Abstract
The title compound was prepared by reaction of the Schiff base ligand N-(2-hydroxy-1-naphthylidene)leucine with dichlorodimethylsilane in the presence of triethylamine as base. The resulting pentacoordinate silicon complex was characterized by NMR, IR, UV-Vis spectroscopy and melting point. The structure was confirmed by single-crystal X-ray diffraction data. It crystallizes in the monoclinic space group Ic with unit cell dimensions a = 7.2030(6), b = 22.9842(14), c = 10.8946(12) Å, β = 96.141(7)°, V = 1793.3(3) Å3, Z = 4.
1. Introduction
The reaction of aromatic ortho-hydroxycarbonyl compounds with amino acids allows access to versatile Schiff base ligands. These act as tridentate O,N,O-ligands in the coordination of a wide range of metal ions; see, for instance, [1,2,3,4,5,6]. The possible applications of these complexes are manifold. They range from catalysts [7], sensors for copper [8] and non-linear optics [9] to the generation of polynuclear magnetic complexes [10,11,12] and different biomedical applications [13,14,15,16,17].
Silicon complexes with tridentate O,N,O ligands have already been investigated with regard to various possible applications. These include use in thin-film solar cells [18], as dyes [19], or as compounds with antibacterial activity [20,21]. Complexes in which the Schiff base ligand is substituted with sulfonamide residues possess, in addition to antimicrobial properties, nematicidal and insecticidal properties [22].
Herein, we wanted to expand the available pentacoordinate silicon complexes to those containing the N-[(2-oxy-1-naphthyl)methylene]leucinato ligand. Previously published related silicon complexes contain Schiff base ligands derived from o-hydroxyacetophenone [4,23], salicyl aldehyde [5], acetylacetone [24,25], or o-hydroxynaphthyl aldehyde [6].
2. Results and Discussion
N-(2-hydroxy-1-naphthylidene)leucine was synthesized from a racemic mixture of leucine and 2-hydroxy-1-naphthaldehyde as shown in Scheme 1. The reaction of the Schiff base ligand with dichlorodimethylsilane was performed in THF as the solvent in the presence of triethylamine as the base. The crude product was recrystallized from chloroform/hexane and provided the silicon complex 1. It was shown via thin-layer chromatography (1:3 mixture of chloroform and hexane) that the ligand molecule was no longer present in the product and that the batch product consisted of only one component.
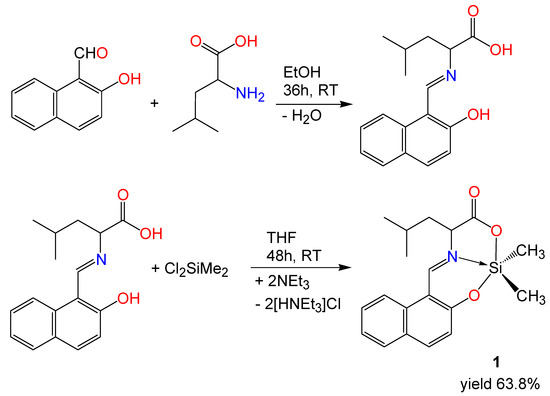
Scheme 1.
Synthesis of the Schiff base ligand N-(2-hydroxy-1-naphthylidene)leucine and the silicon complex 1.
Compound 1 was characterized with spectroscopic methods. The 1H NMR spectrum shows the absence of OH groups, which are present in the NMR spectrum of the ligand molecule. The 13C NMR spectrum shows the presence of the ligand framework plus signals for the silicon-bound methyl groups at 1.6 and 5.2 ppm. The 29Si NMR spectra in solution and in the solid state show chemical shift values of −65.6 and −68.7 ppm, respectively. This indicates the presence of a pentacoordinate silicon complex in solution and in the solid state. UV-Vis and IR data are summarized in the Experimental section. The most striking difference in the IR spectra of the ligand and product 1 is the occurrence of the C=O stretching vibrations at higher wavenumbers in 1. The strong bands at 1692.6 and 1709.2 cm−1 occur due to the coordination with the silicon atom. All spectra are shown in the Supplementary Materials.
