Kinetics and Mechanism of BaLaCuS3 Oxidation
Abstract
1. Introduction
2. Materials and Synthesis
3. Characterization
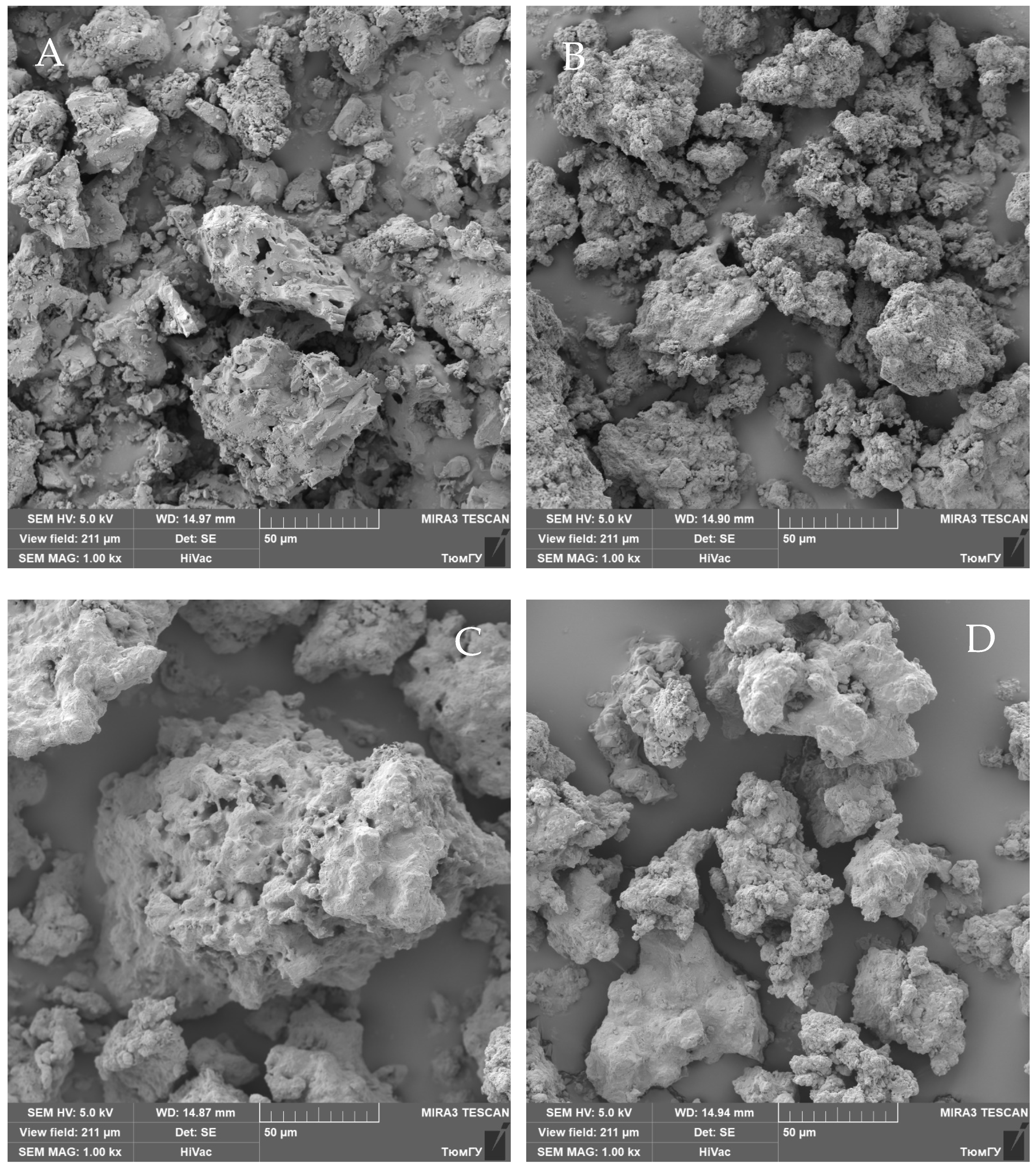
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fthenakis, V.M.; Kim, H.C. CdTe photovoltaics: Life cycle environmental profile and comparisons. Thin Solid Film. 2007, 515, 5961–5963. [Google Scholar] [CrossRef]
- Fraboni, B.; Piana, E.; Cesca, T.; Gasparotto, A.; Longo, M.; Jakomin, R.; Tarricone, L. Deep levels controlling the electrical properties of Fe-implanted GaInP/GaAs. Appl. Phys. Lett. 2007, 90, 182106. [Google Scholar] [CrossRef]
- Reshak, A.H.; Atuchin, V.V.; Auluck, S.; Kityk, I.V. First and second harmonic generation of the optical susceptibilities for the non-centro-symmetric orthorhombic AgCd2GaS4. J. Phys. Condens. Matter 2008, 20, 325234. [Google Scholar] [CrossRef]
- Kang, Z.-H.; Guo, J.; Feng, Z.-S.; Gao, J.-Y.; Xie, J.-J.; Zhang, L.-M.; Atuchin, V.; Andreev, Y.; Lanskii, G.; Shaiduko, A. Tellurium and sulfur doped GaSe for mid-IR applications. Appl. Phys. B 2012, 108, 5251–5255. [Google Scholar]
- Nishinaga, J.; Nagai, T.; Sugaya, T.; Shibata, H.; Niki, S. Single-crystal Cu(In,Ga)Se2 solar cells grown on GaAs substrates. Appl. Phys. Express 2018, 11, 082302. [Google Scholar] [CrossRef]
- Shen, G.; Guyot-Sionnest, P. HgTe/CdTe and HgSe/CdX (X = S, Se, and Te) core/shell mid-infrared quantum dots. Chem. Mater. 2018, 31, 286–293. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Liang, F.; Grazhdannikov, S.; Isaenko, L.I.; Krinitsin, P.G.; Molokeev, M.S.; Prosvirin, I.P.; Jiang, X.; Lin, Z. Negative thermal expansion and electronic structure variation of chalcopyrite type LiGaTe2. RSC Adv. 2018, 8, 9946–9955. [Google Scholar] [CrossRef]
- Suresh, R.; Pandiaraj, M.; Sankaralingam, M.; Giribabu, K. Graphene–metal chalcogenide modified electrochemical sensors. In Graphene-Based Electrochemical Sensors for Biomolecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–153. [Google Scholar] [CrossRef]
