Abstract
The synthesis and X-ray crystal structure analyses of the azido complex [Zn(N3)(Arg)2](N3)·3H2O, where Arg is L-arginine, were presented. The molecular structure of the complex was further studied using FT-IR spectra as well as atoms in molecules (AIM) theory. An analysis of the crystal data indicated monoclinic crystal system and P21 space group with a = 13.0283(5) Å, b = 15.2032(7) Å, c = 13.3633(6) Å, β = 114.3580(10)°, V = 2411.28(18) Å3, and Z = 4. Two of the [Zn(N3)(Arg)2](N3)·3H2O formulae represent the asymmetric unit of this complex where the geometric parameters of both units are slightly different. In [Zn(N3)(Arg)2](N3)·3H2O, the central Zn(II) ion is penta-coordinated with two Arg molecules as a bidentate ligand and one terminally coordinated azide ion. Each of the two Arg molecules are located trans to one another and coordinated with the Zn(II) via the N and O atoms of the amino and carboxylate groups, respectively. Hence, Zn(II) is five-coordinated and has a distorted square pyramidal coordination geometry. The supramolecular structure of the [Zn(N3)(Arg)2](N3)·3H2O complex was inspected using the Hirshfeld analysis. The O···H (26.6–28.4%), H···H (32.3–35.3%), and N···H (30.4–34.0%) contacts are the most significant interactions in the crystal structure of the [Zn(N3)(Arg)2](N3)·3H2O complex. The Zn–N, and Zn–O bonds have slight covalent interactions based on the AIM study.
1. Introduction
Over the past ten years, there has been a lot of interest in the self-assembly of supramolecular structures involving metal coordination compounds because of their potential uses in many domains such as catalysis [1,2,3] and biological systems [4]. In coordination chemistry, multi-dentate ligands play a significant role because they can form metal complexes with extra stability as a result of the chelate effect [5,6,7,8,9,10,11]. First row transition metals have a variety of coordination numbers and can be used to bind ligands to create coordination complexes with diverse of coordination geometries [12,13,14,15,16]. Consider the coordination structure of Zn(II) as an example; it is typically tetra-coordinated with tetrahedral geometry [17,18]. Also, it has the ability to form five coordination numbers with square pyramidal [19], and trigonal bipyramidal geometries [6]. In addition, it could be six coordinated in octahedral geometry [18]. All previous geometries are found for Zn(II) whether it is in an ideal or a distorted geometry.
On the other hand, amino acids are important parts of numerous natural substances and are usually present in food, drugs, physiological fluids, and tissues [20,21,22,23]. Also, amino acids have many uses in different fields such as water treatment [24] and quality control [25]. Metal–amino acid complexes have seen substantial studies in terms of their preparations and structures [21]. A common naturally occurring amino acid called arginine can form salt bridges with anionic side chains because of its guanidinium side chain [21,26]. Similar to other amino acids, L-arginine (Arg) binds to metal ion through the N atom of the amino group, O atom of carboxylate group, or both of them [23].
On the other hand, the azido ligands are a category of organic or inorganic azides [27,28]. The azido ligand has been commonly applied in many scopes such as molecular magnetism [29,30,31,32,33] and metalloenzymes [34]. For instance, azide ion is a simple inorganic ligand and could work as a linker between metal sites leading to versatile coordination modes [35,36,37]. It can act as a terminal monodentate [30], or bridging ligand [34,38,39,40]. Also, it can form 1-D [41,42,43,44,45,46,47], 2-D [36,48,49], and 3-D supramolecular structures [50,51]. In this regard, Hirshfeld surface analysis gained a lot of popularity in terms of crystal structure analysis. It is a rapidly growing tool for the qualitative and quantitative analyses of the different intermolecular interactions in crystalline materials. Hence, Hirshfeld surface analysis is an important tool in crystal engineering in order to understand the molecular packing in the crystal structure [52].
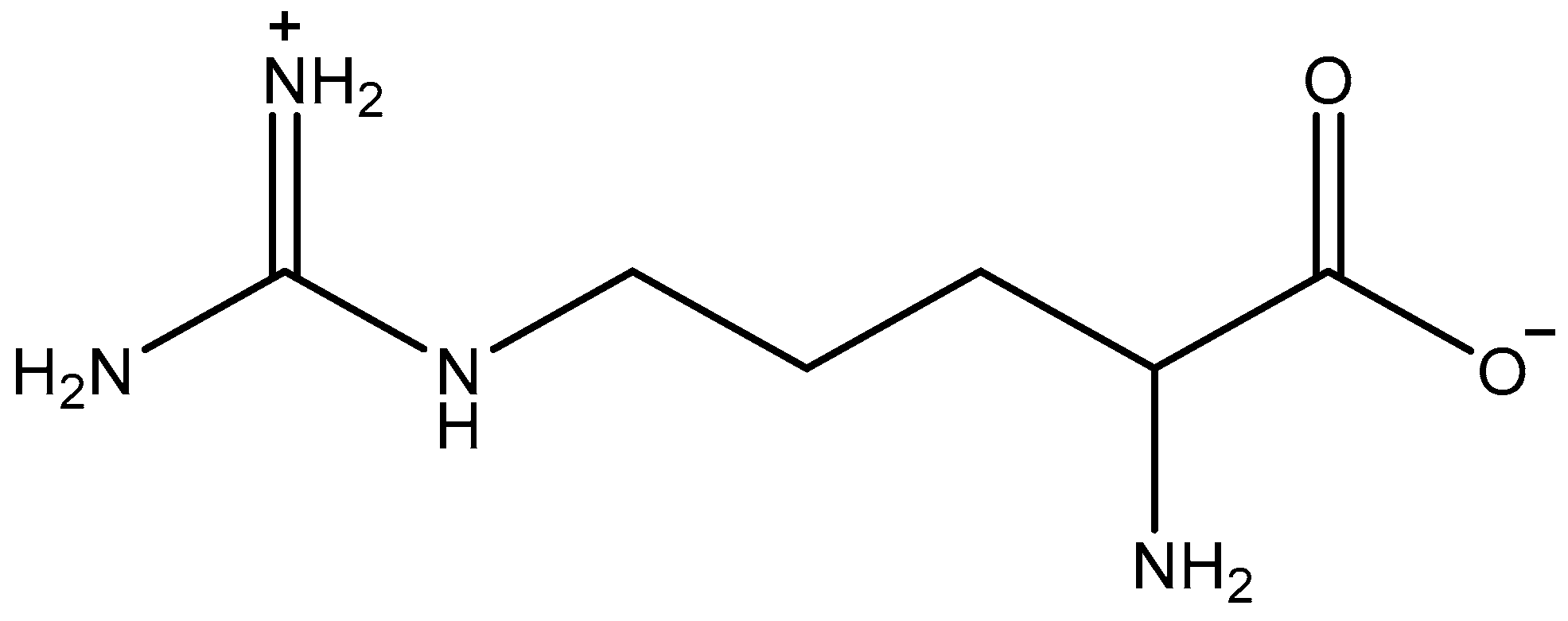
In light of the interesting coordination chemistry of L-arginine (Arg; Figure 1) and the diverse applications of the metal–Arg complexes [23,53,54,55], the current work presents the synthesis of a new Zn(II) azido complex with Arg using a self-assembly technique. An analysis of the molecular and supramolecular structure of the new Zn(II) azido complex is presented. The structural properties of the newly synthesized azido complex were investigated using single-crystal X-ray diffraction and FTIR spectroscopy, as well as theoretically using Hirshfeld and AIM calculations.
Figure 1.
Structure of L-arginine (Arg).
2. Materials and Methods
2.1. Materials and Physical Measurements
Chemicals used were obtained from Sigma-Aldrich without further purifications. In the range of 4000–400 cm−1, the FTIR analysis was carried out by using a Bruker Tensor 37 FTIR instrument (Waltham, MA, USA) in KBr pellets. Using a Perkin-Elmer 2400 Elemental Analyzer (Inc.940 Winter Street, Waltham, MA, USA), the CHN analyses were performed. The amount of zinc was determined with the aid of a Shimadzu atomic absorption spectrophotometer (AA-7000 series, Shimadzu, Ltd., Tokyo, Japan).
2.2. Synthesis of [Zn(N3)(Arg)2](N3)·3H2O Complex
A solution of ZnSO4.7H2O (28.8 mg, 0.1 mmol) in 5 mL distilled water was slowly added to a solution of Arg (34.8 mg, 0.2 mmol) in 5 mL distilled water. As a result, a white precipitate was immediately formed. To the resulting mixture, a solution of NaN3 (13 mg, 0.2 mmol) in 5 mL distilled water was added, and then the resulting solution was stirred for 2 min at 60 °C. The mixture was left to cool and then filtered, and the clear filtrate was left for slow evaporation at room temperature. After 12 days, suitable crystals for X-ray analysis were collected from solution by filtration.
[Zn(N3)(Arg)2](N3)·3H2O: Yield: 68%; Anal. Calc. C24H68Zn2N28O14: C, 26.12; H, 6.21; N, 35.53; Zn, 11.85%. Found: C, 26.23; H, 6.12; N, 35.37; Zn, 11.59%. FTIR cm−1: 3358 br, 2081 m, 1666 m, 1581 m, 1506 s, 1388 s, 1116 s, 1044 m, 950 w, 832 m, 739 w, 703 w, 620 w, 518 w, 471 w, 415 w. Ligand (Arg): 3347 br, 3285 br, 3112 br, 2954 s, 2870 s, 1684 s, 1646 s, 1605 s, 1563 s, 1473 m, 1447 m, 1420 m, 1379 m, 1328 s, 1188 m, 1138 m, 1080 w, 1009 m, 799 m, 754 m, 664 m, 552 m, 493 w (Figure S1, Supplementary Data).
2.3. Crystal Structure Determination
Bruker APEX II diffractometer graphite applying monochromated Mo Kα radiation, the crystal structure of the complex under study was determined. SADABS was used to make absorption corrections [56] and SHELXTL was applied for all calculations [57]. The direct SHELXS methods were used to find the positions of the heavy atoms. The hydrogen atoms that were bound to the carbon atoms were refined using the SHELX riding model. The hydrogen atoms that were bound to oxygen and nitrogen in the crystal structure were refined using DFIX limitations and a constant thermal parameter with a value that was 1.5 times the displacement parameters of the corresponding oxygen atoms. The crystal data are summarized in Table 1.

