Electrowetting and Surface Tension of Chromonic Liquid Crystals
Abstract
:1. Introduction
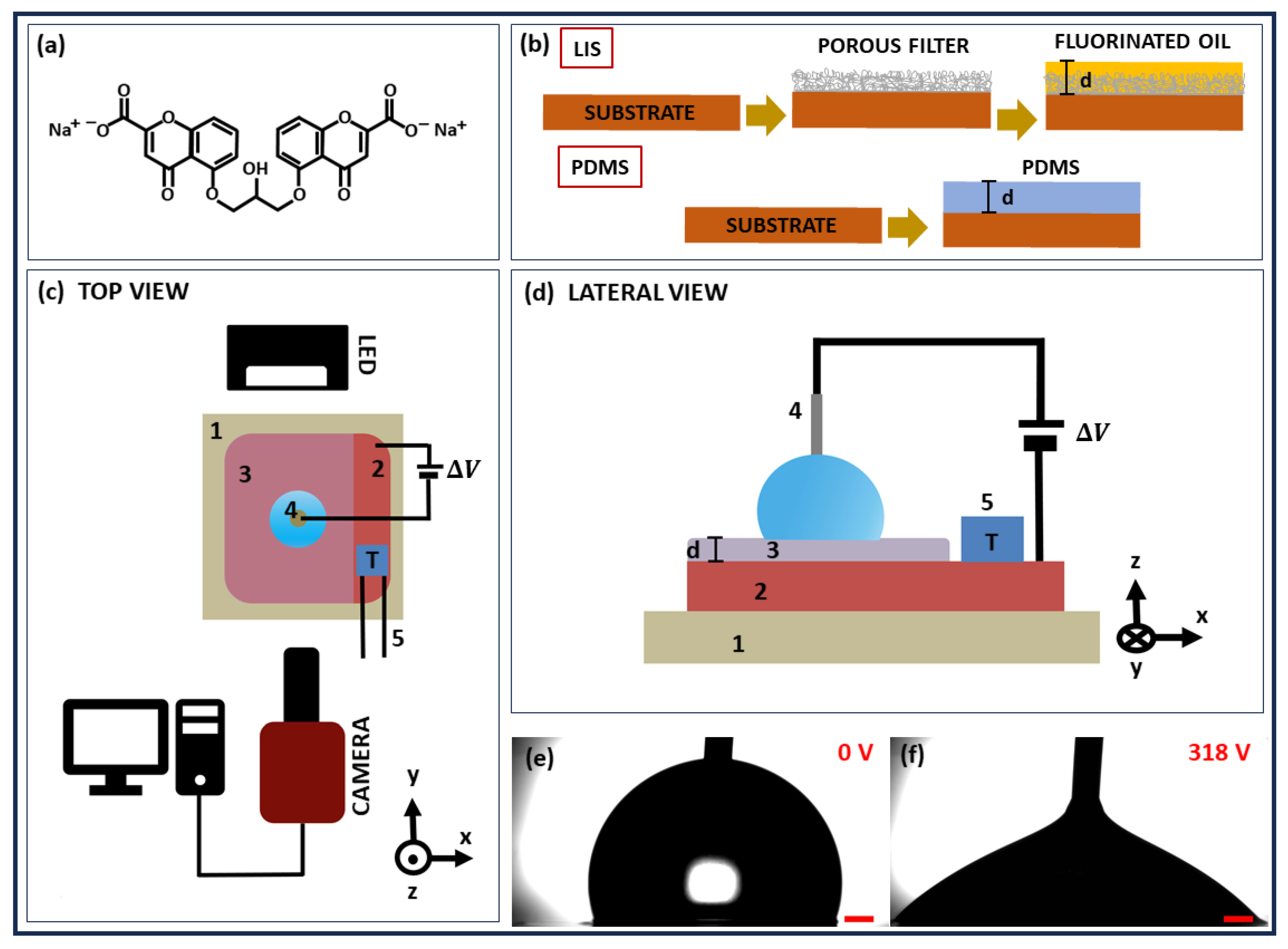
2. Materials and Methods
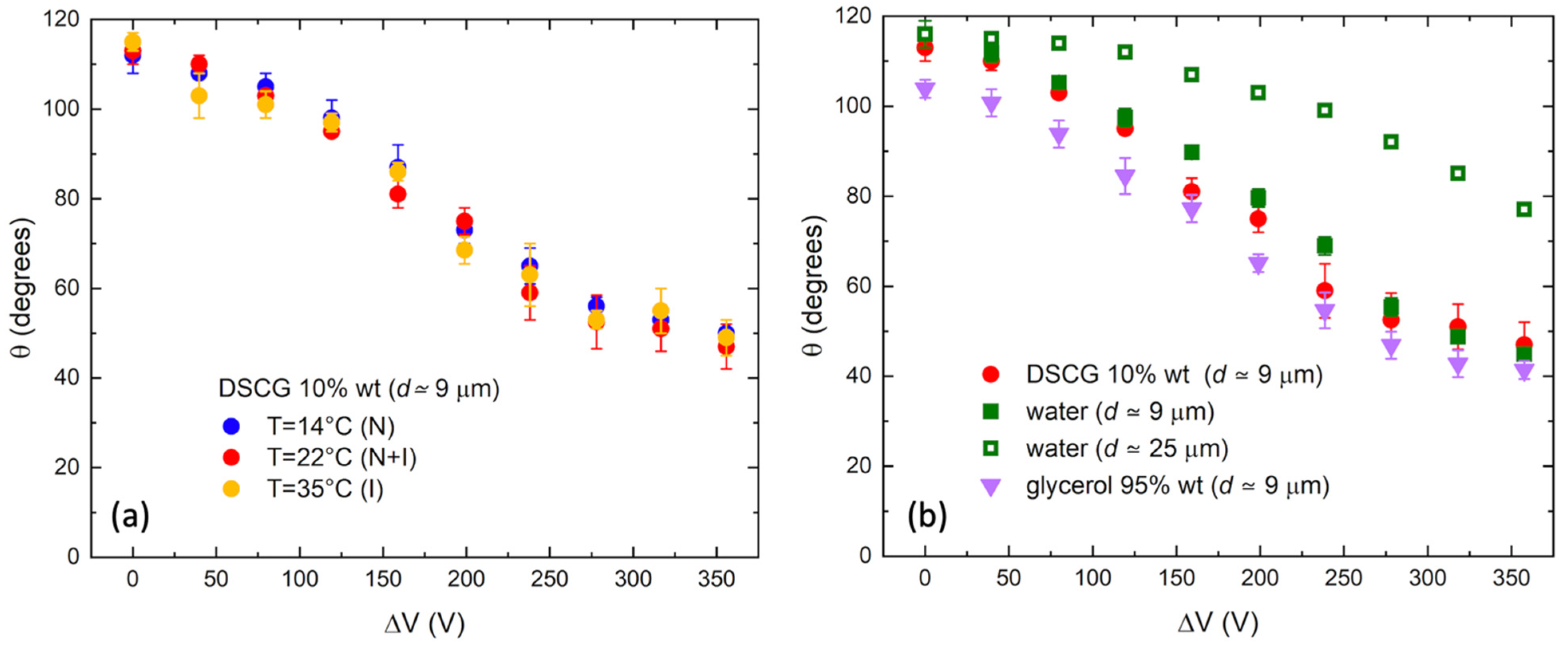
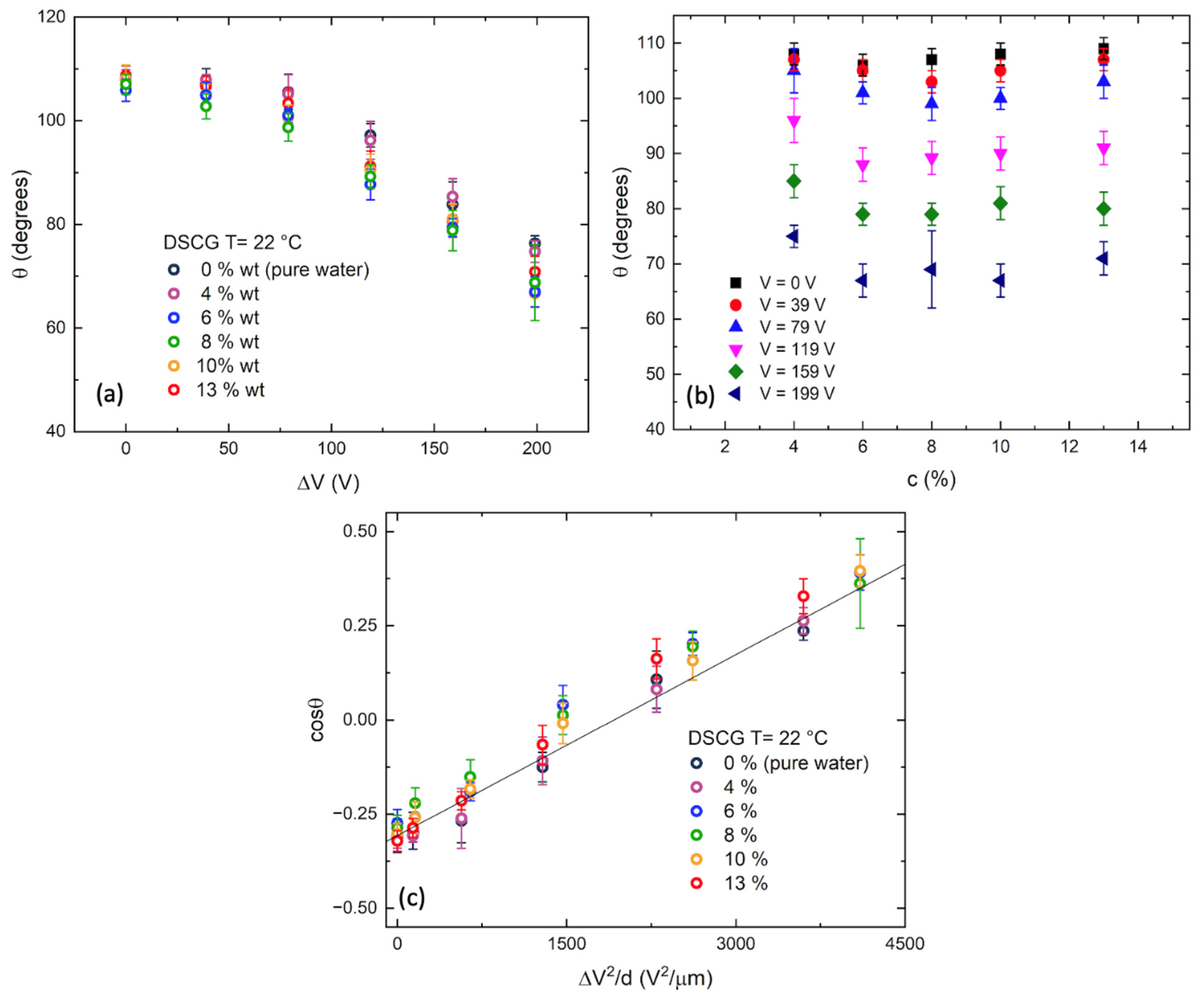
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Gennes, P.G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves; Springer: New York, NY, USA, 2004. [Google Scholar]
- Bocquet, L.; Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 2010, 39, 1073–1095. [Google Scholar] [CrossRef] [PubMed]
- Mistura, G.; Pierno, M. Drop mobility on chemically heterogeneous and lubricant-impregnated surfaces. Adv. Phys.-X 2017, 2, 591–607. [Google Scholar] [CrossRef]
- Malinowski, R.; Parkin, I.P.; Volpe, G. Advances towards programmable droplet transport on solid surfaces and its applications. Chem. Soc. Rev. 2020, 49, 7879–7892. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Mugele, F.; Baret, J.C. Electrowetting: From basics to applications. J. Phys. Condens. Matter 2005, 17, R705–R774. [Google Scholar] [CrossRef]
- Li, J.; Kim, C.J. Current commercialization status of electrowetting-on-dielectric (EWOD) digital microfluidics. Lab Chip 2020, 20, 1705–1712. [Google Scholar] [CrossRef]
- Banpurkar, A.G.; Duits, M.H.G.; van den Ende, D.; Mugele, F. Electrowetting of Complex Fluids: Perspectives for Rheometry on Chip. Langmuir 2009, 25, 1245–1252. [Google Scholar] [CrossRef]
- Mampallil, D.; Eral, H.B.; van den Ende, D.; Mugele, F. Control of evaporating complex fluids through electrowetting. Soft Matter 2012, 8, 10614–10617. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chang, C.A.; Yeh, J.A. Variable focus dielectric liquid droplet lens. Opt. Express 2006, 14, 4101–4106. [Google Scholar] [CrossRef]
