Abstract
In recent years, “Jin Gao Yu” that has been traded as a kind of jade has appeared in areas of the Luonan and Shangnan counties, Shangluo City, Shaanxi Province, attracting the attention of scholars and consumers for its delicate texture and warm color. In this study, infrared spectroscopy, Raman spectroscopy, X-ray diffraction analysis, and electron microprobe analysis were used to conduct a systematic gemmological test and an analysis of “Jin Gao Yu”. The results show that “Jin Gao Yu” is a compact mineral aggregate dominated by dolomite, which contains quartz mineral inclusions. The color of “Jin Gao Yu” is grayish-white to earthy-yellow, the refractive index is about 1.54, and the relative density is about 2.86. Its crystal structure is basically the same as that of dolomite, both of which are trigonal systems with granular crystalloblastic textures. It has good crystallinity. The recrystallization phenomenon can be seen under a polarizing microscope. This study determined the species of “Jin Gao Yu”, improved its gemological basic data, provided a theoretical basis for the identification of “Jin Gao Yu” in the future, and, also, provided a new direction for the use of dolomite.
1. Introduction
Carbonates are thought to be storage depots for carbon in the Earth’s mantle. And, they are both important non-metallic mineral raw materials as well as important mineral sources of metals such as iron, magnesium, manganese, zinc, and copper and radioactive elements such as uranium, thorium, and rare earth elements [1]. In gemmology, carbonate jade, whose main mineral composition comprises calcite or dolomite, is often used to carve or imitate other precious gemstones.
Dolomite is a common carbonate mineral with the chemical formula [CaMg(CO3)2] that often contains Fe and Mn and occasionally contains Pb, Zn, Ni, and Co [2]. When its composition is pure, it is white. When it contains other elements and impurities, it can be gray-green, gray-yellow, and pink [3]. The structure of dolomite is very similar to that of calcite, both of which are metal cations bonded to six O’s from [CO3] to form a sixfold coordination [4,5]. Half of the Ca2+ position in the calcite structure is occupied by Mg2+, Ca2+, and Mg2+, arranged in the direction perpendicular to the c-axis in layers in a regular alternating pattern [6]. The previous research on dolomite mainly focused on the field of geochemistry, which is relatively rare in geomology. Currently, cobalt-bearing dolomite, which is pink to purplish-red, is considered to be the only dolomite in the world to be used as a gem material, and it is mainly found in the Democratic Republic of the Congo [7,8]. Grayish-white to earthy-yellow dolomite is hardly ever used as a gemstone. However, in recent years, “Jin Gao Yu” that has been traded as a kind of jade has appeared in Luonan County and Shangnan County, Shangluo City, Shaanxi Province, China. It is very similar to “Myanmar Yellow Jade”, with silica being its main component. And, it is grayish-white to earthy-yellowish in color, with a fine, clear, and neat surface texture, attracting the attention of many scholars. Some scholars [9] have studied “Jin Gao Yu” as a carbonate mineral, and its main mineral composition is dolomite. At present, there is still insufficient research on the structure, composition, and gemological aspects of “Jin Gao Yu”.
In this paper, we report a systematic gemmological study of “Jin Gao Yu” that used conventional gemmological testing, infrared spectroscopic testing, Raman spectroscopic testing, X-ray diffraction analysis, and electron microprobe analysis. Thus, we can have a more comprehensive understanding of it, make better use of our mineral resources, and enrich the use of dolomite in the direction of gemmology, providing a new direction for the development and utilization of dolomite minerals.
2. Materials and Methods
2.1. Materials
The three samples in this experiment were collected from Shimen Town, Luonan County in the northern foothills of the Qinling Mountains, Shaanxi Province, China. The high-quality dolomite of Luonan County is found in the fourth lithological section of the Longjiayuan Formation, which is a light-gray thin-to-thick laminate dolomite interspersed with flint bands, and part of this geological section is located in Shimen Town. Most of Shimen Town is Neogenic, consisting mainly of a set of terrigenous clast rocks and carbonate sedimentary rock [10]. As shown in Figure 1, all three samples comprise plates of about 3.5 cm × 2.3 cm × 0.7 cm, which are uniform in color (from left to right, they are earthy-yellow, light-yellow, and grayish-white, named JGY1, JGY2, and JGY3 in turn). They have smooth surfaces, fine textures, and clear, neat textures. JGY1 consists of a yellow substrate and a few irregular, short, and thin yellowish-brown mineral strips. JGY2 consists of a light-yellow mineral substrate, and its surface is relatively clean with no impurity minerals visible to the naked eyes. JGY3 consists of a grayish-white mineral base with distinctive laminar mineral bands.