Table 1.
Crystal data and structure refinement for [Zn(N3)(Arg)2](N3)·3H2O complex.
2.4. Computational Details
For the Hirshfeld analysis, the Crystal Explorer 17.5 software was used [58], while Gaussian 09 software was used to perform for DFT single point computations at the X-ray geometry of a single complex [Zn(N3)(Arg)2](N3) molecule [59]. The MPW1PW91 and WB97XD methods were used for single point calculations applying cc-pVTZ basis sets for all atoms. Multiwfn 3.3.6 program was used for the AIM analysis [60].
3. Results and Discussion
3.1. Synthesis and Characterizations
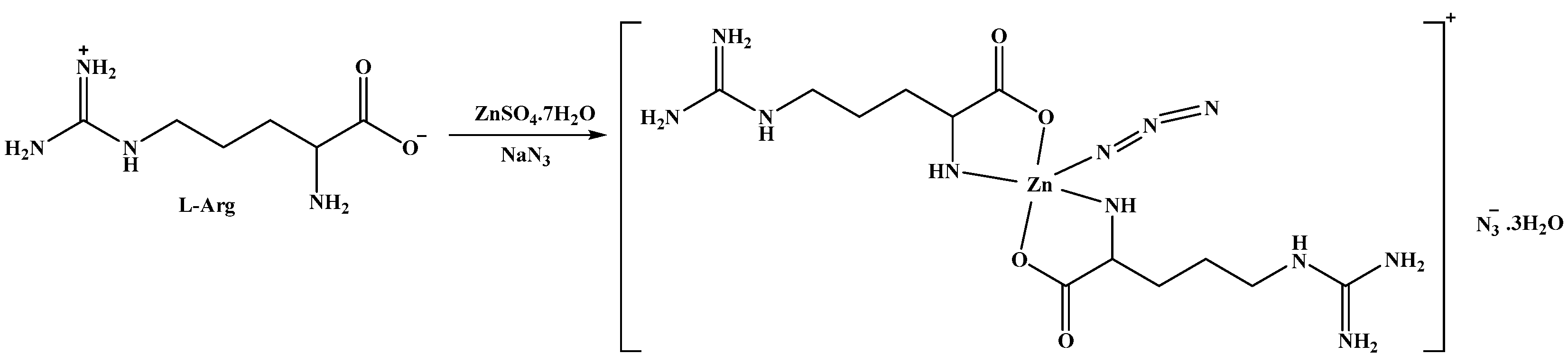
Using self-assembly, the [Zn(N3)(Arg)2](N3)·3H2O complex was synthesized by the reaction between ZnSO4·7H2O and Arg in the presence of NaN3 as a co-ligand (Scheme 1). The FTIR spectra of the free ligand (Arg) and the synthesized [Zn(N3)(Arg)2](N3)·3H2O complex were analyzed within the range 4000–400 cm−1 (Figure S1, Supplementary Data). For Arg, there are stretching vibrations for the O-H in COOH which responsible for the broad absorption band at 3300 cm−1 [23]. In addition, four distinct bands are attributed to C=O (asymmetric), NH2 of guanidyl group (deformation, and bending) vibrations at 1684, 1646, 1605, and 1563 cm−1 [23], where the α-amino acids are characterized by the bands produced by the stretching vibrations of C=O and NH2 [61,62,63,64]. For the [Zn(N3)(Arg)2](N3)·3H2O complex, the broad absorption band at 3500–3000 cm−1 is attributed to crystal H2O molecules in the complex [23]. That a significantly sharp peak at 2081 cm−1 was observed is strong proof of the presence of the azide ion in the complex [65]. The M-N stretching vibrations in the complex appeared as weak absorptions in the range of 518–471 cm−1 [62,65,66,67,68]. Using X-ray diffraction from a single crystal, the structure of the new complex was further confirmed to be [Zn(N3)(Arg)2](N3)·3H2O.
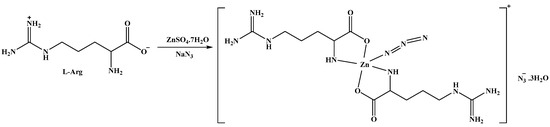
Scheme 1.
The synthesis of the [Zn(N3)(Arg)2](N3)·3H2O complex.
3.2. X-ray Description
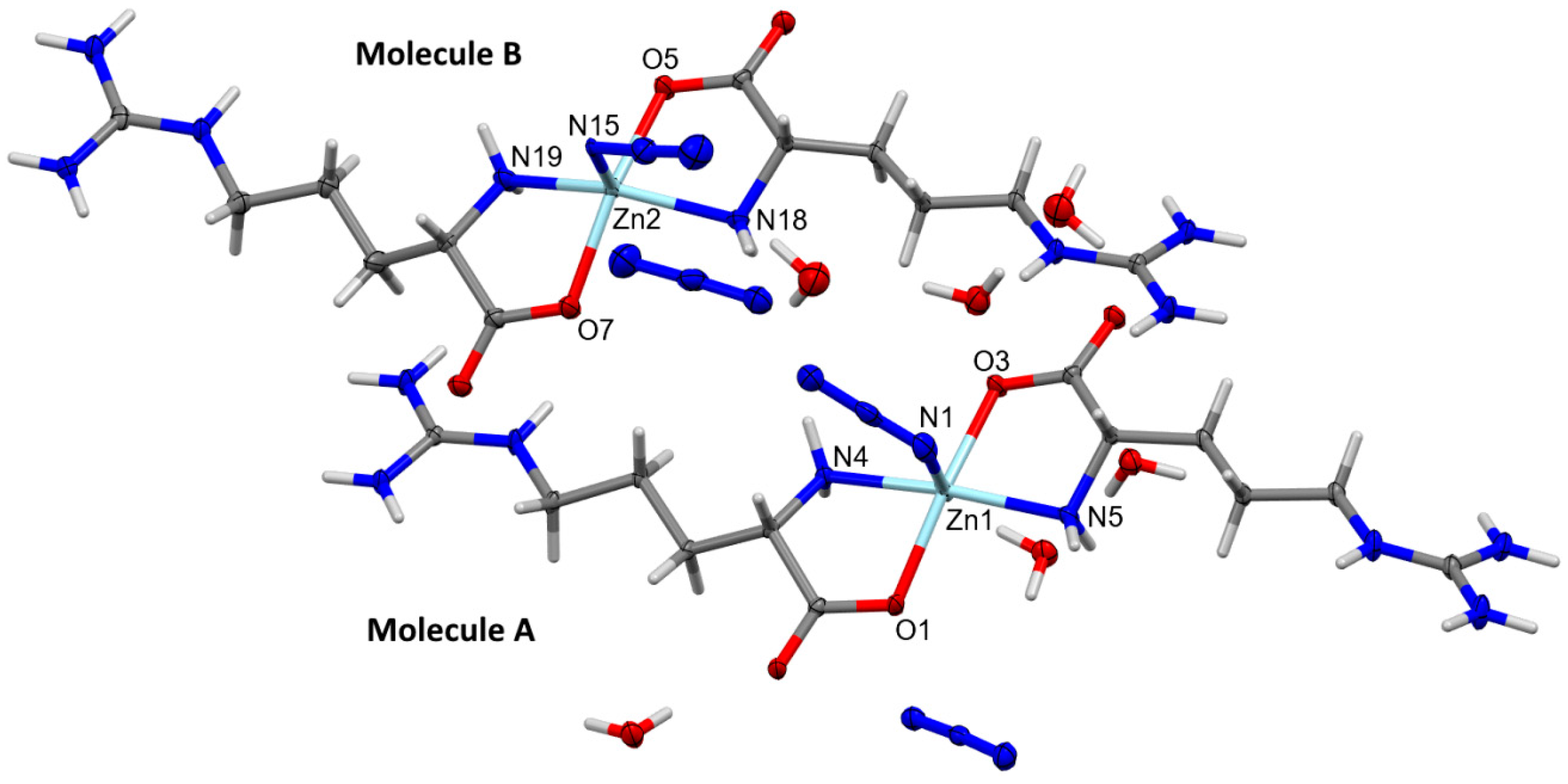
The structure of the [Zn(N3)(Arg)2](N3)·3H2O complex was determined with no doubt using X-ray diffraction from a single crystal. The crystal data are depicted in Table 1. The [Zn(N3)(Arg)2](N3)·3H2O complex crystallized in the monoclinic crystal system and P21 space group. The unit cell parameters are a = 13.0283(5) Å, b = 15.2032(7) Å, c = 13.3633(6) Å, and β = 114.3580(10)°, while the unit cell volume is 2411.28(18) Å3, and there are four [Zn(N3)(Arg)2](N3)·3H2O molecules found in the unit cell, while two of these formulae represent the asymmetric unit of this complex. For simplicity, the two molecules are represented by A and B in Figure 2. The geometric parameters of molecules A and B in the asymmetric unit are slightly different from each other (Table 2). It is worth noting that the complex unit B showed some disorder, with two parts where the major part contributed by 96% while the minor part has a contribution of 6%. For simplicity, only the major part of this complex unit is shown in Figure 2.
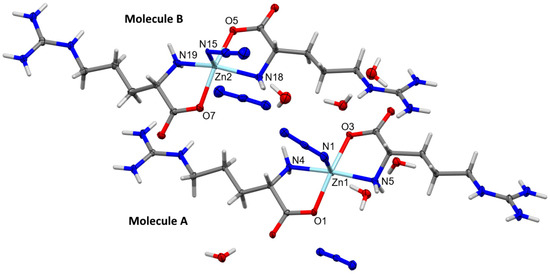
Figure 2.
Structure of the asymmetric unit of [Zn(N3)(Arg)2](N3)·3H2O showing the atom numbering of molecules A and B. Thermal ellipsoids are at the 50% probability level. The disorder of the [Zn(N3)(Arg)2](N3)·3H2O complex at molecule B was omitted from this figure for better clarity and shown in Figure S2 (Supplementary Data).

Table 2.
Selected bond lengths (Å) and angles (°) for [Zn(N3)(Arg)2](N3)·3H2O complex.
