One-Dimensional NaSn2F5 Crystals Inside Single-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Investigation of NaSn2F5 Crystals
2.2. Synthesis and Investigation of NaSn2F5@SWCNT Nanocomposite
3. Results and Discussion
3.1. Investigation of NaSn2F5 Crystals
3.1.1. X-ray Diffraction Phase Analysis
3.1.2. Scanning Electron Microscopy
3.2. Investigation of the NaSn2F5@SWCNT Nanocomposite
3.2.1. Transmission Electron Microscopy and Structure Modelling
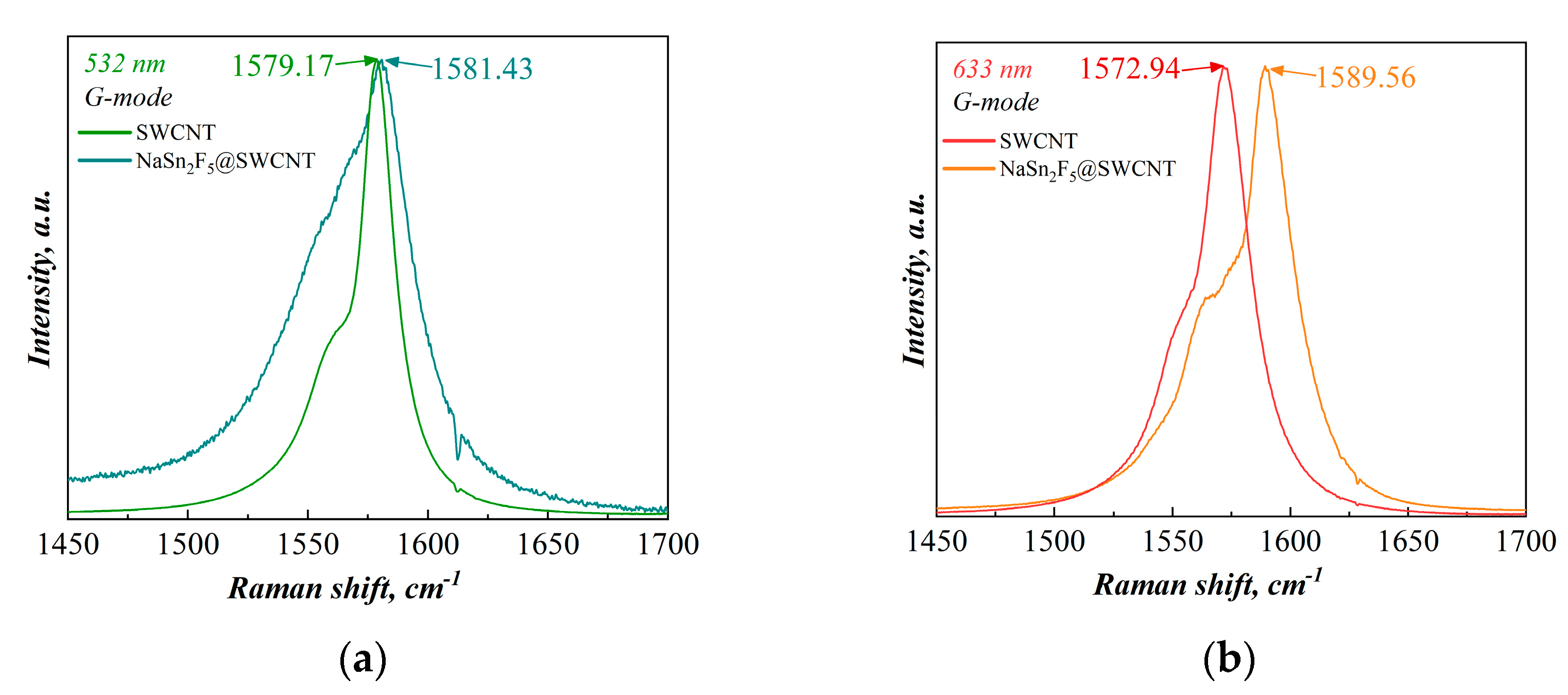
3.2.2. Raman Spectroscopy
- RBM-mode, or first-order mode (radial breathing mode, ~100–350 cm−1), corresponds to radial vibrations of carbon atoms. It is a unique phonon mode, appearing only in carbon nanotubes. It is possible to estimate the diameter distribution of SWCNTs from the positions of the RBM peaks.