Compound 1 crystallizes in the monoclinic space group Ic with one molecule in the asymmetric unit (Figure 1). The space group Ic (containing a glide plane) shows that both enantiomers of 1 are present in the crystal [26]. The powder XRD of the batch material was compared with a powder XRD generated from the single crystal structure data in order to demonstrate the identity of both (see Supplementary Materials). The value of optical rotation of the batch product was determined to be [α]20D = 0°. This confirms the presence of the racemate for the batch product. This was to be expected since leucine was used as the racemate.
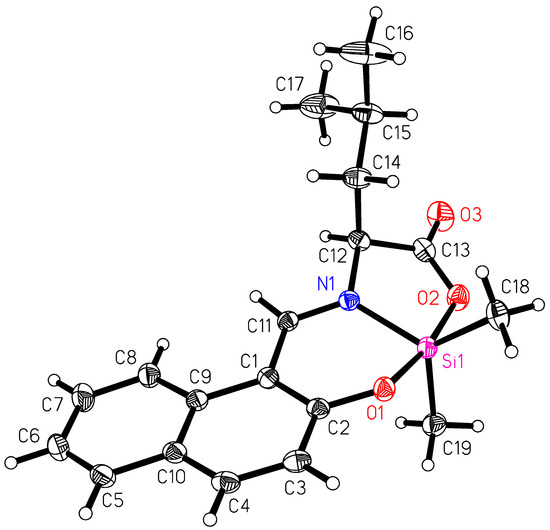
Figure 1.
Molecular structure of 1. Anisotropic displacement ellipsoids are drawn at the 50% probability level.
The silicon complex features a pentacoordinate silicon atom, coordinated by phenoxy-O1, carboxyl-O2, imine-N1 and two carbon atoms from methyl groups (C18 and C19). The parameter τ as defined by Addison et al. [27] is useful for analyzing the coordination geometry of the pentacoordinate silicon atom. The parameter is defined as τ = (β − α)/60°, wherein β is the largest and α is the second-largest angle at the central atom. A perfect square pyramid has a parameter of τ = 0, whereas a perfect trigonal bipyramid has τ = 1. The largest angle at the silicon atom is O1–Si1–O2 with 170.47(8)° and the second-largest is C18–Si1–C19 with 123.77(11)° (see also Table 1). This gives a parameter of τ = 0.78, which corresponds to a distorted trigonal bipyramid. The apical positions are occupied by O1 and O2 of the tridentate ligand, while the atoms N1, C18 and C19 represent the atoms in the trigonal plane.

Table 1.
Selected bond lengths [Å] and angles [°] for 1.
The bond Si–O1 [1.8039(15) Å] is shorter than the bond Si1–O2 [1.8525(17) Å]. This can be explained by the electronegative character of the phenyl-bound O1 and the carboxyl-type oxygen atom O2. The bond lengths Si1–N1 and Si–C (Table 1) are similar as in similar pentacoordinate silicon complexes [4,6,23].
A comparison of 1 with silicon complexes which contain the N-[(2-oxy-1-naphthyl)methylene]valinato ligand (L′) shows some striking differences in the bond angles C-Si-C (see Table 2). Very small bond angles of 78.60(6) and 110.8(2)° are observed for the silacyclobutane and the silacyclohexane derivatives. The compounds L′SiEt2 and L′SiPh2 have larger bond angles of 118.4(4) to 119.7(4)°, respectively. The compound 1, with two methyl groups at the silicon atom, features the largest bond angle in this series with a value of 123.77(11)°. This can be explained by the nature of the CH3 groups, which carry three hydrogen atoms directly bound to the Cα-atom at silicon.

Table 2.
Comparison of bond angles C-Si-C of silicon complexes with comparable ligand environment.
The potential donor atoms O1, O2 and N1 are engaged in the coordination with the silicon atom. Therefore, these atoms do not contribute to intermolecular interactions in the solid-state structure. The oxygen atom O3 from the carboxyl group is not engaged in the coordination of the central atom and forms hydrogen bonds to H8 and H11 from a neighboring molecule (Table 3 and Figure 2). These bifurcated hydrogen bonds form zig-zag chains of molecules parallel to the crystallographic bc-plane.

Table 3.
Selected hydrogen bonds for 1 [Å and °].