- Petrushkov, M.O.; Abramkin, D.S.; Emelyanov, E.A.; Putyato, M.A.; Komkov, O.S.; Firsov, D.D.; Vasev, A.V.; Yesin, M.Y.; Bakarov, A.K.; Loshkarev, I.D.; et al. Dislocation filter based on LT-GaAs layers for monolithic GaAs/Si integration. Nanomaterials 2022, 12, 4449. [Google Scholar] [CrossRef]
- Winton, G.H.; Faraone, L.; Lamb, R. Correlation of x-ray photoelectron spectroscopy and Rutherford backscattering spectroscopy depth profiles on Hg1−xCdxTe native oxides. J. Vac. Sci. Technol. A 1994, 12, 35–43. [Google Scholar] [CrossRef]
- Bando, H.; Koizumi, K.; Oikawa, Y.; Daikohara, K.; Kulbachinskii, V.A.; Ozaki, H. The time-dependent process of oxidation of the surface of Bi2Te3 studied by x-ray photoelectron spectroscopy. J. Phys. Condens. Matter 2000, 12, 5607–5616. [Google Scholar] [CrossRef]
- Vasil’ev, V.V.; Zakhar’yash, T.I.; Kesler, V.G.; Parm, I.O.; Solov’ev, A.P. Investigation of a change in the chemical composition of the surface of CdxHg1−xTe samples a result of treatment by N2O and H2 gases activated in a high-frequency discharge. Semiconductors 2001, 35, 196–198. [Google Scholar] [CrossRef]
- Yashina, L.V.; Tikhonov, E.V.; Neudachina, V.S.; Zyubina, T.S.; Chaika, A.N.; Shtanov, V.I.; Kobeleva, S.P.; Dobrovolsky, Y.A. The oxidation of PbTe(100) surface in dry oxygen. Surf. Interface Anal. 2004, 36, 993–996. [Google Scholar] [CrossRef]
- Andreev, Y.M.; Atuchin, V.V.; Lanskii, G.V.; Morozov, A.N.; Pokrovsky, L.D.; Sarkisov, S.Y.; Voevodina, O.V. Growth, real structure and applications of GaSe1−xSx crystals. Mater. Sci. Eng. B 2006, 128, 205–210. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Ursaki, V.V.; Tezlevan, V.E. Electronic structure of HgGa2S4. Solid State Commun. 2006, 138, 250–254. [Google Scholar] [CrossRef]