- Fan, S.K.; Chiu, C.P.; Lin, J.W. Electrowetting on polymer dispersed liquid crystal. Appl. Phys. Lett. 2009, 94, 164109. [Google Scholar] [CrossRef]
- Wells, G.G.; Matranga, M.A.; Newton, C.J.P.; Taphouse, T.S.; Baig, S.A.; Kitson, S.C. Electrowetting pixels with improved transmittance using dye doped liquid crystals. Appl. Phys. Lett. 2013, 103, 031107. [Google Scholar] [CrossRef]
- Fukuto, M.; Gang, O.; Alvine, K.J.; Ocko, B.M.; Pershan, P.S. Wetting of liquid-crystal surfaces and induced smectic layering at a nematic-liquid interface: An x-ray reflectivity study. Phys. Rev. E 2008, 77, 031607. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.B.; Gharbi, M.A.; Ngo, V.L.; Kamien, R.D.; Yang, S.; Stebe, K.J. Elastocapillary interactions on nematic films. Proc. Natl. Acad. Sci. USA 2015, 112, 6336–6340. [Google Scholar] [CrossRef]
- Teixeira, P.I.C.; Sluckin, T.J.; Sullivan, D.E. Landau-De Gennes theory of anchoring transitions at a nematic liquid-crystal substrate interface. Liq. Cryst. 1993, 14, 1243–1253. [Google Scholar] [CrossRef]
- Delrio, E.M.; Dagama, M.M.T.; Demiguel, E.; Rull, L.F. Surface-induced alignment at model nematic interfaces. Phys. Rev. E 1995, 52, 5028–5039. [Google Scholar] [CrossRef]
- Osipov, M.A.; Sluckin, T.J.; Cox, S.J. Influence of permanent molecular dipoles on surface anchoring of nematic liquid crystals. Phys. Rev. E 1997, 55, 464–476. [Google Scholar] [CrossRef]
- Rey, A.D.; Golmohammadi, M.; Valencia, E.E.H. A model for mesophase wetting thresholds of sheets, fibers and fiber bundles. Soft Matter 2011, 7, 5002–5009. [Google Scholar] [CrossRef]
- Rey, A.D.; Herrera-Valencia, E.E. Dynamic wetting model for the isotropic-to-nematic transition over a flat substrate. Soft Matter 2014, 10, 1611–1620. [Google Scholar] [CrossRef]