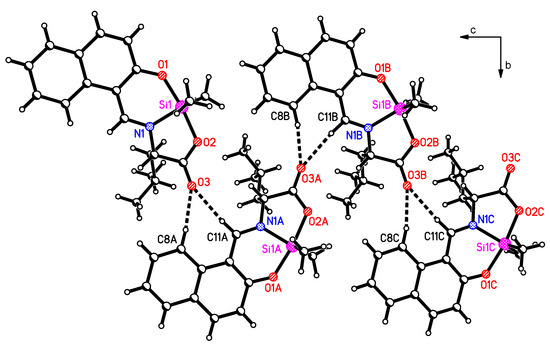
Figure 2.
Intermolecular interactions in 1 between O3 and H8 and H11 from neighboring molecules (view in crystallographic a-direction).
The leucinato group and the methyl groups at the silicon atom are orientated out of the plane of the molecule. These steric requirements hinder the formation of π-π arene stacking interactions which would normally be expected for such a compound containing a naphthyl group. Only one weak C…C contact is observed between C4 and C1A (with symmetry operation x − 0.5, 0.5 − y, z). The distance of this contact is 3.344 Å, which is only 0.056 Å less than the sum of the van der Waals radii of both atoms. The distance of the centroids of the aromatic rings involved (C1, C2, C3, C4, C9, C10) is 3.641 Å. Therefore, we can state that between the layers formed by the chains of molecules we can observe only weak C…C and H…H contacts.
There are only two structural characterized complexes with N-[(2-oxy-1-naphthyl)methylene]leucinato (=L) in the literature. One is a pentacoordinate copper complex with the composition (L)Cu(phenanthroline) [28]. The other one is (L)(methanol)(methanolato)(oxo)vanadium(V). Therein, the vanadium atom reaches hexacoordination with the tridentate Schiff base, oxygen, methanol and methoxide as ligands [29].
In conclusion, we were able to expand the available pentacoordinate silicon complexes to those containing the N-[(2-oxy-1-naphthyl)methylene]leucinato ligand. The dimethylsilyl derivative 1 shows a larger bond angle C-Si-C than comparable pentacoordinate silicon complexes.
3. Experimental Section
3.1. Methods
NMR spectra were measured with a BRUKER AVANCE III 500 MHz spectrometer in DMSO-d6 or CDCl3 with TMS as internal standard. UV/Vis spectra were recorded on a JASCO V-650 UV/Vis photometer in acetonitrile. IR spectra were recorded with a Nicolet 380 (Thermo Fisher) at room temperature. The products were ground together with dry potassium bromide and compressed as a tablet.
The specific rotation values were determined on the Model 241 MC digital polarimeter from Perkin Elmer at a temperature of 20 °C. The cuvette length was 10 cm. The measurements were made at a wavelength of 589 nm (sodium lamp). The powder diffractogram was recorded on the X-ray diffractometer D2 PHASER from Bruker. The melting points were determined with a “Polytherm A” hot-stage microscope from Wagner & Munz. The temperatures determined were not corrected.
3.2. Synthesis of N-(2-Hydroxy-1-naphthylidene)leucine
The ligand was prepared according to a method from the literature [30] and obtained as a pale yellow solid with a melting point of 172 °C [31].
1H-NMR (DMSO-d6, 500 MHz): δ [ppm] = 14.40, 13.31 (br, 2, C-OH), 9.23 (s, 1, CH=N), 6.79–8.12 (mm, 6, Har), 4.52 (d, 1, CH-COO, 3JHH = 7.1 Hz), 1.81 (dd, 2, CH2, 3JHH = 7.1 Hz), 1.62 (m, 1, CH(CH3)2), 0.95 (d, 3, CH3, 3JHH = 4.6 Hz), 0.94 (d, 3, CH3, 3JHH = 4.6 Hz); 13C-NMR (DMSO-d6, 126 MHz): δ [ppm] = 176.0 (COO), 173.2 (Car-OH), 160.0 (CH=N), 137.6, 134.4, 129.4, 128.5, 126.1, 125.0, 123.0, 119.2 (8 Car), 106.8 (Car-CH=N), 63.0 (CH-COO), 42.2 (CH2), 24.9 (CH(CH3)2), 23.2, 21.9 (2 CH3).