- Balitskii, O.A.; Jaegermann, W. XPS study of InTe and GaTe single crystals oxidation. Mater. Chem. Phys. 2006, 97, 98–101. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Parasyuk, O.V. Electronic structure of AgCd2GaS4. Surf. Rev. Lett. 2007, 14, 403–409. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Lobanov, S.I. Core level photoelectron spectroscopy of LiGaS2 and Ga–S bonding in complex sulfides. J. Alloys Compd. 2010, 497, 244–248. [Google Scholar] [CrossRef]
- Bereznaya, S.A.; Korotchenko, Z.V.; Novikov, V.A.; Redkin, R.A.; Sarkisov, S.Y.; Atuchin, V.V. Formation of native oxide crystallites on GaSe(001) surface. Inf. Phys. Technol. 2016, 76, 126–130. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Lobanov, S.I.; Goloshumova, A.A.; Molokeev, M.S.; Zhang, Z.; Zhang, X.; Jiang, X.; Lin, Z. Anisotropic thermal expansion and electronic structure of LiInSe2. Molecules 2022, 27, 5078. [Google Scholar] [CrossRef]
- Nguyen, M.; Ernits, K.; Tai, K.F.; Ng, C.F.; Pramana, S.S.; Sasangka, W.A.; Batabyal, S.K.; Holopainen, T.; Meissner, D.; Neisser, A.; et al. ZnS buffer layer for Cu2ZnSn(SSe)4 monograin layer solar cell. Solar Energy 2015, 111, 344–349. [Google Scholar] [CrossRef]
- Sengar, B.S.; Garg, V.; Awasthi, V.; Aaryashree; Kumar, S.; Mukherjee, C.; Gupta, M.; Mukherjee, S. Growth and characterization of dual ion beam sputtered Cu2ZnSn(S, Se)4 thin films for cost-effective photovoltaic application. Solar Energy 2016, 139, 1–12. [Google Scholar] [CrossRef]
- Sengar, B.S.; Garg, V.; Kumar, A.; Awasthi, V.; Kumar, S.; Atuchin, V.V.; Mukherjee, S. Band alignment of Cd-free (Zn, Mg)O layer with Cu2ZnSn(S,Se)4 and its effect on the photovoltaic properties. Opt. Mater. 2018, 84, 748–756. [Google Scholar] [CrossRef]
- Azarapin, N.O.; Aleksandrovsky, A.S.; Atuchin, V.V.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Mukherjee, S.; Oreshonkov, A.S.; Andreev, O.V. Synthesis, structural and spectroscopic properties of orthorhombic compounds BaLnCuS3 (Ln = Pr, Sm). J. Alloys Compd. 2020, 832, 153134. [Google Scholar] [CrossRef]
- Garg, V.; Sengar, B.S.; Siddharth, G.; Kumar, S.; Atuchin, V.V.; Mukherjee, S. Insights into the sputter-instigated valence plasmon oscillations in CIGSe thin films. Surf. Interfaces 2021, 25, 101146. [Google Scholar] [CrossRef]