The central Zn(II) ion is penta-coordinated with two nitrogen atoms and two oxygen atoms from Arg, and one nitrogen atom from the terminally coordinated azide ion. Both Arg molecules act as a bidentate ligand, where each Arg unit is located trans to one another. It is clear from the X-ray structure of the [Zn(N3)(Arg)2](N3)·3H2O complex shown in Figure 2 that the Arg ligand is coordinated with the Zn(II) ion in its zwitterion form where the guanidyl group is positively charged while the carboxylate group is negatively charged. In unit A, the Zn1-N1 (2.151(2) Å), Zn1-N4 (2.121(2) Å) Zn1-N5 (2.098 (2) Å), Zn1-O1 (2.078(2) Å), and Zn1-O3 (2.058(2) Å) are almost to the same as the respective values in unit B. In the latter, the Zn2-N15 (2.019(2) Å), Zn2-N18 (2.111(2) Å), Zn2-N19 (2.100(2) Å), Zn2-O5 (2.080(2) Å), and Zn2-O7 (2.068(2) Å) are slightly different. On the other hand, the O3-Zn1-O1, N5-Zn1-N1, O7-Zn2-O5, and N15-Zn2-O7 angles are found to be 166.00(8)°, 92.92(8)°, 154.16(11)°, and 101.94(9)°, respectively. These values are found to deviate from the ideal angles of the perfect square pyramidal configuration (180° and 90°), indicating a distorted square pyramidal geometry for [Zn(N3)(Arg)2](N3)·3H2O complex. For better description of the distortion in this penta-coordinated system, the τ5 geometry index is calculated. The values of the τ5 parameter are calculated to be 0.006 for Zn(1) and 0.115 for Zn(2) [69]. These values deviated from the ideal value for a perfect square pyramidal (0 for square pyramidal and 1 for trigonal bipyramidal). Hence, the Zn(II) ion centers in both molecules A and B have a slightly distorted square pyramidal geometry. For related Cu(II)–Arg complexes, the τ5 parameter values are also used for describing the distortion from the square pyramidal coordination geometry [70,71]. The values of τ5 are determined to be 0.11 for [CuCl(Arg)(2,2′-bipyridine)]Cl·3H2O complex [70], while for [Cu(Arg)(phenanthroline)Cl]Cl·2H2O complex the τ5 values are in the range of 0.04–0.05 [71]. On the other hand, the calculated τ5 values of the [Zn(N3)(Arg)2](N3)·3H2O complex are low compared to that obtained from the [Zn(glycine)2] complex (τ5 = 0.326–0.458) indicated distorted trigonal bipyramidal coordination geometry [19].
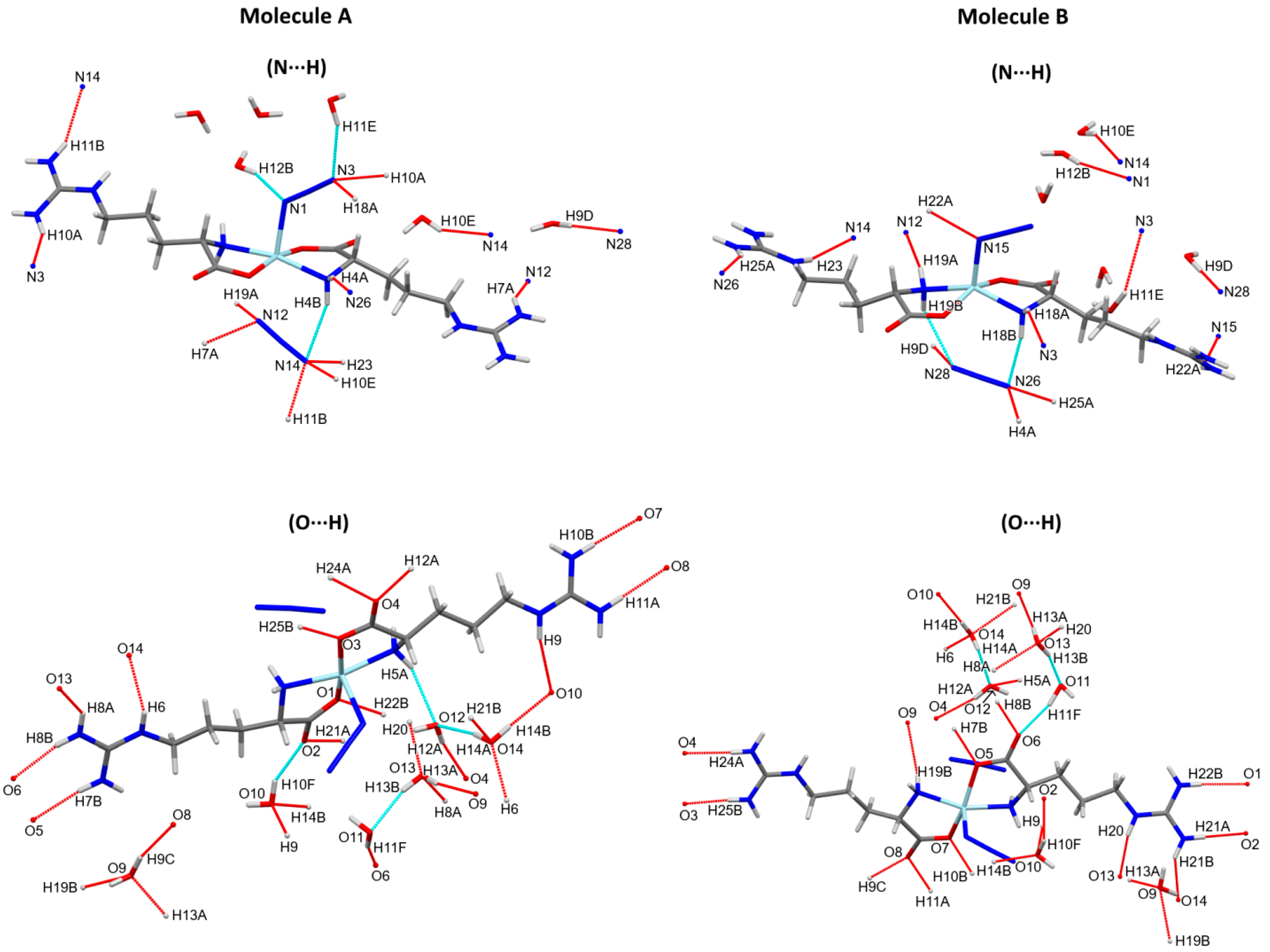
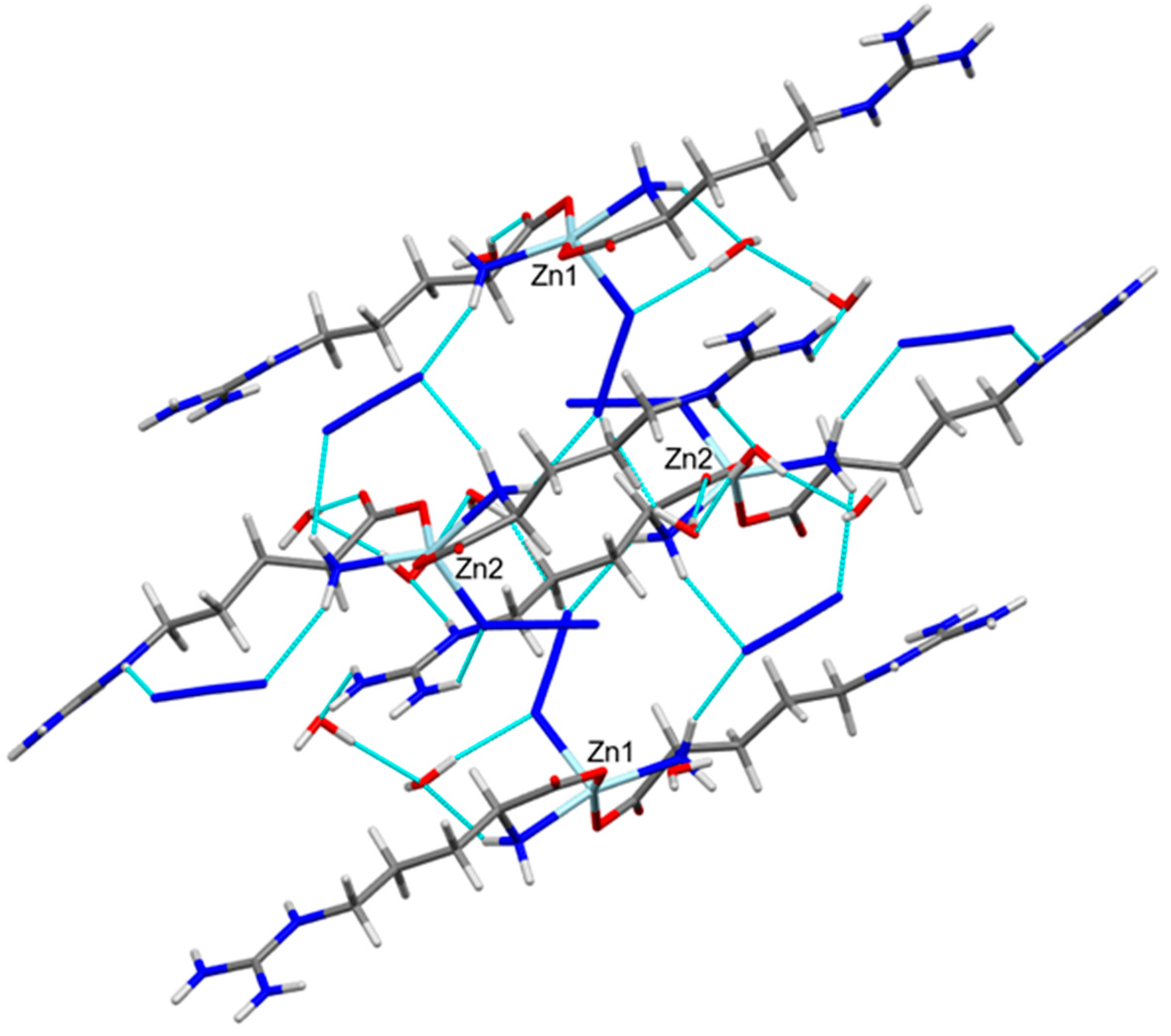
The different intramolecular and intermolecular contacts contributed in the supramolecular structure of the [Zn(N3)(Arg)2](N3)·3H2O complex are presented in Figure 3 and summarized in Table 3. The presence of hydrogen bonding interactions is responsible for the stabilization of the crystal structure of the [Zn(N3)(Arg)2](N3)·3H2O complex. It was found that there are various classical and non-classical hydrogen bonds in the supramolecular structure of the [Zn(N3)(Arg)2](N3)·3H2O complex. Its molecular structure was found to be stabilized by different intramolecular hydrogen bonds originated between the N-H of amino group with N atoms of the un-coordinated azide ion (N4-H4B···N14 and N19-H19B···N28), and between the N-H of amino group with the O atom of H2O molecules (N5-H5A···O12, O11-H11E···N3, and O12-H12B···N1). In addition, intermolecular hydrogen bonds were observed between the O-H groups of H2O molecule and the O atom from another H2O molecule (O13-H13B···O11 and O14-H14A···O12), and between O atom of Arg and the H2O molecule (O10-H10F···O2 and O11-H11F···O6) within the asymmetric unit of the complex. The donor–acceptor distances of these interactions ranged from 2.752(4) Å (O13-H13B···O11) to 3.333(4) Å (O11-H11E···N3). Additionally, various intermolecular hydrogen bonds were found. The donor–acceptor distances of these interactions ranged from 2.750(3) Å (O13-H13A···O9) to 3.309(4) Å (N19-H19A···N12). Also, there are few non-classical hydrogen bonding interactions, which involve the coordinated N and O atoms as hydrogen bond acceptors, while the methyl group of the Arg ligand acts as a hydrogen bond donor. These non-classical hydrogen bonding interactions are typically weak, where their donor-acceptor distances are ranging from 3.482(3) Å (C16-H16B···N3) to 3.567(3) Å (C16-H16B···N2). The weak non-classical C-H···N interactions were omitted from Figure 3 for better clarity. Figure 4 shows the complex unit’s packing view along ac plane.