- Vanzo, D.; Ricci, M.; Berardi, R.; Zannoni, C. Wetting behaviour and contact angles anisotropy of nematic nanodroplets on flat surfaces. Soft Matter 2016, 12, 1610–1620. [Google Scholar] [CrossRef]
- Hiltrop, K.; Stegemeyer, H. Contact angles and alignment of liquid crystals on lecithin monolayers. Mol. Cryst. Liq. Cryst. 1978, 49, 61–65. [Google Scholar] [CrossRef]
- Gvozdovskyy, I.; Kurioz, Y.; Reznikov, Y. Exposure and temperature dependences of contact angle of liquid crystals on photoaligning surface. Opto-Electron. Rev. 2009, 17, 116–119. [Google Scholar] [CrossRef]
- Barboza, R.; Marni, S.; Ciciulla, F.; Mir, F.A.; Nava, G.; Caimi, F.; Zaltron, A.; Clark, N.A.; Bellini, T.; Lucchetti, L. Explosive electrostatic instability of ferroelectric liquid droplets on ferroelectric solid surfaces. Proc. Natl. Acad. Sci. USA 2022, 119, e2207858119. [Google Scholar] [CrossRef] [PubMed]
- Marni, S.; Barboza, R.; Zaltron, A.; Lucchetti, L. Optical control of mass ejection from ferroelectric liquid droplets: A possible tool for the actuation of complex fluids. J. Mol. Liq. 2023, 384, 122287. [Google Scholar] [CrossRef]
- Marni, S.; Nava, G.; Barboza, R.; Bellini, T.G.; Lucchetti, L. Walking ferroelectric liquid droplets with light. Adv. Mater. 2023, 35, 2212067. [Google Scholar] [CrossRef] [PubMed]
- Kolacz, J.; Wei, Q.H. Self-Localized Liquid Crystal Micro-Droplet Arrays on Chemically Patterned Surfaces. Crystals 2022, 12, 13. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Sengupta, A. Spatio-temporal programming of lyotropic phase transition in nanoporous microfluidic confinements. J. Colloid Interface Sci. 2023, 649, 302–312. [Google Scholar] [CrossRef]