- Bakhadur, A.M.; Uralbekov, B.M.; Atuchin, V.V.; Mukherjee, S.; Kokh, K.A. Single-phase CZTSe via isothermal recrystallization in a KI–KCl flux. CrystEngComm 2022, 24, 2291–2296. [Google Scholar] [CrossRef]
- Andreev, O.V.; Atuchin, V.V.; Aleksandrovsky, A.S.; Denisenko, Y.G.; Zakharov, B.A.; Tyutyunnik, A.P.; Habibullayev, N.N.; Velikanov, D.A.; Ulybin, D.A.; Shpindyuk, D.D. Synthesis, structure, and properties of EuLnCuSe3 (Ln = Nd, Sm, Gd, Er). Crystals 2022, 12, 17. [Google Scholar] [CrossRef]
- Dubey, M.; Siddharth, G.; Singh, R.; Patel, C.; Kumar, S.; Htay, M.T.; Atuchin, V.V.; Mukherjee, S. Influence of substrate temperature and sulfurization on sputtered Cu2SnGe(S,Se)3 thin films for solar cell application. IEEE Trans. Elect. Devices 2022, 69, 2488–2493. [Google Scholar] [CrossRef]
- Christuk, A.E.; Wu, P.; Ibers, J.A. New quaternary chalcogenides BaLnMQ3 (Ln = Rare earths; M = Cu, Ag; Q = S, Se), I. Structure and grinding-induced phase transition in BaLnMQ3. J. Solid State Chem. 1994, 110, 330–336. [Google Scholar] [CrossRef]
- Yang, Y.; Ibers, J.A. Synthesis and characterization of a series of quaternary chalcogenides BaLnMQ3 (Ln = Rare earth, M = Coinage metal, Q = Se or Te). J. Solid State Chem. 1999, 147, 366–371. [Google Scholar] [CrossRef]
- Huang, F.Q.; Mitchell, K.; Ibers, J.A. New Layered Materials: Syntheses, structures, and optical and magnetic properties of CsGdZnSe3, CsZrCuSe3, CsUCuSe3, and BaGdCuSe3. Inorg. Chem. 2001, 40, 5123–5126. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Solov’ev, L.A.; Andreev, O.V. Crystal structures and properties of SrLnCuS3 (Ln = La, Pr). Russ. J. Inorg. Chem. 2014, 59, 196–201. [Google Scholar] [CrossRef]
- Oreshonkov, A.; Azarapin, N.; Shestakov, N.; Adichtchev, S. Experimental and DFT study of BaLaCuS3: Direct band gap semiconductor. J. Phys. Chem. Solids 2021, 148, 109670. [Google Scholar] [CrossRef]