- G-mode (tangential C-C stretch mode, ~1550–1600 cm−1) characterizes the sp2 vibrational mode. Unlike graphene/graphite (G~1580 cm−1), the G band of carbon nanotubes consists of several peaks due to twisting effects. The two main components are G+ (oscillations of atoms parallel to the nanotube axis—LO phonon mode) and G- (oscillations of atoms in the direction perpendicular to the axis of the nanotube tangentially to its wall—TO phonon mode) [31,32,33].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monthioux, M. Carbon Meta-Nanotubes: Synthesis, Properties and Applications; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential applications of carbon nanotubes. In Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 13–62. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Applications of filled single-walled carbon nanotubes: Progress, challenges, and perspectives. Nanomaterials 2021, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kim, U.; Yoo, Y.; Byeon, J.; Lee, S.-K.; Nam, J.-S.; Kim, K.; Zhang, Q.; Kauppinen, E.I.; Maruyama, S.; et al. Foldable Perovskite Solar Cells Using Carbon Nanotube-Embedded Ultrathin Polyimide Conductor. Adv. Sci. 2021, 8, 2004092. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sheng, J.; Chen, Y.; Ni, J.; Li, Y. Carbon nanotubes for flexible batteries: Recent progress and future perspective. Natl. Sci. Rev. 2021, 8, nwaa261. [Google Scholar] [CrossRef] [PubMed]
- Halankar, K.K.; Mandal, B.P.; Tyagi, A.K. Superior electrochemical performance of MoS2 decorated on functionalized carbon nanotubes as anode material for sodium ion battery. Carbon Trends 2021, 5, 100103. [Google Scholar] [CrossRef]