- Chen, X.; Korblova, E.; Dong, D.P.; Wei, X.Y.; Shao, R.F.; Radzihovsky, L.; Glaser, M.A.; Maclennan, J.E.; Bedrov, D.; Walba, D.M.; et al. First -principles experimental demonstration of ferroelectricity in a thermotropic nematic liquid crystal: Polar domains and striking electro-optics. Proc. Natl. Acad. Sci. USA 2020, 117, 14021–14031. [Google Scholar] [CrossRef]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef]
- Carlton, R.J.; Hunter, J.T.; Miller, D.S.; Abbasi, R.; Mushenheim, P.C.; Tan, L.N.; Abbott, N.L. Chemical and biological sensing using liquid crystals. Liq. Cryst. Rev. 2013, 1, 29–51. [Google Scholar] [CrossRef]
- Lydon, J. Chromonic review. J. Mater. Chem. 2010, 20, 10071–10099. [Google Scholar] [CrossRef]
- Tam-Chang, S.W.; Huang, L.M. Chromonic liquid crystals: Properties and applications as functional materials. Chem. Commun. 2008, 17, 1957–1967. [Google Scholar] [CrossRef]
- Smith, G.P.; Fraccia, T.P.; Todisco, M.; Zanchetta, G.; Zhu, C.H.; Hayden, E.; Bellini, T.; Clark, N.A. Backbone-free duplex-stacked monomer nucleic acids exhibiting Watson-Crick selectivity. Proc. Natl. Acad. Sci. USA 2018, 115, E7658–E7664. [Google Scholar] [CrossRef]
- Shiyanovskii, S.V.; Lavrentovich, O.D.; Schneider, T.; Ishikawa, T.; Smalyukh, I.I.; Woolverton, C.J.; Niehaus, G.D.; Doane, K.J. Lyotropic chromonic liquid crystals for biological sensing applications. Mol. Cryst. Liq. Cryst. 2005, 434, 259–587. [Google Scholar] [CrossRef]
- Shiyanovskii, S.V.; Schneider, T.; Smalyukh, I.I.; Ishikawa, T.; Niehaus, G.D.; Doane, K.J.; Woolverton, C.J.; Lavrentovich, O.D. Real-time microbe detection based on director distortions around growing immune complexes in lyotropic chromonic liquid crystals. Phys. Rev. E 2005, 71, 020702. [Google Scholar] [CrossRef] [PubMed]
