Effects of Fe and Ni Doping on the Electronic Structure and Optical Properties of Cu2ZnSnS4
Abstract
1. Introduction
2. Theoretical Models and Calculation Methods
3. Results and Discussion
3.1. Geometric Structure Optimization
3.2. Electronic Structure
3.2.1. Energy Band Structure
3.2.2. Electronic Density of States
3.3. Mulliken Population Analysis
3.4. Optical Properties
3.4.1. Complex Dielectric Function
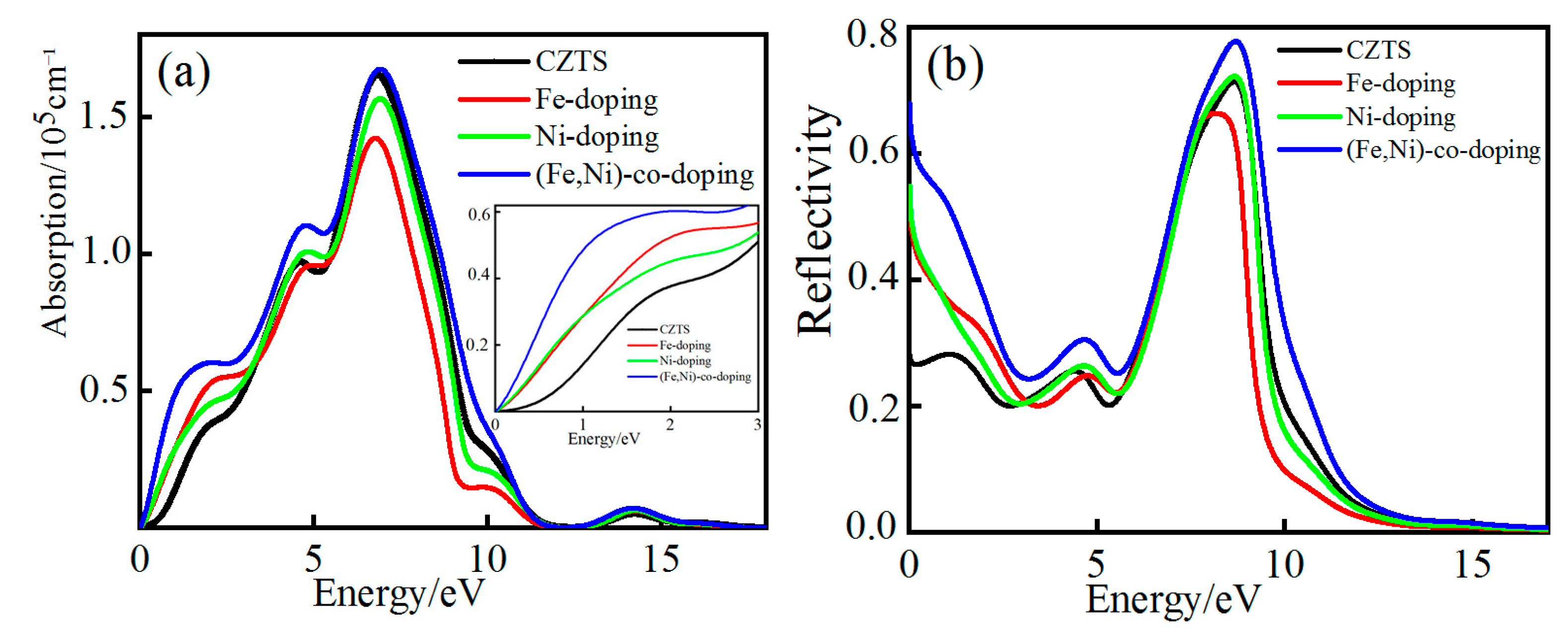
3.4.2. Absorption and Reflection Spectra
3.4.3. Complex Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thirunavukkarasu, M.; Sawle, Y.; Lala, H. A comprehensive review on optimization of hybrid renewable energy systems using various optimization techniques. Renew. Sustain. Energy Rev. 2023, 176, 113192. [Google Scholar] [CrossRef]
- Das, B.; Hossain, S.M.; Nandi, A.; Samanta, D.; Pramanick, A.K.; Chapa, S.O.M.; Ray, M. Spectral conversion by silicon nanocrystal dispersed gel glass: Efficiency enhancement of silicon solar cell. J. Phys. D Appl. Phys. 2021, 55, 025106. [Google Scholar] [CrossRef]
- Okil, M.; Salem, M.S.; Abdolkader, T.M.; Shaker, A. From Crystalline toLow-cost Silicon-based Solar Cells: A Review. Silicon 2021, 14, 1895–1911. [Google Scholar] [CrossRef]
- Koltsov, M.; Gopi, S.V.; Raadik, T.; Krustok, J.; Josepson, R.; Gržibovskis, R.; Vembris, A.; Spalatu, N. Development of Bi2S3 thin film solar cells by close-spaced sublimation and analysis of absorber bulk defects via in-depth photoluminescence analysis. Sol. Energy Mater. Sol. Cells 2023, 254, 112292. [Google Scholar] [CrossRef]
- Eka, C.P.; Jessie, M.; Endi, S.; Andhy, S.; Ganes, S.; Mohammad, K.A.; Brian, Y. The effect of CuZn+ZnCu defect complex on Cu2ZnSnS4 thin film solar cell:A density functional theory study. Mater. Chem. Phys. 2023, 296, 127192. [Google Scholar]