Figure 1.
Samples of “Jin Gao Yu”.
2.2. Methods
This paper mainly focuses on the systematic gemmological study conducted on the “Jin Gao Yu” samples through conventional gemmological tests, infrared spectroscopy, Raman spectroscopy, X-ray diffraction analysis, and electron microprobe tests.
Conventional gemmological characteristics were determined at the Gemmological Experimental Teaching Center, School of Gemmology, China University of Geosciences, Beijing. The refractive indices were measured through point measurement using a gemstone refractometer. The specific gravity was determined by hydrostatic weighing method. Microscopic observations were made with a GI-MP22 gemmological photomicrographic microscope. Polarizing microscope observations were made using a BX51-type polarizing microscope.
Infrared spectra were obtained by using FT-IR Spectrometer Tensor 27, produced by Bruker, Germany, in the Gem Testing Laboratory at the School of Gemmology, China University of Geosciences, Beijing. Since KBr crystals absorb very little in the infrared region, the KBr compression method is widely used in infrared characterization and structural analysis. In this test, the infrared spectrum (transmission) of a sample of “Jin Gao Yu” was collected by using the KBr compression transfer method.
Raman spectra were collected with the HR Evolution micro-Raman spectroscope, produced by HORIBA, Japan, in the Gem Testing Laboratory at the School of Gemmology, China University of Geosciences, Beijing. The analytical conditions used for measurement included a range of 100–4000 cm−1, an accumulation time of 3 s, a laser wavelength of 532 nm, and a grating of 600 (500 nm).
The X-ray powder diffraction (XRD) tests were performed using Dmax12KW in the School of Materials Sciences and Technology, China University of Geosciences, Beijing. The tests were carried out using CuKα rays at a scanning speed of 0.17°/s.
Electron Probe Microanalysis (EPMA) test data were collected with an EPMA-1720, produced by Shimadzu, Japan, in the Geoscience Test Center at China University of Geosciences, Beijing. The main experimental conditions were as follows: an accelerating voltage of 15 KV; a beam current of 50.4 nA; and a beam diameter of 5 μm.
3. Results
3.1. Conventional Gemological Features
Basic gemmological tests were conducted on three samples of “Jin Gao Yu”. The test results show that “Jin Gao Yu” is opaque, with a refractive index (spot measurement) of about 1.54 and a relative density of about 2.85, and is inert under both long- and short-wave UV fluorescent lamp irradiation. Under a gemological microscope, it can be observed that it has a dense granular texture, with vein-like structures, and that there are obvious colorless quartz veins interspersed with it (as shown in Figure 2).
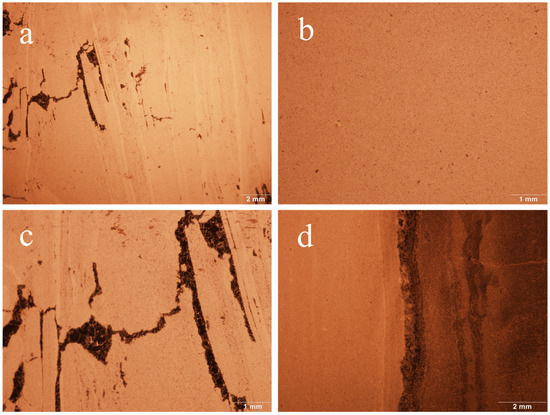
Figure 2.
Characteristics of “Jin Gao Yu” under the gemstone microscope. (a) JGY1, magnified 0.63×; vein structure and a few black minerals are visible. (b) JGY2, magnified 1.5×; dense granular structure is visible. (c) JGY1, magnified 1.5×; black minerals are visible. (d) JGY3, magnified 1.0×; black quartz veins are visible.