[α]20D = 0° (c = 1 g/100 mL in DMSO), UV/Vis (in DMSO; c = 0.196 mmol/L; d = 1 cm) λ [nm] (ε [lmol−1cm−1]) = 424 (11,443), 403 (11,271), 307 (13,948).
IR (KBr): ν (cm−1) = 423.9(w), 458.7(w), 489.4(w), 507.2(m), 524.5(m), 643.9(m), 669.7(m), 708(m), 746.8(s), 828.7(s), 969.9(m), 1011.4(m), 1036.9(m), 1100.4(m), 1149.3(m), 1213(m), 1270.9(m), 1340.1(m), 1366.9(s), 1425.6(m), 1456.3(m), 1469.9(m), 1506.7(m), 1576.1(m), 1632.1(s), 1642(vs), 1684.3(s), 1700.6(w), 1718.1(m), 1733.7(m), 2870.7(w), 2927.6(w), 2957.2(m), 3048.1(w).
3.3. Synthesis of Dimethyl{N-[(2-Oxy-1-Naphthyl)Methylene]Leucinato}Silicon (1)
To a solution of 1.19 g (4.17 mmol) of N-(2-hydroxy-1-naphthylidene)leucine in 30 mL of dry THF, 1.27 g (12.55 mmol, 50% excess) of triethylamine was added and the mixture was cooled in an ice bath to 0 °C. Next, 0.57 g (4.42 mmol) of SiCl2Me2 was mixed with 20 mL of THF. This mixture was added via a dropping funnel into the solution. The reaction mixture was stirred for 48 h at room temperature. A white precipitate of triethylammonium chloride formed during that time, which was separated by filtration. The solvent was removed from the filtrate in a vacuum and the pale-yellow residue was dissolved in 25 mL of chloroform. The obtained suspension was filtered again and 5 mL of n-hexane was added. The resulting solution was stored for 3 weeks in a refrigerator at 8 °C. Yellow crystals were obtained, which were suitable for crystal structure analysis. A powder XRD was recorded from the batch product and compared with a powder XRD generated from the single crystal structure data. The comparison of the data sets showed a very good agreement (see Supplementary Materials).
The purity of the product was further verified with TLC. For this purpose, chloroform and hexane (1:3) were used as eluent and the ligand was applied to the TLC plate as a reference substance. The analysis showed that the free ligand was no longer present and that the batch product consisted of only one component.
Yield: 0.91 g (63.8%), melting point: 158 °C under decomposition.
1H-NMR (CDCl3, 500 MHz): δ [ppm] = 8.93 (s, 1, CH=N), 7.00–8.01 (mm, 6, Har), 4.26 (m, 1, CH-COO), 1.70–2.04 (mm, 3, CH2-CH(CH3)2), 1.03 (m, 6, CH(CH3)2), 0.62 (s, 3, Si-CH3), 0.24 (s, 3, Si-CH3); 13C-NMR (CDCl3, 126 MHz): δ [ppm] = 171.5 (COO), 168.9 (Car-O), 163.2 (CH=N), 141.8, 132.3, 129.9, 129.7, 127.5, 124.9, 122.5, 118.5 (8 Car), 108.9 (Car-CH=N), 66.6 (CH-COO), 44.4 (CH2), 24.0 (CH3-CH-CH3), 22.9, 22.1 (CH3-CH-CH3), 5.2 (Si-CH3), 1.6 (Si-CH3); 29Si-NMR (CDCl3, 79.5 MHz): δ [ppm] = −65.6; 29Si-CP/MAS-NMR (79.5 MHz, υrot = 5 kHz): δ [ppm] = −68.7.
[α]20D = 0° (c = 1 g/100 mL CHCl3), UV/Vis (CHCl3; c = 0.439 mmol/L; d = 1 cm) λ [nm] (ε [lmol−1cm−1]) = 411 (9152), 341 (7189), 338 (6925), 303 (2565), 260 (10,299).