- Pan, B.; Wei, M.; Liu, W.; Jiang, G.; Zhu, C. Fabrication of Cu2ZnSnS4 absorber layers with adjustable Zn/Sn and Cu/Zn+Sn ratios. J. Mater. Sci. Mater. Electron. 2014, 25, 3344–3352. [Google Scholar] [CrossRef]
- Su, Z.H.; Liang, G.X.; Fan, P.; Luo, J.; Zheng, Z.; Xie, Z.; Wang, W.; Chen, S.; Hu, J.; Wei, Y.; et al. Device Postannealing Enabling over 12% Efficient Solution-Processed Cu2ZnSnS4 Solar Cells with Cd2+ Substitution. Adv. Mater. 2020, 32, 2000121. [Google Scholar] [CrossRef]
- Ki, W.; Hillhouse, H.W. Earth-Abundant Element Photovoltaics Directly from Soluble Precursors with High Yield Using a Non-Toxic Solvent. Adv. Energy Mater. 2011, 1, 732–735. [Google Scholar] [CrossRef]
- Crovetto, A.; Hansen, O. What is the band alignment of Cu2ZnSn(S,Se)4 solar cells? Sol. Energy Mater. Sol. Cells. 2017, 169, 177–194. [Google Scholar] [CrossRef]
- Bao, W.; Ichimura, M. Prediction of the Band Offsets at the CdS/Cu2ZnSnS4 Interface Based on the First-Principles Calculation. Jpn. J. Appl. Phys. 2012, 51, 10NC31. [Google Scholar] [CrossRef]
- Chen, S.; Walsh, A.; Gong, X.G.; Wei, S.H. Classification of Lattice Defects in the kesterite Cu2ZnSnS4 and Cu2ZnSnSe4 Earth-Abundant Solar Cell Absorbers. Adv. Mater. 2013, 25, 1522–1539. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Tan, J.M.; Li, X.; Zeng, X.; Batabyal, S.K.; Wong, L.H. Cation Substitution of Solution-Processed Cu2ZnSnS4 Thin Film Solar Cell with over 9% Efficiency. Adv. Energy Mater. 2015, 5, 1500682. [Google Scholar] [CrossRef]
- Choubrac, L.; Bär, M.; Kozina, X.; Félix, R.; Wilks, R.G.; Brammertz, G.; Levcenko, S.; Arzel, L.; Barreau, N.; Harel, S.; et al. Sn Substitution by Ge: Strategies to Overcome the Open-Circuit Voltage Deficit of Kesterite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 5830–5839. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Xu, X.; Duan, B.W.; Shi, J.J.; Luo, Y.H.; Wu, H.J.; Li, D.M.; Meng, Q.B. Research Progress of Metal(I) Substitution in Cu2ZnSn(S,Se)4 Thin Film Solar Cells. Acta Chim. Sinica. 2021, 79, 303–318. [Google Scholar] [CrossRef]
- Neuschitzer, M.; Rodriguez, M.E.; Guc, M.; Marquez, J.A.; Giraldo, S.; Forbes, I.; Perez-Rodriguez, A.; Saucedo, E. Revealing the beneficial effects of Ge doping on Cu2ZnSnSe4 thin film solar cells. J. Mater. Chem. A 2018, 6, 11759. [Google Scholar] [CrossRef]
- Yuan, Z.-K.; Chen, S.; Xiang, H.; Gong, X.-G.; Walsh, A.; Park, J.-S.; Repins, I.; Wei, S.-H. Engineering Solar Cell Absorbers by Exploring the Band Alignment and Defect Disparity: The Case of Cu- and Ag-Based Kesterite Compounds. Adv. Funct. Mater. 2015, 25, 6733–6743. [Google Scholar] [CrossRef]
- Sravani, L.; Routray, S.; Courel, M.; Radhan, K.P. Loss mechanisms in CZTS and CZTSe kesteritethin-film solarcells: Understanding the complexity of defect density. Sol. Energy. 2021, 227, 56–66. [Google Scholar] [CrossRef]
- Bencherif, H. Towards a high efficient Cd-free double CZTS layers kesterite solar cell using an optimized interface band alignment. Sol. Energy 2022, 238, 114–125. [Google Scholar] [CrossRef]
- Kaur, K.; Nisika; Chowdhury, A.H.; Qiao, Q.; Kumar, M. Nanoscale charge transport and local surface potential distribution toprobe the defect passivation in Cr-substituted earth abundant CZTS absorber layer. J. Alloys Compd. 2021, 854, 157160. [Google Scholar] [CrossRef]
- Muska, K.; Timmo, K.; Pilvet, M.; Kaupmees, R.; Raadik, T.; Mikli, V.; Grossberg-Kuusk, M.; Krustok, J.; Josepson, R.; Lange, S.; et al. Impact of Li and K co-doping on the optoelectronic properties of CZTS monograin powder. Sol. Energy Mater. Sol. Cells 2023, 252, 112182. [Google Scholar] [CrossRef]