- Shahid, O.; Yadav, S.; Maity, D.; Deepa, M.; Niranjan, M.K.; Prakash, J. Synthesis, crystal structure, DFT, and photovoltaic studies of BaCeCuS3. New J. Chem. 2023, 47, 5378–5389. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Molokeev, M.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Atuchin, V.V.; Azarapin, N.O.; Plyusnin, P.E.; Sal’nikova, E.I.; Andreev, O.V. High-temperature oxidation of europium (II) sulfide. J. Indust. Eng. Chem. 2019, 79, 62–70. [Google Scholar] [CrossRef]
- Llanos, J.; Sánchez, V.; Mujica, C.; Buljan, A. Synthesis, physical and optical properties, and electronic structure of the rare-earth oxysulfides Ln2O2S (Ln = Sm, Eu). Mater. Res. Bull. 2002, 37, 2285–2291. [Google Scholar] [CrossRef]
- Andreev, P.O.; Sal’nikov, E.I.; Kovenskii, I.M. Preparation of Ln2O2S (Ln = Gd, Dy, Y, Er, Lu) in flowing hydrogen and hydrogen sulfide. Inorg. Mater. 2014, 50, 1018–1023. [Google Scholar] [CrossRef]
- Andreev, O.V.; Denisenko, Y.G.; Osseni, S.A.; Bamburov, V.G.; Sal’nikova, E.I. Rare Earth Sulfates and Oxysulfides; Monograph; Tyumen State University: Tyumen, Russia, 2017; ISBN 978-5-400-01341-6. [Google Scholar]
- Mukherjee, S. The Science of Clays; Springer Science and Business Media: Dordrecht, The Netherlands, 2013; ISBN 0-471-03288-3. [Google Scholar]
- Evans, H.T. Djurleite (Cu1.94S) and low chalcocite (Cu2S): New crystal structure studies. Science 1979, 203, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna Rao, V.V.V.N.S.; Abraham, K.P. Kinetics of oxidation of copper sulfide. Metall. Trans. 1971, 2, 2463–2470. [Google Scholar] [CrossRef]
- Asaki, Z.; Ueguchi, A.; Tanabe, T.; Kondo, Y. Oxidation of Cu2S pallet. Trans. Jpn. Inst. Metals 1986, 27, 361–371. [Google Scholar] [CrossRef]
- Asaki, Z.; Ando, S.; Kondo, Y. Oxidation of molten copper matte. Metall. Trans. 1988, 19B, 47–52. [Google Scholar] [CrossRef]
- Liu, J.; Xue, D. Thermal oxidation strategy towards porous metal oxide hollow architectures. Adv. Mater. 2008, 20, 2622–2627. [Google Scholar] [CrossRef]
