parameters, protocols, equipment, etc. The data from these microgravity experiments are critical for the full understanding of crystallization in a low-gravity environment.
5. Conclusions
Utilizing the microgravity environment for crystallization experiments has clear benefits for successful experimental results. The databases that have been created and curated that contain data from published reports provide an easy-to-use resource for researchers to evaluate information across different crystal types and applications. In this study, crystals formed in the microgravity environment were improved on the metrics of size, uniformity, structure, mosaicity, resolution limit, and/or performance. Improvements have been made over time, and the technology is now mature. Large biological systems can be expected to crystallize and perform as well as simple systems in the microgravity environment, as molecular weight, corresponding size, and molecular complexity appear to have no impact on crystallization success. It is incumbent upon the scientific establishment to exploit the merits of crystallization in the microgravity environment, utilize the lessons learned, capitalize on the benefits of this unique domain, and share their results with the greater scientific community. With such dissemination of research, compilations of information, such as the databases utilized here, will be useful tools for future investigations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst14010012/s1, where links to databases utilized for this study are provided.
Author Contributions
Conceptualization, L.H. and A.M.W.; methodology, K.J., L.H. and A.M.W.; software, K.J.; investigation, F.B., A.W. (Ashley Wilkinson), A.W. (Amari Williams), B.W. and H.W.; data curation, F.B., A.W. (Ashley Wilkinson), A.W. (Amari Williams), B.W. and H.W.; writing—original draft preparation, A.M.W.; writing—review and editing, K.J. and L.H.; project administration, A.M.W.; funding acquisition, A.M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Aerospace Corporation, contract number WR 120622.
Data Availability Statement
The databases utilized for these studies can be found at the following links: macromolecules/organic compounds—https://docs.google.com/spreadsheets/d/1JrXe4Drne7JKcU4EKG7ObQDfrm6EzrZkneTCrPyYZos/edit#gid=1461894258, accessed 31 October 2023; inorganic compounds—https://docs.google.com/spreadsheets/d/1PZ8AAZlXYAln4d2UNR4x_zu-rpkWK8ByG04KiprAAXM/edit#gid=1518976417, accessed 31 October 2023.
Acknowledgments
The authors would like to acknowledge Jessica Frick and Ken Savin for helpful discussions; Kevin Engelbert, the NASA/InSpace Production Applications Portfolio Manager, for funding the study; and Butler University for sabbatical support of AMW.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ding, C.; Jia, H.; Sun, Q.; Yao, Z.; Yang, H.; Liu, W.; Pang, X.; Li, S.; Liu, C.; Minari, T.; et al. Wafer-scale single crystals: Crystal growth mechanisms, fabrication methods, and functional applications. J. Mater. Chem. C 2021, 9, 7829–7851. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Jiao, J.; Lin, C. Recent research progress on crystallization strategies for difficult-to-crystallize organic molecules. Results Chem. 2023, 5, 100859. [Google Scholar] [CrossRef]
- Govada, L.; Chayen, N. Crystallisation and characterisation of muscle proteins: A mini-review. J. Muscle Res. Cell Motil. 2023, 44, 209–215. [Google Scholar] [CrossRef]