- Duong, H.M.; Tran, T.Q.; Kopp, R.; Myint, S.M.; Peng, L. Chapter 1—Direct spinning of horizontally aligned carbon nanotube fibers and films from the floating catalyst method. In Nanotube Superfiber Materials (Second Edition); Schulz, M.J., Shanov, V., Yin, Z., Cahay, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 3–29. [Google Scholar]
- Duong, H.M.; Myint, S.M.; Tran, T.Q.; Le, D.K. Chapter 6—Post-spinning treatments to carbon nanotube fibers. In Carbon Nanotube Fibers and Yarns; Miao, M., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 103–134. [Google Scholar]
- Kamedulski, P.; Lukaszewicz, J.P.; Witczak, L.; Szroeder, P.; Ziolkowski, P. The importance of structural factors for the electrochemical performance of graphene/carbon nanotube/melamine powders towards the catalytic activity of oxygen reduction reaction. Materials 2021, 14, 2448. [Google Scholar] [CrossRef]
- Hu, Z.; Breeze, B.; Kashtiban, R.; Sloan, J.; Lloyd-Hughes, J. Zigzag HgTe nanowires modify the electron–phonon interaction in chirality-refined single-walled carbon nanotubes. ACS Nano 2022, 16, 6789–6800. [Google Scholar] [CrossRef]
- Faulques, E.; Kalashnyk, N.; Slade, C.A.; Sanchez, A.M.; Sloan, J.; Ivanov, V.G. Vibrational and electronic structures of tin selenide nanowires confined inside carbon nanotubes. Synth. Met. 2022, 284, 116968. [Google Scholar] [CrossRef]
- Vorfolomeeva, A.A.; Pushkarevsky, N.A.; Koroteev, V.O.; Surovtsev, N.V.; Chuvilin, A.L.; Shlyakhova, E.V.; Plyusnin, P.E.; Makarova, A.A.; Okotrub, A.V.; Bulusheva, L.G. Doping of carbon nanotubes with encapsulated phosphorus chains. Inorg. Chem. 2022, 61, 9605–9614. [Google Scholar] [CrossRef]
