Effect of Machine Pin-Manufacturing Process Parameters by Plasma Nitriding on Microstructure and Hardness of Working Surfaces
Abstract
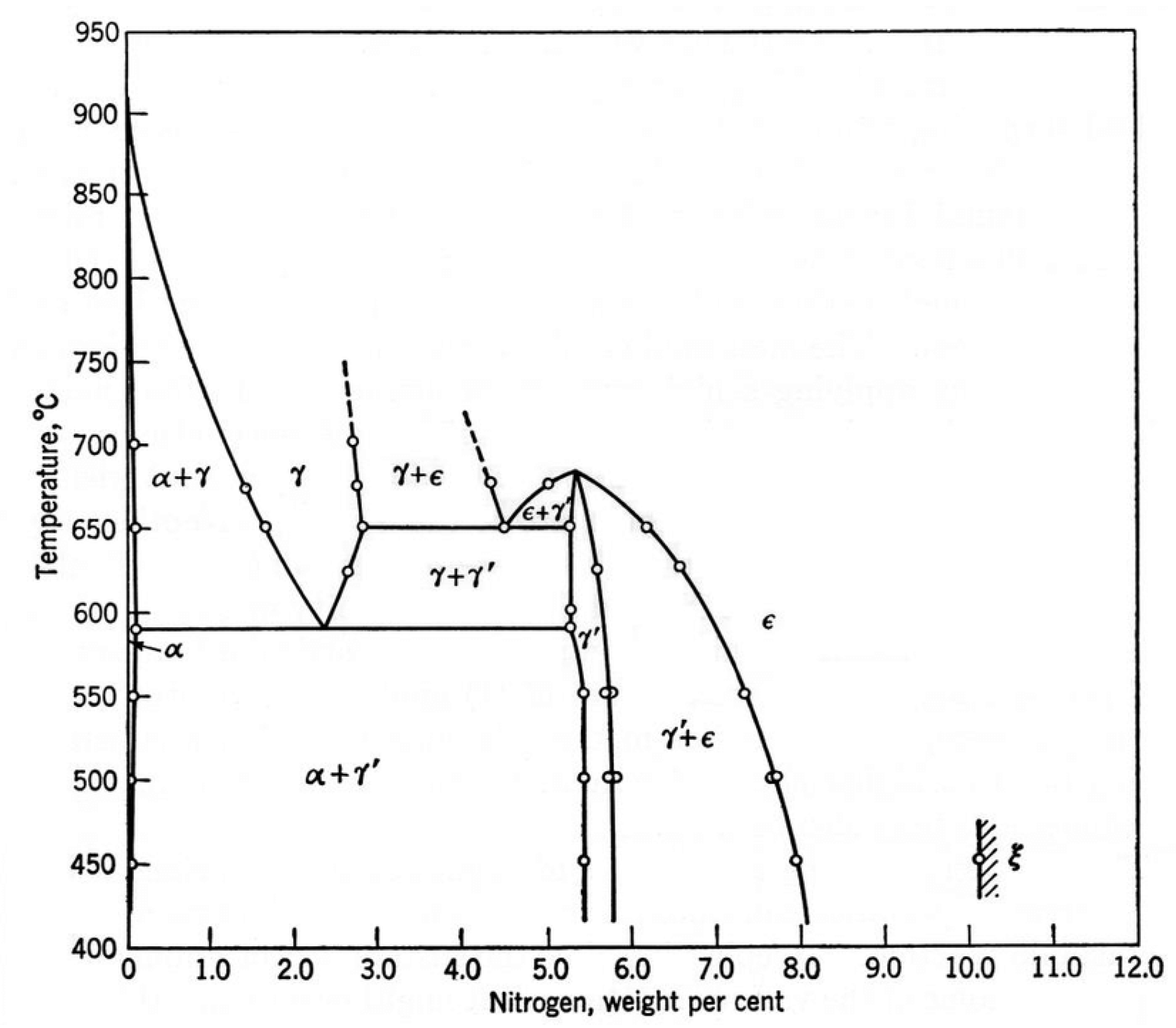
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Testing of Currently Produced Pins—38HMJ Steel
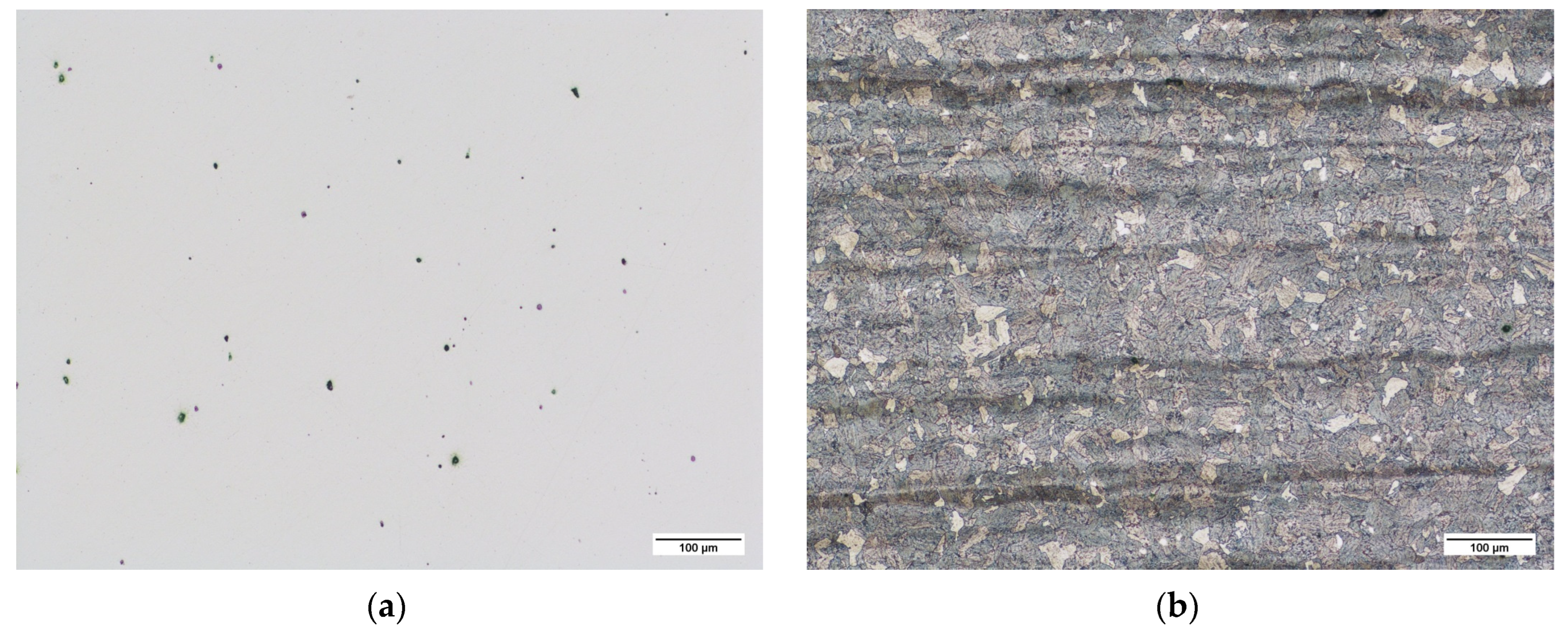
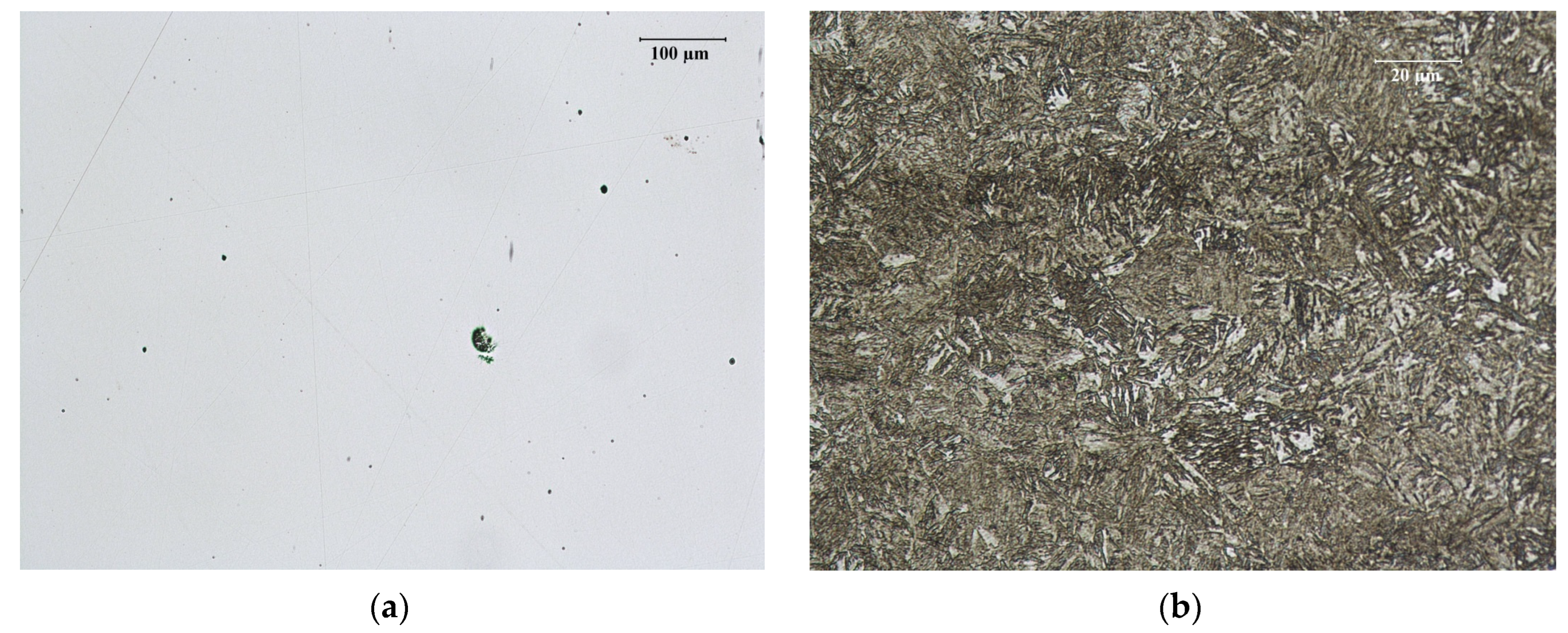
3.1.1. Microscopic Observations of Pins in Raw State
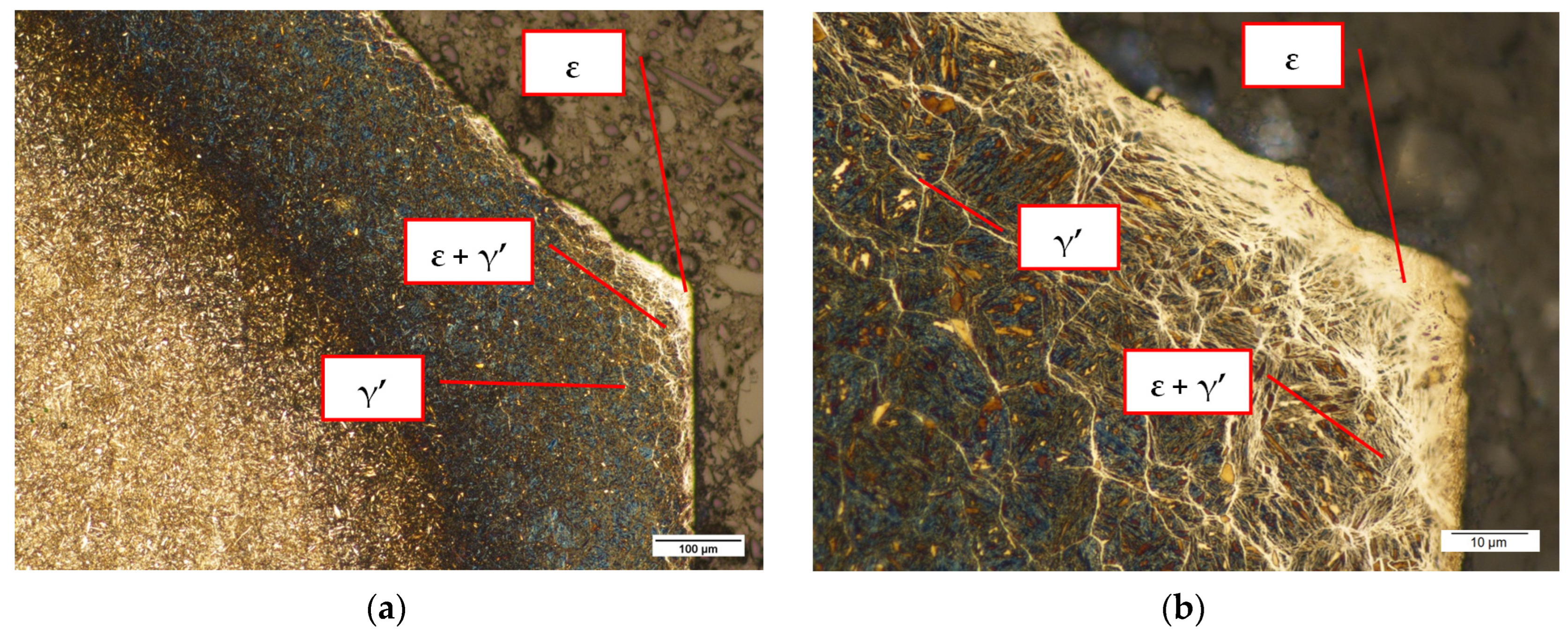
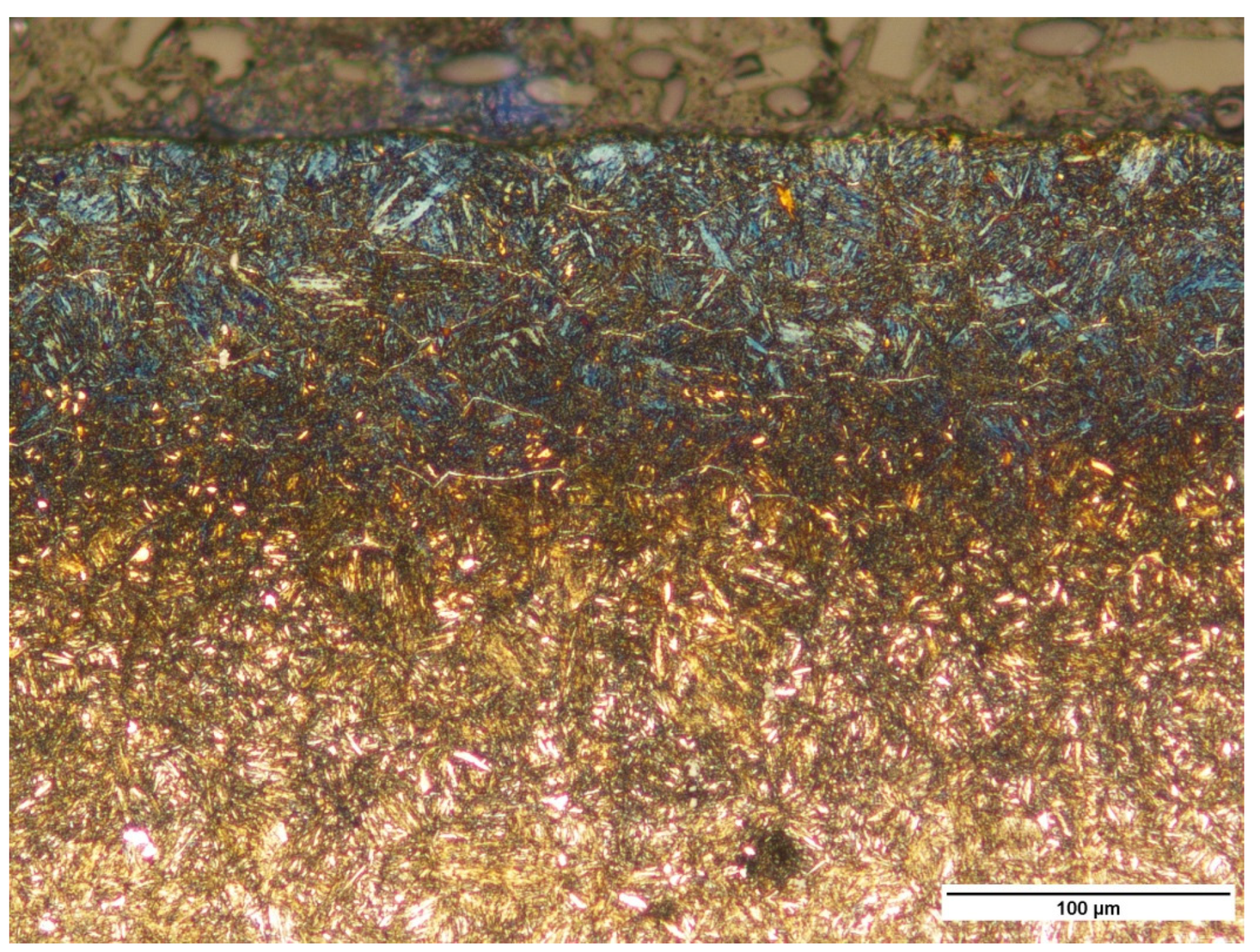
3.1.2. Microscopic Observations of Pins after Nitriding
3.1.3. Pin Hardness Measurements before and after Nitriding
3.2. Study of Pins Manufactured from Alternative Steel—42CrMo4
3.2.1. Pins before Nitriding
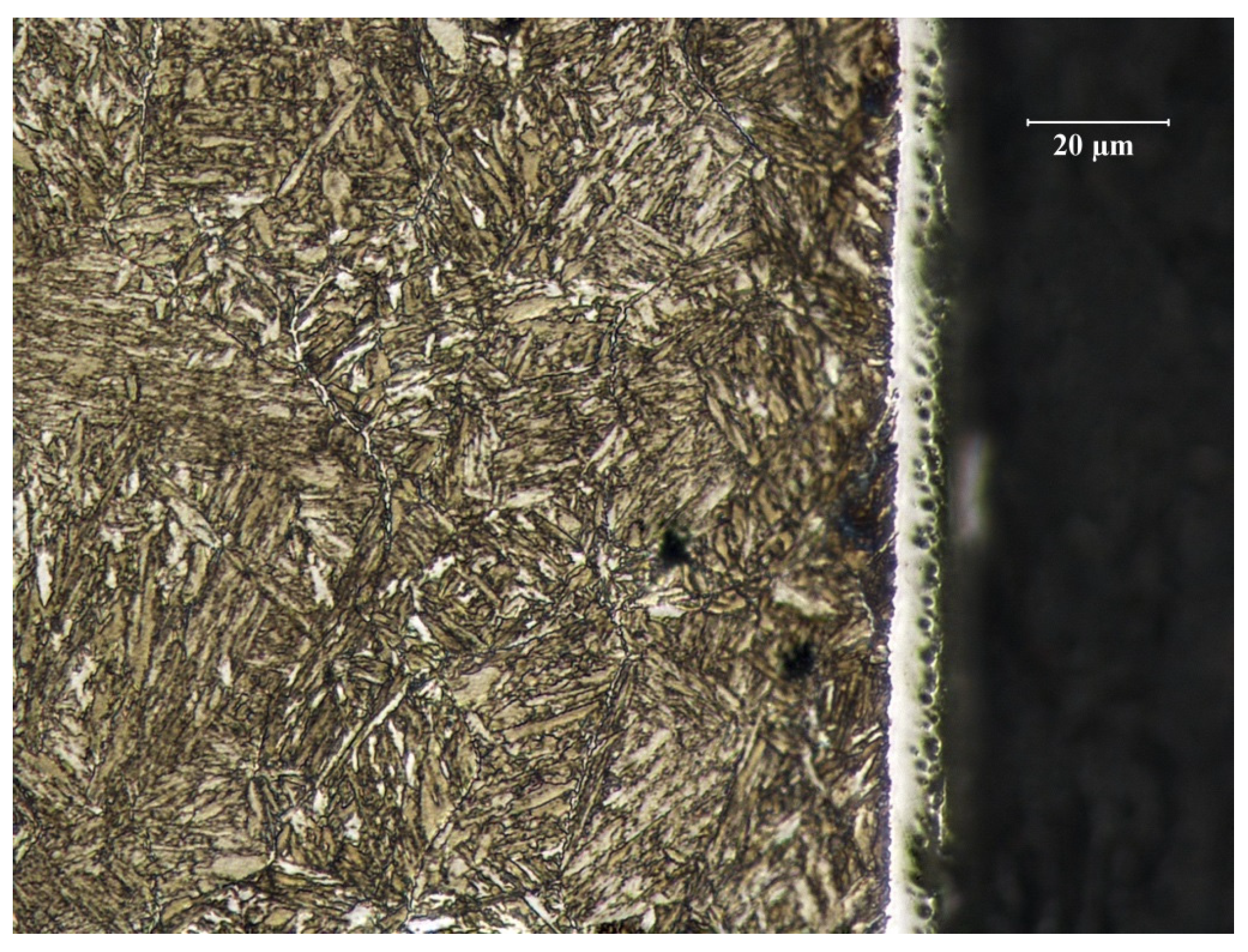
3.2.2. Pin Surface after Nitriding
3.2.3. Measurements of Hardness of Samples Core after Nitriding
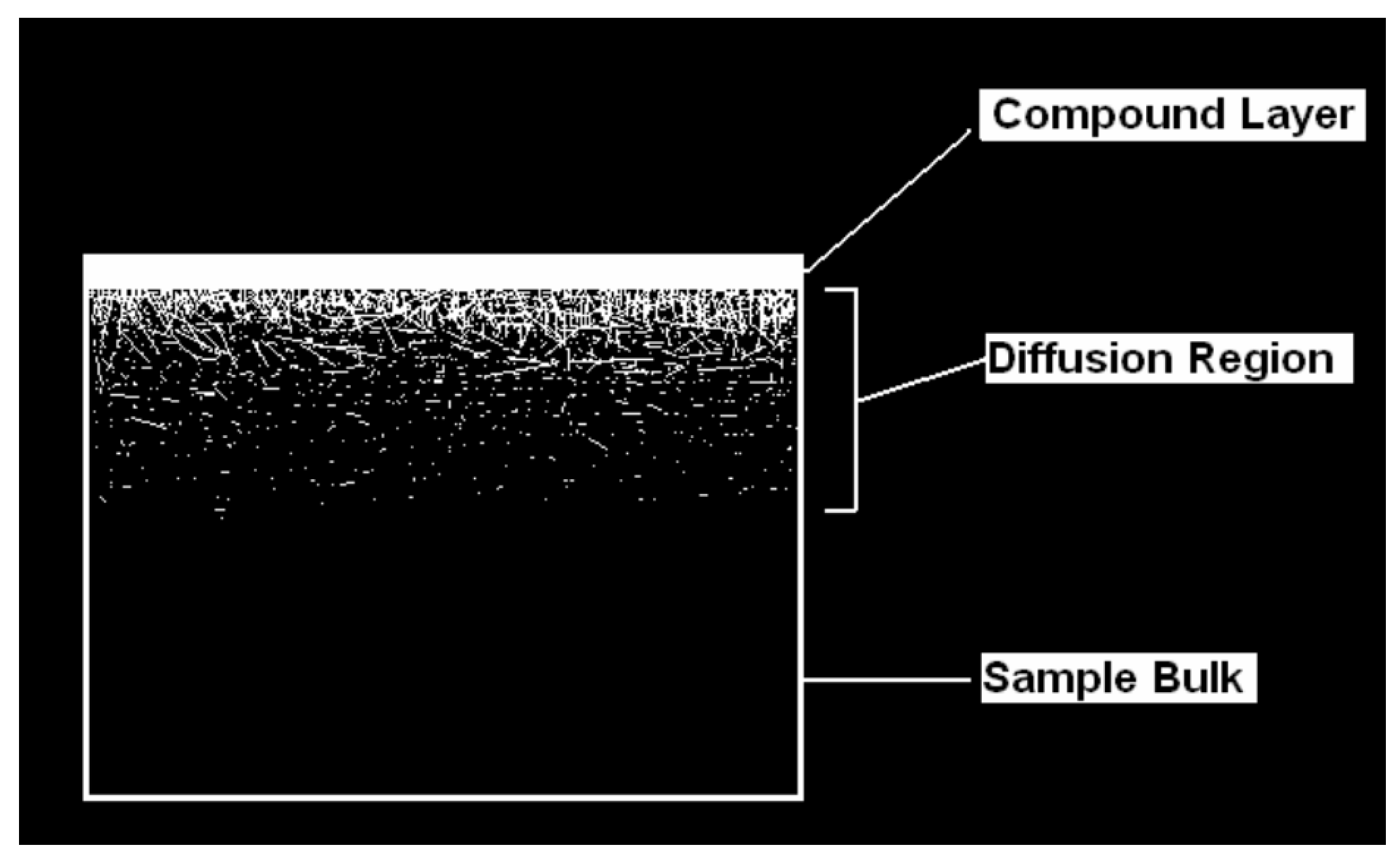
3.2.4. Determination of Nitrided Layer Thickness
3.3. Testing of Samples Taken from Pins Made of Nitrided 42CrMo4 Steel at Various Process Parameters
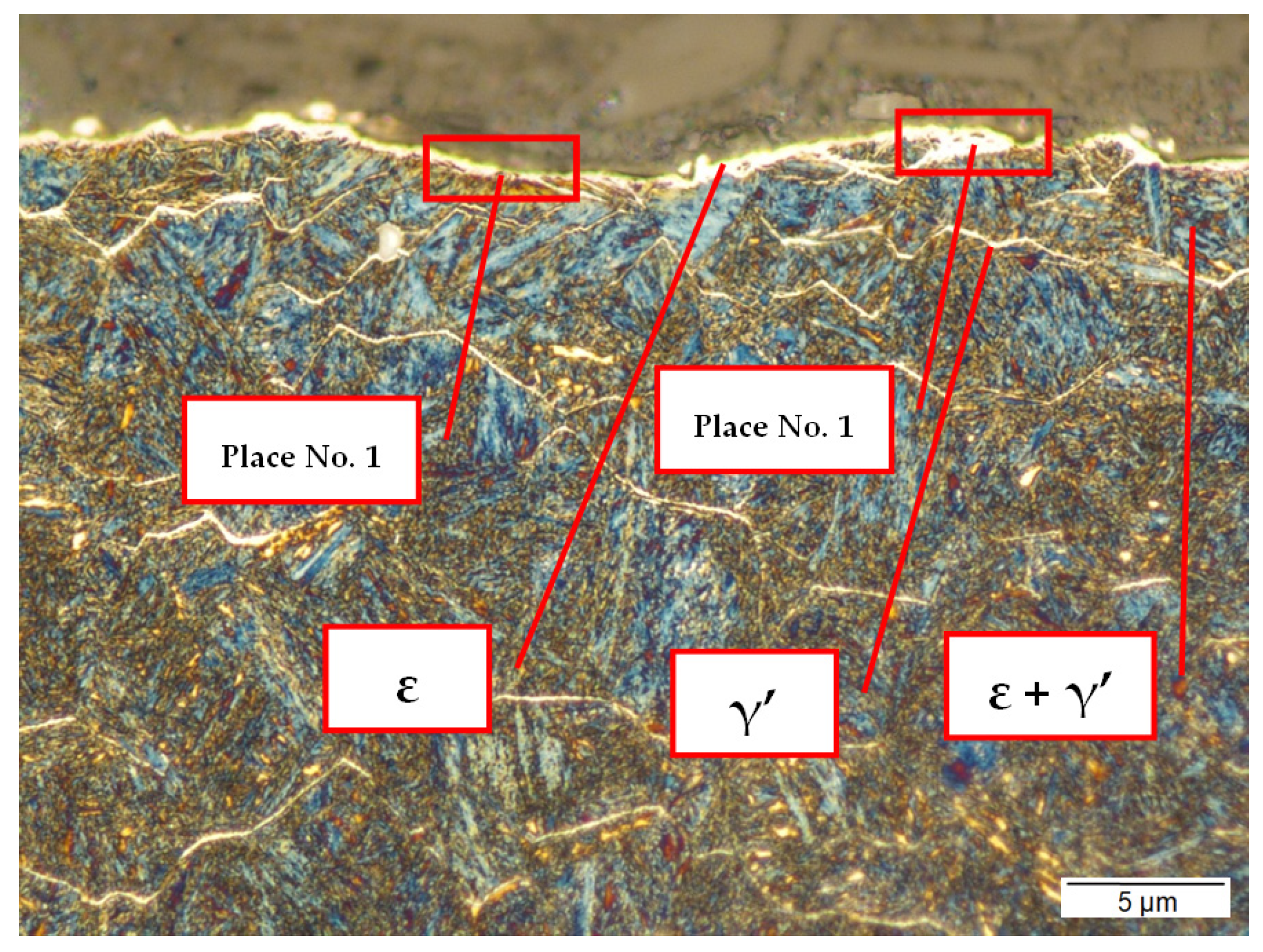
3.3.1. Sample No. 1—Microscopic Observations
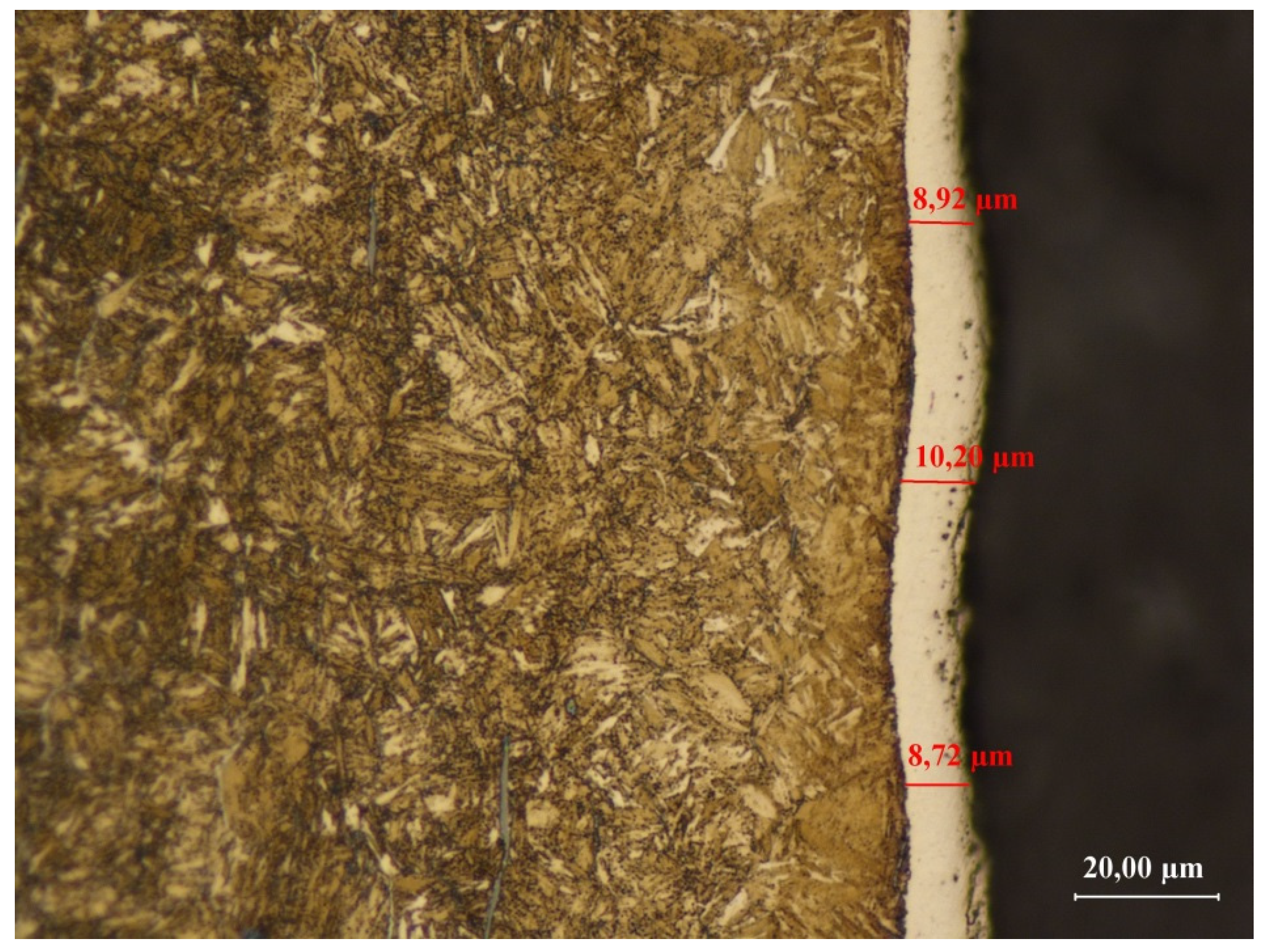
3.3.2. Sample No. 2—Microscopic Observations

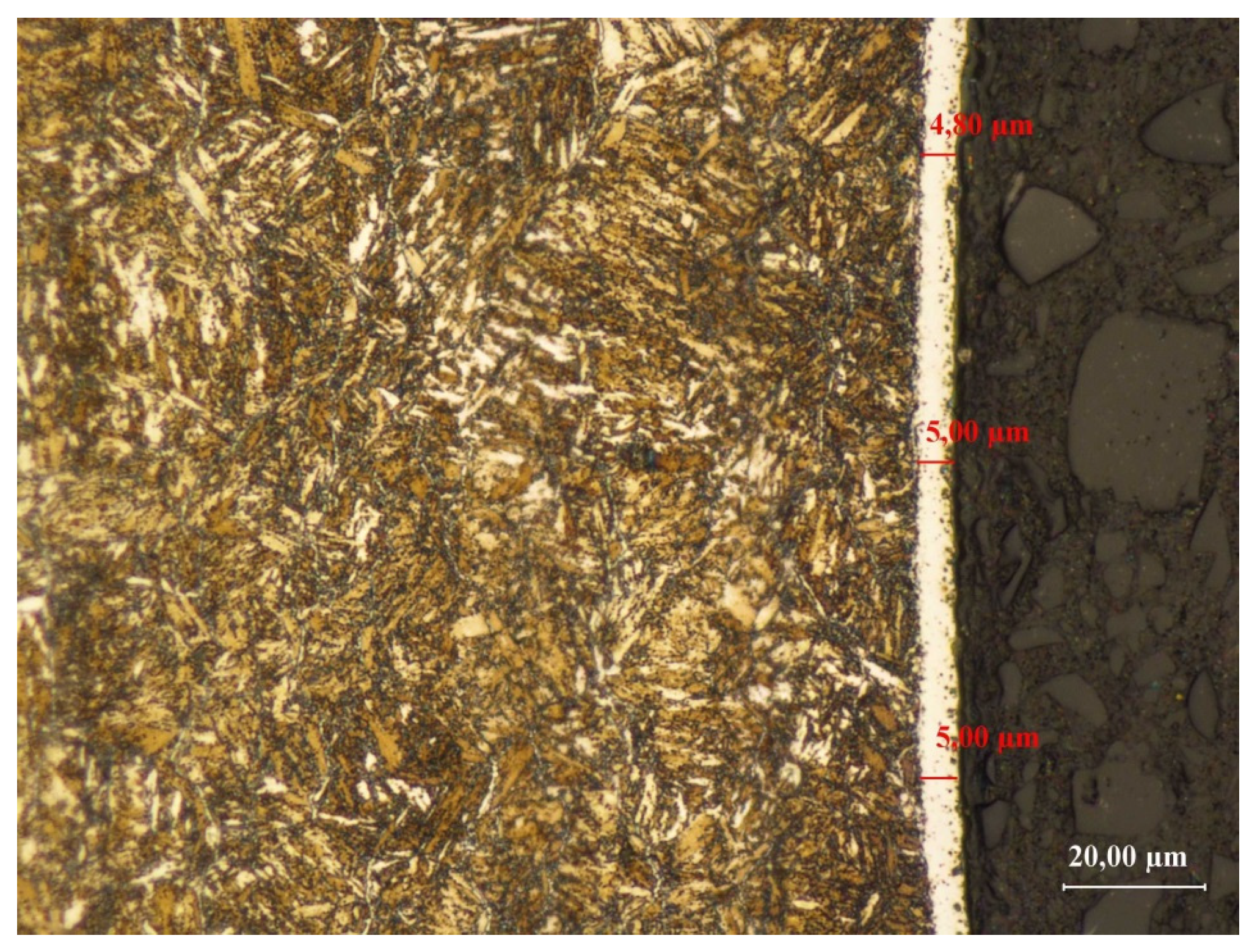
3.3.3. Sample No. 3—Microscopic Observations
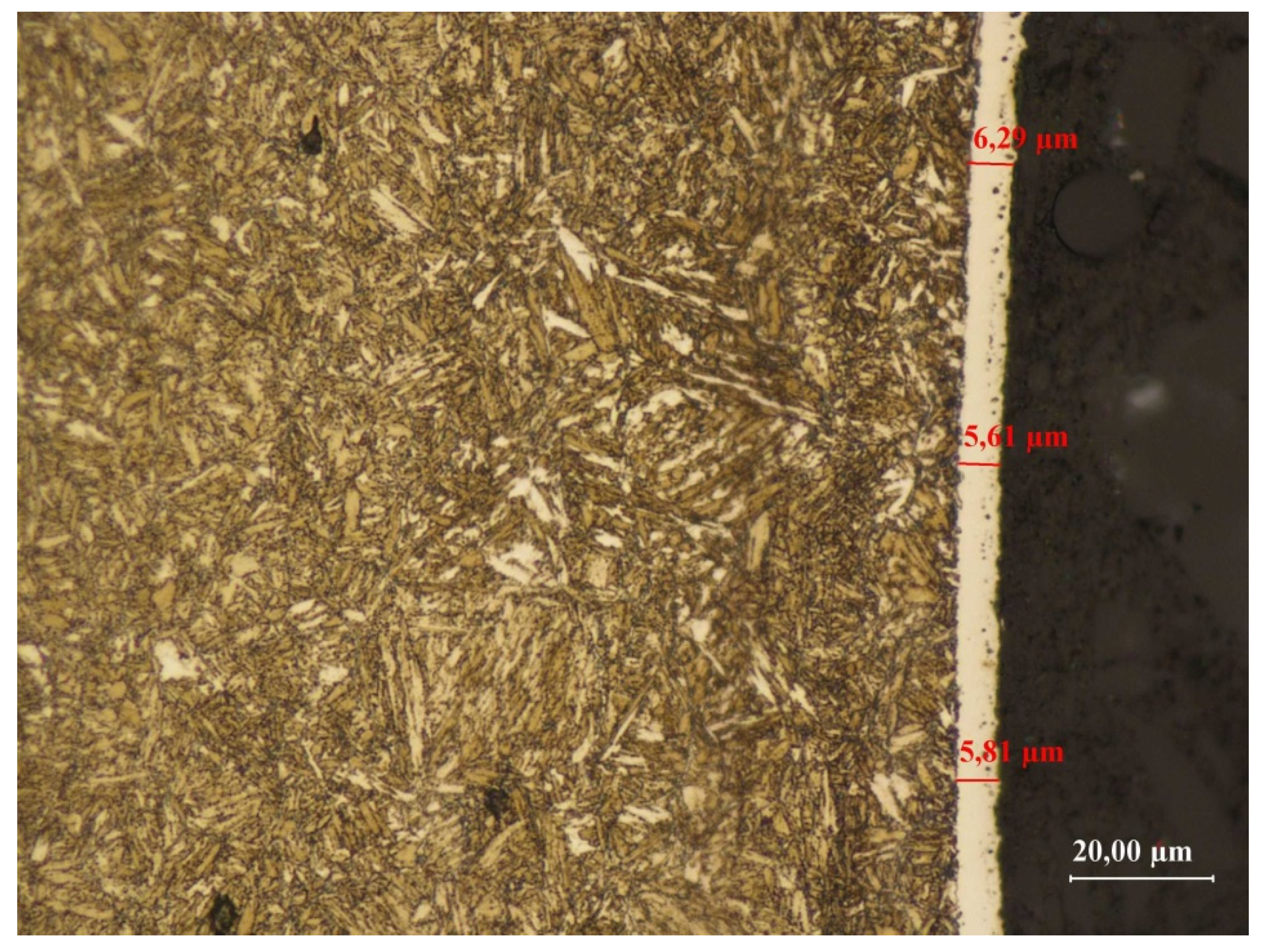
3.3.4. Sample No. 4—Microscopic Observations
3.3.5. Samples from 1 to 4-Hardness Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dan, J.; Boving, H.; Hintermann, H. Hard coatings. HAL Open Sci. J. Phys. IV Proc. 1993, 3, 933–941. [Google Scholar] [CrossRef]
- Bell, T. Surface engineering: Its current and future impact on tribology. J. Phys. D Appl. Phys. 1992, 25, A297. [Google Scholar] [CrossRef]
- Bell, T. Engineering the Surface to Combat Wear; Tribology Series; Elsevier: Amsterdam, The Netherlands, 1993; Volume 25, pp. 27–37. [Google Scholar]
- Ren, C.X.; Wang, Q.; Zhang, Z.J.; Zhang, P.; Zhang, Z.F. Surface strengthening behaviors of four structural steels processed by surface spinning strengthening. Mater. Sci. Eng. A 2017, 704, 262–273. [Google Scholar] [CrossRef]