- Liu, B.; Guo, J.; Hao, R.; Wang, L.; Gu, K.; Sun, S.; Aierken, A. Effect of Na doping on the performance and the band alignment of CZTS/CdS thin film solar cell. Sol. Energy. 2020, 201, 219–226. [Google Scholar] [CrossRef]
- Heydar, H.N.; Tara, P.D. Influence of Ag-doping on the performance of Cu2ZnSnS4 solar cells. Sol. Energy. 2023, 253, 321–331. [Google Scholar]
- Ashfaq, A.; Jacob, J.; Amami, M.; Al-Harbi, F.; Ali, A.; Mahmood, K.; Rehman, U.; Amin, N.; Ikram, S.; Akbar, S.T.; et al. Effect of Al-doping on the thermoelectric properties of CZTS thin film grown by sol-gel method. Solid State Commun. 2022, 345, 114701. [Google Scholar] [CrossRef]
- Pandey, A.; Yadav, P.; Kumar, P.; Singh, M.K. Synthesis, morphological and optical properties of hydrothermally synthesized Bi and Mn co-doped Cu2ZnSnS4(CZTS). Mater. Today Proc. 2022, 11, 407. [Google Scholar] [CrossRef]
- Gupta, A.K.S.; Farhad, S.F.U.; Habib, S.; Hossan, M.R.; Hossain, K.; Das, N.K.; Quamruzzaman, M.; Matin, M.; Amin, N. Characterizations of extrinsically doped CZTS thin films for solar cell absorbers fabricated by sol-gel spin coating method. Appl. Surf. Sci. Adv. 2023, 13, 100352. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, D.; Wu, Z.; Wang, D. The Fe-promoted MoP catalyst with high activity for water splitting. Appl. Catal. A Gen. 2016, 524, 134–138. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Ismael, M. One-step ultrasonic-assisted synthesis of Ni-doped g-C3N4 photocatalyst for enhanced photocatalytic hydrogen evolution. Inorg. Chem. Commun. 2023, 151, 110607. [Google Scholar] [CrossRef]