- Cao, K.; Biskupek, J.; Stoppiello, C.T.; McSweeney, R.L.; Chamberlain, T.W.; Liu, Z.; Suenaga, K.; Skowron, S.T.; Besley, E.; Khlobystov, A.N.; et al. Atomic mechanism of metal crystal nucleus formation in a single-walled carbon nanotube. Nat. Chem. 2020, 12, 921–928. [Google Scholar] [CrossRef]
- Fan, X.; Dickey, E.C.; Eklund, P.C.; Williams, K.A.; Grigorian, L.; Buczko, R.; Pantelides, S.T.; Pennycook, S.J. Atomic arrangement of iodine atoms inside single-walled carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4621–4624. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gómez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, M.; Hayashi, T.; Endo, M.; Young Hong, S.; et al. Conducting linear chains of sulphur inside carbon nanotubes. Nat. Commun. 2013, 4, 2162. [Google Scholar] [CrossRef] [PubMed]
- Lusk, M.T.; Hamm, N. Ab initio study of toroidal carbon nanotubes with encapsulated atomic metal loops. Phys. Rev. B 2007, 76, 125422. [Google Scholar] [CrossRef]
- Medeiros, P.V.C.; Marks, S.; Wynn, J.M.; Vasylenko, A.; Ramasse, Q.M.; Quigley, D.; Sloan, J.; Morris, A.J. Single-atom scale structural selectivity in Te nanowires encapsulated inside ultranarrow single-walled carbon nanotubes. ACS Nano 2017, 11, 6178–6185. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.; Kirkland, A.I.; Hutchison, J.L.; Green, M.L.H. Integral atomic layer architectures of 1D crystals inserted into single walled carbon nanotubes. Chem. Commun. 2002, 13, 1319–1332. [Google Scholar] [CrossRef]