- Śliwa, A.; Mikuła, J.; Gołombek, K.; Tański, T.; Kwaśny, W.; Bonek, M.; Brytan, Z. Prediction of the properties of PVD/CVD coatings with the use of FEM analysis. Appl. Surf. Sci. 2016, 388, 281–287. [Google Scholar] [CrossRef]
- Kıvak, T. Optimization of surface roughness and flank wear using the Taguchi method in milling of Hadfield steel with PVD and CVD coated inserts. Measurement 2014, 50, 19–28. [Google Scholar] [CrossRef]
- Vetter, J.; Barbezat, G.; Crummenauer, J.; Avissar, J. Surface treatment selections for automotive applications. Surf. Coat. Technol. 2005, 200, 1962–1968. [Google Scholar] [CrossRef]
- Ferreira, A.A.; Silva, F.J.; Pinto, A.G.; Sousa, V.F. Characterization of thin chromium coatings produced by PVD sputtering for optical applications. Coatings 2021, 11, 215. [Google Scholar] [CrossRef]
- Frohn-Sörensen, P.; Cislo, C.; Paschke, H.; Stockinger, M.; Engel, B. Dry friction under pressure variation of PACVD TiN surfaces on selected automotive sheet metals for the application in unlubricated metal forming. Wear 2021, 476, 203750. [Google Scholar] [CrossRef]
- Baptista, A.; Pinto, G.; Silva, F.J.; Ferreira, A.A.; Pinto, A.G.; Sousa, V.F. Wear characterization of chromium PVD coatings on polymeric substrate for automotive optical components. Coatings 2021, 11, 555. [Google Scholar] [CrossRef]
- ChandraYadaw, R.; Singh, S.K.; Chattopadhyaya, S.; Kumar, S.; Singh, R.C. Comparative study of surface coating for automotive application—A review. Int. J. Appl. Eng. Res. 2018, 13, 17–22. [Google Scholar]
- Ginting, A.; Skein, R.; Cuaca, D.; Masyithah, Z. The characteristics of CVD-and PVD-coated carbide tools in hard turning of AISI 4340. Measurement 2018, 129, 548–557. [Google Scholar] [CrossRef]
- Martinho, R.P.; Silva, F.J.G.; Martins, C.; Lopes, H. Comparative study of PVD and CVD cutting tools performance in milling of duplex stainless steel. Int. J. Adv. Manuf. Technol. 2019, 102, 2423–2439. [Google Scholar] [CrossRef]
- He, Q.; Paiva, J.M.; Kohlscheen, J.; Beake, B.D.; Veldhuis, S.C. An integrative approach to coating/carbide substrate design of CVD and PVD coated cutting tools during the machining of austenitic stainless steel. Ceram. Int. 2020, 46, 5149–5158. [Google Scholar] [CrossRef]