The three samples of “Jin Gao Yu” were made into thin sections and magnified 20 times under the polarizing microscope, and the results are shown in Figure 3. Figure 3a–d show the images of sample JGY1 under the polarizing microscope, and in these, dolomite is colorless and turbid-gray under the monoscope, and it has an advanced white interference color under the orthoscope (similar to a pearl-halo-like advanced white). The dynamic recrystallization is visible in Figure 3a,b, which show the gradual change in particle size from coarse grains to fine grains. And, Figure 3c,d show a rhombic cleavage with bicrystalline lines parallel to the short diagonal of the rhombus. The grain size of the dolomite shown in sample JGY2 (Figure 3e,f) is much smaller than 0.2 mm, with a uniform distribution and a crystalloblastic texture. Sample JGY3 (Figure 3g,h) is turbid-gray-white under the monoscope, and it shows the phenomenon of having a pearl-like halo color under the orthoscope, which indicates a fine-grained crystalloblastic texture, and it also shows the appearance of the dynamic recrystallization phenomenon. In addition, quartz minerals and some calcite can be seen, consistent with the quartz veins observed under the gemstone microscope, and the quartz has a large grain size and no cleavage and is of positive low relief under the monoscope.
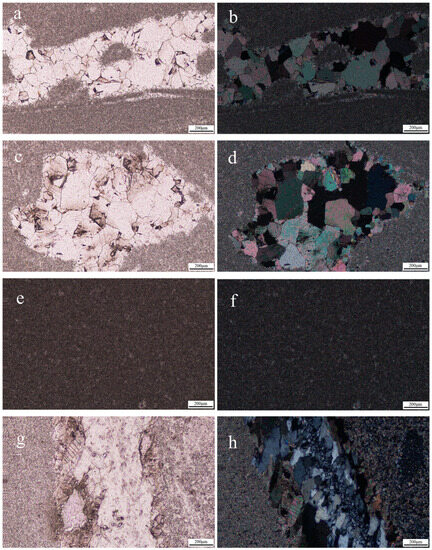
Figure 3.
The “Jin Gao Yu” samples, magnified 20× under the polarizing microscope. (a,c,e,g) are from under the monoscope; (b,d,f,h) are from under the orthoscope. (a–d) show the granular crystalloblastic texture of dolomite, rhombic cleavage, and the dynamic recrystallization phenomenon in the JGY1 sample; (e,f) shows the crystalloblastic texture of dolomite in the JGY2 sample; (g,h) show that there are large quartz particles and some calcite in the sample JGY3.
3.2. Infrared Spectra
The infrared spectra was measured using the infrared KBr pressing method (transmission) (Figure 4). The results show that the tested samples indicate the absorption of dolomite-group minerals. The Ca2+ and Mg2+ ions inside the dolomite crystals have less influence on the vibration frequency of the groups, and what the infrared spectra present is mainly determined by the vibration of the [CO3]2− groups. The absorption valleys around 1444 cm−1 show [CO3]2− anti-symmetric telescopic vibration; the absorption valleys around 881 cm−1 and 852 cm−1 show [CO3]2− out-of-plane bending vibrational absorption; and the absorption valleys around 729 cm−1 show [CO3]2− in-plane bending vibrational absorption. Moreover, the tested spectrum also shows the absorption valleys around 1051 cm−1, belonging to cerussite, which indicate [CO3]2− in-plane bending vibrational absorption. In addition, an absorption valley is present around 1051 cm−1, caused by [CO3]2− symmetric telescoping vibrations, and it is Raman-active and less frequent in the infrared spectra [11].
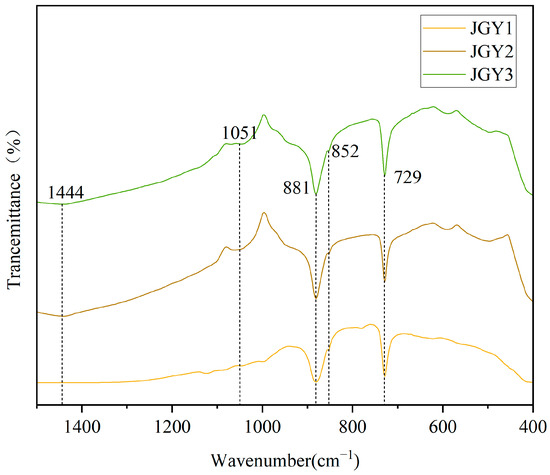
Figure 4.
