The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Calcium Oxalate Extraction Protocols
2.3. Experimental Techniques Applied for Phytolith Characterization
2.3.1. Flame Atomic Absorption Spectroscopy (FAAS)
2.3.2. X-ray Fluorescence (XRF)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. 13C-Nuclear Magnetic Resonance (NMR)
3. Results and Discussion
3.1. Bulk Chemical Composition of Plant Materials
3.2. Mineralogical Composition of Plant Materials
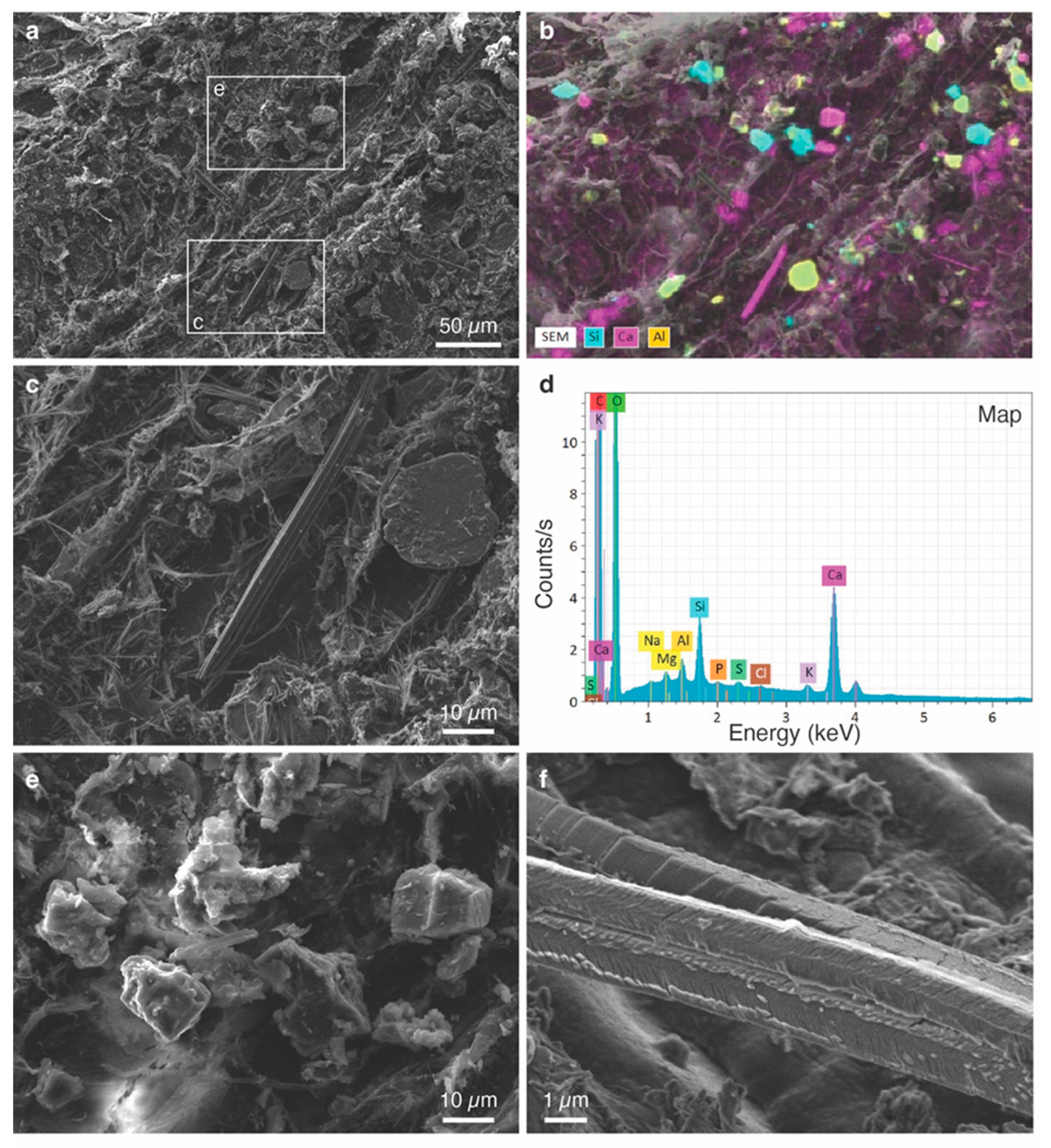
3.3. Characterization of Individual Phytoliths
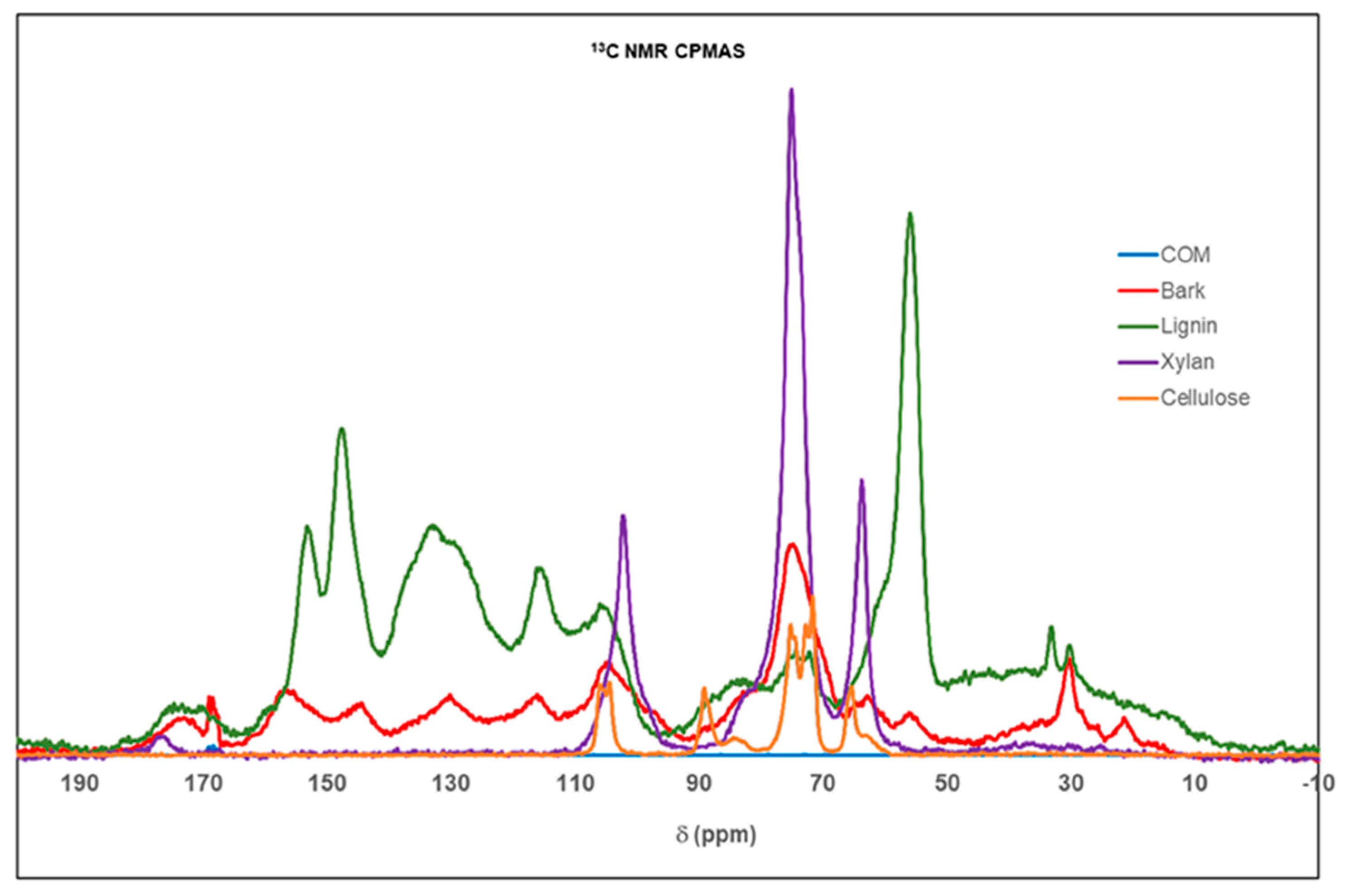
3.4. 13C-NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, R.-J.; Luan, S. Regulation of Calcium and Magnesium Homeostasis in Plants: From Transporters to Signaling Network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. CALCIUM OXALATE IN PLANTS: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Pérez Cuadra, V.; Hermann, P. Characterization and Macropattern of Calcium Oxalate Phytoliths in Argentinean Endemic Species of Chenopodioideae (Amaranthaceae). Quat. Int. 2013, 287, 83–88. [Google Scholar] [CrossRef]
- Crutcher, E.; Crutcher, H. Calcium Oxalate Phytoliths in Environmental Samples 1. Microscope 2020, 67, 3–11. [Google Scholar]
- Franceschi, V.R.; Horner, H.T. Calcium Oxalate Crystals in Plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Gębura, J.; Winiarczyk, K. A Study on Calcium Oxalate Crystals in Tinantia Anomala (Commelinaceae) with Special Reference to Ultrastructural Changes during Anther Development. J. Plant Res. 2016, 129, 685–695. [Google Scholar] [CrossRef]
- Stephens, W.E. Whewellite and Its Key Role in Living Systems. Geol. Today 2012, 28, 180–185. [Google Scholar] [CrossRef]
- Vigliaturo, R.; Kehrli, D.; Garra, P.; Dieterlen, A.; Trouvé, G.; Dietze, V.; Wilson, J.P.; Gieré, R. Opaline Phytoliths in Miscanthus Sinensis and Its Cyclone Ash from a Biomass-Combustion Facility. Ind. Crops Prod. 2019, 139, 111539. [Google Scholar] [CrossRef]
- DataCite Search. Available online: https://search.datacite.org/works/10.17632/w86x7fbdhz (accessed on 30 May 2023).