- Thakur, A.; Gangopadhyay, S. Influence of tribological properties on the performance of uncoated, CVD and PVD coated tools in machining of Incoloy 825. Tribol. Int. 2016, 102, 198–212. [Google Scholar] [CrossRef]
- Besley, N.A.; Johnston, R.L.; Stace, A.J.; Uppenbrink, J. Theoretical study of the structures and stabilities of iron clusters. J. Mol. Struct. 1995, 341, 75–90. [Google Scholar] [CrossRef]
- Friehling, P.B.; Poulsen, F.W.; Somers, M.A. Nucleation of iron nitrides during gaseous nitriding of iron; effect of a preoxidation treatment. Int. J. Mater. Res. 2022, 92, 589–595. [Google Scholar]
- Shakhnazarov, K.Y. Chernov’s iron–carbon diagram, the structure and properties of steel. Met. Sci. Heat Treat. 2009, 51, 3–6. [Google Scholar] [CrossRef]
- Davydov, S.V. Phase Equilibria in the Carbide Region of Iron–Carbon Phase Diagram. Steel Transl. 2020, 50, 888–896. [Google Scholar] [CrossRef]
- Shohoji, N. Uncracked Gaseous Ammonia NH3 as a Powerful Nitriding Medium; Lambert Academic Publishing: London, UK, 2017. [Google Scholar]
- Cahn, R.W.; Haasen, P. Physical Metallurgy, 4th ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Brandes, E.A.; Brook, G.B. Smithells Metals Reference Book, 7th ed.; Butterworth Heinemann: Oxford, UK, 1999. [Google Scholar]
- Kovacs, W.; Russell, W. Ion Nitriding. In Proceedings of the an International Conference, Cleveland, OH, USA, 15–17 September 1986. [Google Scholar]
- Jack, D.H.; Jack, K.H. Invited review: Carbides and nitrides in steel. Mater. Sci. Eng. 1973, 11, 1–27. [Google Scholar] [CrossRef]
- Menthe, E.; Bulak, A.; Olfe, J.; Zimmermann, A.; Rie, K.T. Improvement of the mechanical properties of austenitic stainless steel after plasma nitriding. Surf. Coat. Technol. 2000, 133, 259–263. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Amrutwar, S. Effect of plasma nitriding pretreatment on the mechanical properties of AlCrSiN-coated tool steels. Materials 2019, 12, 795. [Google Scholar] [CrossRef]