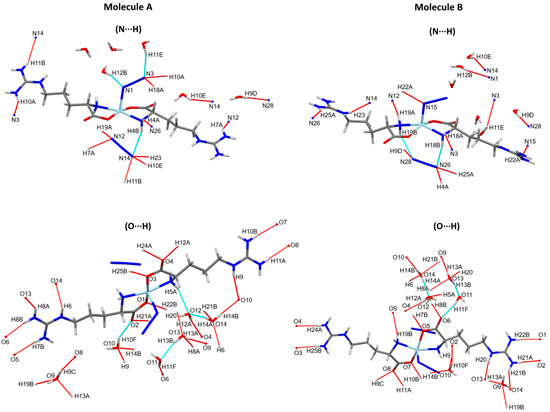
Figure 3.
Intra- and inter-molecular hydrogen bond contacts for molecules A and B. The non-classical C-H···N interactions were omitted from this figure for better clarity.

Table 3.
Hydrogen bond parameters (Å, °) in the [Zn(N3)(Arg)2](N3)·3H2O complex.
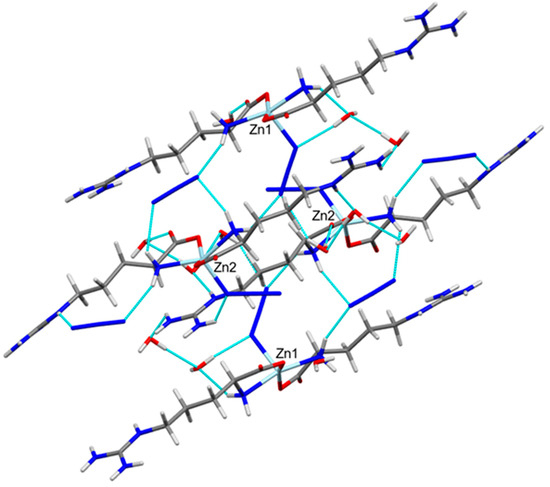
Figure 4.
View of the hydrogen bond packing scheme along ac plane.
3.3. Molecular Packing Analysis
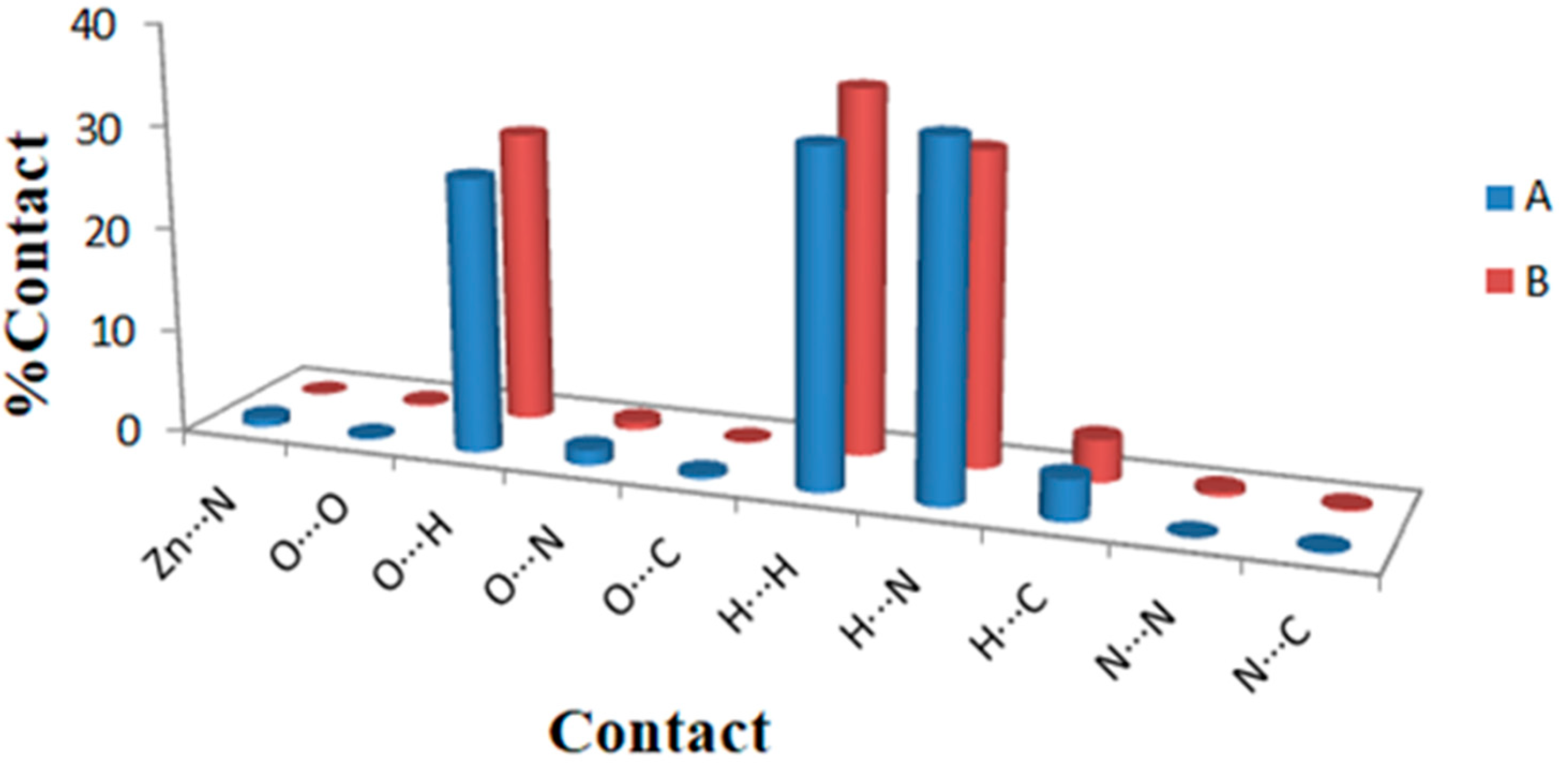
Hirshfeld maps and fingerprint plots have great potential to estimate all intermolecular interactions in the crystal structure of the studied complex. Figure 5 displays various intermolecular interactions and their percentages in the crystal structure of the [Zn(N3)(Arg)2](N3)·3H2O complex. The O···H, H···H, and N···H contacts are the most significant interactions in the crystal structure of the [Zn(N3)(Arg)2](N3)·3H2O complex. The percentages of these interactions for molecule A are 26.6, 32.3, and 34.0%, respectively, while for molecule B, the percentages of these interactions are 28.4, 35.3, and 30.4%, respectively. In contrast, the O···O and C···H interactions contributed little in the molecular packing. Their percentages are 0.2 and 4.0%, respectively (for molecule A), and 0.2 and 4.1%, respectively (for molecule B).
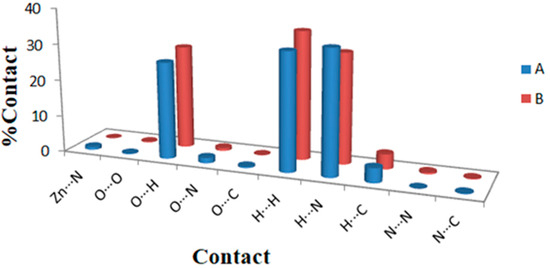
Figure 5.
The potential intermolecular contacts and their percentages in the two molecules A and B of the [Zn(N3)(Arg)2](N3)·3H2O complex.