- Senko, J.M.; Campbell, B.S.; Henriksen, J.; Elshahed, M.S.; Dewers, T.A.; Krumholz, L.R. Barite deposition resulting from phototrophic sulfide-oxidizing bacterial activity. Geochim. Cosmochim. Acta 2004, 68, 773–780. [Google Scholar] [CrossRef]
- Plummer, L.N. Barite deposition in central Kentucky. Econ. Geol. 1971, 66, 252–258. [Google Scholar] [CrossRef]
- NETZSCH. Proteus 6, Thermic Analyses e User’s and Software Manuals; NETZSCH: Selb, Germany, 2012. [Google Scholar]
- Denisenko, Y.; Khritokhin, N.; Andreev, O.; Basova, S.; Sal’nikova, E.; Polkovnikov, A. Thermal decomposition of europium sulfates Eu2(SO4)3·8H2O and EuSO4. J. Solid State Chem. 2017, 255, 219–224. [Google Scholar] [CrossRef]
- Bruker AXS. TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual; Bruker, AXS: Karlsruhe, Germany, 2008. [Google Scholar]
- Azarapin, N.O.; Atuchin, V.V.; Maximov, N.G.; Aleksandrovsky, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; Krylov, A.S.; Burkhanova, T.M.; Mukherjee, S.; et al. Synthesis, structure, melting and optical properties of three complex orthorhombic sulfides BaDyCuS3, BaHoCuS3 and BaYbCuS3. Mater. Res. Bull. 2021, 140, 111314. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Aleksandrovsky, A.; Atuchin, V.; Krylov, A.; Molokeev, M.; Oreshonkov, A.; Shestakov, N.; Andreev, O. Exploration of structural, thermal and spectroscopic properties of self-activated sulfate Eu2(SO4)3 with isolated SO4 groups. J. Indust. Eng. Chem. 2018, 68, 109–116. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Wang, N.; Jiang, X.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Sedykh, A.E.; Volkova, S.S.; et al. Negative thermal expansion in one-dimension of a new double sulfate AgHo(SO4)2 with isolated SO4 tetrahedra. J. Mater. Sci. Technol. 2021, 76, 111–121. [Google Scholar] [CrossRef]