Infrared spectra (transmission method) of “Jin Gao Yu”.
3.3. Raman Spectra
The results of Raman spectroscopic tests on the basal portion of the “Jin Gao Yu” samples are shown in Figure 5. The results of the sample have the characteristic peaks of dolomite (the data on dolomite Raman peaks are from the RRUFF standard card R040030). The antisymmetric stretching vibration of the C-O bond is 1444 cm−1, 1097 cm−1 is the symmetric telescopic vibration of internal CO32−, 725 cm−1 is the symmetric vibration of the in-plane bending of CO32−, 340 cm−1 is the out-of-plane bending vibration of CO32−, and 175 and 300 cm−1 are the internal vibration values of carbonate mineral lattice [12,13,14,15,16,17]. The Raman peaks of carbonate minerals are very similar [18]. The two Raman displacements within the range of 250–350 cm−1 are the characteristic Raman peaks distinguishing the calcite group minerals, and these are caused by the regular alternating arrangement of Ca2+ octahedra and Mg2+ octahedra along the direction of the tertiary axes of the dolomite’s own structure, leading to the splitting of its internal vibrational modes νob into νob and νaob [19]. This indicates that “Jin Gao Yu” belongs to the dolomite group of minerals.
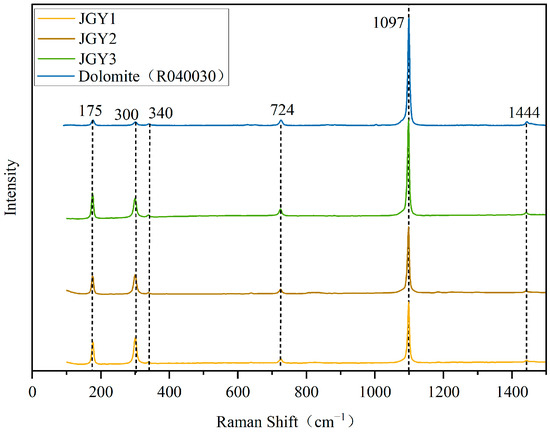
Figure 5.
Raman spectra of “Jin Gao Yu” and dolomite.
As can be seen, the surface of the sample of “Jin Gao Yu” shows obvious speckled and vein-like impurities. Therefore, the impurities were further tested using Raman spectroscopy. The test results are shown in Figure 6. From Figure 6, one notes that the tested point of JGY3-1 has the characteristic Raman peaks of quartz (the Raman data for quartz are from the RRUFF standard card R050125), and the strongest peak, near 464 cm−1, is caused by the symmetric bending vibration of Si-O, and it is the characteristic Raman peak for identifying quartz [20]. The Raman peaks in the 200–300 cm−1 interval are related to the rotational or translational vibrations of the silica–oxygen tetrahedron [SiO4], those in the 300–600 cm−1 interval are related to the Si-O bending vibrations, those in the 600–800 cm−1 interval are related to the Si-O-Si symmetric telescoping vibrations, and those in the 1000–1200 cm−1 interval are related to the Si-O asymmetric stretching vibration. The rest of the Raman peaks are related to the stretching vibration of M-O, which has an ionic bonding nature, and its coupling vibration, with the bending vibration of Si-O-Si and the stretching vibration of Si-O [21]. These results show that the sample of “Jin Gao Yu” contains quartz impurities.
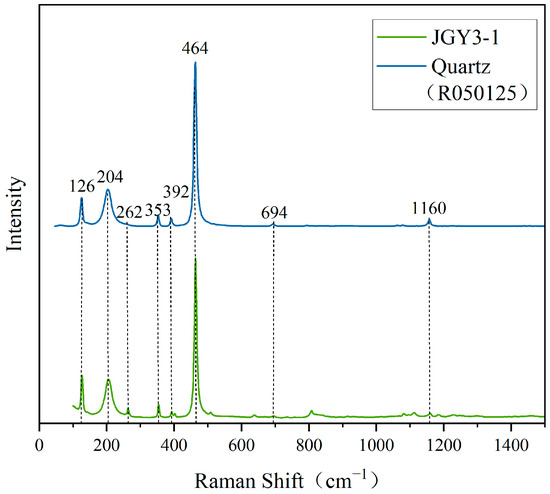
Figure 6.