- Strelov, V.L.; Kuranova, I.P.; Zakherov, B.G.; Voloshin, A.E. Crystallization in Space: Results and Prospects. Crystallogr. Rep. 2014, 59, 781–806. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Furukawa, E.; Dold, P.; Yamamoto, M.; Tachibana, M.; Kojima, K.; Yoshizaki, I.; Vlieg, E.; Gonzalez-Ramirez, L.A.; Garcia-Ruiz, J.M. Higher growth rate of protein crystals in space than on the Earth. J. Cryst. Growth 2023, 603, 127016. [Google Scholar] [CrossRef]
- Pettit, D.; Fontana, P. Comparison of sodium chloride hopper cubes grown under microgravity and terrestrial conditions. NPJ Microgravity 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Reichert, P.; Prosise, W.; Fischmann, T.O.; Scapin, G.; Narasimhan, C.; Spinale, A.; Polniak, R.; Yang, X.; Walsh, E.; Patel, D.; et al. Pembrolizumab microgravity crystallization experimentation. NPJ Microgravity 2019, 5, 28. [Google Scholar] [CrossRef]
- Yue, J.T.; Voltmer, F.W. Influence of gravity-free solidification on solute microsegregation. J. Cryst. Growth 1975, 29, 329–341. [Google Scholar] [CrossRef]
- Maes, D.; Decanniere, K.; Segers, I.; Vanhee, C.; Sleutel, M.; Willaert, R.; van der Woerd, C.; Martial, J.; Declercq, J.-P.; Evrard, C.; et al. Protein crystallization under microgravity conditions: What did we learn on TIM crystallization from the Soyuz missions? Microgravity Sci. Technol. 2007, XIX, 90–94. [Google Scholar]
- Vergera, A.; Lorber, B.; Sagari, A.; Geigé, R. Physical aspect of protein crystal growth investigated with the Advanced Protein Crystallization Facility in reduced-gravity environments. Acta Cryst. D 2003, 59, 2–15. [Google Scholar] [CrossRef]
- Judge, R.A.; Snell, E.H.; van der Woerd, M.J. Extracting trends from two decades of microgravity macromolecular crystallization history. Acta Cryst. D 2005, 61, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Boyko, K.M.; Timofeev, V.I.; Samygina, V.R.; Kuranova, I.P.; Popov, V.O.; Koval’chuk, M.V. Protein crystallization under microgravity conditions. Analysis of the results of Russian experiments performed on the International Space Station in 2005–2015. Crystallogr. Rep. 2016, 61, 718–729. [Google Scholar]
- Snell, E.H.; Helliwell, J.R. Microgravity as an environment for macromolecular crystallization—An outlook in the era of space stations and commercial space flight. Crystallogr. Rev. 2021, 27, 3–46. [Google Scholar] [CrossRef]
- Regel, L. Materials Science Research in Space: Theory—Experiments—Technology; Halstead Press: New York, NY, USA, 1987. [Google Scholar]
- Wilkinson, A. Investigation of Microgravity Grown Organic Crystals by Diffusion Techniques of the Course of Thirty-One Years. Undergraduate Thesis, Butler University, Indianapolis, IN, USA, 2023. Available online: https://digitalcommons.butler.edu/ugtheses/678/ (accessed on 30 May 2023).
- Wilkinson, A.; Brewer, F.; Wright, H.; Whiteside, B.; Williams, A.; Harper, L.; Wilson, A.M. Semiconductor Materials Fabricated in Microgravity; A Meta-Analysis. Discov. Mater. 2023; manuscript under review. [Google Scholar]
- Wright, H.; Williams, A.; Wilkinson, A.; Harper, L.; Savin, K.; Wilson, A.M. An analysis of publicly available microgravity crystallization data: Emergent themes across crystal types. Cryst. Growth Des. 2022, 22, 6849–6851. [Google Scholar] [CrossRef]