- Hernandez, M.H.S.M.; Staia, M.H.; Puchi-Cabrera, E.S. Evaluation of microstructure and mechanical properties of nitrided steels. Surf. Coat. Technol. 2008, 202, 1935–1943. [Google Scholar] [CrossRef]
- Raj, V.R.; Vijayaramnathb, B.; Ramanan, N.; Ponshanmugakumar, A. Mechanical and corrosion resistant properties of nitrided low carbon steel. Mater. Today Proc. 2021, 46, 3288–3291. [Google Scholar] [CrossRef]
- Özkan, D.; Yilmaz, M.A.; Karakurt, D.; Szala, M.; Walczak, M.; Bakdemir, S.A.; Türküz, C.; Sulukan, E. Effect of AISI H13 Steel Substrate Nitriding on AlCrN, ZrN, TiSiN, and TiCrN Multilayer PVD Coatings Wear and Friction Behaviors at a Different Temperature Level. Materials 2023, 16, 1594. [Google Scholar] [CrossRef]
- Lin, N.; Liu, Q.; Zou, J.; Guo, J.; Li, D.; Yuan, S.; Ma, Y.; Wang, Z.; Wang, Z.; Tang, B. Surface texturing-plasma nitriding duplex treatment for improving tribological performance of AISI 316 stainless steel. Materials 2016, 9, 875. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Wear and corrosion properties of cold-sprayed AISI 316L coatings treated by combined plasma carburizing and nitriding at low temperature. Coatings 2018, 8, 456. [Google Scholar] [CrossRef]
- Mateescu, A.O.; Mateescu, G.; Balan, A.; Ceaus, C.; Stamatin, I.; Cristea, D.; Samoila, C.; Ursutiu, D. Stainless steel surface nitriding in open atmosphere cold plasma: Improved mechanical, corrosion and wear resistance properties. Materials 2021, 14, 4836. [Google Scholar] [CrossRef]
- Xu, M.; Kang, S.; Lu, J.; Yan, X.; Chen, T.; Wang, Z. Properties of a plasma-nitrided coating and a CrN x coating on the stainless steel bipolar plate of PEMFC. Coatings 2020, 10, 183. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, H.; Fan, H.; Liu, Q.; Lv, C.; Zhao, X.; Tang, M.; Wu, J.; Cao, X. Corrosion resistance of modified hexagonal boron nitride (h-BN) nanosheets doped acrylic acid coating on hot-dip galvanized steel. Materials 2020, 13, 2340. [Google Scholar] [CrossRef]
- Sumiya, K.; Tokuyama, S.; Nishimoto, A.; Fukui, J.; Nishiyama, A. Application of active-screen plasma nitriding to an austenitic stainless steel small-diameter thin pipe. Metals 2021, 11, 366. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Zhang, X.; Li, W.; Ma, A.; Fan, K.; Xing, W. Effect of Alloying Elements and Low Temperature Plasma Nitriding on Corrosion Resistance of Stainless Steel. Materials 2022, 15, 6575. [Google Scholar] [CrossRef]