- Golyeva, A. Biomorphic Analysis as a Part of Soil Morphological Investigations. CATENA 2001, 43, 217–230. [Google Scholar] [CrossRef]
- Madella, M.; Lancelotti, C.; Osterrieth, M. Comprehensive Perspectives on Phytolith Studies in Quaternary Research. Quat. Int. 2013, 287, 1–2. [Google Scholar] [CrossRef]
- Pető, Á. Studying Modern Soil Profiles of Different Landscape Zones in Hungary: An Attempt to Establish a Soil-Phytolith Identification Key. Quat. Int. 2013, 287, 149–161. [Google Scholar] [CrossRef]
- Leszczuk, A.; Wydrych, J.; Szczuka, E. The Occurrence of Calcium Oxalate Crystals and Distribution of Arabinogalactan Proteins (AGPs) in Ovary Cells During Fragaria x Ananassa (Duch.) Development. J. Plant Growth Regul. 2019, 38, 1028–1036. [Google Scholar] [CrossRef]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Alarm Photosynthesis: Calcium Oxalate Crystals as an Internal CO2 Source in Plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef]
- Tütüncü Konyar, S.; Öztürk, N.; Dane, F. Occurrence, Types and Distribution of Calcium Oxalate Crystals in Leaves and Stems of Some Species of Poisonous Plants. Bot. Stud. 2014, 55, 32. [Google Scholar] [CrossRef] [PubMed]
- Calmés, J.; Piquemal, M. Variation Saisonnière Des Cristaux d’oxalate de Calcium Des Tissus de Vigne Vierge. Can. J. Bot. 1977, 55, 2075–2078. [Google Scholar] [CrossRef]
- Webb, M.A. Cell-Mediated Crystallization of Calcium Oxalate in Plants. Plant Cell 1999, 11, 751–761. [Google Scholar] [CrossRef]
- Arnott, H.J.; Webb, M.A. Twinned Raphides of Calcium Oxalate in Grape (Vitis): Implications for Crystal Stability and Function. Int. J. Plant Sci. 2000, 161, 133–142. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Wu, J.; Huang, Z.; Chang, S.X.; Jiang, P. Intensive Management Increases Phytolith-Occluded Carbon Sequestration in Moso Bamboo Plantations in Subtropical China. Forests 2019, 10, 883. [Google Scholar] [CrossRef]
- Rajendiran, S.; Coumar, M.V.; Kundu, S.; Dotaniya, M.; Rao, A. Role of Phytolith Occluded Carbon of Crop Plants for Enhancing Soil Carbon Sequestration in Agro-Ecosystems. Curr. Sci. 2012, 103, 911–920. [Google Scholar]
- Verrecchia, E.P.; Braissant, O.; Cailleau, G. The Oxalate–Carbonate Pathway in Soil Carbon Storage: The Role of Fungi and Oxalotrophic Bacteria. In Fungi in Biogeochemical Cycles; Gadd, G.M., Ed.; British Mycological Society Symposia; Cambridge University Press: Cambridge, UK, 2006; pp. 289–310. ISBN 978-0-521-84579-3. [Google Scholar]
- Sun, X.; Liu, Q.; Zhao, G.; Chen, X.; Tang, T.; Xiang, Y. Comparison of Phytolith-Occluded Carbon in 51 Main Cultivated Rice (Oryzasativa) Cultivars of China. RSC Adv. 2017, 7, 54726–54733. [Google Scholar] [CrossRef]
- Minocha, R.; Chamberlain, B.; Long, S.; Turlapati, S.A.; Quigley, G. Extraction and Estimation of the Quantity of Calcium Oxalate Crystals in the Foliage of Conifer and Hardwood Trees. Tree Physiol. 2015, 35, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.F.; Dolic, V.; Lancaster, G.; Boyd, W.E. A Microwave Digestion Method for the Extraction of Phytoliths from Herbarium Specimens. Rev. Palaeobot. Palynol. 2001, 116, 203–212. [Google Scholar] [CrossRef]