- Woolverton, C.J.; Gustely, E.; Li, L.; Lavrentovich, O.D. Liquid crystal effects on bacterial viability. Liq. Cryst. 2005, 32, 417–423. [Google Scholar] [CrossRef]
- Helfinstine, S.L.; Lavrentovich, O.D.; Woolverton, C.J. Lyotropic liquid crystal as a real-time detector of microbial immune complexes. Lett. Appl. Microbiol. 2006, 43, 27–32. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.F.; Pontes, K.D.; Alves, T.F.R.; Amaral, V.A.; Rebelo, M.D.; Hausen, M.A.; Chaud, M.V. Spotlight on biomimetic systems based on lyotropic liquid crystal. Molecules 2017, 22, 419. [Google Scholar] [CrossRef] [PubMed]
- Oton, E.; Oton, J.M.; Cano-Garcia, M.; Escolano, J.M.; Quintana, X.; Geday, M.A. Rapid detection of pathogens using lyotropic liquid crystals. Opt. Express 2019, 27, 10098–10107. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Labes, M.M. Phase diagram and thermodynamic properties of disodium cromoglycate-water lyomesophases. Mol. Cryst. Liq. Cryst. 1983, 91, 53–58. [Google Scholar] [CrossRef]
- Lafuma, A.; Quere, D. Slippery pre-suffused surfaces. Europhys. Lett. 2011, 96, 56001. [Google Scholar] [CrossRef]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Ueda, E.; Paulssen, D.; Levkin, P.A. Slippery lubricant-infused surfaces: Properties and emerging applications. Adv. Funct. Mater. 2019, 29, 1802317. [Google Scholar] [CrossRef]
- Sartori, P.; Ferraro, D.; Dassie, M.; Meggiolaro, A.; Filippi, D.; Zaltron, A.; Pierno, M.; Mistura, G. Oscillatory motion of viscoelastic drops on slippery lubricated surfaces. Commun. Phys. 2022, 5, 81. [Google Scholar] [CrossRef]
- Carneri, M.; Ferraro, D.; Azarpour, A.; Meggiolaro, A.; Cremaschini, S.; Filippi, D.; Pierno, M.; Zanchetta, G.; Mistura, G. Sliding and rolling of yield stress fluid droplets on highly slippery lubricated surfaces. J. Colloid Interface Sci. 2023, 644, 487–495. [Google Scholar] [CrossRef]