- Schorr, S.; Hoebler, H.-J.; Tovar, M. A neutron diffraction study of the stannite-kesterite solid solution series. Eur. J. Miner. 2007, 19, 65–73. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr.Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B. 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, J.; Zhang, Y. First-principles calculation of compensated (2N, W) codoping impacts on band gap engineering in anatase TiO2. Chem. Phys. Lett. 2012, 527, 63–66. [Google Scholar] [CrossRef]
- Long, R.; English, N.J. Synergistic Effects of Bi/S Codoping on Visible Light-Activated Anatase TiO2 Photocatalysts from First Principles. J. Phys. Chem. C 2009, 113, 8373–8377. [Google Scholar] [CrossRef]
- Paier, J.; Asahi, R.; Nagoya, A.; Kresse, G. Cu2ZnSnS4 as a potential photovoltaic material: A hybrid Hartree-Fockdensity functional theory study. Phys. Rev. B. 2009, 79, 115126. [Google Scholar] [CrossRef]
- Sun, D.; Ding, Y.; Kong, L.; Zhang, Y.; Guo, X.; Wei, L.; Zhang, L.; Zhang, L. First-principles Study on Mg Doping in Cu2ZnSnS4. J. Inorg. Mater. 2020, 35, 1290–1294. [Google Scholar] [CrossRef]
- Digraskar, R.V.; Mali, S.M.; Tayade, S.B.; Ghule, A.V.; Sathe, B.R. Overall noble metal free Ni and Fe doped Cu2ZnSnS4 (CZTS) bifunctional electrocatalytic systems for enhanced water splitting reactions. Int. J. Hydrogen Energy. 2019, 44, 8144–8155. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, Q.; Guo, W.; Zhang, W.; Ya, Y.; Xia, Y. Novel Platycladus orientalis–shaped Fe-doped ZnO hierarchical nanoflowerdecorated with Ag nanoparticles for photocatalytic application. J. Alloys Compd. 2021, 880, 160501. [Google Scholar] [CrossRef]
- Mo, M.; Zeng, J.-S.; He, H.; Zhang, L.; Du, L.; Fang, Z.-J. The first-principle study on the formation energies of Be, Mg and Mn doped CuInO2. Acta Phys. Sin. 2019, 68, 106102. [Google Scholar] [CrossRef]
- Zhao, H.; Persson, C. Optical properties of Cu(In,Ga)Se2 and Cu2ZnSn(S,Se)4. Thin Solid Film. 2011, 519, 7508–7512. [Google Scholar] [CrossRef]