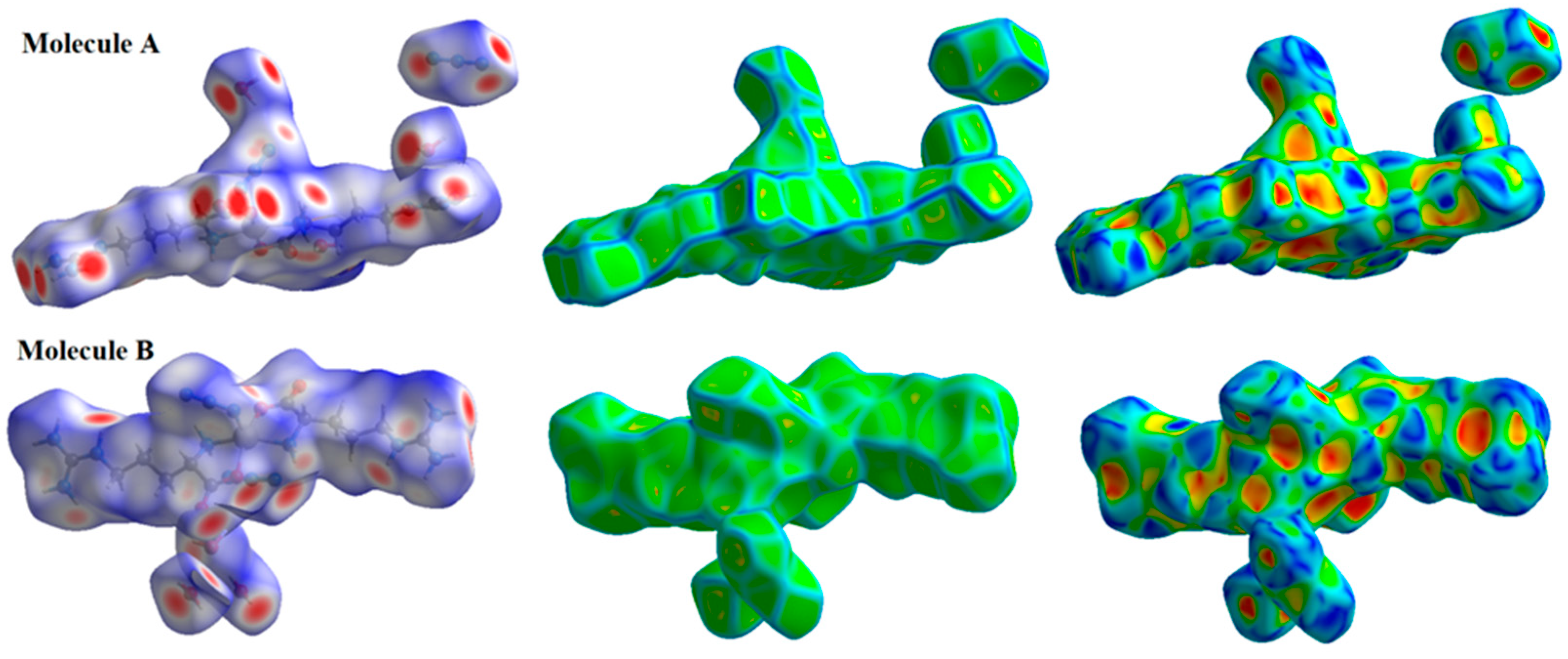
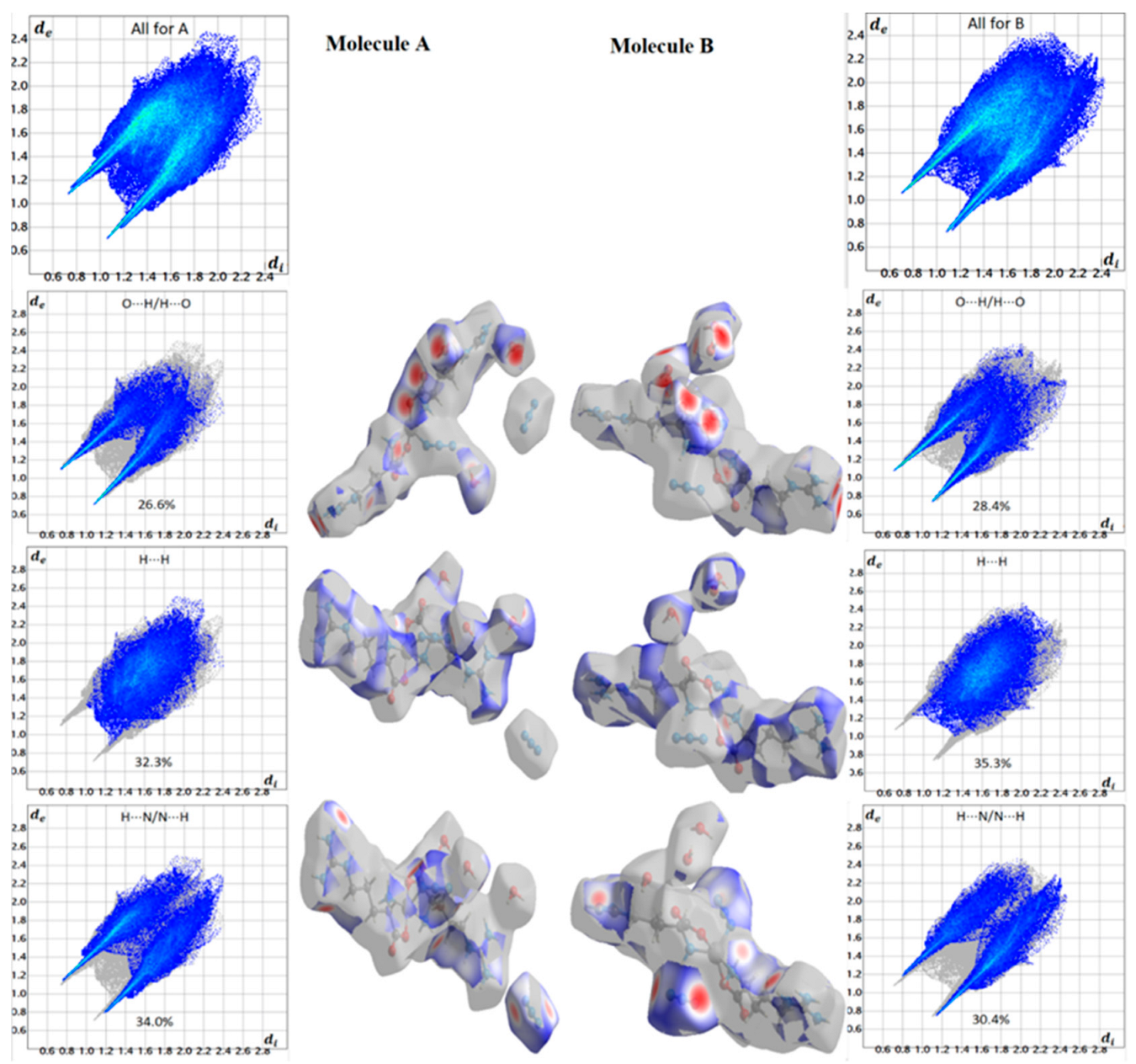
The Hirshfeld surfaces of the [Zn(N3)(Arg)2](N3)·3H2O complex are depicted in Figure 6. Hirshfeld surface analyses display close contacts in the crystal structure based on the van der Waals radii of the interacting atoms. Red spots in the dnorm map are indicative of close contacts with an interaction distance shorter than the van der Waals radii sum. In addition, white and blue spots indicate contacts with equal and longer interaction distances, respectively, compared to the van der Waals radii sum. Also, a fingerprint plot can be used to represent these contacts in a quantitative manner. The absence of red and blue triangles in the shape index map and curvature in the curvedness map of the [Zn(N3)(Arg)2](N3)·3H2O complex indicated no important π-π stacking contacts. The Zn···N, O···H, N···H and C···H contacts appeared as red zones in the dnorm map revealing strong intermolecular interactions between these atoms. As a result, these atoms form shorter distances than their van der Waals radii sum (Table 4). In addition, fingerprint plots confirm the previous result by displaying sharp spikes for the previously mentioned interactions. All dnorm maps and fingerprint plots for the important contacts are depicted in Figure 7.
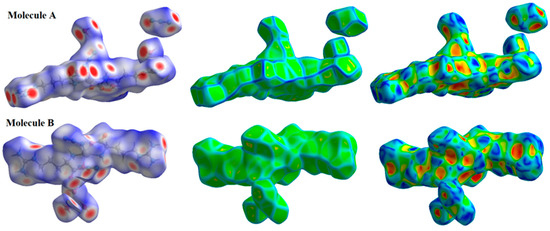
Figure 6.
The Hirshfeld surfaces of the [Zn(N3)(Arg)2](N3)·3H2O complex for molecules A and B.

Table 4.
Contacts and their distances (Å) in the [Zn(N3)(Arg)2](N3)·3H2O complex.
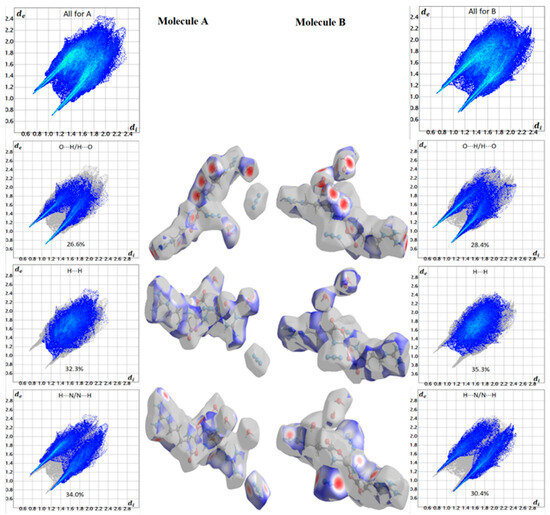
Figure 7.
Important dnorm maps and fingerprint plots for the [Zn(N3)(Arg)2](N3)·3H2O complex for molecules A and B.
3.4. AIM Studies
In the AIM concept, the existence of a bond path indicates the presence of physical interactions between atoms [72,73,74]. By identifying the critical points (CP) of the bonds, the topological parameters can be predicted, which enabled us to study the type and strength of interaction in molecular systems with the aid of AIM theory [73]. The AIM topological parameters are characteristics determining the stability or instability of the structure [73]. These significant topological parameters are the electron density (ρ), the total energy density (H(r)), the laplacian of electron density (∇2ρ(r)), and the bond critical point’s ratio of local electron potential energy density to local electron kinetic energy density (|V(r)|/G(r)). All these topological parameters for the molecular units A and B are calculated for each coordinate bond at its bond critical point (BCP) and the results are summarized in Table 5.

Table 5.
The topological parameters (a.u.) at the Zn-N, Zn-O and N-N bond critical points (BCPs) using MPW1PW91 method a and the cc-pVTZ basis set.