- Edenhofer, B. The Ionitriding Process-Thermochemical Treatment of Steel and Cast Iron Materials. Metall. Mater. Technol. 1976, 8, 421–426. [Google Scholar]
- D’Haen, J.; Quaeyhaegens, C.; Knuyt, G.; D’Olieslaeger, M.; Stals, L.M. Structure analysis of plasma-nitrided pure iron. Surf. Coat. Technol. 1995, 74–75, 405. [Google Scholar] [CrossRef]
- Lengauer, W.; Binder, S.; Aigner, K.; Ettmayer, P.; Guillou, A.; Debuigne, J.; Groboth, G. Solid state properties of group IVb carbonitrides. J. Alloys Compd. 1995, 217, 137–147. [Google Scholar] [CrossRef]
- Taneike, M.; Sawada, K.; Abe, F. Effect of carbon concentration on precipitation behavior of M23C6 carbides and MX carbonitrides in martensitic 9Cr steel during heat treatment. Metall. Mater. Trans. A 2004, 35, 1255–1262. [Google Scholar] [CrossRef]
- Thelning, K.E. Steel and Its Heat Treatment; Butterworth: London, UK, 1984. [Google Scholar]
- Akhlaghi, M.; Meka, S.R.; Jägle, E.A.; Kurz, S.J.; Bischoff, E.; Mittemeijer, E.J. Formation mechanisms of alloying element nitrides in recrystallized and deformed ferritic Fe-Cr-Al alloy. Metall. Mater. Trans. A 2016, 47, 4578–4593. [Google Scholar] [CrossRef]
- Dossett, J.; Totten, G.E. Fundamentals of nitriding and nitrocarburizing. In ASM Handbook: Steel Heat Treating Fundamentals and Processes; ASM International: Materials Park, OH, USA, 2013; Volume 619. [Google Scholar]
- Morita, Z.; Tanaka, T.; Yanai, T. Equilibria of nitride forming reactions in liquid iron alloys. Metall. Trans. B 1987, 18, 195–202. [Google Scholar] [CrossRef]
- Babaskin, Y.Z.; Shipitsyn, S.Y. Microalloying of structural steel with nitride-forming elements. Steel Transl. 2009, 39, 1119–1121. [Google Scholar] [CrossRef]
- Bell, T.; Birch, B.J.; Korotchenko, V.; Evans, S.P. Controlled nitriding in ammonia-hydrogen mixtures. In Heat Treatment’73; The Metals Society: London, UK, 1975. [Google Scholar]