- Islam, S.; Majumdar, P.; Hossain, M.A.; Ahmed, F. Effect of Fe3+ Doping concentration in Cu2ZnSnS4 thin film: Structural and Optical Analysis. J. Mater. Sci. Eng. A. 2022, 12, 97–106. [Google Scholar]
- Chen, H.J.; Fu, S.W.; Wu, S.H.; Tsai, T.C.; Wu, H.T.; Shih, C.F. Structural and photoelectron spectroscopic studies of band alignment at the Cu2ZnSnS4/CdS heterojunction with slight Ni doping in Cu2ZnSnS4. J. Phys. D Appl. Phys. 2016, 49, 335102. [Google Scholar] [CrossRef]
- Lebègue, S.; Arnaud, B.; Alouani, M. Calculated quasiparticle and optical properties of orthorhombic and cubic Ca2Si. Phys. Rev. B. 2005, 72, 085103. [Google Scholar] [CrossRef]
- Borlido, P.; Schmidt, J.; Huran, A.W.; Tran, F.; Marques, M.A.L.; Botti, S. Exchange-correlation functionals for band gaps of solids: Benchmark, reparametrization and machine learning. NPJ Comput. Mater. 2020, 6, 96. [Google Scholar] [CrossRef]
- Xiao, C.; Li, K.; Zhang, J.; Tong, W.; Liu, Y.; Li, Z.; Huang, P.; Pan, B.; Su, H.; Xie, Y. Magnetic ions in wide band gap semiconductor nanocrystals for optimized thermoelectric properties. Mater. Horizons. 2014, 1, 81–86. [Google Scholar] [CrossRef]
- Prokopidis, K.; Kalialakis, C. Physical interpretation of amodified Lorentz dielectric function for metals based on the Lorentz–Diracforce. Appl. Phys. B 2014, 117, 25–32. [Google Scholar] [CrossRef]
- Li, C.R.; Li, Y.F.; Yao, B.; Yang, G.; Zhan, H.D.; Deng, R.; Liu, L. Electronic and optical properties of kesteriteCu2ZnSnS4 under in-plane biaxial strains: First-principles calculations. Phys. Lett. A 2013, 377, 2398–2402. [Google Scholar] [CrossRef]
- Behera, N.; Mohan, D.B. Investigation of broad-band optical absorption and electrical properties in vacuum annealed CZTS/Ag multi-layered stack structure for plasmonic solar cell application. Opt. Mater. 2022, 127, 112316. [Google Scholar] [CrossRef]