Raman spectra of impurities at the tested point of JGY3-1 and quartz.
3.4. Electron Microprobe
Electron microprobe spectroscopy is one of the effective means of identifying carbonate mineral species. The electron microprobe spectra of the samples of “Jin Gao Yu” were tested separately using an electron microprobe wave analyzer (shown in Table S1). From Table S1, we can see that the chemical composition data of the five test points are generally the same, and it is clear that the main components of the sample are CaO (27.86~29.83 wt%) and MgO (20.72~22.73 wt%) and the minor components are Cr2O3 (0.00~0.01 wt%), FeO (0.19~2.37 wt%), Na2O (0.01~0.03 wt%), SiO2 (0.02~0.03 wt%), and MnO (0.02~0.08 wt%). The CaO and MgO contents of “Jin Gao Yu” samples are in general agreement with the theoretical values of dolomite (the theoretical values of the chemical composition of dolomite minerals are: 30.41 wt% CaO, 47.33 wt% CO2, and 21.86 wt% MgO [22]). In the test results, the total amount of composition was less than 100%. Referring to the previous research on dolomite, it is believed that the total composition was less than 100% mainly due to the inability to collect the content of light elements in the process of testing. This is because the results of the above tests show that the main component of “Jin Gao Yu” is dolomite, and the theoretical value of CO2 in dolomite is 47.33%, close to 100% when added to the total amount of components measured. It is presumed that the insufficient part mainly comprises the content of CO2.
3.5. XRD Analysis
The X-ray diffraction spectrum often reflects information about the composition of a mineral. Three samples of “Jin Gao Yu” were tested using X-ray diffraction spectroscopy, and the results are shown in Figure 7. The XRD results of the sample are consistent with those of dolomite PDF#36-0426. The strongest 2-Theta peak at 30.938° correspond to the crystal plane 104 of dolomite, and the following stronger peaks at 41.127°, 50.526°, 51.068°, and 44.949° correspond to the crystal planes 113, 018, 116, and 202 of dolomite, respectively. Meanwhile, the characteristic peak d values of 0.28845 nm, 0.28831 nm, and 0.28871 nm for the sample of “Jin Gao Yu” (104) are within the range of the characteristic peak d values of dolomite 104 (0.2854–0.2912 nm) [23], indicating that the different colors of the “Jin Gao Yu” and dolomite basically have the same crystal structure. In addition, there is a weak diffraction peak at a d value of 0.33445 nm (as shown in gray bar of Figure 7), which is weakly expressed in JGY1 and JGY2, while the peak is more pronounced in JGY3. This peak was matched to be the diffraction peak of quartz (the standard card of quartz in Figure 7 is PDF#46-1045).
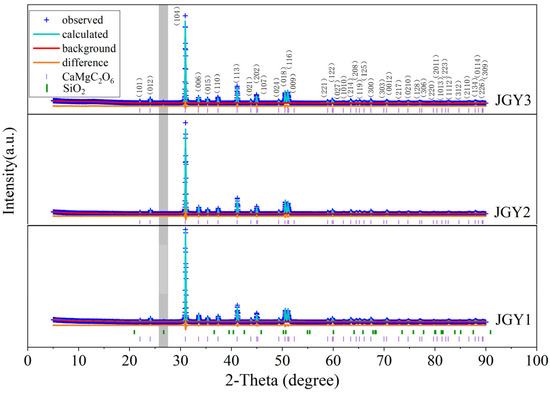
Figure 7.
X-ray diffraction patterns of “Jin Gao Yu”.
The Rietveld method is used to refine the XRD test data to obtain the unit cell parameters of the measured “Jin Gao Yu” (as shown in Table 1). As shown in Table 1, the relative density of the measured sample is 2.86, which is consistent with the density measured using the hydrostatic method, and the unit cell parameters of the sample are slightly smaller than that of standard dolomite.