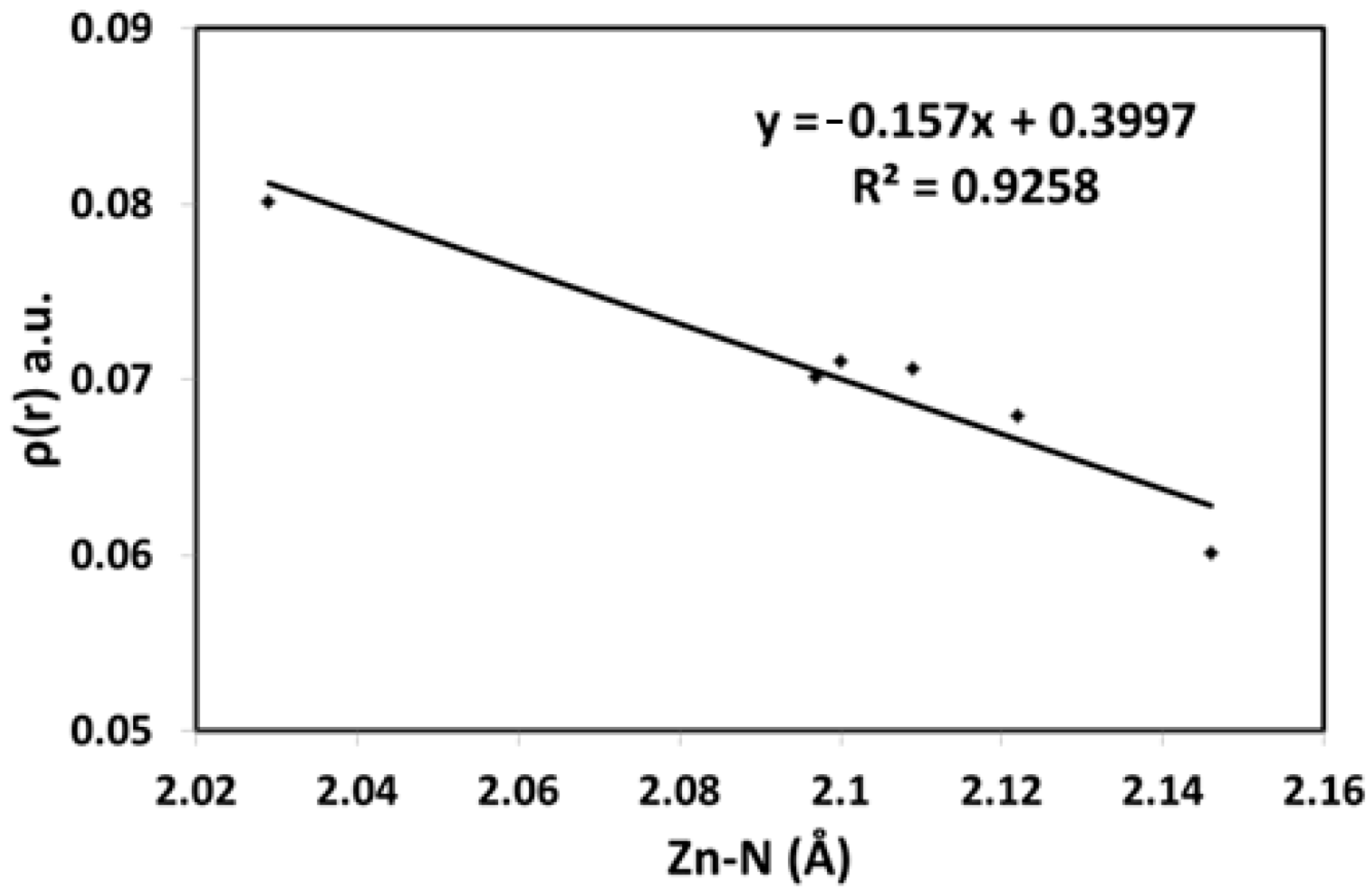
For unit A, the calculated ρ(r) values are within the range of 0.0601–0.0711 and 0.0748–0.0786 a.u. for Zn-N and Zn-O, respectively. In the same way, the calculated ρ(r) values for Zn-N and Zn-O bonds of unit B are within the range of 0.0701–0.0803 and 0.0717–0.0733 a.u., respectively. All ρ(r) values are close to 0.1 a.u. but generally less than this limit, which is the main feature of closed shell interactions involving few covalent characteristics [73]. The total energy density H(r) is another topological parameter that is useful to explain the bond nature. Its sign is negative for all Zn-N and Zn-O bonds, which reveals that these interactions have some covalent characteristics. Additionally, for typical covalent bonding, |V(r)|/G(r) exceeds two. Generally, covalent interaction is predominant when the |V(r)|/G(r) ratio is greater than one [75]. Also, covalent interactions have ∇2ρ(r) values smaller than zero, whereas closed-shell interactions have ∇2ρ(r) values greater than zero [73]. This observation is valid for elements in the second row, whereas longer bonds have a tendency to have a positive ∇2ρ(r) [76]. The positive ∇2ρ(r) results could be considered another piece of evidence for the few covalent characteristics of the Zn-N and Zn-O bonds. Also, the ρ(r) values are a good indication of the bond strength (Figure 8). In this figure, there is an inverse relationship between these two parameters.
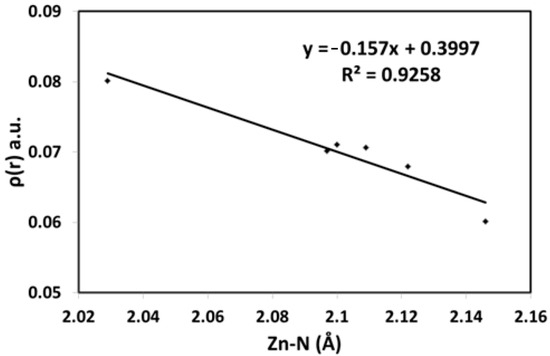
Figure 8.
The correlation between Zn-N distances and electron density.
In addition, the comparison between the coordinated and ionic azide groups in the [Zn(N3)(Arg)2](N3)·3H2O complex was performed based on AIM parameters. It is well known that the free azide ion has two equidistant N-N bonds. For unit A, the N12-N13 and N13-N14 bond distances are 1.177 and 1.173 Å, respectively. Similarly, the N26-N27 and N27-N28 bond distances are 1.169 and 1.187 Å for unit B, respectively. The differences between the two N-N bonds are marginal (not exceeds 0.019 Å). In contrast, the coordinated azide of unit A has N1-N2 (1.130 Å) and N2-N3 (1.199 Å) bonds which differ by 0.069 Å. For unit B, the N15-N16 and N16-N17 bond distances are 1.213 and 1.172 Å, respectively, which differ by 0.041 Å. It was found that the coordination between the Zn(II) ion and one of the azide N atoms increased the asymmetry of the azide ion. The AIM topological parameters presented in Table 5 are agree with this conclusion to a great extent. The ρ(r) was found to be larger for the N1-N2 (0.5859 a.u.) than N2-N3 (0.4871 a.u.) for unit A. In contrast, the ρ(r) was found to be smaller for the N15-N16 (0.4689 a.u.) than N16-N17 (0.5289 a.u.). Also, the |V(r)|/G(r) ratios were found to be greater than two for all N-N bonds, and the H(r) values were negative. As a result, the covalent interaction was confirmed. It is worth noting that the results obtained from both DFT methods (MPW1PW91 and WB97XD) are almost the same and gave the same physical meaning (Table S1, Supplementary Data).
4. Conclusions
The synthesis of the [Zn(N3)(Arg)2](N3)·3H2O complex was performed based on the reaction between ZnSO4·7H2O, Arg, and sodium azide at room temperature. With the use of the X-ray diffraction of a single crystal and FT-IR, the structure of this complex was identified. The coordination environment of the [Zn(N3)(Arg)2](N3)·3H2O complex has a distorted square pyramidal geometry. Additionally, theoretical analyses for the crystallographic structure of [Zn(N3)(Arg)2](N3)·3H2O were performed with the aid of Hirshfeld topology analysis. The most important interactions in the crystal structure of [Zn(N3)(Arg)2](N3)·3H2O are the O···H, H···H, and N···H contacts. The AIM studies predicted the topological parameters of the Zn-N and Zn-O bonds in order to inspect the nature and strength of theses coordination interactions. It was found that the Zn-N and Zn-O bonds have few covalent interactions. In addition, the asymmetry of the azide ion is increased by the coordination of the Zn(II) ion with one of the azide N atoms which is revealed by AIM analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13091375/s1, Figure S1: FTIR spectra of the [Zn(N3)(Arg)2](N3)·3H2O complex; (upper) and the free ligand (Arg); (lower); Figure S2: The disorder of the [Zn(N3)(Arg)2](N3)·3H2O complex at molecule B at Zn2, H18B, H18C, H19B, and H19C atoms which are split into two parts (major and minor). The probability of finding Zn2 and Zn2A parts in space are 94 and 6%, respectively; Table S1: The topological parameters (a.u.) at the Zn–N, Zn–O and N-N bond critical points (BCPs) using WB97XD method and the cc-pVTZ basis set.