- Zaltron, A.; Ferraro, D.; Meggiolaro, A.; Cremaschini, S.; Carneri, M.; Chiarello, E.; Sartori, P.; Pierno, M.; Sada, C.; Mistura, G. Optofluidic platform for the manipulation of water droplets on engineered LiNbO3 surfaces. Adv. Mater. Interfaces 2022, 9, 2200345. [Google Scholar] [CrossRef]
- Meggiolaro, A.; Cremaschini, S.; Ferraro, D.; Zaltron, A.; Carneri, M.; Pierno, M.; Sada, C.; Mistura, G. Determination of the dielectrophoretic force induced by the photovoltaic effect on lithium niobate. Micromachines 2022, 13, 316. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef]
- Arakawa, K.; Tonooka, M.; Sakamoto, K. Membrane stabilizing action of NCO-650 and its congeners. Jpn. J. Pharmacol. 1984, 36, 311–318. [Google Scholar] [CrossRef]
- Davidson, Z.S.; Huang, Y.; Gross, A.; Martinez, A.; Still, T.; Zhou, C.; Collings, P.J.; Kamien, R.D. Nat. Commun. 2017, 8, 15642. [Google Scholar]
- Gannon, M.G.J.; Faber, T.E. The surface tension of nematic liquid crystals. Philos. Mag. A 1978, 37, 117–135. [Google Scholar] [CrossRef]
- Korjenevsky, V.A.; Tomilin, M.G. Experimental investigation of the surface energy of a nematic liquid crystal. Liq. Cryst. 1993, 385, 643–649. [Google Scholar] [CrossRef]
- Tortora, L.; Lavrentovich, O.D. Chiral symmetry breaking by spatial confinement in tactoidal droplets of lyotropic chromonic liquid crystals. Proc. Natl. Acad. Sci. USA 2011, 108, 5163–5168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, T.; Noel, A.; Chen, Y.-C.; Liu, T. Applications of liquid crystals in biosensing. Soft Matter 2021, 17, 4675. [Google Scholar] [CrossRef]