- Anuchin, N.M.; Enyashin, A.N. Electronic and mechanical properties of endohedral composites of carbon nanotubes with potassium iodide: DFT study. J. Struct. Chem. 2023, 64, 662–673. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Falaleev, N.S.; Verbitskiy, N.I.; Volykhov, A.A.; Yashina, L.V.; Kumskov, A.S.; Zhigalina, V.G.; Vasiliev, A.L.; Lukashin, A.V.; Sloan, J.; et al. Size-dependent structure relations between nanotubes and encapsulated nanocrystals. Nano Lett. 2017, 17, 805–810. [Google Scholar] [CrossRef]
- Kashtiban, R.J.; Patrick, C.E.; Ramasse, Q.; Walton, R.I.; Sloan, J. Picoperovskites: The smallest conceivable isolated halide perovskite structures formed within carbon nanotubes. Adv. Mater. 2023, 35, 2208575. [Google Scholar] [CrossRef]
- Zakalyukin, R.M.; Levkevich, E.A.; Kumskov, A.S.; Orekhov, A.S. Superionic conductor PbSnF4 in the inner channel of SWNT. AIP Conf. Proc. 2018, 1957, 030001. [Google Scholar] [CrossRef]
- Zakalyukin, R.M.; Levkevich, E.A.; Kumskov, A.S. Nanocomposite E(PbSnF4;SnF2)@SWCNT—Promising material for fluoride-ion batteries. Ceram. Int. 2022, 48, 26565–26574. [Google Scholar] [CrossRef]
- Zakalyukin, R.M.; Mavrin, B.N.; Dem’yanets, L.N.; Kiselev, N.A. Synthesis and characterization of single-walled carbon nanotubes filled with the superionic material SnF2. Carbon 2008, 46, 1574–1578. [Google Scholar] [CrossRef]
- Zakalyukin, R.M.; Demyanets, L.; Kiselev, N.; Kumskov, A.S.; Kislov, M.; Krestinin, A.; Hutchison, J. One-dimensional SnF2 single crystals in the inner channels of single-wall carbon nanotubes: I. Preparation and basic characterization. Crystallogr. Rep. 2010, 55, 507–512. [Google Scholar] [CrossRef]