Author Contributions
Conceptualization, S.M.S., M.S.A. (Mohammed Salah Ayoup) and M.A.M.A.-Y.; methodology, A.Y.; software, S.M.S., J.H.A. and R.C.F.; validation, J.H.A. and R.C.F.; formal analysis, A.Y.; investigation, A.Y. and M.S.A. (Mohammed Salah Ayoup); resources, M.A.M.A.-Y., M.S.A. (Mezna Saleh Altowyan) and A.B.; data curation, A.B. and A.Y.; writing—original draft preparation, A.Y., S.M.S., J.H.A., R.C.F. and A.B.; writing—review and editing, A.Y., M.A.M.A.-Y., S.M.S., M.S.A. (Mohammed Salah Ayoup) and A.B.; visualization, A.Y., S.M.S. and A.B.; supervision, M.A.M.A.-Y., M.S.A. (Mohammed Salah Ayoup) and S.M.S.; project administration, M.A.M.A.-Y., S.M.S. and A.B.; funding acquisition, M.S.A. (Mezna Saleh Altowyan) and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R86), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R86), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kitagawa, S.; Kitaura, R.; Noro, S.I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Beyazit, N.; Çakmak, D.; Demetgül, C. Chromone-based schiff base metal complexes as catalysts for catechol oxidation: Synthesis, kinetics and electrochemical studies. Tetrahedron 2017, 73, 2774–2779. [Google Scholar] [CrossRef]
- Li, X.-Y.; Chen, L.; Gao, L.; Zhang, Y.; Akogun, S.F.; Dong, W.-K. Syntheses, crystal structures and catalytic activities of two solvent-induced homotrinuclear Co(II) complexes with a naphthalenediol-based bis(salamo)-type tetraoxime ligand. RSC Adv. 2017, 7, 35905–35916. [Google Scholar] [CrossRef]
- Swiegers, G.F.; Malefetse, T.J. New self-assembled structural motifs in coordination chemistry. Chem. Rev. 2000, 100, 3483–3538. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.; Elsilk, S.E.; El-Faham, A. Synthesis, structure and biological activity of zinc(II) pincer complexes with 2,4-bis(3,5-dimethyl-1H-pyrazol-1-yl)-6-methoxy-1,3,5-triazine. Inorg. Chim. Acta 2020, 508, 119627. [Google Scholar] [CrossRef]
- Chai, L.-Q.; Hu, Q.; Zhang, K.-Y.; Zhou, L.; Huang, J.-J. Synthesis, structural characterization, spectroscopic, and DFT studies of two penta-coordinated zinc(II) complexes containing quinazoline and 1,10-phenanthroline as mixed ligands. J. Lumin. 2018, 203, 234–246. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, H.-C. A metal-organic framework with entatic metal centers exhibiting high gas adsorption affinity. J. Am. Chem. Soc. 2006, 128, 11734–11735. [Google Scholar] [CrossRef]
- Chae, H.K.; Kim, J.; Friedrichs, O.D.; O’Keeffe, M.; Yaghi, O.M. Design of frameworks with mixed triangular and octahedral building blocks exemplified by the structure of [Zn4O(TCA)2] having the pyrite topology. Angew. Chem. 2003, 115, 4037–4039. [Google Scholar] [CrossRef]
- Kepert, C.J.; Rosseinsky, M.J. Zeolite-like crystal structure of an empty microporous molecular framework. Chem. Commun. 1999, 375–376. [Google Scholar] [CrossRef]
- Yousri, A.; El-Faham, A.; Haukka, M.; Ayoup, M.S.; Ismail, M.M.; Menofy, N.G.E.; Soliman, S.M.; Öhrström, L.; Barakat, A.; Abu-Youssef, M.A. A novel Na(I) coordination complex with s-triazine pincer ligand: Synthesis, X-ray structure, Hirshfeld analysis, and antimicrobial activity. Crystals 2023, 13, 890. [Google Scholar] [CrossRef]
- Yousri, A.; Haukka, M.; Abu-Youssef, M.A.; Ayoup, M.S.; Ismail, M.M.; El Menofy, N.; Soliman, S.M.; Barakat, A.; Noa, F.M.A.; Öhrström, L.R. Synthesis, structure diversity, and antimicrobial studies of Ag(I) complexes with quinoline-type ligands. CrystEngComm 2023, 25, 3922–3930. [Google Scholar] [CrossRef]
- Zhou, H.-P.; Gan, X.-P.; Li, X.-L.; Liu, Z.-D.; Geng, W.-Q.; Zhou, F.-X.; Ke, W.-Z.; Wang, P.; Kong, L.; Hao, F.-Y. Anion-induced assembly of five-coordinated mercury(II) complexes and density functional theory calculations to study bond dissociation energies of long Hg−N bonds. Cryst. Growth Des. 2010, 10, 1767–1776. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Liang, X.-X.; Cui, Y.-H.; Wu, J.; Kirillov, A.M. Exploring 4-(3-carboxyphenyl) picolinic acid as a semirigid building block for the hydrothermal self-assembly of diverse metal–organic and supramolecular networks. CrystEngComm 2017, 19, 117–128. [Google Scholar] [CrossRef]
- Dahlous, K.A.; Soliman, S.M.; El-Faham, A.; Massoud, R.A. Synthesis and X-ray structure combined with Hirshfeld and aim studies on a new trinuclear Zn(II)-azido complex with s-triazine pincer ligand. Crystals 2022, 12, 1786. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Chen, C.; Zhang, J.; Bai, Y.; Shi, F.; Wang, X. DNA-binding studies and antioxidant activities of two-, three- and four-coordinate silver(I) complexes containing bis(2-benzimidazolyl) aniline derivatives. New J. Chem. 2014, 38, 3688–3698. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Chen, C.; Zhang, H.; Peng, H.; Wang, F.; Yang, Z. Synthesis, crystal structure, and DNA-binding studies of different coordinate binuclear silver(I) complexes with benzimidazole open-chain ether ligands. New J. Chem. 2015, 39, 7172–7181. [Google Scholar] [CrossRef]
- Zhao, K.; Qu, Y.; Wu, Y.; Wang, C.; Shen, K.; Li, C.; Wu, H. Synthesis, structures and properties of copper(II) and zinc(II) complexes with 1,2-bis(benzimidazol-2-yl) ethane ligands. Transit. Met. Chem. 2019, 44, 713–720. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Tetrahedral vs. octahedral zinc complexes with ligands of biological interest: A DFT/CDM study. J. Am. Chem. Soc. 2000, 122, 11146–11153. [Google Scholar] [CrossRef]
- Abendrot, M.; Chęcińska, L.; Kusz, J.; Lisowska, K.; Zawadzka, K.; Felczak, A.; Kalinowska-Lis, U. Zinc(II) complexes with amino acids for potential use in dermatology: Synthesis, crystal structures, and antibacterial activity. Molecules 2020, 25, 951. [Google Scholar] [CrossRef]
- Stenberg, M.; Marko-Varga, G.; Öste, R. Enantioseparation of D-and L-amino acids by a coupled system consisting of an ion-exchange column and a chiral column and determination of D-aspartic acid and D-glutamic acid in soy products. Food Chem. 2002, 79, 507–512. [Google Scholar] [CrossRef]
- Alagha, A.; Brown, D.A.; Elawad, M.; Müller-Bunz, H.; Nimir, H.; Yanovsky, A.I.; Nolan, K.B. The preparation and crystal structure of acetatobis(L-arginine)zinc(II) acetate trihydrate, the first reported X-ray structure of a zinc(II)–arginine complex. Inorg. Chim. Acta 2011, 377, 185–187. [Google Scholar] [CrossRef]
- Lekakis, J.P.; Papathanassiou, S.; Papaioannou, T.G.; Papamichael, C.M.; Zakopoulos, N.; Kotsis, V.; Dagre, A.G.; Stamatelopoulos, K.; Protogerou, A.; Stamatelopoulos, S.F. Oral L-arginine improves endothelial dysfunction in patients with essential hypertension. Int. J. Cardiol. 2002, 86, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Kochel, A.; Zierkiewicz, W. 1–D framework L-arginine zinc(II) units bridged by oxalate: Synthesis, structure, properties, and theoretical studies. J. Coord. Chem. 2016, 69, 886–900. [Google Scholar] [CrossRef]
- Guo, S.; Jiao, P.; Dan, Z.; Duan, N.; Chen, G.; Zhang, J. Preparation of L-arginine modified magnetic adsorbent by one-step method for removal of Zn(II) and Cd(II) from aqueous solution. Chem. Eng. J. 2017, 317, 999–1011. [Google Scholar] [CrossRef]
- Qi, L.; Chen, Y.; Xie, M.; Guo, Z.; Wang, X. Separation of dansylated amino acid enantiomers by chiral ligand-exchange CE with a zinc(II) L-arginine complex as the selecting system. Electrophoresis 2008, 29, 4277–4283. [Google Scholar] [CrossRef]
- Schug, K.A.; Lindner, W. Noncovalent binding between guanidinium and anionic groups: Focus on biological-and synthetic-based arginine/guanidinium interactions with phosph[on]ate and sulf[on]ate residues. Chem. Rev. 2005, 105, 67–114. [Google Scholar] [CrossRef]
- Mueller, T.G.; Buchner, M.R.; Scheubeck, T.J.; Korber, N.; Kraus, F. Ammine complexes of Na-, Ag-, Mn-, and Zn-azides. Z. Anorg. Allg. Chem. 2016, 642, 796–803. [Google Scholar] [CrossRef]
- Šima, J. Photochemistry of azide-moiety containing inorganic compounds. Coord. Chem. Rev. 2006, 250, 2325–2334. [Google Scholar] [CrossRef]
- Zeng, Y.-F.; Hu, X.; Liu, F.-C.; Bu, X.-H. Azido-mediated systems showing different magnetic behaviors. Chem. Soc. Rev. 2009, 38, 469–480. [Google Scholar] [CrossRef]
- Saha, S.; Mal, D.; Koner, S.; Bhattacherjee, A.; Gütlich, P.; Mondal, S.; Mukherjee, M.; Okamoto, K.-I. Synthesis, X-ray structure and magnetic properties of the azido adducts of quadridentate schiff base manganese(III) complexes. Polyhedron 2004, 23, 1811–1817. [Google Scholar] [CrossRef]
- Koner, S.; Iijima, S.; Watanabe, M.; Sato, M. Μ-azido-and μ-oxo-complexes of Fe(III) with schiff bases. J. Coord. Chem. 2003, 56, 103–111. [Google Scholar] [CrossRef]
- Goher, M.A.; Abu-Youssef, M.A.; Mautner, F.A.; Popitsch, A. Preparation and structural characterization of catena-μ(1,3)-azido-μ(O,N-picolinato)-aquamanganese(II), Mn(NC5H4CO2)(N3)(H2O). Polyhedron 1992, 11, 2137–2141. [Google Scholar] [CrossRef]
- Maji, T.K.; Mukherjee, P.S.; Koner, S.; Mostafa, G.; Tuchagues, J.-P.; Chaudhuri, N.R. 1D coordination polymer of copper(II) containing μ-1,1,3 azido ligand with alternating ferro–antiferromagnetic interactions. Inorg. Chim. Acta 2001, 314, 111–116. [Google Scholar] [CrossRef]
- McKee, V.; Zvagulis, M.; Dagdigian, J.V.; Patch, M.G.; Reed, C.A. Hemocyanin models: Synthesis, structure, and magnetic properties of a binucleating copper(II) system. J. Am. Chem. Soc. 1984, 106, 4765–4772. [Google Scholar] [CrossRef]
- Goher, M.A.; Mautner, F.A.; Sodin, B.; Bitschnau, B. Synthesis, spectral and structural characterization of three zinc(II) azide complexes with aminopyrazine. J. Mol. Struct. 2008, 879, 96–101. [Google Scholar] [CrossRef]