- Yin, Q.; Kelsall, G.H.; Vaughan, D.J.; England, K.E.R. Atmospheric and electrochemical oxidation of the surface of chalcopyrite (CuFeS2). Geochim. Cosmochim. Acta 1995, 59, 1091–1100. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, X.; Ding, J.; Zhou, D. Leaching mechanism for chalcopyrite in hydrochloric acid. Hydrometallurgy 2012, 113, 109–118. [Google Scholar] [CrossRef]
- Nathans, M.; Wendlandt, W. The thermal decomposition of the rare-earth sulphates: Thermogravimetric and differential thermal analysis studies up to 1400 °C. J. Inorg. Nucl. Chem. 1962, 24, 869–879. [Google Scholar] [CrossRef]
- Zhu, Y.; Mimura, K.; Isshiki, M. Oxidation mechanism of Cu2O to CuO at 600–1050 °C. Oxid. Met. 2004, 62, 207–222. [Google Scholar] [CrossRef]
- Enhessari, M.; Shaterian, M.; Esfahani, M.J.; Motaharian, M.N. Motaharian, Synthesis, characterization and optical band gap of La2CuO4 nanoparticles. Mater. Sci. Semicond. Proc. 2013, 16, 1517–1520. [Google Scholar] [CrossRef]
- Imai, Y.; Kato, M.; Takarabe, Y.; Noji, T.; Koike, Y. Low-temperature synthesis of La2CuO4 with the T‘-structure from molten hydroxides. Chem. Mater. 2007, 19, 3584–3585. [Google Scholar] [CrossRef]
- Abboudi, M.; Messali, M.; Kadiri, N.; Ben Ali, A.; Moran, E. Synthesis of CuO, La2O3, and La2CuO4 by the thermal-decomposition of oxalates precursors using a new method. Synth. React. Inorg. Met. Organ. Nano-Met. Chem. 2011, 41, 683–688. [Google Scholar] [CrossRef]
- Tholence, J. Superconductivity of La2CuO4 and YBa2Cu3O7. In Proceedings of the Yamada Conference XVIII on Superconductivity in Highly Correlated Fermion Systems; Elsevier: Amsterdam, The Netherlands, 1987; pp. 353–356. [Google Scholar] [CrossRef]
- Cheong, S.-W.; Thompson, J.; Fisk, Z. Properties of La2CuO4 and related compounds. Phys. C Supercond. 1989, 158, 109–126. [Google Scholar] [CrossRef]
- Shishkin, R.A.; Suntsov, A.Y. La2CuO4 as a promising oxygen carrier for CLOU process. AIP Conf. Proc. 2020, 2313, 060024. [Google Scholar] [CrossRef]
- Gao, P.; He, Y.; Lu, S.; He, M.; Liu, Z.; Deng, Y.; Xu, T.; Zhang, H. Activation of peroxymonosulfate by La2CuO4 perovskite for synergistic removal of Microcystis aeruginosa and microcystin-LR in harmful algal bloom impacted water. Appl. Catal. B Environ. 2023, 328, 122511. [Google Scholar] [CrossRef]