| Solution | T (°C) | γ (mN/m) Pendant Drop | Surface | γ (mN/m) Linear Fit |
|---|---|---|---|---|
| Water | 22 | 72.7 ± 0.3 | LIS | 71 ± 6 |
| Glycerol–water 95% | 22 | 60 ± 1 | LIS | 67 ± 7 |
| DSCG–water 10% | 14 | 75 ± 1 | LIS | 68 ± 7 |
| DSCG–water 10% | 22 | 71.0 ± 0.5 | LIS | 64 ± 6 |
| DSCG–water 10% | 35 | 69 ± 1 | LIS | 68 ± 6 |
| Water | 22 | 72.7 ± 0.3 | PDMS | 74 ± 5 |
| DSCG–water 4% | 22 | 72.6 ± 0.7 | PDMS | 72 ± 4 |
| DSCG–water 6% | 22 | 72.0 ± 0.7 | PDMS | 68 ± 6 |
| DSCG–water 8% | 22 | 71.7 ± 0.3 | PDMS | 75 ± 6 |
| DSCG–water 10% | 22 | 71.0 ± 0.5 | PDMS | 71 ± 5 |
| DSCG–water 13% | 22 | 68.5 ± 0.8 | PDMS | 64 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinello, F.; Ferraro, D.; Meggiolaro, A.; Cremaschini, S.; Zaltron, A.; Pierno, M.; Mistura, G.; Zanchetta, G.; Lucchetti, L. Electrowetting and Surface Tension of Chromonic Liquid Crystals. Crystals 2024, 14, 1. https://doi.org/10.3390/cryst14010001
Marinello F, Ferraro D, Meggiolaro A, Cremaschini S, Zaltron A, Pierno M, Mistura G, Zanchetta G, Lucchetti L. Electrowetting and Surface Tension of Chromonic Liquid Crystals. Crystals. 2024; 14(1):1. https://doi.org/10.3390/cryst14010001
Chicago/Turabian StyleMarinello, Filippo, Davide Ferraro, Alessio Meggiolaro, Sebastian Cremaschini, Annamaria Zaltron, Matteo Pierno, Giampaolo Mistura, Giuliano Zanchetta, and Liana Lucchetti. 2024. "Electrowetting and Surface Tension of Chromonic Liquid Crystals" Crystals 14, no. 1: 1. https://doi.org/10.3390/cryst14010001
APA StyleMarinello, F., Ferraro, D., Meggiolaro, A., Cremaschini, S., Zaltron, A., Pierno, M., Mistura, G., Zanchetta, G., & Lucchetti, L. (2024). Electrowetting and Surface Tension of Chromonic Liquid Crystals. Crystals, 14(1), 1. https://doi.org/10.3390/cryst14010001