- Gao, E.-Q.; Bai, S.-Q.; Wang, Z.-M.; Yan, C.-H. Two-dimensional homochiral manganese(II)-azido frameworks incorporating an achiral ligand: Partial spontaneous resolution and weak ferromagnetism. J. Am. Chem. Soc. 2003, 125, 4984–4985. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Kircher, P.; Pritzkow, H. Tetranuclear nickel(II) complexes with genuine μ3-1,1,3 and μ4-1,1,3,3 azide bridges. Chem. Commun. 2003, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, C.G.; Hendrickson, D.N.; Duggan, D.M.; Wagner, F.; Barefield, E.K. Crystal and molecular structure of di-μ-azido-bis(2,2′,2″-triaminotriethylamine)dinickel(II) tetraphenylborate. Magnetic exchange between azide-bridged octahedral nickel(II) centers. Di-μ-azido and mono-μ-azido cases. Inorg. Chem. 1975, 14, 604–610. [Google Scholar] [CrossRef]
- Escuer, A.; Castro, I.; Mautner, F.; El Fallah, M.S.; Vicente, R. Magnetic studies on μ-azido polynuclear nickel(II) compounds with the 222-tet ligand. Crystal structure of (μ-N3)2[Ni(222-tet)]2(BPh4)2 (222-tet = triethylenetetramine) and EXAFS structural characterization of the triangular compounds (μ-N3)3[Ni(222-tet)]3(X)3 (X = PF6−, ClO4−). Inorg. Chem. 1997, 36, 4633–4640. [Google Scholar]
- Goher, M.A.; Mak, T.C. Synthesis and characterization of a 3:2 complex of copper(II) azide with 2-benzoylpyridine: A polymeric structure containing an end-on triply bridging azido ligand. Inorg. Chim. Acta 1985, 99, 223–229. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.; Escuer, A.; Gatteschi, D.; Goher, M.A.; Mautner, F.A.; Vicente, R. Synthesis, structural characterization, magnetic behavior, and single crystal EPR spectra of three new one-dimensional manganese azido systems with FM, alternating FM-AF, and AF coupling. Inorg. Chem. 1999, 38, 5716–5723. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.; Escuer, A.; Goher, M.A.; Mautner, F.A.; Reiß, G.J.; Vicente, R. Can a homometallic chain be ferrimagnetic? Angew. Chem. Int. Ed. 2000, 39, 1624–1626. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.; Drillon, M.; Escuer, A.; Goher, M.A.; Mautner, F.A.; Vicente, R. Topological ferrimagnetic behavior of two new [Mn(L)2(N3)2]n chains with the new AF/AF/F alternating sequence (L = 3-methylpyridine or 3,4-dimethylpyridine). Inorg. Chem. 2000, 39, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Escuer, A.; Vicente, R.; El Fallah, M.S.; Goher, M.A.; Mautner, F.A. Synthesis and structural characterization of the one-dimensional [Cu(3-Clpy)2(N3)2]n complex (3-Clpy = 3-chloropyridine): A singular ferrimagnetic chain with local SA= SB. Inorg. Chem. 1998, 37, 4466–4469. [Google Scholar] [CrossRef] [PubMed]
- Ribas, J.; Monfort, M.; Diaz, C.; Bastos, C.; Mer, C.; Solans, X. Two new one-dimensional antiferromagnetic nickel(II) complexes bridged by azido ligands in cis positions. Effect of the counteranion on the magnetic properties. Inorg. Chem. 1995, 34, 4986–4990. [Google Scholar] [CrossRef]
- Hong, C.S.; Do, Y. Canted ferromagnetism in a NiII chain with a single end-to-end azido bridge. Angew. Chem. Int. Ed. 1999, 38, 193–195. [Google Scholar] [CrossRef]
- Maji, T.K.; Mukherjee, P.S.; Mostafa, G.; Mallah, T.; Cano-Boquera, J.; Chaudhuri, N.R. First observation of a ferromagnetic interaction through an end-to-end azido bridging pathway in a 1D copper(II) system. Chem. Commun. 2001, 1012–1013. [Google Scholar] [CrossRef]
- Escuer, A.; Cano, J.; Goher, M.A.; Journaux, Y.; Lloret, F.; Mautner, F.A.; Vicente, R. Synthesis, structural characterization, and monte carlo simulation of the magnetic properties of two new alternating MnII azide 2-D honeycombs. Study of the ferromagnetic ordered phase below 20 K. Inorg. Chem. 2000, 39, 4688–4695. [Google Scholar] [CrossRef]
- Ribas, J.; Monfort, M.; Resino, I.; Solans, X.; Rabu, P.; Maingot, F.; Drillon, M. A unique NiII complex with three different azido bridges: Magneto-structural correlations in the first triply alternating S = 1 chain. Angew. Chem. Int. Ed. Engl. 1996, 35, 2520–2522. [Google Scholar] [CrossRef]
- Liu, F.-C.; Zeng, Y.-F.; Li, J.-R.; Bu, X.-H.; Zhang, H.-J.; Ribas, J. Novel 3-D framework nickel(II) complex with azide, nicotinic acid, and nicotinate (1−) as coligands: Hydrothermal synthesis, structure, and magnetic properties. Inorg. Chem. 2005, 44, 7298–7300. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, J.; Li, N.; Dai, J.; Ru, C.; Ye, Y.; Shen, R.; Zhang, W. A micro-initiator realized by in-situ synthesis of three-dimensional porous copper azide and its ignition performance. Chem. Eng. J. 2017, 326, 1116–1124. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Janczak, J.; Rytlewski, P.; Sarewicz, M.; Santos, A.C.; Salgueiro, L.; Korabik, M. The influence of ancillary NCS− ions on structural, spectroscopic, magnetic and biological properties of copper(II) L-argininato complex. J. Mol. Struct. 2023, 1276, 134776. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Bregier, R.; Komarnicka, U.K.; Kozieł, S.; Szuster, A.; Sztandera, M.; Jarząb, A.; Staszak, Z.; Witkowska, D.; Bojarska, A. Isothiocyanate L−argininato copper(II) complexes–solution structure, DNA interaction, anticancer and antimicrobial activity. Chem. Biol. Interact. 2021, 348, 109636. [Google Scholar] [CrossRef]
- Chow, S.; McAuliffe, C. Metal complexes of amino acids and derivatives—XIII some nickel(II) complexes of L-arginine zwitterions or L-argininate anions. A novel ionic → bidentate perchlorate rearrangement brought about by pressure [1–5]. J. Inorg. Nucl. Chem. 1975, 37, 1059–1064. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program for empirical absorption correction of area detector data. Sadabs 1996. [Google Scholar]
- Bruker, A.S.; Bruker, A.X.S. Inc., Madison, Wisconsin, USA, 2004, (b) Sheldrick. Acta Cryst. A 1990, 46, 467–473. [Google Scholar]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystalexplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Foresman, J.; Frish, E. Exploring Chemistry; Gaussian Inc.: Pittsburg, PA, USA, 1996; Volume 21. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Aruna, S.; Anuradha, A.; Thomas, P.C.; Mohamed, M.G.; Rajasekar, S.; Vimalan, M.; Mani, G.; Sagayaraj, P. Growth, optical and thermal studies of L-arginine perchlorate—A promising non-linear optical single crystal. Indian J. Pure Appl. Phys. 2007, 45, 524–528. [Google Scholar]
- Montazerozohori, M.; Yadegari, S.; Naghiha, A.; Veyseh, S. Synthesis, characterization, electrochemical behavior, thermal study and antibacterial/antifungal properties of some new zinc(II) coordination compounds. J. Ind. Eng. Chem. 2014, 20, 118–126. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, S. Spectroscopic studies of L-arginine molecule. Indian J. Pure Appl. Phys. 2010, 48, 251–255. [Google Scholar]
- Govani, J.R.; Durrer, W.G.; Manciu, M.; Botez, C.; Manciu, F.S. Spectroscopic study of L-arginine interaction with potassium dihydrogen phosphate crystals. J. Mater. Res. 2009, 24, 2316–2320. [Google Scholar] [CrossRef][Green Version]
- Montazerozohori, M.; Jahromi, S.M.; Masoudiasl, A.; McArdle, P. Nano structure zinc(II) schiff base complexes of a N3-tridentate ligand as new biological active agents: Spectral, thermal behaviors and crystal structure of zinc azide complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Montazerozohori, M.; Musavi, S.A. Synthesis and spectral characterization of a new symmetric bidentate schiff-base and its zinc complexes. J. Coord. Chem. 2008, 61, 3934–3942. [Google Scholar] [CrossRef]
- Samanta, B.; Chakraborty, J.; Choudhury, C.R.; Dey, S.; Dey, D.; Batten, S.R.; Jensen, P.; Yap, G.P.; Mitra, S. New Cu(II) complexes with polydentate chelating schiff base ligands: Synthesis, structures, characterisations and biochemical activity studies. Struct. Chem. 2007, 18, 33–41. [Google Scholar] [CrossRef]
- Montazerozohori, M.; Musavi, S.A.; Naghiha, A.; Zohour, M.M. Some new nano-structure zinc(II) coordination compounds of an imidazolidine schiff base: Spectral, thermal, antimicrobial properties and DNA interaction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 382–391. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Rojek, T.; Misiaszek, T.; Gągor, A.; Rytlewski, P. The supramolecular hybrid inorganic–organic L-argininato-based copper(II) materials–preparation, structural, spectroscopic and thermal properties. Inorg. Chim. Acta 2023, 557, 121698. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Janczak, J.; Zierkiewicz, W.; Rytlewski, P.; Rojek, T.; Duczmal, M. Copper(II) complex with L-arginine–crystal structure, DFT calculations, spectroscopic, thermal and magnetic properties. Mater. Chem. Phys. 2019, 228, 272–284. [Google Scholar] [CrossRef]
- Pendás, A.M.; Francisco, E.; Blanco, M.A.; Gatti, C. Bond paths as privileged exchange channels. Chem. Eur. J. 2007, 13, 9362–9371. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.; Pereira, F.; de Araújo, R.; Ramos, M. The hydrogen bond strength: New proposals to evaluate the intermolecular interaction using DFT calculations and the aim theory. Chem. Phys. Lett. 2006, 427, 181–184. [Google Scholar] [CrossRef]
- Matta, C.F.; Hernández-Trujillo, J.; Tang, T.H.; Bader, R.F. Hydrogen–hydrogen bonding: A stabilizing interaction in molecules and crystals. Chem. Eur. J. 2003, 9, 1940–1951. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Soliman, S.M.; Albering, J.; Abu-Youssef, M.A. Structural analyses of two new highly distorted octahedral copper(II) complexes with quinoline-type ligands; Hirshfeld, aim and NBO studies. Polyhedron 2017, 127, 36–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).