- Drago, V.N.; Devos, J.M.; Blakeley, M.P.; Forsyth, V.T.; Kovalevsky, A.Y.; Schall, C.A.; Mueser, T.C. Microgravity crystallization of perdeuterated tryptophan synthase for neutron diffraction. NPJ Microgravity 2022, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Duffar, T.; Boiton, P.; Dusserre, P.; Abadie, J. Crucible de-wetting during Bridgman growth in microgravity. II. Smooth crucibles. J. Cryst. Growth 1997, 179, 397–409. [Google Scholar] [CrossRef]
- Morimoto, Y.; Kamo, M.; Furubayashi, N.; Higashino, Y.; Inaka, K. Crystal Structure Analysis of the 20S Proteasome Grown in Space: Comparison between Space and Ground Crystals. Int. J. Microgravity Sci. Appl. 2020, 37, 370404. [Google Scholar]
- Vojtěch, D.; Barta, C.; Barta, C., Jr.; Görler, G.P.; Otto, G.; Wittmann, K. Non-equilibrium primary crystallisation in silver–germanium alloy under microgravity conditions. Mater. Sci. Technol. 1999, 15, 1266–1272. [Google Scholar] [CrossRef]
- Sabirov, M.; Popovich, A.; Boyko, K.; Nikolaeva, A.; Kyrchanova, O.; Maksimenko, O.; Popov, V.; Georgiev, P.; Bonchuk, A. Mechanisms of CP190 Interaction with Architectural Proteins in Drosophila Melanogaster. Int. J. Mol. Sci. 2021, 22, 12400. [Google Scholar] [CrossRef]
- Tian, H.; Tan, P.; Meng, X.; Hu, C.; Shi, G.; Zhou, Z.; Wang, X. Effects of Growth Temperature on Crystal Morphology and Size Uniformity in KTa1–xNbxO3 and K1–yNayNbO3 Single Crystal. Cryst. Growth Des. 2016, 16, 325–330. [Google Scholar] [CrossRef]
- Datta, S.; Grant, D. Crystal structures of drugs: Advances in determination, prediction and engineering. Nat. Rev. Drug Discov. 2004, 3, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Quan, Z.; Yang, J.; Yang, P.; Lin, J. Highly Uniform and Monodisperse β-NaYF4:Ln3+ (Ln = Eu, Tb, Yb/Er, and Yb/Tm) Hexagonal Microprism Crystals: Hydrothermal Synthesis and Luminescent Properties. Inorg. Chem. 2007, 46, 6329–6337. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A. Protein Crystallization. In Protein Crystallography. Methods in Molecular Biology; Wlodawer, A., Dauter, Z., Jaskolski, M., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1607. [Google Scholar]
- Rathore, I.; Mishra, V.; Bhaumik, P. Advancements in macromolecular crystallography: From past to present. Emerg. Top Life Sci. 2021, 5, 127–149. [Google Scholar] [PubMed]
- Geiger, T.; Wehner, A.; Schaab, C.; Cox, J.; Mann, M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol. Cell. Proteom. 2012, 11, M111.014050. [Google Scholar] [CrossRef]
- McPherson, A.; Gavira, J.A. Introduction to protein crystallization. Acta Crystallogr. 2014, F70, 2–20. [Google Scholar] [CrossRef]
- Saha, A.; Chellappan, K.V.; Narayan, K.S.; Ghatak, J.; Datta, R.; Viswanatha, R. Near-Unity Quantum Yield in Semiconducting Nanostructures: Structural Understanding Leading to Energy Efficient Applications. J. Phys. Chem. Lett. 2013, 4, 3544–3549. [Google Scholar] [CrossRef]
- Esposito, E.; Sica, F.; Sorrentino, G.; Berisio, R.; Carotenuto, L.; Giordano, A.; Raia, C.A.; Rossi, M.; Lamzin, V.S.; Wilson, K.S.; et al. Protein Crystal Growth in the Advanced Protein Crystallization Facility on the LMS Mission: A Comparison of Sulfolobus solfataricus Alcohol Dehydrogenase Crystals Grown on the Ground and in Microgravity. Acta Crystallogr. 1998, D54, 386–390. [Google Scholar] [CrossRef]
- Delucas, L.J.; (The Aerospace Corporation, El Segundo, CA, USA). Personal Communication, 2022.