- Georges, J. Nitriding Process and Nitriding Furnace Therefore. Plasma Metal SA. U.S. Patent No. 5,989,363, 23 November 1999. [Google Scholar]
- Malvos, H.; Ricard, A.; Szekely, J.; Michel, H.; Gantois, M.; Ablitzer, D. Modelling of a microwave postdischarge nitriding reactor. Surf. Coat. Technol. 1993, 59, 59–66. [Google Scholar] [CrossRef]
- Aghajani, H.; Behrangi, S. Plasma Nitriding of Steels; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Georges, J.; Cleugh, D. Active screen plasma nitriding. In Stainless Steel 2000; CRC Press: Boca Raton, FL, USA, 2020; pp. 377–387. [Google Scholar]
- Ohtsu, N.; Miura, K.; Hirano, M.; Kodama, K. Investigation of admixed gas effect on plasma nitriding of AISI316L austenitic stainless steel. Vacuum 2021, 193, 110545. [Google Scholar] [CrossRef]
- Priest, J.M.; Baldwin, M.J.; Fewell, M.P. The action of hydrogen in low-pressure rf-plasma nitriding. Surf. Coat. Technol. 2001, 145, 152–163. [Google Scholar] [CrossRef]
- Zdravecká, E.; Slota, J.; Solfronk, P.; Kolnerová, M. Evaluation of the effect of different plasma-nitriding parameters on the properties of low-alloy steel. J. Mater. Eng. Perform. 2017, 26, 3588–3596. [Google Scholar] [CrossRef]
- Kovacı, H.; Baran, Ö.; Yetim, A.F.; Bozkurt, Y.B.; Kara, L.; Çelik, A. The friction and wear performance of DLC coatings deposited on plasma nitrided AISI 4140 steel by magnetron sputtering under air and vacuum conditions. Surf. Coat. Technol. 2018, 349, 969–979. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Kim, M.H. Effects of the process parameters on the layer formation behavior of plasma nitrided steels. Surf. Coat. Technol. 2001, 141, 182–186. [Google Scholar] [CrossRef]
- Yazdani, S.; Mahboubi, F. Comparison between microstructure, wear behavior, and corrosion resistance of plasma-nitrided and vacuum heat-treated electroless Ni–B coating. J. Bio-Tribo-Corros. 2019, 5, 69. [Google Scholar] [CrossRef]
- Ricard, A.; Czerwiec, T.; Belmonte, T.; Bockel, S.; Michel, H. Detection by emission spectroscopy of active species in plasma–surface processes. Thin Solid Film. 1999, 341, 1–8. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.X.; Dong, H.; Bell, T. Study on the active screen plasma nitriding and its nitriding mechanism. Surf. Coat. Technol. 2006, 201, 2320–2325. [Google Scholar] [CrossRef]