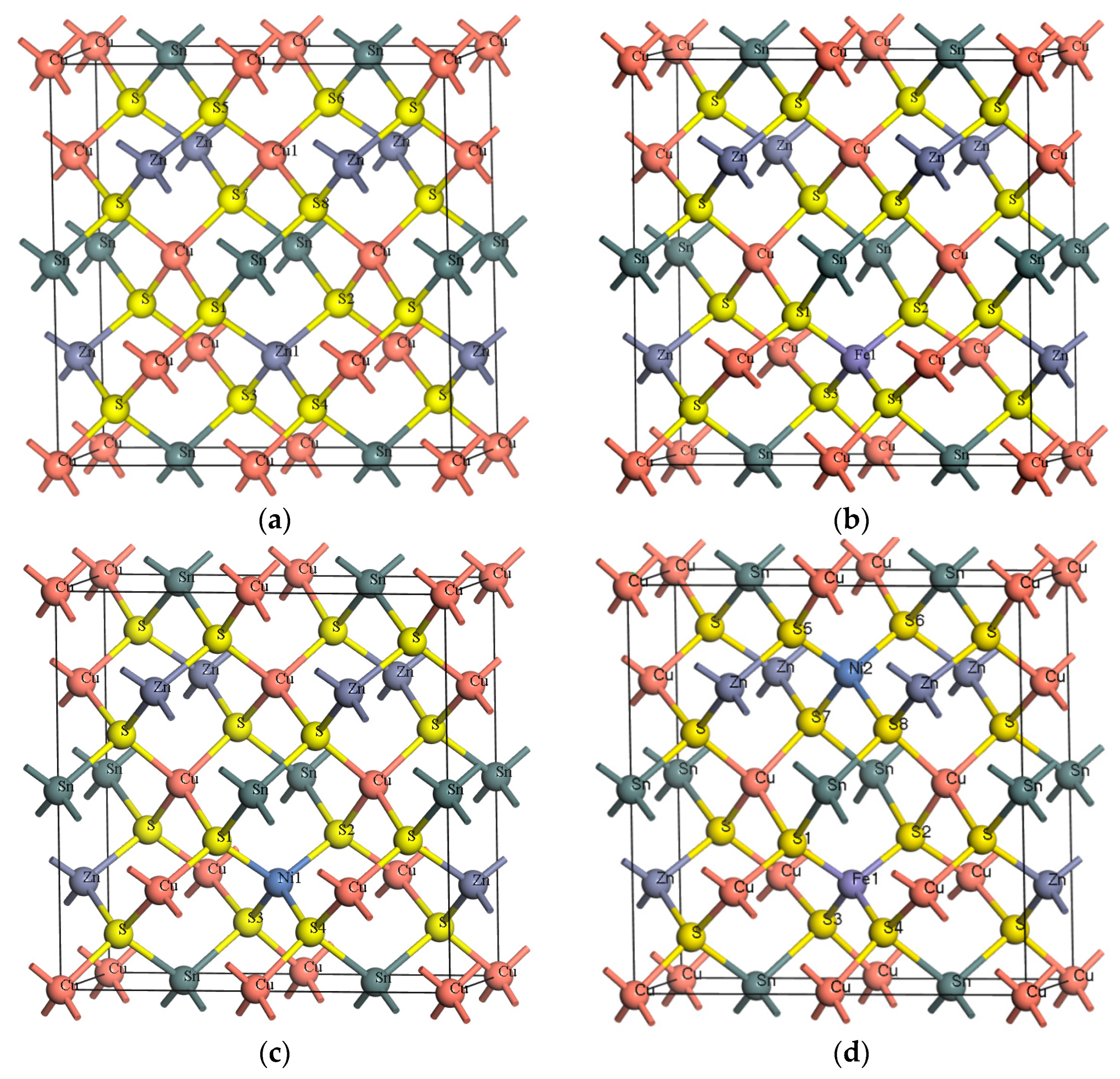
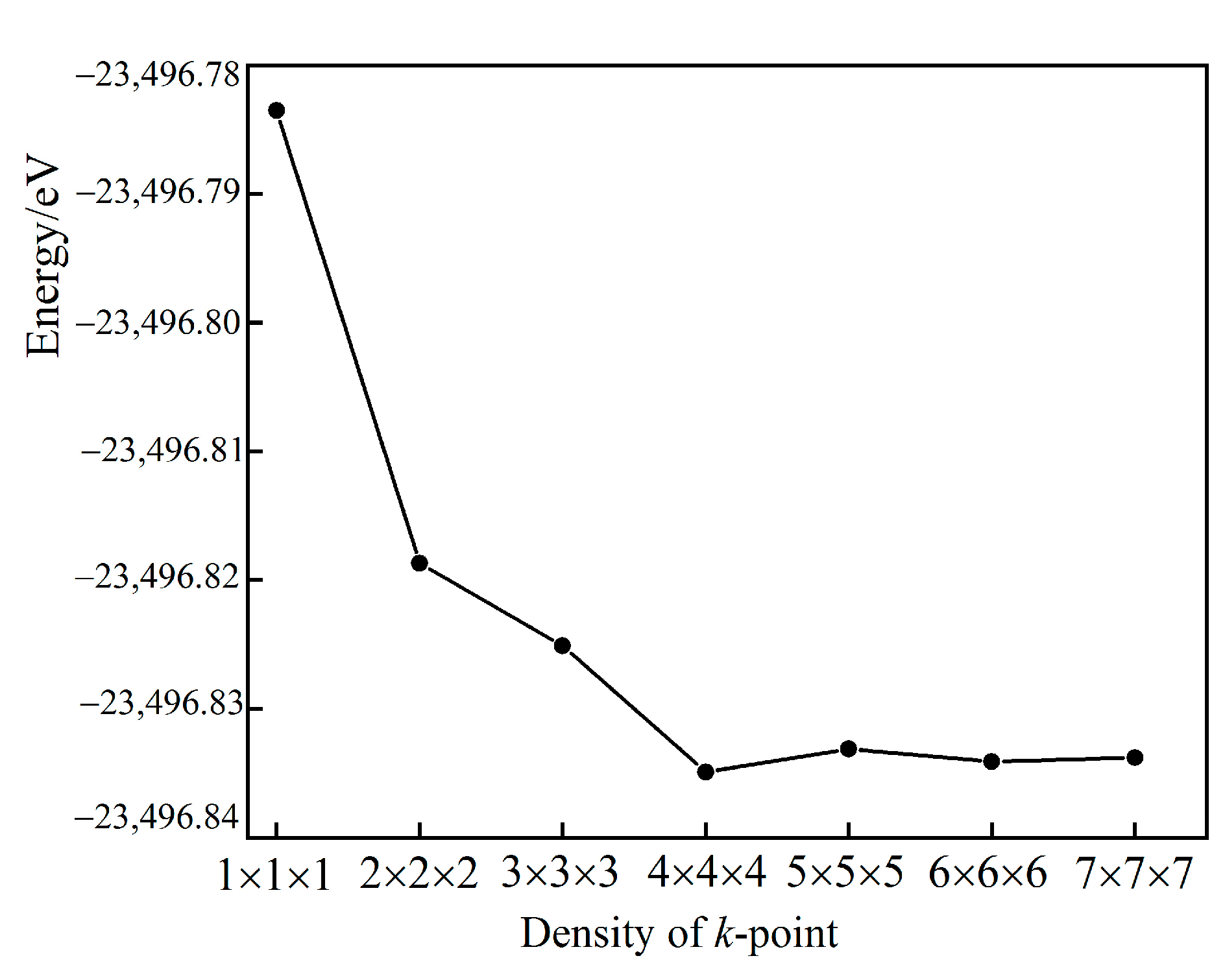

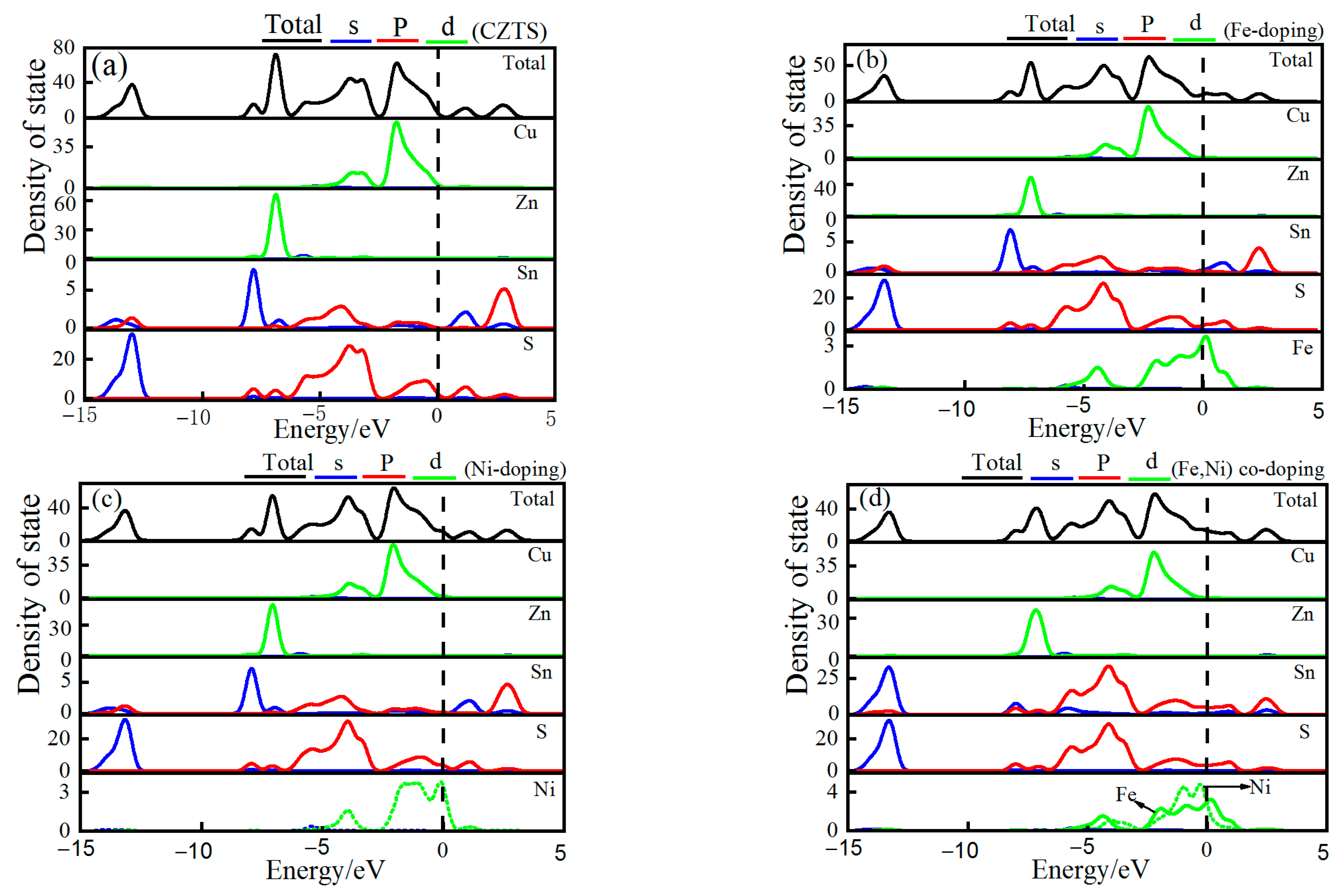
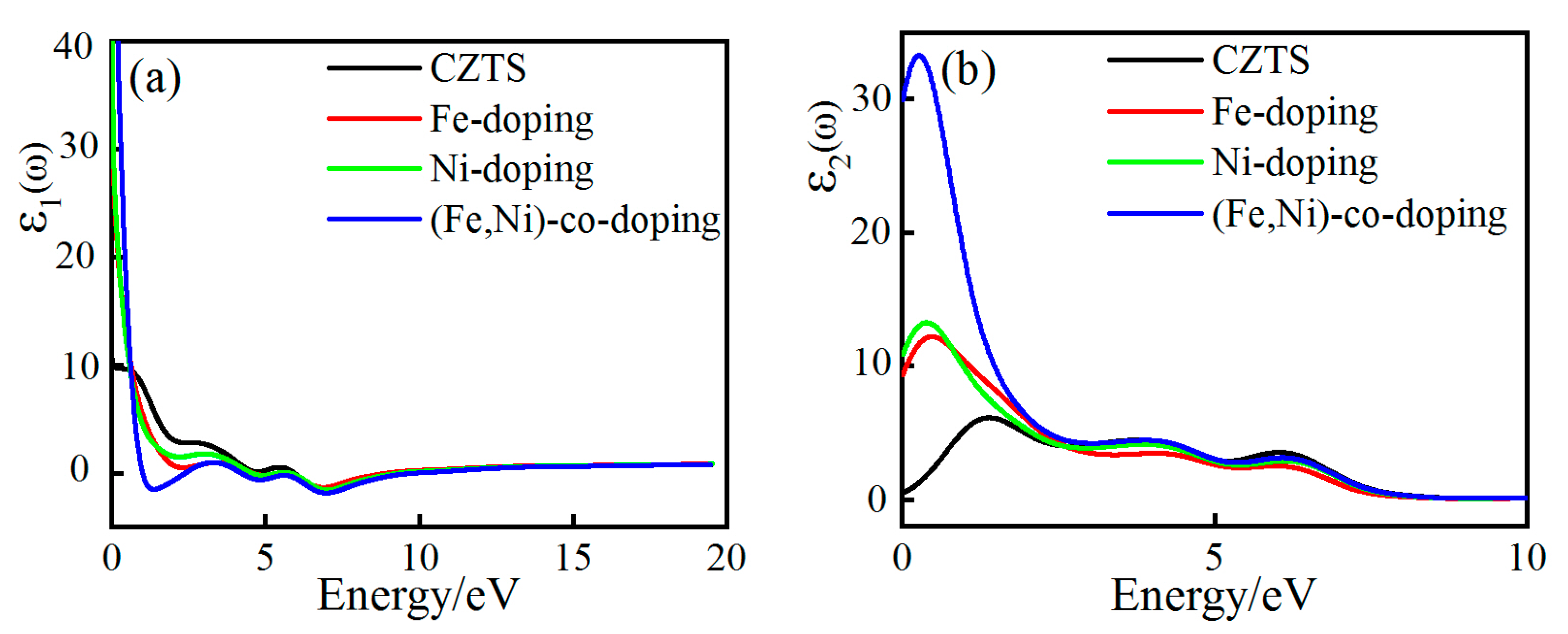

| Sample | a/Å | a% | c/Å | c% | v/Å3 | Formation Energy/eV |
|---|---|---|---|---|---|---|
| Un-doping Cu2ZnSnS4(experiment) [33] | 5.4270 | — | 10.8710 | — | — | — |
| Un-doping Cu2ZnSnS4(caculated) [34] | 5.4710 | — | 10.9440 | — | — | — |
| Geometry optimization Cu2ZnSnS4 | 5.4690 | 0.77% | 10.9460 | 0.69% | 655.0330 | — |
| Fe-doping Cu2ZnSnS4 | 5.4415 | 0.27% | 10.9194 | 0.45% | 647.9225 | 1.00 |
| Ni-doping Cu2ZnSnS4 | 5.4460 | 0.35% | 10.9085 | 0.34% | 647.0102 | 0.58 |
| (Fe,Ni)-co-doping Cu2ZnSnS4 | 5.3665 | −1.11% | 11.1363 | 2.44% | 652.0260 | 0.78 |
| High Symmetry Point | x | y | z |
|---|---|---|---|
| G | 0.000 | 0.000 | 0.000 |
| F | 0.000 | 0.500 | 0.000 |
| Q | 0.000 | 0.500 | 0.500 |
| Z | 0.000 | 0.000 | 0.500 |
| G | 0.000 | 0.000 | 0.000 |
| Sample | Atom | s | p | d | Total | Charge/e |
|---|---|---|---|---|---|---|
| Cu2ZnSnS4 | Zn 1 | 0.42 | 0.94 | 9.98 | 11.34 | 0.66 |
| S 1, S 2, S 3, S 4 | 1.84 | 4.54 | 0 | 6.37 | −0.37 | |
| Cu 1 | 0.6 | 0.64 | 9.81 | 11.06 | −0.06 | |
| S 5, S 6, S 7, S 8 | 1.84 | 4.54 | 0 | 6.37 | −0.37 | |
| Fe-doping Cu2ZnSnS4 | Fe 1 | 0.4 | 0.62 | 6.94 | 7.96 | 0.04 |
| S 1, S 2 | 1.83 | 4.41 | 0 | 6.24 | −0.24 | |
| S 3, S 4 | 1.83 | 4.43 | 0 | 6.26 | −0.26 | |
| Ni-doping Cu2ZnSnS4 | Ni 1 | 0.5 | 0.69 | 8.84 | 10.03 | −0.03 |
| S 1, S 2 | 1.83 | 4.45 | 0 | 6.29 | −0.29 | |
| S 3, S 4 | 1.83 | 4.45 | 0 | 6.28 | −0.28 | |
| (Fe,Ni)-co-doping Cu2ZnSnS4 | Fe 1 | 0.41 | 0.62 | 6.91 | 7.94 | 0.06 |
| S 1, S 2, S 3 | 1.84 | 4.42 | 0 | 6.26 | −0.26 | |