- Zakalyukin, R.M.; Demyanets, L.; Kiselev, N. One-Dimensional SnF2 single crystals in the inner channels of single-wall carbon nanotubes: II. Structure and nanocomposite construction modeling. Crystallogr. Rep. 2010, 55, 688–694. [Google Scholar] [CrossRef]
- Donaldson, J.D.; O’Donoghue, J.D. Complex tin(II) fluorides. J. Chem. Soc. 1964, 44, 271–275. [Google Scholar] [CrossRef]
- McDonald, R.; Larson, A.; Cromer, D.T. The crystal structure of sodium pentafluorodistannate (II), NaSn2F5. Acta Crystallogr. 1964, 17, 1104–1108. [Google Scholar] [CrossRef]
- Zakalyukin, R.M.; Levkevich, E.A.; Nikolaeva, A.V. Synthesis and X-ray-graphical characteristics of the MeSn2F5 (Me = Na, K, Rb, Cs) fluoride-ion conductors. Fine Chem. Technol. 2021, 16, 426–437. [Google Scholar] [CrossRef]
- Birchall, T.; Dénès, G. A 19F, 119Sn nuclear magnetic resonance and 119Sn Mössbauer study of the SnF2–MF–H2O system. Can. J. Chem. 1984, 62, 591–595. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Eklund, P.C. Phonons in carbon nanotubes. Adv. Phys. 2000, 49, 705–814. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Kojima, N.; Maniwa, Y.; Umezu, I.; Masubuchi, S.; Kazama, S.; Zhao, X.; Ando, Y.; Ohtsuka, Y.; et al. Optical absorption and resonance Raman scattering of carbon nanotubes. AIP Conf. Proc. 1999, 486, 328–332. [Google Scholar] [CrossRef]
- Jorio, A.; Dresselhaus, G.; Dresselhaus, M.S.; Souza, M.; Dantas, M.S.S.; Pimenta, M.A.; Rao, A.M.; Saito, R.; Liu, C.; Cheng, H.M. Polarized Raman study of single-wall semiconducting carbon nanotubes. Phys. Rev. Lett. 2000, 85, 2617–2620. [Google Scholar] [CrossRef] [PubMed]
