Salts of S-(+)-Ibuprofen Formed via Its Reaction with the Antifibrinolytic Agents Aminocaproic Acid and Tranexamic Acid: Synthesis and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis via Liquid-Assisted Grinding and Co-Precipitation
2.3. X-ray Powder Diffraction
2.4. 1H-NMR Spectroscopy
2.5. Single-Crystal X-ray Diffraction (SCXRD)
2.6. Thermal Analysis
2.7. Fourier Transform Infrared (FT-IR) Spectroscopy
2.8. Solubility Measurements
3. Results and Discussion
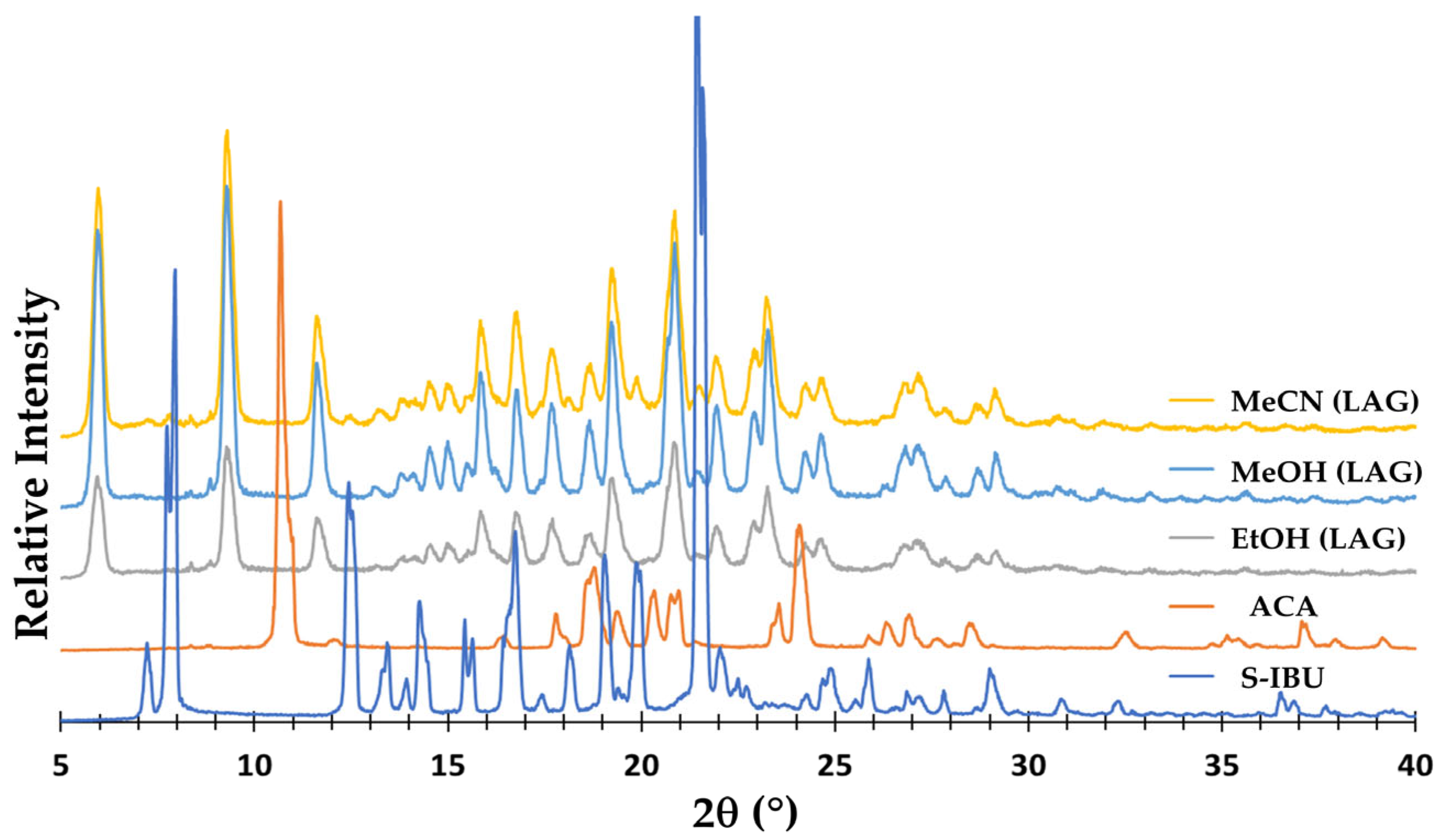
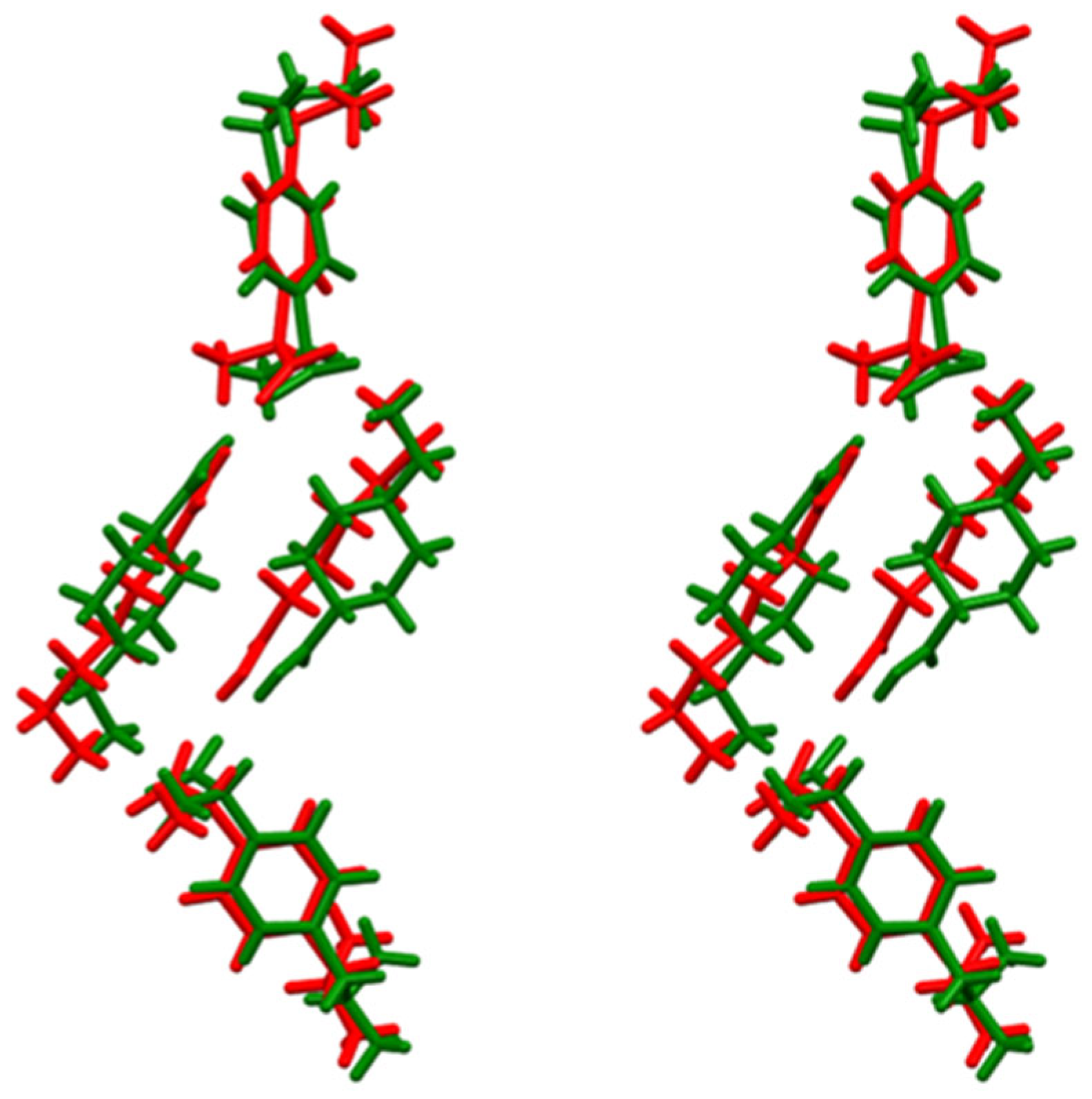
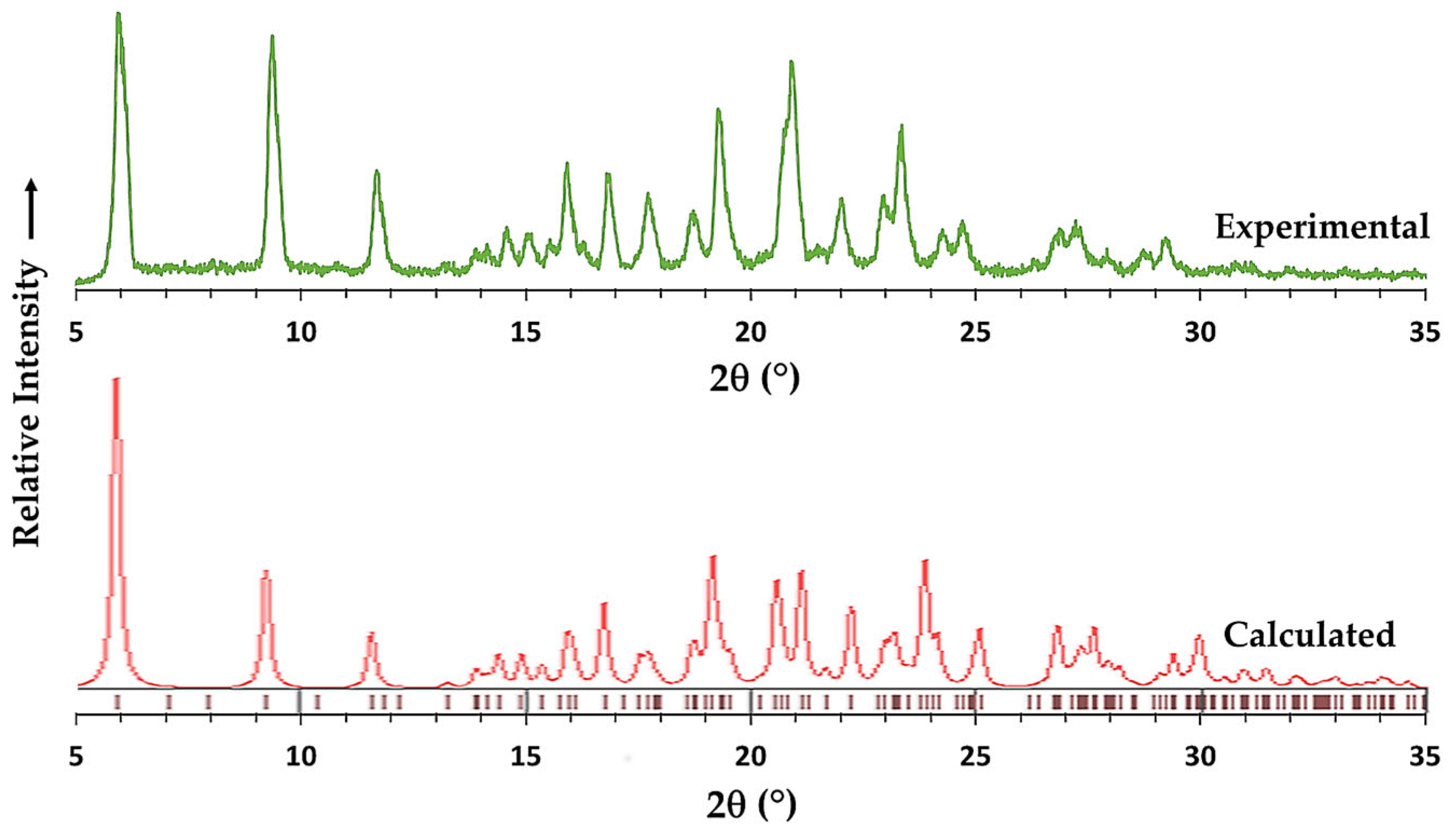
3.1. Synthesis and Characterization of the Products by XRD Methods and 1H-NMR Spectroscopy
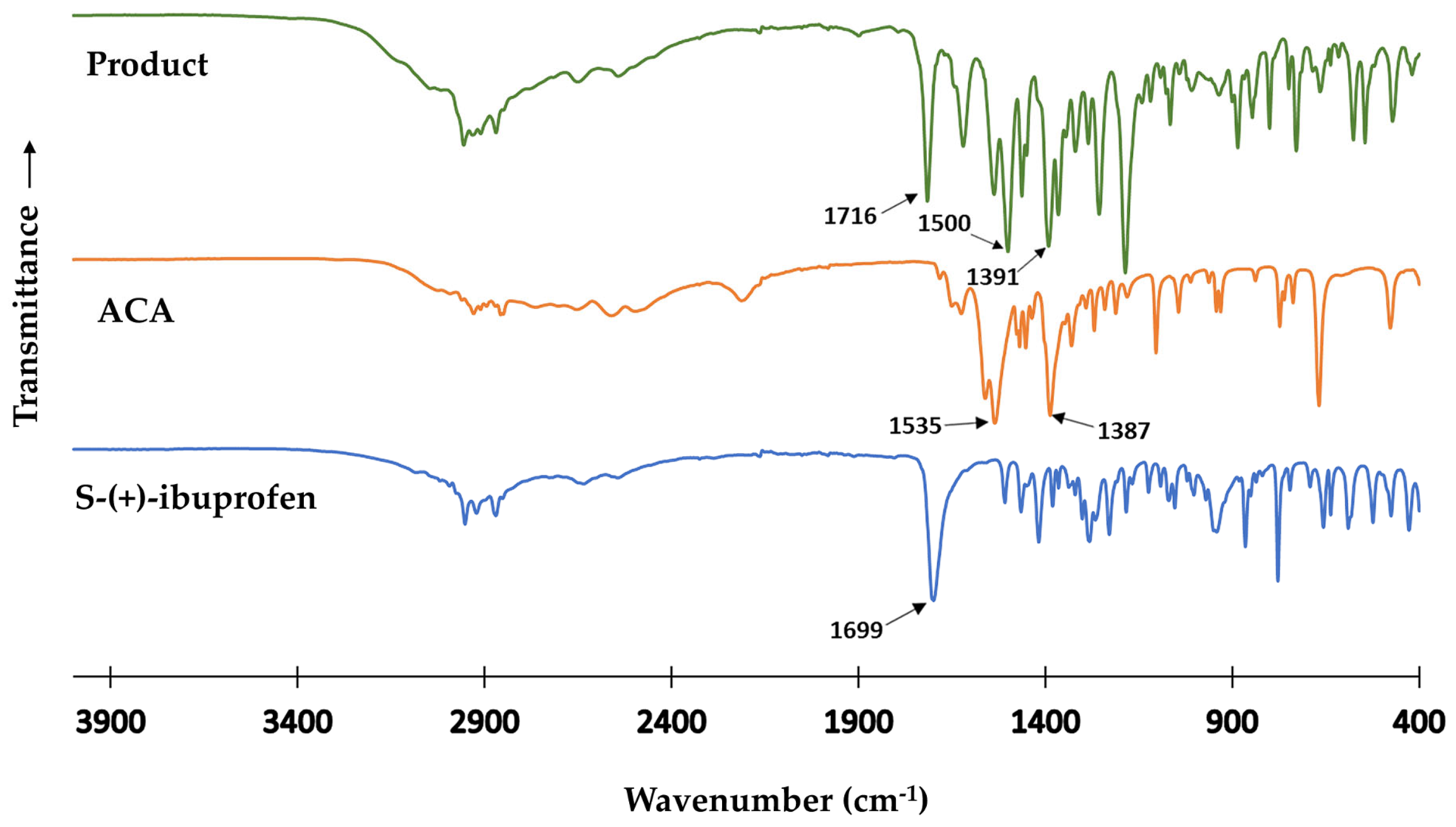
3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
3.3. Thermal Analysis