Table 1.
Cell parameters of “Jin Gao Yu” dolomites.
4. Discussion
The test results show that the infrared spectra, Raman spectra, and XRD patterns of “Jin Gao Yu” are consistent with those of dolomite. Based on the electron microprobe test data, and referring to the previous calculations of the chemical formula of dolomite [10], the crystal formula of the “Jin Gao Yu” sample was calculated by using the cation method and found to be Ca0.94–0.98 Mg0.97–1.03 Fe0.00–0.60 (CO3)2, which is basically the same as the ideal chemical formula CaMg(CO3)2 for the dolomite group of minerals [24]. And, the molar ratio of Ca2+ to Mg2+ is close to 1:1, which satisfies the conditions of ideal dolomite [25]. It is also necessary to demonstrate that the crystal structure is highly ordered, meaning that CaCO3-MgCO3 is regularly interlayered in the c-axis direction. The presence of three peaks (101, 015, and 021) in the XRD test results indicates that the sample has an ordered dolomite crystal structure [2]. And, it has been found that the higher the degree of ordering of dolomite is, the closer the CaCO3 molar fraction in the lattice is to the 50% theoretical value [26,27]. Using the test data from XRD, the molar fraction of CaCO3 was calculated by using the method of LUMMSDEN (NCaCO3 = 333.33d104 − 911.11, N-mole fraction content (%); d104-strongest diffraction-peak crystal-plane spacing of dolomite). The values of JGY1, JGY2, and JGY3 were calculated to be 50.38%, 49.91%, and 51.25% in order, and the order of the three samples was JGY2, JGY1, and JGY3 in descending order. The results show that the molar fractions of CaCO3 in JGY1, JGY2, and JGY3 are 50.38%, 49.91%, and 51.25% in that order, which means that the three samples have crystal structures with a high degree of order, and their degree of ordering is JGY2, JGY1, and JGY3 in the order of high to low. Therefore, it is believed that the main component of “Jin Gao Yu” is dolomite.
Comparing the crystal chemical formula of “Jin Gao Yu” with the ideal chemical formula of dolomite, “Jin Gao Yu” has a higher iron content. In previous studies, some scholars [9] found that “Jin Gao Yu” contains hematite and limonite. However, this experiment did not detect hematite or limonite. Combined with the geological structure of Shimen Town [28], it is thought that the Fe ions may come from terrestrial clastic rocks near the dolomite. The Fe2+ substituted Mg2+ and Ca2+ in an isomorphic replacement manner, resulting in a higher iron content within the samples.
In addition, the composition of quartz was detected in XRD tests, by using Raman spectroscopy, and in electron probe test results, indicating that “Jin Gao Yu” contains quartz impurities. Combined with the geological characteristics of Shimen Town, it is believed that the quartz may come from the flint band or flint layer near the dolomite, and the quartz veins presented in JGY3 may be part of the flint band.
There are many factors affecting the crystal constants such as the ordering of the crystal structure and the substitution of ions of different radii [19]. The order of the samples has been calculated in the previous section and is, in descending order, JGY2, JGY1, JGY3. In addition, in combination with the results of electron microprobe tests, it is believed that the reason for the decreases in the sample cell volume is related to the isomorphous substitution of Fe2+ for Mg2+ and Ca2+, and the difference in the proportions of its substitution caused the decreases in the cell volume to different degrees. From the analysis and calculation (Table 1), it can be seen that the crystallinity of the sample of “Jin Gao Yu” is good, except for the highest diffraction peaks corresponding to the lower crystallinity (about 68–78%), and the crystallinity values of the rest of the diffraction peaks correspond to the higher crystallinity (most of the crystallinity is in the range of 98–99%). Its high crystallinity indicates that the number of crystals in the tested samples of “Jin Gao Yu” is large and that the crystals are well organized. It shows that the “Jin Gao Yu” has a dense granular structure, which is consistent with the microscopic observation.
In summary, “Jin Gao Yu” is a dense granular mineral aggregate, dominated by dolomite, that can contain quartz mineral inclusions inside, with high crystal structure order and good crystallinity.