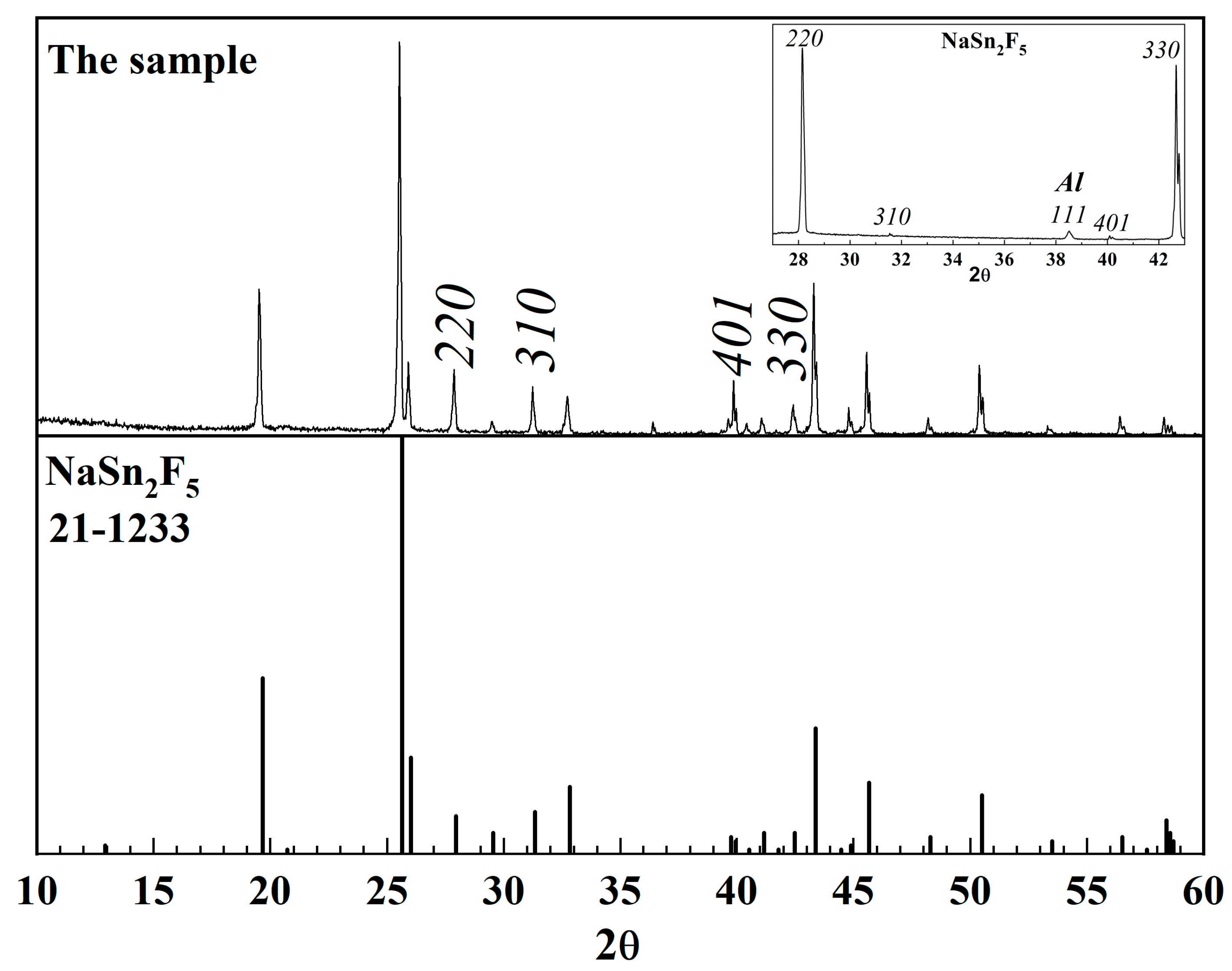
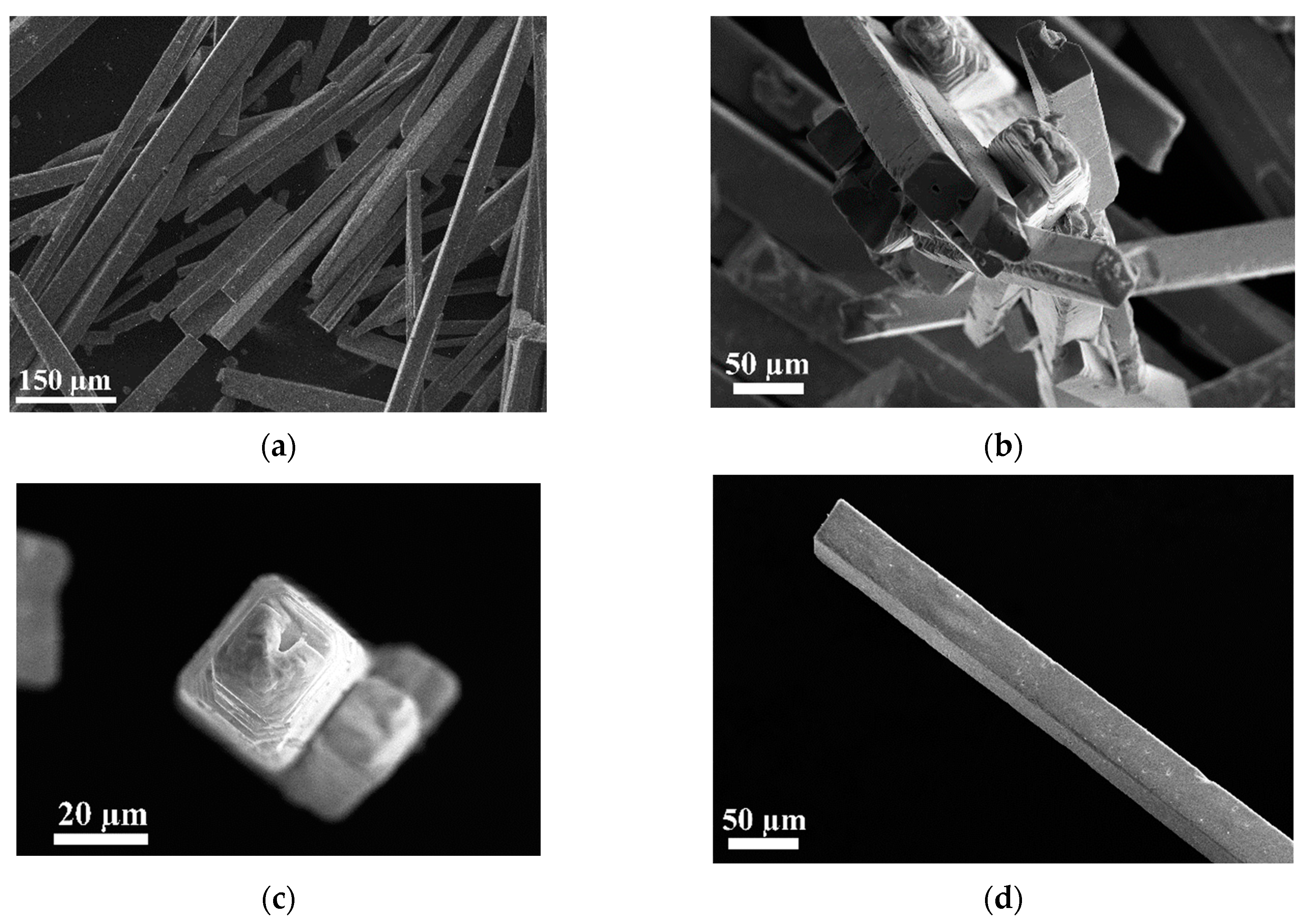
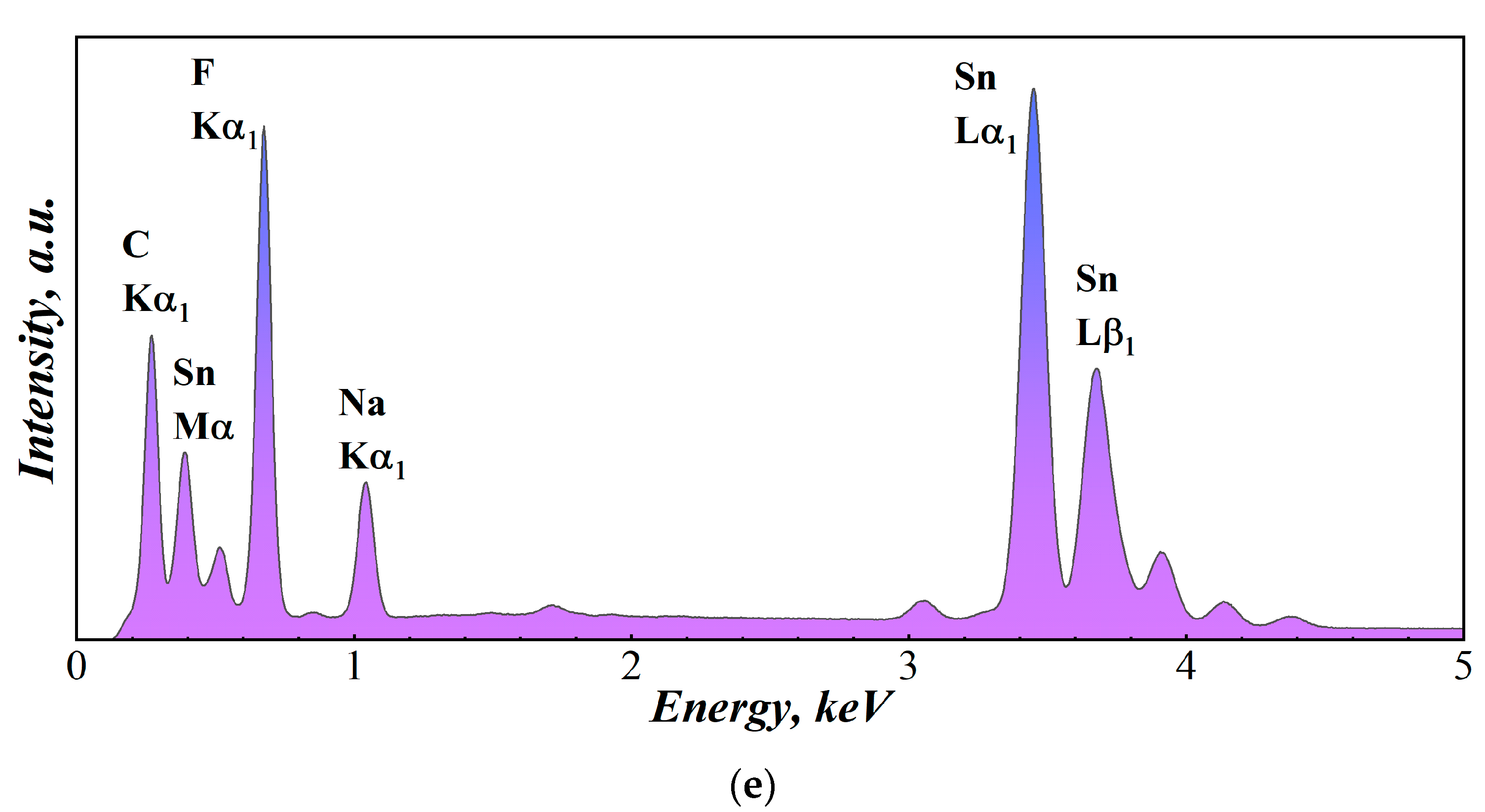
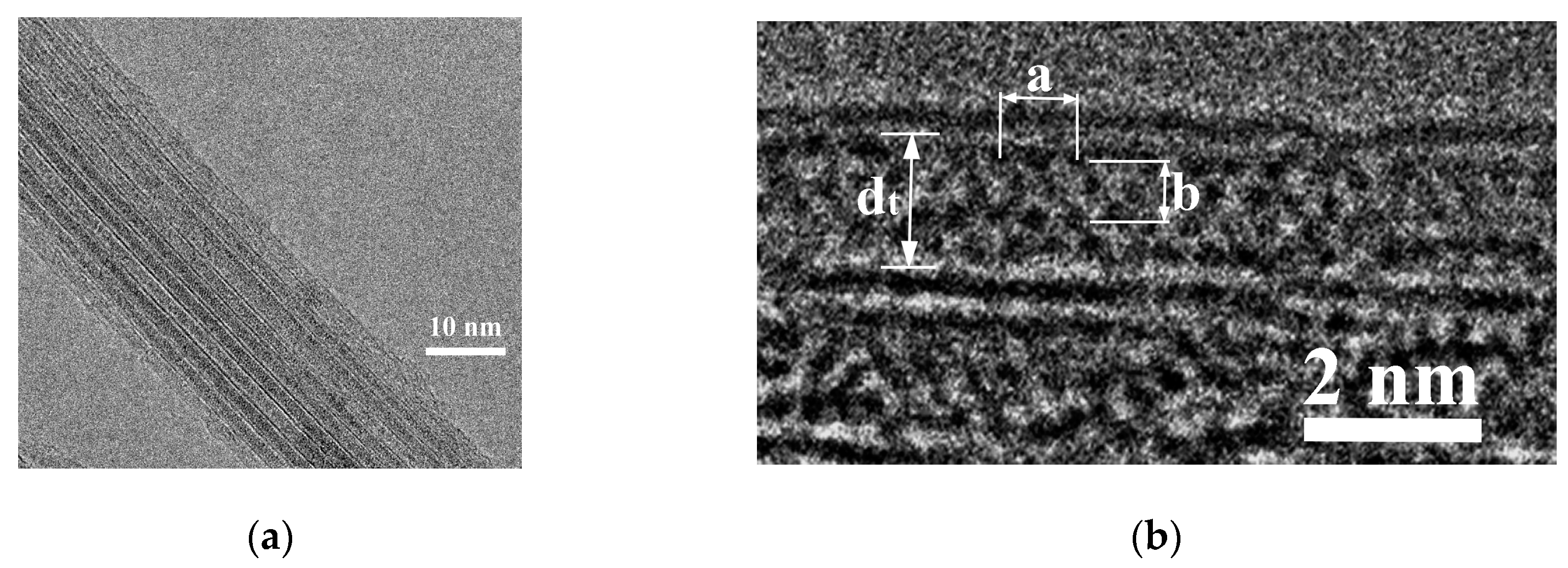
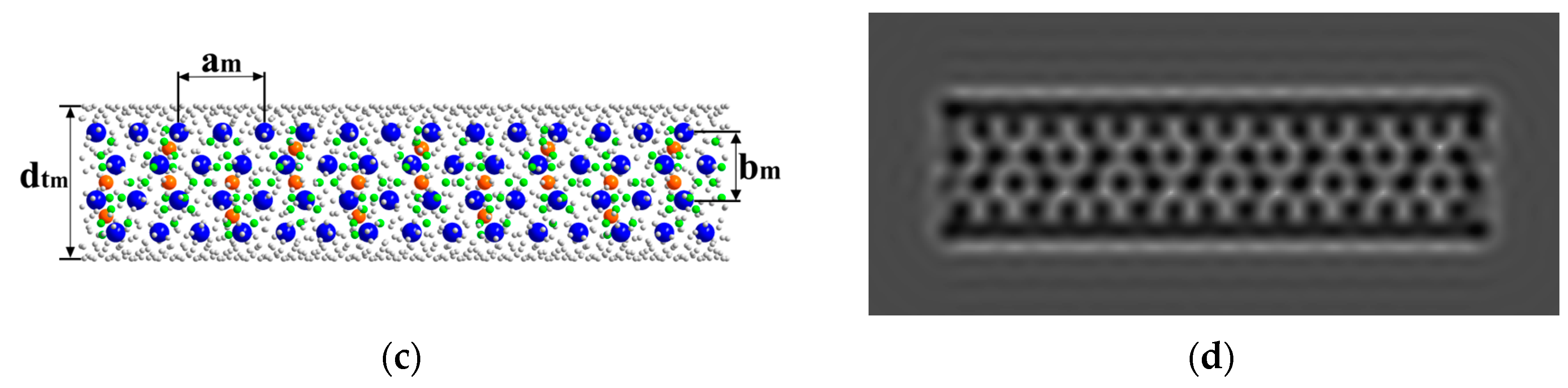
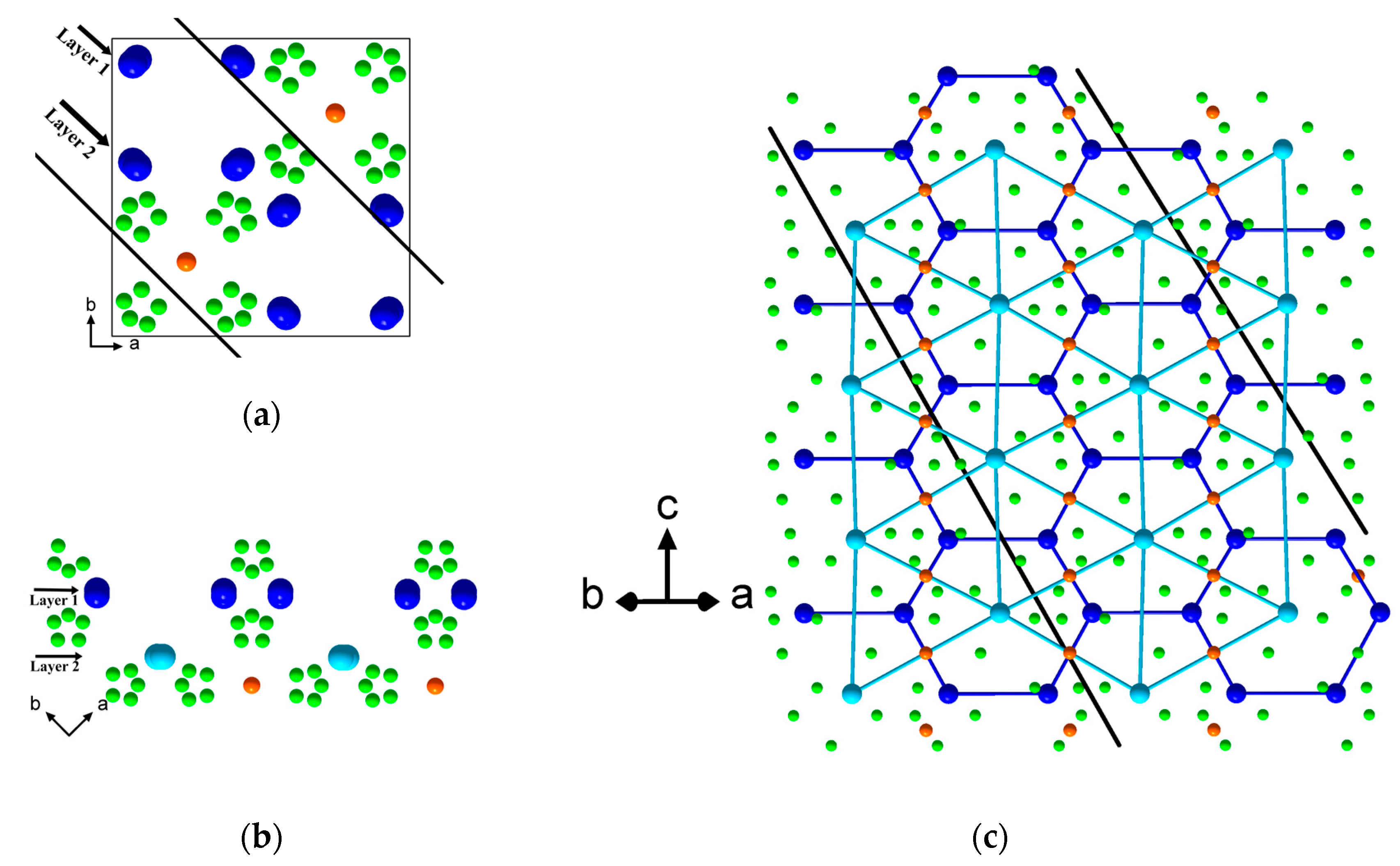

| № | 2θ | d, Å | Qexp | I/Imax | h k l | Qcalc | ΔQ |
|---|---|---|---|---|---|---|---|
| 1 | 19.593 | 4.5272 | 487.91 | 33 | 2 0 0 | 490.03 | −2.12 |
| 2 | 20.582 | 4.3118 | 537.88 | 4 | 2 0 1 | 543.22 | −5.34 |
| 3 | 25.532 | 3.4860 | 822.90 | 100 | 2 1 2 | 825.31 | −2.41 |
| 4 | 25.918 | 3.4349 | 847.56 | 14 | 0 0 4 | 851.10 | −3.54 |
| 5 | 27.867 | 3.1990 | 977.17 | 15 | 2 2 0 | 980.05 | −2.88 |
| 6 | 29.512 | 3.0243 | 1093.33 | 5 | 2 1 3 | 1091.28 | 2.05 |
| 7 | 31.264 | 2.8587 | 1223.67 | 12 | 3 1 0 | 1225.07 | −1.40 |
| 8 | 32.741 | 2.7330 | 1338.82 | 15 | 2 0 4 | 1341.13 | −2.31 |
| 9 | 39.672 | 2.2701 | 1940.48 | 5 | 2 1 5 | 1942.38 | −1.90 |
| 10 | 39.869 | 2.2593 | 1959.08 | 3 | 4 0 0 | 1960.11 | −1.03 |
| 11 | 40.422 | 2.2297 | 2011.44 | 2 | 4 0 1 | 2013.30 | −1.86 |
| 12 | 41.085 | 2.1952 | 2075.16 | 7 | 3 1 4 | 2076.17 | −1.01 |
| 13 | 42.405 | 2.1299 | 2204.36 | 8 | 3 3 0 | 2205.12 | −0.77 |
| 14 | 43.311 | 2.0874 | 2295.03 | 51 | 4 1 2 | 2295.39 | −0.36 |
| 15 | 44.836 | 2.0199 | 2450.98 | 4 | 4 2 0 | 2450.13 | 0.85 |
| 16 | 45.579 | 1.9887 | 2528.49 | 25 | 2 1 6 | 2527.51 | 0.98 |
| 17 | 48.213 | 1.8860 | 2811.36 | 6 | 4 0 4 | 2811.21 | 0.15 |
| 18 | 50.427 | 1.8082 | 3058.49 | 23 | 3 3 4 | 3056.22 | 2.27 |
| 19 | 53.426 | 1.7136 | 3405.50 | 3 | 0 0 8 | 3404.41 | 1.09 |