- Li, C.X. Active screen plasma nitriding—An overview. Surf. Eng. 2010, 26, 135–141. [Google Scholar] [CrossRef]
- Nishimoto, A.; Nagatsuka, K.; Narita, R.; Nii, H.; Akamatsu, K. Effect of the distance between screen and sample on active screen plasma nitriding properties. Surf. Coat. Technol. 2010, 205, S365–S368. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, D.; Shen, L. Influence of bias voltage on the formation and properties of iron-based nitrides produced by plasma nitriding. J. Alloys Compd. 2010, 497, 285–289. [Google Scholar] [CrossRef]
- Ahangarani, S.; Mahboubi, F.; Sabour, A.R. Effects of various nitriding parameters on active screen plasma nitriding behavior of a low-alloy steel. Vacuum 2006, 80, 1032–1037. [Google Scholar] [CrossRef]


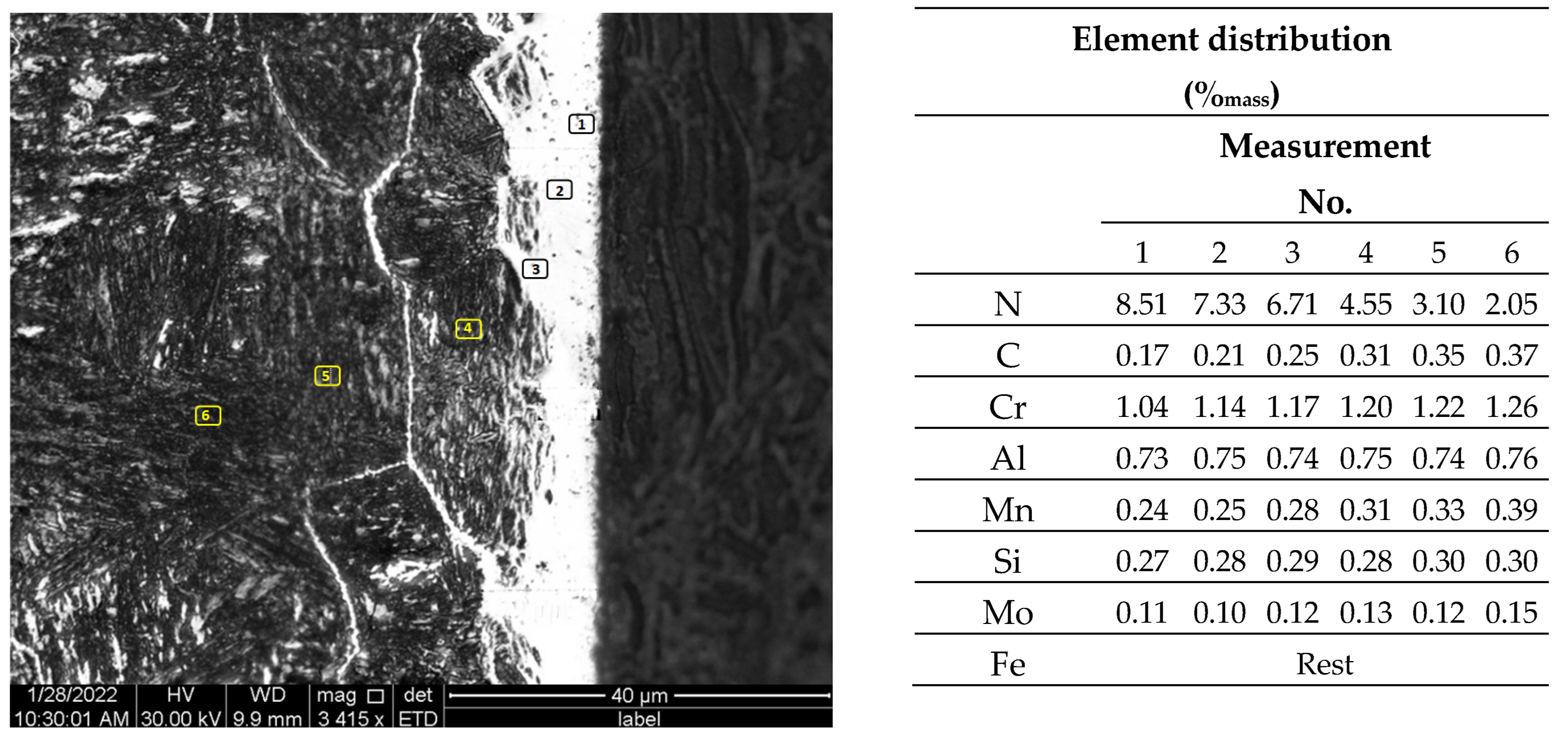


| Ordinal Number | Element | Chemical Composition of Steel (%mass) |
|---|---|---|
| 1 | C | 0.392 |
| 2 | Cr | 1.270 |
| 3 | Al | 0.763 |
| 4 | Mn | 0.401 |
| 5 | Si | 0.308 |
| 6 | Mo | 0.166 |
| 7 | Ni | 0.086 |
| 8 | Ti | 0.004 |
| 9 | P | 0.006 |
| 10 | S | 0.001 |
| Distance from the Surface (µm) | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 | 550 | 600 | 650 | 700 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardness HV0.5 | 732.0 | 663.9 | 442.7 | 373.1 | 369.9 | 364.7 | 372.8 | 369.4 | 365 | 384.7 | 376.4 | 378.1 | 377.9 | 366.4 |
| Distance from the Surface [µm] | 50 | 100 | 150 | 200 | 250 |
|---|---|---|---|---|---|
| Hardness HV0.5 | 761.2 | 729.9 | 567.9 | 396.9 | 390.1 |
| Distance from the Surface (µm) | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 | 550 | 600 | 650 | 700 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardness HV 0.5 | 614.0 | 619.4 | 556.1 | 526.1 | 526.0 | 512.2 | 492.2 | 485.1 | 468.4 | 468.6 | 425.5 | 447.2 | 436.8 | 442.0 |
| Samples No | Gas Flow Rate (L/min) | Currents | Time (h/min) | Temperature (°C) | |||
|---|---|---|---|---|---|---|---|
| N | H | Ar | BIAS | SCREEN | |||