5. Conclusions
The following conclusions can be drawn from the tests on the composition and structure of “Jin Gao Yu”. “Jin Gao Yu” from areas in Luonan County and Shangnan County, Shangluo City, Qinling Mountains, Shaanxi Province, China is a kind of dense granular mineral aggregate, dominated by dolomite, that contains quartz mineral inclusions inside it, with a granular metamorphic structure, high crystal structure order, and good crystallinity, and the recrystallization phenomenon can be seen under polarizing microscopy. The color of “Jin Gao Yu” is grayish-white to earthy-yellow, the refractive index is about 1.54, and the relative density is about 2.86, and it is inert under both short- and long-wave ultraviolet fluorescence. Preliminary calculations suggest that its chemical formula is Ca0.94~0.98 Mg0.97~1.03 Fe0.00~0.60 (CO3)2. Its crystal structure is basically the same as that of dolomite, both of which are trigonal systems. The Fe2+ isomorphous substitution of Mg2+ and Ca2+ in the crystal makes its unit cell volume slightly smaller than that of dolomite. These conclusions improve the gemological basic data on “Jin Gao Yu” and provide a theoretical basis for the identification of “Jin Gao Yu” in the future.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst13091399/s1, Table S1: EPMA test results of “Jin Gao Yu”.
Author Contributions
L.L. (Liangyu Liu) and N.L.—doing the experiment, conducting analyses, and writing the original manuscript; Q.G.—review and editing; S.Z., Y.R.—translating; Y.L.—drawing pictures; L.L. (Libing Liao)—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by both the National Science and Technology Infrastructure—The National Infrastructure of Mineral, Rock and Fossil Resources for Science and Technology (http//www.nimrf.net.cn, accessed on 25 December 2021) as well as the Program of Data Integration and Standardization in Geological Science and Technology from MOST, China, grant number 2013FY110900-3.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, T.X.; Cui, H.J.; Sun, Y.B.; Huang, T.H. Quantitative analysis of carbonate minerals by electron probe full element method. Acta Geol. Sichuan 2021, 41, 310–314. (In Chinese) [Google Scholar]
- Zheng, Z.; Wang, Q. Research progress on ordering and rheological properties of dolomite. Geol. J. China Univ. 2020, 26, 197–208. (In Chinese) [Google Scholar] [CrossRef]
- Chen, N.X.; Chen, C.; Li, G.G.; Cao, S.Q.; Lu, Y.; Zhang, H. Study on gemological characteristics of pink-purplish red dolomite. Superhard Mater. Eng. 2021, 33, 47–52. (In Chinese) [Google Scholar]
- Barber, D.J.; Reeder, R.J.; Smith, D.J. A tem microstructural study of dolomite with curved faces saddle dolomite. Contrib. Mineral. Petrol. 1985, 91, 82–92. [Google Scholar] [CrossRef]
- Graf, D.L.; Eardley, A.J.; Shimp, N.F. A preliminary report on magnesium carbonate formation in Glacial Lake Bonneville. J. Geol. 1961, 69, 219–223. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, Z.Y. Crystal structure characterization and X-ray studies of dolomite. Mineral. Petrol. 1988, 28–33. (In Chinese) [Google Scholar] [CrossRef]
- Du, J.M.; Zhao, X.Z. Geological characteristics and distribution of copper-cobalt deposits in Congo. Geol. Prospect 2010, 46, 165–174. (In Chinese) [Google Scholar]
- Yan, Y.; Yu, X.Y. Gemology, Mineralogy, and Coloration Mechanism of Pinkish-Purple Cobaltoan Dolomite from the Democratic Republic of Congo. Crystals 2022, 12, 639. [Google Scholar] [CrossRef]
- Zhang, C.S.W.; Pan, N.; Zhang, J.; Song, Y.J. Gem mineralogy characteristics of carbonate jade produced in Shaanxi. In Proceedings of the 2021 International Jewelry Academic Exchange Conference Proceedings, Beijing, China, 19–21 November 2021; pp. 280–284. [Google Scholar]