- Webb, M.A.; Cavaletto, J.M.; Carpita, N.C.; Lopez, L.E.; Arnott, H.J. The Intravacuolar Organic Matrix Associated with Calcium Oxalate Crystals in Leaves of Vitis. Plant J. 1995, 7, 633–648. [Google Scholar] [CrossRef]
- Subashini, S.; Sathishkumar, K. Physico-Chemical Characteristics and Thermal Stability of Calcium Oxalate Crystals Isolated from Beta Vulgaris Root. J. Environ. Biol. 2019, 40, 775–783. [Google Scholar] [CrossRef]
- Ragland, K.W.; Aerts, D.J.; Baker, A.J. Properties of Wood for Combustion Analysis. Bioresour. Technol. 1991, 37, 161–168. [Google Scholar] [CrossRef]
- Yu, C.; Thy, P.; Wang, L.; Anderson, S.N.; VanderGheynst, J.S.; Upadhyaya, S.K.; Jenkins, B.M. Influence of Leaching Pretreatment on Fuel Properties of Biomass. Fuel Process. Technol. 2014, 128, 43–53. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Dhahak, A.; Bounaceur, R.; Le Dreff-Lorimier, C.; Schmidt, G.; Trouve, G.; Battin-Leclerc, F. Development of a Detailed Kinetic Model for the Combustion of Biomass. Fuel 2019, 242, 756–774. [Google Scholar] [CrossRef]
- Orfão, J.J.M.; Antunes, F.J.A.; Figueiredo, J.L. Pyrolysis Kinetics of Lignocellulosic Materials—Three Independent Reactions Model. Fuel 1999, 78, 349–358. [Google Scholar] [CrossRef]
- El May, Y.; Jeguirim, M.; Dorge, S.; Trouvé, G.; Said, R. Study on the Thermal Behavior of Different Date Palm Residues: Characterization and Devolatilization Kinetics under Inert and Oxidative Atmospheres. Energy 2012, 44, 702–709. [Google Scholar] [CrossRef]
- Popova, E.; Chernov, A.; Maryandyshev, P.; Brillard, A.; Kehrli, D.; Trouvé, G.; Lyubov, V.; Brilhac, J.-F. Thermal Degradations of Wood Biofuels, Coals and Hydrolysis Lignin from the Russian Federation: Experiments and Modeling. Bioresour. Technol. 2016, 218, 1046–1054. [Google Scholar] [CrossRef]
- Likar, M.; Vogel-Mikuš, K.; Potisek, M.; Hančević, K.; Radić, T.; Nečemer, M.; Regvar, M. Importance of Soil and Vineyard Management in the Determination of Grapevine Mineral Composition. Sci. Total Environ. 2015, 505, 724–731. [Google Scholar] [CrossRef]
- Rabaçal, M.; Fernandes, U.; Costa, M. Combustion and Emission Characteristics of a Domestic Boiler Fired with Pellets of Pine, Industrial Wood Wastes and Peach Stones. Renew. Energy 2013, 51, 220–226. [Google Scholar] [CrossRef]
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Beech (#797). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Beech (#2142). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Fir (#791). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Spruce (#163). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Spruce (#2402). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Scurfield, G.; Michell, A.J.; Silva, S.R. Crystals in Woody Stems. Bot. J. Linn. Soc. 1973, 66, 277–289. [Google Scholar] [CrossRef]
- Weiner, S.; Pinkas, I.; Kossoy, A.; Feldman, Y. (Isai) Calcium Sulfate Hemihydrate (Bassanite) Crystals in the Wood of the Tamarix Tree. Minerals 2021, 11, 289. [Google Scholar] [CrossRef]