IR (KBr): ν (cm−1) = 422(s), 476.4(m), 492.6(s), 507.9(s), 519.8(w), 538.2(w), 576.1(vs), 658.7(s), 670.5(s), 712.4(s), 747.4(s), 761.9(s), 796.9(vs), 826.4(vs), 865.3(vs), 925.5(w), 953.3(m), 963.7(m), 981.4(s), 1012.1(s), 1033.9(w), 1046.3(w), 1088.2(m), 1100.6(m), 1117.1(w), 1133.2(s), 1143.3(s), 1170.8(s), 1206.8(vs), 1257.8(vs), 1291(s), 1343.8(s), 1370.5(s), 1402.4(vs), 1423.7(vs), 1435.9(vs), 1467(vs), 1490.3(m), 1507.1(m), 1547.7(s), 1586.8(vs), 1603.6(vvs), 1624.6(vs), 1653.1(vs), 1692.6(vs), 1709.2(vs), 1771.7(w), 1922.6(w), 1952.7(w), 2020.3(w), 2842.4(m), 2867.2(m), 2915.6(s), 2955.7(s), 3045.2(m), 3375.8(m). The last band was caused by traces of water in the air during the measurement procedure.
3.4. X-ray Data Collection and Structure Refinement
Crystallographic data of 1 were recorded on a STOE IPDS-2T diffractometer using Mo Kα radiation (λ = 0.71073 Å) at 93 K using ω and φ scans. The crystal data and details of structure refinement are summarized in Table 4. X-AREA was used as software for data collection and for cell refinement. X-RED was used for data reduction [32]. The structure was solved by direct methods [33] and refined with full-matrix least-squares refinement on F2 for all reflections with SHELXL [34]. The non-hydrogen atoms were refined anisotropically. All hydrogen atoms were included in the structure model in calculated positions and were refined as constrained to their bonded atoms.

Table 4.
Crystal data and structure refinement for 1.
CCDC 2291941 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures (accessed on 1 September 2023).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13091407/s1, Figure S1: Powder XRD, Figure S2: 1H and 13C NMR spectra of the ligand N-(2-hydroxy-1-naphthylidene)leucine, Figure S3: 1H and 13C NMR spectra of 1, Figure S4: 29Si NMR spectra of 1, Figure S5: IR Spectra, Figure S6: UV-Vis spectra.
Author Contributions
U.B.: Formal Analysis, Investigation, Data Curation, Writing—Original Draft, Writing—Review and Editing, Visualization. S.F.: Formal Analysis, Investigation, Data Curation, Writing—Original Draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the TU Bergakademie Freiberg (Freiberg, Germany) for financial support. The authors gratefully acknowledge help from Beate Kutzner (NMR), Katrin Fuchs and Betty Günther (IR, UV-Vis). Erica Brendler (Institut für Analytische Chemie, TU Bergakademie Freiberg) is acknowledged for the solid-state NMR measurement. Erik Schumann (Institut für Physikalische Chemie, TU Bergakademie Freiberg) is acknowledged for powder XRD.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siedzielnik, M.; Pantazis, D.A.; Bruniecki, J.; Kaniewska-Laskowska, K.; Dołęga, A. The Reactivity of the Imine Bond within Polynuclear Nickel(II) Complexes. Crystals 2021, 11, 512. [Google Scholar] [CrossRef]
- Novoa-Ramírez, C.S.; Silva-Becerril, A.; Olivera-Venturo, F.L.; García-Ramos, J.C.; Flores-Alamo, M.; Ruiz-Azuara, L. N/N Bridge Type and Substituent Effects on Chemical and Crystallographic Properties of Schiff-Base (Salen/Salphen) Niii Complexes. Crystals 2020, 10, 616. [Google Scholar] [CrossRef]
- Basu Baul, T.S.; Chaurasiya, A.; Rabha, M.; Khatua, S.; Lyčka, A.; Schollmeyer, D.; Jurkschat, K. Diorganotin Compounds Containing α-Aminoacidato Schiff Base Ligands Derived from Functionalized 2-Hydroxy-5-(aryldiazenyl)benzaldehyde. Syntheses, Structures and Sensing of Hydrogen Sulfide. Eur. J. Inorg. Chem. 2020, 2020, 1803–1813. [Google Scholar] [CrossRef]