| 1 | 1.5 | 0.4 | 0.3 | 410V 4.0A | 320V 3.0A | 26/35 | 530 |
| 2 | 1.3 | 0.7 | 0.2 | 440V 6.0A | 330V 3.0A | 26/35 | 510 |
| 3 | 1.3 | 0.7 | 0.2 | 410V 4.0A | 330V 3.0A | 30/38 | 510 |
| 4 | 1.3 | 0.7 | 0.2 | 410V 4.0A | 330V 4.0A | 26/35 | 550 |
| Sample No. 1 | ||||||||||
| Distance from the surface [µm] | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 |
| Hardness HV 0.5 | 621.0 | 638.7 | 605.2 | 539.1 | 512.1 | 478.7 | 444.9 | 419.3 | 416.8 | 366.7 |
| Sample No. 2 | ||||||||||
| Distance from the surface [µm] | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 |
| Hardness HV 0.5 | 529.7 | 499.3 | 461.9 | 438.9 | 420.0 | 386.1 | 375.6 | 359.5 | 359.0 | 330.3 |
| Sample No. 3 | ||||||||||
| Distance from the surface [µm] | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 |
| Hardness HV 0.5 | 568.5 | 529.9 | 483.7 | 434.3 | 409.7 | 383.5 | 356.6 | 347.6 | 335.5 | 341.7 |
| Sample No. 4 | ||||||||||
| Distance from the surface [µm] | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 |
| Hardness HV 0.5 | 554.0 | 527.8 | 481.5 | 454.0 | 421.8 | 417.6 | 398.7 | 366.7 | 355.2 | 337.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudziński, W.; Medyński, D.; Sacher, P. Effect of Machine Pin-Manufacturing Process Parameters by Plasma Nitriding on Microstructure and Hardness of Working Surfaces. Crystals 2023, 13, 1091. https://doi.org/10.3390/cryst13071091
Dudziński W, Medyński D, Sacher P. Effect of Machine Pin-Manufacturing Process Parameters by Plasma Nitriding on Microstructure and Hardness of Working Surfaces. Crystals. 2023; 13(7):1091. https://doi.org/10.3390/cryst13071091
Chicago/Turabian StyleDudziński, Włodzimierz, Daniel Medyński, and Paweł Sacher. 2023. "Effect of Machine Pin-Manufacturing Process Parameters by Plasma Nitriding on Microstructure and Hardness of Working Surfaces" Crystals 13, no. 7: 1091. https://doi.org/10.3390/cryst13071091
APA StyleDudziński, W., Medyński, D., & Sacher, P. (2023). Effect of Machine Pin-Manufacturing Process Parameters by Plasma Nitriding on Microstructure and Hardness of Working Surfaces. Crystals, 13(7), 1091. https://doi.org/10.3390/cryst13071091