3.4. Solubility Measurements in FaSSIF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choquesillo-Lazarte, D.; Domínguez-Martín, A. Editorial: Multicomponent Pharmaceutical Solids. Crystals 2023, 13, 570. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Sánchez-López, E. Dexibuprofen Therapeutic Advances: Prodrugs and Nanotechnological Formulations. Pharmaceutics 2021, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M. Comparative Pharmacology of S(+)-Ibuprofen and (RS)-Ibuprofen. Clin. Rheumatol. 2001, 20, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bonabello, A.; Galmozzi, M.R.; Canaparo, R.; Isaia, G.C.; Serpe, L.; Muntoni, E.; Zara, G.P. Dexibuprofen (S+-isomer ibuprofen) reduces gastric damage and improves analgesic and antiinflammatory effects in rodents. Anesth. Anal. 2003, 97, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef] [PubMed]
- Dexibuprofen: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB09213 (accessed on 29 June 2023).
- Friščić, T.; Jones, W. Cocrystal architecture and properties: Design and building of chiral and racemic structures by solid–solid reactions. Faraday Discuss. 2007, 136, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friščić, T.; et al. Applying Hot-Stage Microscopy to Co-Crystal Screening: A Study of Nicotinamide with Seven Active Pharmaceutical Ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Dash, S.G.; Thakur, T.S. Computational Screening of Multicomponent Solid Forms of 2-Aryl Propionate Class of NSAID, Zaltoprofen, and Their Experimental Variation. Cryst. Growth Des. 2021, 21, 449–461. [Google Scholar] [CrossRef]
- Springuel, G.; Robeyns, K.; Norberg, B.; Wouters, J.; Leyssens, T. Cocrystal Formation between Chiral Compounds: How Cocrystals Differ from Salts. Cryst. Growth Des. 2014, 14, 3996–4004. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kleemiss, F.; Puylaert, P.; Duvinage, D.; Fugel, M.; Sugimoto, K.; Beckmann, J.; Grabowskya, S. Ibuprofen and sil-ibuprofen: Polarization effects in the crystal and enzyme environments. Acta Cryst. 2021, B77, 892–905. [Google Scholar] [CrossRef]
- Tilborg, A.; Springuel, F.; Norberg, B.; Wouters, B.; Leyssens, T. On the influence of using a zwitterionic coformer for co-crystallization: Structural focus on naproxen-proline cocrystals. CrystEngComm 2013, 15, 3341–3350. [Google Scholar] [CrossRef]
- Al Rahal, O.; Williams, P.; Hughes, C.; Kariuki, B.; Harris, K. Structure Determination of Multicomponent Crystalline Phases of (S)-Ibuprofen and L-Proline from Powder X-ray Diffraction Data, Augmented by Complementary Experimental and Computational Techniques. Cryst. Growth Des. 2021, 21, 2498–2507. [Google Scholar] [CrossRef]
- Cajaraville, J.P. Ibuprofen Arginate for Rapid-Onset Pain Relief in Daily Practice: A Review of Its Use in Different Pain Conditions. J. Pain Res. 2021, 14, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Markowski, A.R.; Jarocka-Karpowicz, I. The Importance of 6-Aminohexanoic Acid as a Hydrophobic, Flexible Structural Element. Int. J. Mol. Sci. 2021, 22, 12122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Geng, P.; Shi, L.; Wang, Q.; Wang, P. Tranexamic acid versus aminocaproic acid for blood management after total knee and total hip arthroplasty: A systematic review and meta-analysis. Int. J. Surg. 2018, 54 Pt A, 105–112. [Google Scholar] [CrossRef]
- Prudovsky, I.; Kacer, D.; Zucco, V.V.; Palmeri, M.; Falank, C.; Kramer, R.; Carter, D.; Rappold, J. Tranexamic acid: Beyond antifibrinolysis. Transfusion 2022, 62, S301–S312. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Reddy, I.R.; Tarafder, K.; Trivedi, D.R. Salt/Cocrystal of Anti-Fibrinolytic Hemostatic Drug Tranexamic acid:Structural, DFT, and Stability Study of Salt/Cocrystal with GRAS Molecules. Cryst. Growth Des. 2019, 19, 347–361. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Grepioni, F.; De Cian, A.; Félix, O.; Fischer, J.; Hosseini, M.W. Charge-assisted N-H(+)⋅⋅⋅O(−) and O-H(+)⋅⋅⋅O(−) hydrogen bonds control the supramolecular aggregation of ferrocenedicarboxylic acid and bis-amidines. New J. Chem. 2000, 24, 547–553. [Google Scholar] [CrossRef]
- Gomes dos Reis, L.; Ghadiri, M.; Young, P.; Traini, D. Nasal Powder Formulation of Tranexamic Acid and Hyaluronic Acid for the Treatment of Epistaxis. Pharm. Res. 2020, 37, 186. [Google Scholar] [CrossRef] [PubMed]
- Dexibuprofen|C13H18O2|CID 39912—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dexibuprofen#section=Experimental-Properties (accessed on 29 June 2023).

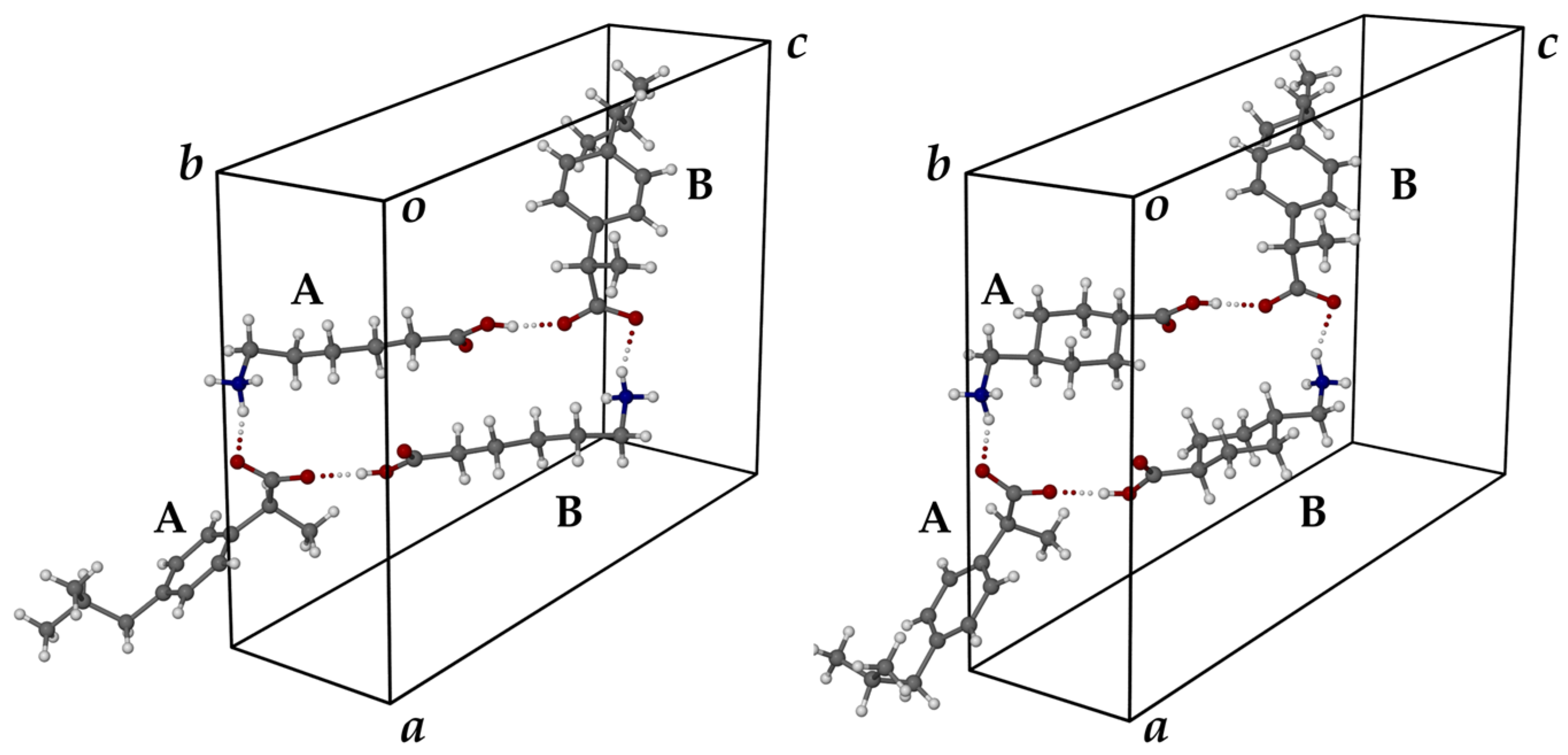
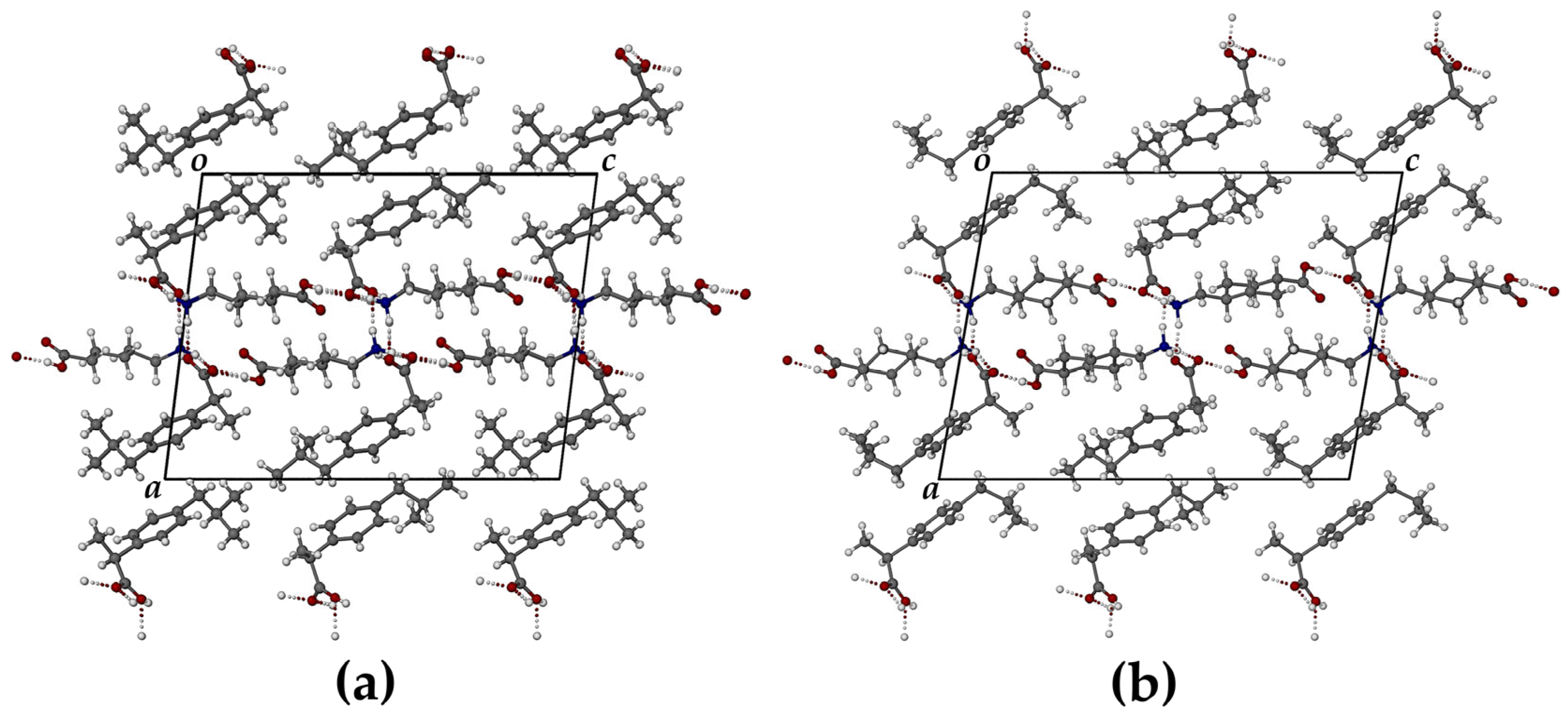
| SALT | N⋅⋅⋅O/Å | N-H⋅⋅⋅O/° | O⋅⋅⋅O/Å | O-H⋅⋅⋅O/° |
|---|---|---|---|---|
| (S-IBU)−(ACA)+ | 2.764(4) | 164 | 2.565(4) | 172 |
| 2.857(4) | 159 | 2.611(4) | 177 | |
| (S-IBU)−(TXA)+ | 2.756(6) | 160 | 2.622(6) | 178 |
| 2.847(7) | 151 | 2.576(6) | 175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frösler, H.M.; Ramulumo, H.S.; Edmonds-Smith, C.; Caira, M.R. Salts of S-(+)-Ibuprofen Formed via Its Reaction with the Antifibrinolytic Agents Aminocaproic Acid and Tranexamic Acid: Synthesis and Characterization. Crystals 2023, 13, 1222. https://doi.org/10.3390/cryst13081222
Frösler HM, Ramulumo HS, Edmonds-Smith C, Caira MR. Salts of S-(+)-Ibuprofen Formed via Its Reaction with the Antifibrinolytic Agents Aminocaproic Acid and Tranexamic Acid: Synthesis and Characterization. Crystals. 2023; 13(8):1222. https://doi.org/10.3390/cryst13081222
Chicago/Turabian StyleFrösler, Hannah M., Humbelani S. Ramulumo, Cesarina Edmonds-Smith, and Mino R. Caira. 2023. "Salts of S-(+)-Ibuprofen Formed via Its Reaction with the Antifibrinolytic Agents Aminocaproic Acid and Tranexamic Acid: Synthesis and Characterization" Crystals 13, no. 8: 1222. https://doi.org/10.3390/cryst13081222
APA StyleFrösler, H. M., Ramulumo, H. S., Edmonds-Smith, C., & Caira, M. R. (2023). Salts of S-(+)-Ibuprofen Formed via Its Reaction with the Antifibrinolytic Agents Aminocaproic Acid and Tranexamic Acid: Synthesis and Characterization. Crystals, 13(8), 1222. https://doi.org/10.3390/cryst13081222