| 20 | 56.443 | 1.6289 | 3768.87 | 4 | 5 2 2 | 3765.47 | 3.40 |
| Laser | SWCNT | NaSn2F5@SWCNT | ||
|---|---|---|---|---|
| RBM, cm−1 | dt, nm | RBM, cm−1 | dt, nm | |
| 532 nm (2.33 eV) | 118.48 | 2.16 | 136.95 | 1.84 |
| 136.89 | 1.84 | 166.29 | 1.50 | |
| 149.28 | 1.68 | 203.56 | 1.21 | |
| 168.19 | 1.48 | 241.74 | 1.01 | |
| 633 nm (1.96 eV) | 116.55 | 2.20 | 116.32 | 2.20 |
| 131.60 | 1.92 | 134.10 | 1.89 | |
| 145.03 | 1.73 | 161.64 | 1.54 | |
| Laser | G Mode | SWCNT | NaSn2F5@SWCNT | ΔG, cm−1 |
|---|---|---|---|---|
| 532 nm (2.33 eV) | G+, cm−1 | 1579.17 | 1581.43 | +2.26 |
| G−, cm−1 | 1557.86 | 1553.67 | −4.19 | |
| 633 nm (1.96 eV) | G+, cm−1 | 1572.94 | 1589.56 | +16.62 |
| G−, cm−1 | 1550.06 | 1563.86 | +13.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakalyukin, R.M.; Levkevich, E.A.; Kumskov, A.S. One-Dimensional NaSn2F5 Crystals Inside Single-Walled Carbon Nanotubes. Crystals 2023, 13, 1065. https://doi.org/10.3390/cryst13071065
Zakalyukin RM, Levkevich EA, Kumskov AS. One-Dimensional NaSn2F5 Crystals Inside Single-Walled Carbon Nanotubes. Crystals. 2023; 13(7):1065. https://doi.org/10.3390/cryst13071065
Chicago/Turabian StyleZakalyukin, Ruslan M., Ekaterina A. Levkevich, and Andrey S. Kumskov. 2023. "One-Dimensional NaSn2F5 Crystals Inside Single-Walled Carbon Nanotubes" Crystals 13, no. 7: 1065. https://doi.org/10.3390/cryst13071065
APA StyleZakalyukin, R. M., Levkevich, E. A., & Kumskov, A. S. (2023). One-Dimensional NaSn2F5 Crystals Inside Single-Walled Carbon Nanotubes. Crystals, 13(7), 1065. https://doi.org/10.3390/cryst13071065