- Zhu, D.-W.; Zhou, M.; Mao, Y.; Labrie, F.; Lin, S.-X. Crystallization of human estrogenic 17β-hydroxysteroid dehydrogenase under microgravity. J. Cryst. Growth 1995, 156, 108–111. [Google Scholar] [CrossRef]
- Helliwell, J.R.; Snell, E.; Weisgerber, S. An investigation of the perfection of lysozyme protein crystals grown in microgravity and on earth. In Materials and Fluids under Low Gravity. Lecture Notes in Physics; Ratke, L., Walter, H., Feuerbacher, B., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 464. [Google Scholar]
- Fiederle, M.; Duffar, T.; Babentsov, V.; Benz, K.W.; Dusserre, P.; Corregidor, V.; Dieguez, E.; Delaye, P.; Roosen, G.; Chevrier, V.; et al. Dewetted growth of CdTe in microgravity (STS-95). Cryst. Res. Technol. 2004, 39, 481–490. [Google Scholar] [CrossRef]
- Smirnova, E.A.; Kislitsyn, Y.A.; Sosfenov, N.I.; Lyashenko, A.V.; Popov, A.N.; Baīdus’, A.N.; Timofeev, V.I.; Kuranova, I.P. Protein crystal growth on the Russian segment of the International Space Station. Crystallogr. Rep. 2009, 54, 901–911. [Google Scholar] [CrossRef]
- Akparov, V.; Sokolenko, N.; Timofeev, V.I.; Kuranova, I. Structure of the complex of carboxypeptidase B and N-sulfamoyl-L-arginine. Acta Crystallogr. 2015, F71, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ciszak, E.; Pangborn, W. A novel complex of a phenolic derivative with insulin: Structural features related to the T → R transition. Protein Sci. 1996, 5, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, V.I.; Abramchik, Y.A.; Zhukhlistova, N.E.; Muravieva, T.I.; Esipov, R.S.; Kuranova, I.P. Three-dimensional structure of phosphoribosyl pyrophosphate synthetase from E. coli at 2.71 Å resolution. Crystallogr. Rep. 2016, 61, 44–54. [Google Scholar] [CrossRef]
- Timofeev, V.I.; Zhukhlistova, N.E.; Abramchik, Y.A.; Fateev, I.I.; Kostromina, M.A.; Muravieva, T.I.; Esipov, R.S.; Kuranova, I.P. Crystal structure of Escherichia coli purine nucleoside phosphorylase in complex with 7-deazahypoxanthin. Acta Crystallogr. 2018, F74, 355–362. [Google Scholar]
- Timofeev, V.I.; Sinitsyna, E.V.; Kostromina, M.A.; Muravieva, T.I.; Makarov, D.A.; Mikheeva, O.O.; Kuranova, I.P.; Esipov, R.S. Crystal structure of recombinant phosphoribosylpyrophosphate synthetase-2 from Thermus thermophilus HB27 complexed with ADP and sulfate ions. Acta Cryst. 2017, F73, 369–375. [Google Scholar]
- Timofeev, V.I.; Abramchik, Y.A.; Fateev, I.V.; Zhukhlistova, N.E.; Murav’eva, T.I.; Kuranova, I.P.; Esipov, R.S. Three-dimensional structure of thymidine phosphorylase from E. coli in complex with 3′-azido-2′-fluoro-2′,3′-dideoxyuridine. Crystallogr. Rep. 2013, 58, 842–853. [Google Scholar] [CrossRef]
- Debaerdemaeker, T.; Evrard, C.; Declercq, J.P.; Claus, H.; Akca, E.; König, H. The first crystallization of the outer surface (S-layer)glycoprotein of the mesophilic bacterium Bacillus sphaericus and the hyperthermophilic archaeon Methanothermus fervidus. In Proceedings of the 2nd European Workshop on Exo/Astro-Biology, Graz, Austria, 16–19 September 2002; pp. 441–442, ESA SP-518. [Google Scholar]
- DeLucas, L.J.; Moore, K.M.; Bray, T.L.; Rosenblum, W.M.; Einspahr, H.M.; Clancy, L.L.; Rao, G.S.J.; Harris, B.G.; Munson, S.H.; Finzel, B.C. Protein crystal growth results from the United States Microgravity Laboratory-1 mission. J. Phys. D Appl. Phys. 1993, 26, B100. [Google Scholar] [CrossRef]
- Kjeld Flemming, N.; Lind, M.D. Results of the TTF-TCNQ and the calcium carbonate crystallization on the Long Duration Exposure Facility. In LDEF: 69 Months in Space. First Post-Retrieval Symposium, Part 3; SEE N92-27083 17-99; NASA Langley Research Center: Hampton, VA, USA, 1992; pp. 1675–1685. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).