- Sun, B.; Liu, Y.Y. Distribution and physical characteristics of metallurgical grade dolomite in northern Luonan County, Shaanxi Province. Miner. Explor. 2020, 11, 954–958. (In Chinese) [Google Scholar]
- Yang, N.; Kuang, S.Y.; Yue, Y.H. Infrared spectra analysis of several common anhydrous carbonate minerals. Mineral. Petrol. 2015, 35, 37–42. (In Chinese) [Google Scholar] [CrossRef]
- Edwards, H.G.; Villar, S.E.J.; Jehlicka, J.; Munshi, T. FT–Raman spectroscopic study of calcium-rich and magnesium-rich carbonate minerals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2273–2280. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Nicola, J.; Scott, J.; Couto, R.; Correa, M. Raman spectra of dolomite [CaMg(CO3)2]. Phys. Rev. B 1976, 14, 4676. [Google Scholar] [CrossRef]
- Rutt, H.; Nicola, J. Raman spectra of carbonates of calcite structure. J. Phys. C Solid State Phys. 1974, 7, 4522. [Google Scholar] [CrossRef]
- Herman, R.G.; Bogdan, C.E.; Sommer, A.J.; Simpson, D.R. Discrimination among carbonate minerals by Raman spectroscopy using the laser microprobe. Appl. Spectrosc. 1987, 41, 437–440. [Google Scholar] [CrossRef]
- Gao, R. Study of Raman Spectroscopy of Common Carbonate Minerals at High Pressures and High Temperatures and Its Constrains to Their Thermal Properties. Master’s Thesis, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang, China, 2012. [Google Scholar]
- Chai, C.; Wang, L.J. Raman spectroscopy studies of siderite. West-China Explor. Eng. 2012, 24, 156–158. (In Chinese) [Google Scholar]
- Du, G.P.; Fan, J.L. Characteristics of raman spectral of calcite group minerals. Mineral. Petrol. 2010, 30, 32–35. (In Chinese) [Google Scholar] [CrossRef]
- Su, L.; Fan, J.L.; Guo, S.G. Study on mineralogical characteristics and coloration mechanism of purple chalcedony. Conserv. Util. Miner. Resour. 2008, 10, 21–26. (In Chinese) [Google Scholar]
- Chen, X.J.; Wang, Y.Q.; Mao, J. Spectral characteristics and coloration mechanism of natural and synthetic amethyst. J. East China Univ. Sci. Technol. 2011, 37, 320–324. (In Chinese) [Google Scholar] [CrossRef]
- Hao, Y.F.; Zhao, A.L. A simple method of quantitative analysis for calcite and dolomite in rock by X-ray diffraction. Non-Ferr. Min. Metall. 2005, 58–60. (In Chinese) [Google Scholar]
- Shen, J.Y.; Yan, Z.Y.; Fu, H.P.; Ma, X.; Zhang, H.Y.; Zhao, Q.; Xu, H. Spatial variation and genesis of miocene ankerite in well xiyong 2. Mar. Geol. Front. 2021, 37, 39–48. (In Chinese) [Google Scholar] [CrossRef]
- Li, S.R. Crystallography & Mineralogy; Geological Publishing House: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Huang, S.J. Diagenesis of Carbonate Rocks; Geological Publishing House: Beijing, China, 2010; pp. 1–288. (In Chinese) [Google Scholar]
- Kaczmarek, S.E.; Sibley, D.F. On the evolution of dolomite stoichiometry and cation order during hightemperature synthesis experiments: An alternative model for the geochemical evolution of natural dolomites. Sediment. Geol. 2011, 240, 30–40. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Qiao, Z.F.; Shou, F.Y.; Meng, S.X.; Lv, X.J. Origin and formation mechanism of dolomite in Penglaiba Formation of Yonganba outcrop, Tarim Basin: Evidence from ordering degree and unit cell parameters. Nat. Gas Geosci. 2020, 31, 602–611. (In Chinese) [Google Scholar]
- Zhang, Y.L.; Zhou, C.Y.; Li, Y.Z.; Lu, A.H.; Ding, H.R. Analysis of mineralogical characteristics and formation environment of dolomitic carbonate rocks in western Shandong. Acta Petrol. Mineral. 2023, 42, 365–378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).