| S 4 | 1.81 | 4.43 | 0 | 6.26 | −0.26 | |
| Ni 2 | 0.48 | 0.66 | 8.91 | 10.05 | −0.05 | |
| S 5, S 6 | 1.83 | 4.49 | 0 | 6.32 | −0.32 | |
| S 7, S 8 | 1.84 | 4.49 | 0 | 6.34 | −0.34 |
| Sample | Bond | Population | Length (Å) |
|---|---|---|---|
| Cu2ZnSnS4 | S 1—Zn 1, S 2 —Zn 1 | 0.40 | 2.3659 |
| S 3—Zn 1, S 4—Zn 1 | 0.41 | 2.3653 | |
| Fe-doping Cu2ZnSnS4 | S 1 Fe 1 | 0.64 | 2.1321 |
| S 2—Fe 1 | 0.64 | 2.1322 | |
| S 3—Fe 1 | 0.60 | 2.1598 | |
| S 4—Fe 1 | 0.60 | 2.1597 | |
| Ni-doping Cu2ZnSnS4 | S 3—Ni 1 | 0.49 | 2.2298 |
| S 4—Ni 1 | 0.49 | 2.2299 | |
| S 1—Ni 1, S 2—Ni 1 | 0.50 | 2.2341 | |
| (Fe,Ni)-co-doping Cu2ZnSnS4 | S 1—Fe 1 | 0.64 | 2.1451 |
| S 2—Fe 1 | 0.64 | 2.1433 | |
| S 3—Fe 1 | 0.58 | 2.1666 | |
| S 4—Fe 1 | 0.58 | 2.1679 | |
| S 5—Ni 2 | 0.47 | 2.2055 | |
| S 6—Ni 2 | 0.47 | 2.2038 | |
| S 7—Ni 2 | 0.48 | 2.2778 | |
| S 8—Ni 2 | 0.48 | 2.2794 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Qin, X.; Yan, W.; Zhang, C.; Zhang, D. Effects of Fe and Ni Doping on the Electronic Structure and Optical Properties of Cu2ZnSnS4. Crystals 2023, 13, 1082. https://doi.org/10.3390/cryst13071082
Yang X, Qin X, Yan W, Zhang C, Zhang D. Effects of Fe and Ni Doping on the Electronic Structure and Optical Properties of Cu2ZnSnS4. Crystals. 2023; 13(7):1082. https://doi.org/10.3390/cryst13071082
Chicago/Turabian StyleYang, Xiufan, Xinmao Qin, Wanjun Yan, Chunhong Zhang, and Dianxi Zhang. 2023. "Effects of Fe and Ni Doping on the Electronic Structure and Optical Properties of Cu2ZnSnS4" Crystals 13, no. 7: 1082. https://doi.org/10.3390/cryst13071082
APA StyleYang, X., Qin, X., Yan, W., Zhang, C., & Zhang, D. (2023). Effects of Fe and Ni Doping on the Electronic Structure and Optical Properties of Cu2ZnSnS4. Crystals, 13(7), 1082. https://doi.org/10.3390/cryst13071082