- Böhme, U.; Wiesner, S.; Günther, B. Easy access to chiral penta- and hexacoordinate silicon compounds. Inorg. Chem. Commun. 2006, 9, 806–809. [Google Scholar] [CrossRef]
- Paul, L.E.H.; Foehn, I.C.; Schwarzer, A.; Brendler, E.; Böhme, U. Salicylaldehyde-(2-hydroxyethyl)imine—A flexible ligand for group 13 and 14 elements. Inorg. Chim. Acta 2014, 423, 268–280. [Google Scholar] [CrossRef]
- Schwarzer, S.; Böhme, U.; Fels, S.; Günther, B.; Brendler, E. (S)-N-[(2-hydroxynaphthalen-1-yl)methylidene]valine—A valuable ligand for the preparation of chiral complexes. Inorg. Chim. Acta 2018, 483, 136–147. [Google Scholar] [CrossRef]
- Shraim, A.M.; Salih, K.S.M.; Al-Soufi, R.E.; Al-Mhini, S.R.; Ahmad, M.I.; Warad, I. Synthesis of Novel Aqua ƞ4-NNNO/Cu(II) Complexes as Rapid and Selective Oxidative Catalysts for O-Catechol: Fluorescence, Spectral, Chromotropism and Thermal Analyses. Crystals 2021, 11, 1072. [Google Scholar] [CrossRef]
- Sanmartín-Matalobos, J.; García-Deibe, A.; Zarepour-Jevinani, M.; Aboal-Somoza, M.; Bermejo-Barrera, P.; Fondo, M. Exploring the Chelating Potential of an Easily Synthesized Schiff Base for Copper Sensing. Crystals 2020, 10, 235. [Google Scholar] [CrossRef]
- Kamaal, S.; Mehkoom, M.; Muslim, M.; Afzal, S.M.; Alarifi, A.; Afzal, M.; Alowais, A.; Muddassir, M.; Albalwi, A.N.; Ahmad, M. Crystal Structure, Topological and Hirshfeld Surface Analysis of a Zn(II) Zwitterionic Schiff Base Complex Exhibiting Nonlinear Optical (NLO) Properties Using Z-Scan Technique. Crystals 2021, 11, 508. [Google Scholar] [CrossRef]
- You, Z.; Luo, Y.; Herringer, S.; Li, Y.; Decurtins, S.; Krämer, K.W.; Liu, S.-X. Formation of Tetranuclear Nickel(II) Complexes with Schiff-Bases: Crystal Structures and Magnetic Properties. Crystals 2020, 10, 592. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Heterometallic Complexes Containing the NiII-LnIII-NiII Moiety—Structures and Magnetic Properties. Crystals 2020, 10, 1117. [Google Scholar] [CrossRef]
- Dermitzaki, D.; Panagiotopoulou, A.; Pissas, M.; Sanakis, Y.; Psycharis, V.; Raptopoulou, C.P. Synthesis, Crystal Structures and Magnetic Properties of Trinuclear {Ni2Ln} (LnIII = Dy, Ho) and {Ni2Y} Complexes with Schiff Base Ligands. Crystals 2022, 12, 95. [Google Scholar] [CrossRef]
- Soroceanu, A.; Bargan, A. Advanced and Biomedical Applications of Schiff-Base Ligands and Their Metal Complexes: A Review. Crystals 2022, 12, 1436. [Google Scholar] [CrossRef]
- Nath, M.; Saini, P.K. Chemistry and applications of organotin(IV) complexes of Schiff bases. Dalton Trans. 2011, 40, 7077–7121. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Shah, D.; Khan, I.; Ahmad, S.; Ali, U.; Rahman, A. Synthesis and Antioxidant Activities of Schiff Bases and Their Complexes: An Updated Review. Biointerface Res. Appl. Chem. 2020, 10, 6936–6963. [Google Scholar] [CrossRef]
- Shi, X.; Kong, G.; Liu, L.; Gao, H.; Gao, X.; Tian, L. Synthesis, characterization and antibacterial activity of cyclohexyltin N-(salicylidene)valinates. Commun. Inorg. Synth. 2016, 4, 12–16. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Cui, G.; Tian, L. Synthesis, structure, and cytotoxicity of some triorganotin(IV) complexes of 3-aminobenzoic acid-based Schiff bases. Main Group Met. Chem. 2022, 45, 242–254. [Google Scholar] [CrossRef]