- Hourlier, D. Thermal Decomposition of Calcium Oxalate: Beyond Appearances. J. Therm. Anal. Calorim. 2019, 136, 2221–2229. [Google Scholar] [CrossRef]
- Szekely, A.; Varhegyi, G.; Till, F.; Szabo, P.; Jakab, E. The effects of Heat and mass Transport on the Results of Thermal Decomposition Studies. J. Anal. Appl. Pyrolysis 1987, 11, 83–92. [Google Scholar] [CrossRef]
- Aquilano, D.; Franchini-Angela, M. Twin Laws of Whewellite, CaC2O4·H2O. A Structural and Growth Approach. Phys. Chem. Miner. 1981, 7, 124–129. [Google Scholar] [CrossRef]
- Wooten, J.B. 13C CPMAS NMR of Bright and Burley Tobaccos. J. Agric. Food Chem. 1995, 43, 2858. [Google Scholar] [CrossRef]






| Trunk | Root | Bark | Bark Calcined at 250 °C | Bark Calcined at 300 °C | Bark Calcined at 350 °C | |
|---|---|---|---|---|---|---|
| Organic component | 98.0 | 96.6 | 95.0 | 89.1 | 82.7 | 49.8 |
| Na2O | b.d.a | 0.05 | b.d.a | 0.16 | 0.57 | 1.62 |
| MgO | 0.08 | 0.08 | 0.17 | 1.08 | 1.77 | 4.61 |
| Al2O3 | 0.02 | 0.23 | 0.08 | 0.91 | 3.51 | 10.46 |
| SiO2 | 0.04 | 0.41 | 0.17 | 0.92 | 3.71 | 11.68 |
| P2O5 | 0.04 | 0.2 | 0.02 | 0.08 | 0.55 | 1.55 |
| K2O | 0.44 | 0.20 | 0.31 | 1.00 | 1.15 | 2.89 |
| CaO | 0.34 | 1.33 | 3.81 | 6.89 | 6.29 | 18.48 |
| TiO2 | 0.03 | 0.07 | b.d.a | b.d.a | 0.05 | 0.18 |
| Cr2O3 | b.d.a | b.d. | b.d.a | b.d.a | 0.08 | b.d.a |
| MnO | b.d.a | b.d. | b.d.a | 0.10 | 0.09 | 0.28 |
| Fe2O3 | b.d.a | 0.11 | 0.06 | 0.20 | 1.06 | 2.26 |
| Ni | b.d.a | b.d. | b.d.a | b.d.a | b.d.a | 0.02 |
| Cu | b.d.a | 0.008 | b.d.a | 0.03 | 0.04 | 0.08 |
| Zn | 0.003 | 0.009 | 0.006 | 0.02 | 0.08 | 0.23 |
| Rb | b.d.a | b.d.a | b.d.a | b.d.a | 0.005 | 0.009 |
| Sr | b.d.a | 0.004 | 0.009 | 0.02 | 0.02 | 0.05 |
| Pb | b.d.a | b.d. | b.d. | b.d.a | 0.006 | 0.008 |
| Zr | 0.78 | 0.36 | 0.30 | b.d.a | b.d.a | b.d.a |
| S | 0.02 | 0.08 | 0.04 | 0.14 | 0.27 | 0.71 |
| Cl | 0.01 | 0.009 | 0.02 | 0.04 | b.d.a | 0.08 |
| Calcium oxalate monohydrate b | 0.9 | 3.5 | 9.9 | 17.9 | 16.4 | 48.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trouvé, G.; Michelin, L.; Kehrli, D.; Josien, L.; Rigolet, S.; Lebeau, B.; Gieré, R. The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination. Crystals 2023, 13, 967. https://doi.org/10.3390/cryst13060967
Trouvé G, Michelin L, Kehrli D, Josien L, Rigolet S, Lebeau B, Gieré R. The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination. Crystals. 2023; 13(6):967. https://doi.org/10.3390/cryst13060967
Chicago/Turabian StyleTrouvé, Gwenaëlle, Laure Michelin, Damaris Kehrli, Ludovic Josien, Séverinne Rigolet, Bénédicte Lebeau, and Reto Gieré. 2023. "The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination" Crystals 13, no. 6: 967. https://doi.org/10.3390/cryst13060967
APA StyleTrouvé, G., Michelin, L., Kehrli, D., Josien, L., Rigolet, S., Lebeau, B., & Gieré, R. (2023). The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination. Crystals, 13(6), 967. https://doi.org/10.3390/cryst13060967