- Nagao, Y.; Ooki, T.; Kozawa, K. Absorption Spectra and Photovoltaic Characteristics of Silicon Chelate Complexes with Azomethine Dye Ligand. J. -Jpn. Soc. Colour Mater. Shikizai Kyokaishi 2004, 77, 51–56. [Google Scholar] [CrossRef][Green Version]
- Nagao, Y.; Kimura, F.; Kozawa, K.; Uchida, T. Crystal Structures of Diethylamino Substituted Benzylideneanilines and Absorption Spectra of Their Related Derivatives. J. -Jpn. Soc. Colour Mater. Shikizai Kyokaishi 2002, 75, 415–420. [Google Scholar] [CrossRef][Green Version]
- Nath, M.; Goyal, S.; Goyal, S. Synthesis, spectral and biological studies of organosilicon (IV) complexes of Schiff bases derived from amino acids. Synth. React. Inorg. Met.-Org. Chem. 2000, 30, 1791–1804. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Biradar, N.S.; Patil, S.B.; Roddabasanagoudar, V.L.; Rudzinski, W.E. Amino acid schiff base complexes of dimethyldichlorosilane. Inorg. Chim. Acta 1985, 107, 231–234. [Google Scholar] [CrossRef]
- Jain, M.; Gaur, S.; Singh, V.P.; Singh, R.V. Organosilicon (IV) and organotin (IV) complexes as biocides and nematicides: Synthetic, spectroscopic and biological studies of NN donor sulfonamide imine and its chelates. Appl. Organometal. Chem. 2004, 18, 73–82. [Google Scholar] [CrossRef]
- Böhme, U.; Günther, B. Five and six-coordinate silicon complexes with an O,N,O′-chelating ligand derived from ortho-hydroxyacetophenone-N-(2-hydroxyethyl)imine. Inorg. Chem. Commun. 2007, 10, 482–484. [Google Scholar] [CrossRef]
- Böhme, U.; Fels, S. A new type of chiral pentacoordinated silicon compounds with azomethine ligands made from acetylacetone and amino acids. Inorg. Chim. Acta 2013, 406, 251–255. [Google Scholar] [CrossRef]
- Schwarzer, A.; Fels, S.; Böhme, U. Two reversible enantiotropic phase transitions in a pentacoordinate silicon complex with an O,N,O′-tridentate valinate ligand. Acta Cryst. 2015, C71, 511–516. [Google Scholar] [CrossRef]
- Suh, I.-H.; Park, K.H.; Jensen, W.P.; Lewis, D.E. Molecules, Crystals, and Chirality. J. Chem. Educ. 1997, 74, 800. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis (N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]-copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Dong, J.; Li, L.; Li, L.; Xu, T.; Wang, D. Synthesis, Characterization, DNA-binding Properties and DNA Cleavage of a New Ternary Copper(II) Complex with Mixed-ligands of Tridentate Schiff Base and 1,10-Phenanthroline. Chin. J. Chem. 2011, 29, 259–266. [Google Scholar] [CrossRef]
- Liang, H.; Kang, J.-J.; Guo, Q.; Zhang, Q.; Fei, R.-L.; Guo, Z.-D.; Li, L.-Z. Synthesis, crystal structure and spectroscopic characterization of two chiral oxovanadium complexes [VO(Naph-Leu)(OMe)(CH3OH)] and [VO(Naph-Phe)(OMe)(CH3OH)]. Chin. J. Inorg. Chem. (Huaxue Xuebao) 2013, 29, 1819–1824. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 30 August 2023).
- Nitta, H.; Yu, D.; Kudo, M.; Mori, A.; Inoue, S. Peptide-titanium complex as catalyst for asymmetric addition of hydrogen cyanide to aldehydes. J. Amer. Chem. Soc. 1992, 114, 7969–7975. [Google Scholar] [CrossRef]
- Fels, S. Höherkoordinierte Komplexverbindungen des Siliciums, Germaniums und Zinns Mit Chiralen O, N, O′-Liganden. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2015. [Google Scholar]
- STOE & Cie GmbH. X-RED (Version 1.53) and X-AREA (Version 1.55); STOE & Cie GmbH: Darmstadt, Germany, 2009. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).