- Parida, S.; Brahma, S.S.; Nanda, J.; Sethy, S.K.; Sankaran, K. Spin coated La2CuO4 thin film: An extensive study on optical dispersion parameters. Optik 2023, 278, 170728. [Google Scholar] [CrossRef]
- Melchiorre, E.B.; Criss, R.E.; Rose, T.P. Rose, Oxygen and carbon isotope study of natural and synthetic malachite. Econ. Geol. 1999, 94, 245–259. [Google Scholar] [CrossRef]
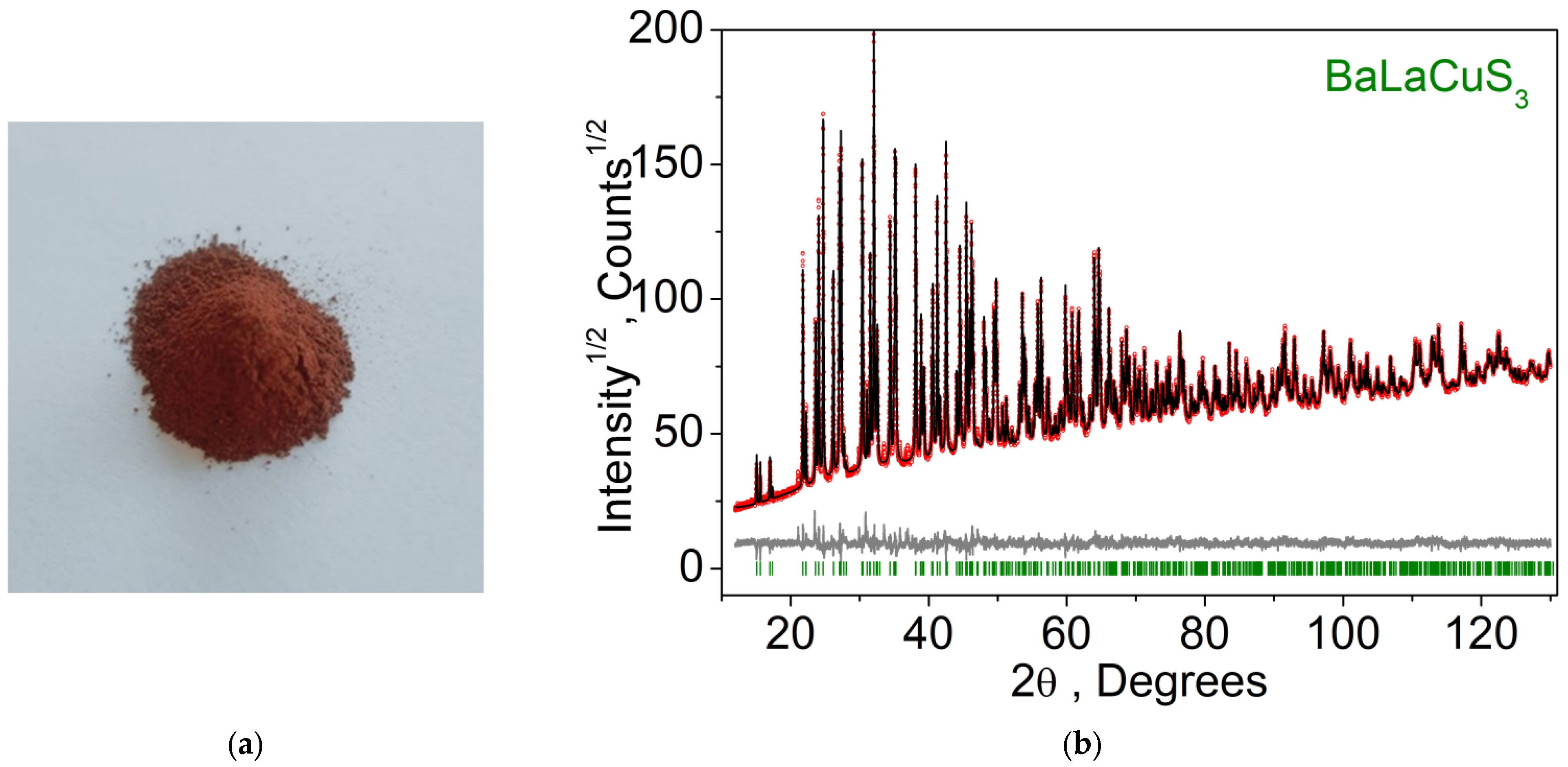




| Compound | BaLaCuS3 | BaLaCuS3 [29] |
|---|---|---|
| Phase type | powder | single crystal |
| Space group | Pnma | Pnma |
| a (Å) | 11.34724(3) | 11.316(2) |
| b (Å) | 4.249621(12) | 4.236(1) |
| c (Å) | 11.73683(3) | 11.724(2) |
| V (Å3) | 565.967(3) | 562.0(2) |
| Z | 4 | 4 |
| 2θ-interval, ° | 12–130 | - |
| Rwp, % | 3.51 | - |
| Rp, % | 2.46 | - |
| χ2 | 2.22 | - |
| RB, % | 1.3 | 5.9 |
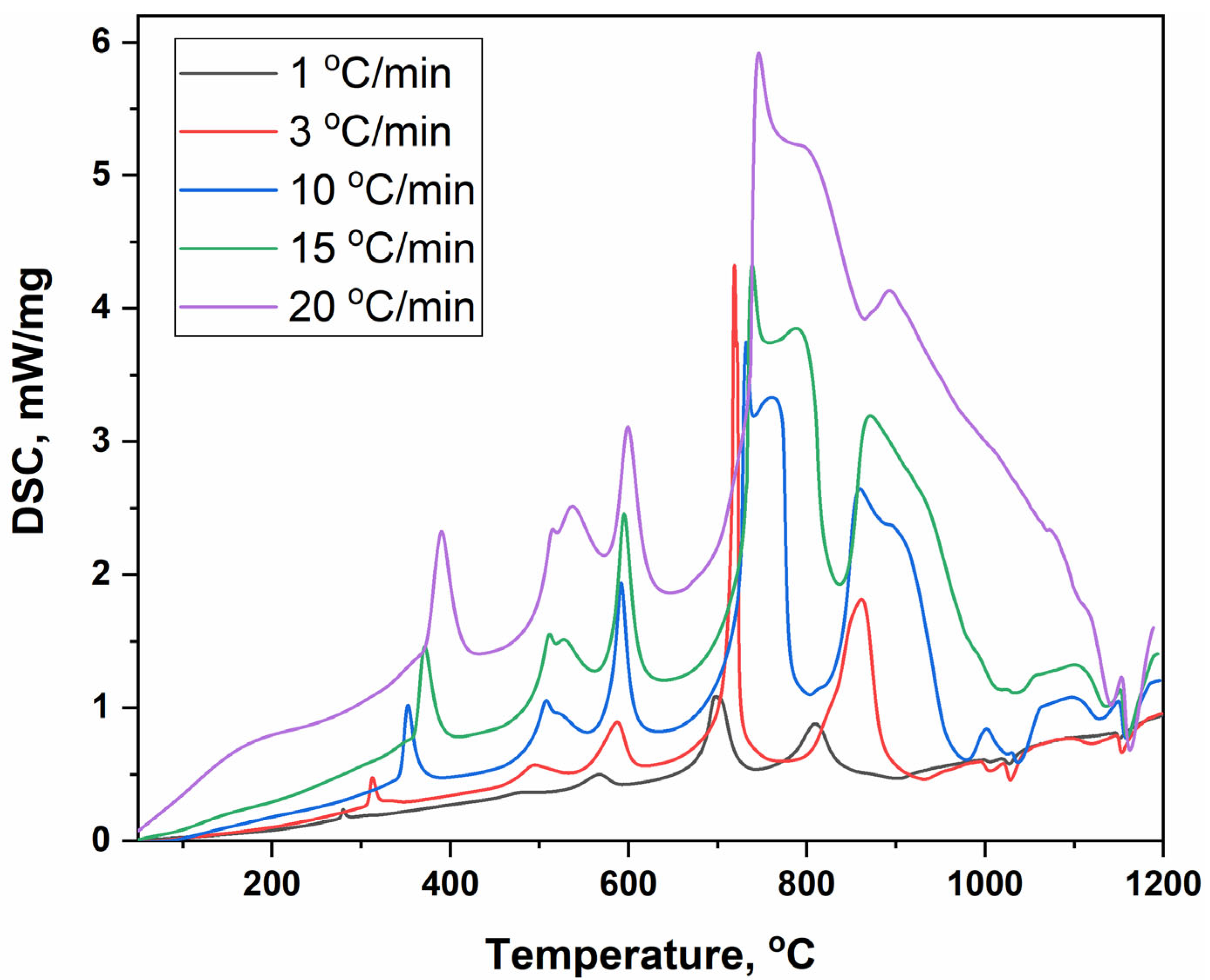
| Effect | Exo | Exo | Exo | Exo | Exo | Exo | Exo | Exo | Endo | Endo | Endo | Endo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heating Rate, °C/min | Number of Peak/Temperatures, °C | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 20 | 390.3 | 515.1 | 537.3 | 599.2 | 746.2 | 805.1 | 892.9 | - | 1069.7 | 1098.5 | 1141.2 | 1162.5 |
| 15 | 371.4 | 511.7 | 527.4 | 595.2 | 738.8 | 788.3 | 871.3 | 937.8 | 1012.9 | 1032.1 | 1135 | 1159.4 |
| 10 | 352.6 | 508.1 | 523.5 | 591.8 | 732.2 | 760.9 | 859.7 | 908.5 | 1024.8 | 1036.8 | 1128.3 | 1158.2 |
| 3 | 312.9 | 495.2 | - | 587.2 | 718.9 | 721.7 | 847.9 | 861.8 | 1004.4 | 1028.3 | 1118.8 | 1153.5 |
| 1 | 280 | 479.2 | - | 567.2 | 697.4 | 703.3 | 810.6 | - | 1005.3 | 1027.1 | - | 1150.2 |
| Ea, kJ/mol | 77 | 417 | 270 | 625 | 526 | 244 | 435 | 233 | 1000 | 1020 | 1493 | 4348 |
| A | 1·104 | 8·1025 | 5·1015 | 8·1035 | 2·1025 | 7·109 | 4·1017 | 6·107 | 1·1038 | 2·1038 | 5·1053 | 2·10158 |
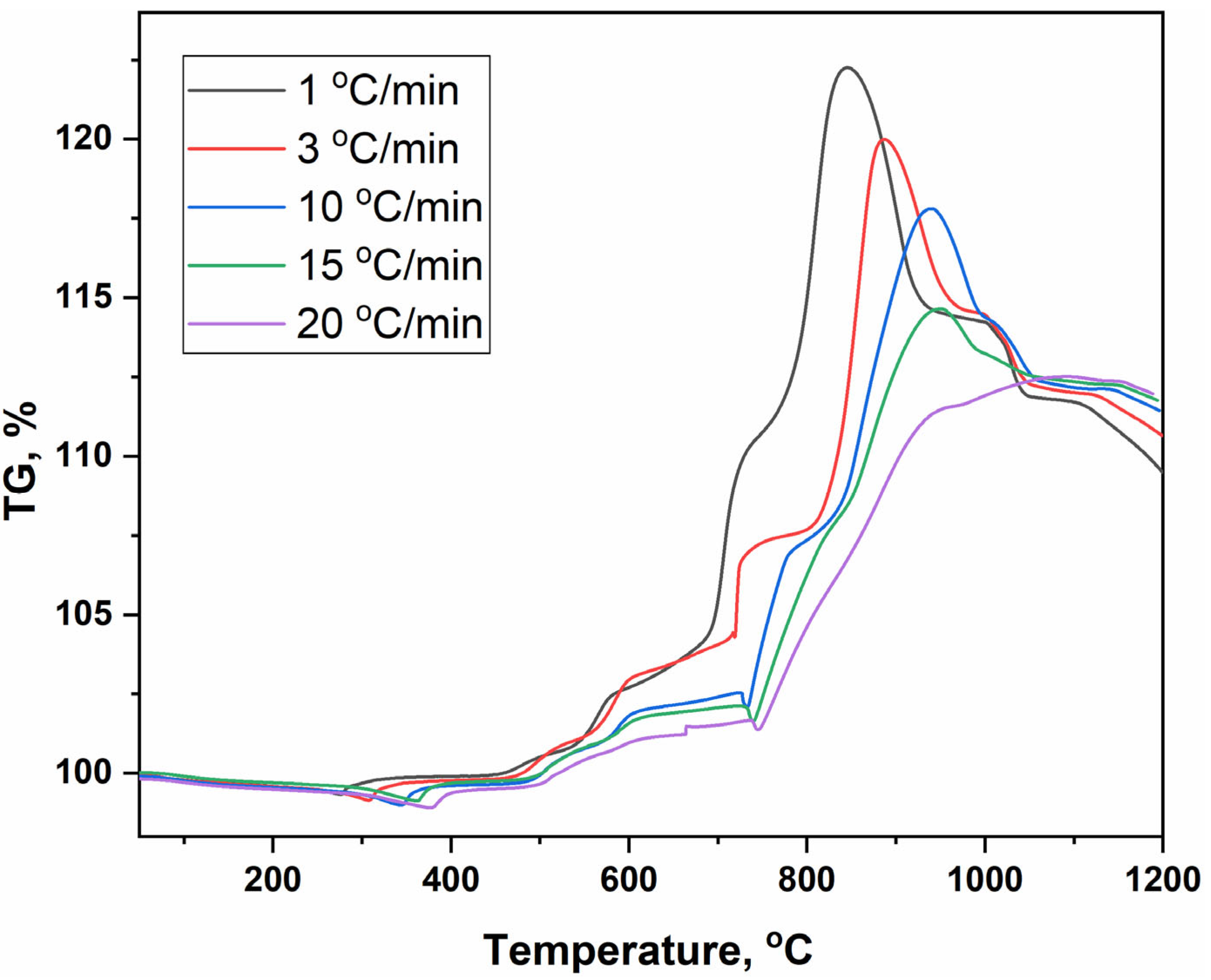
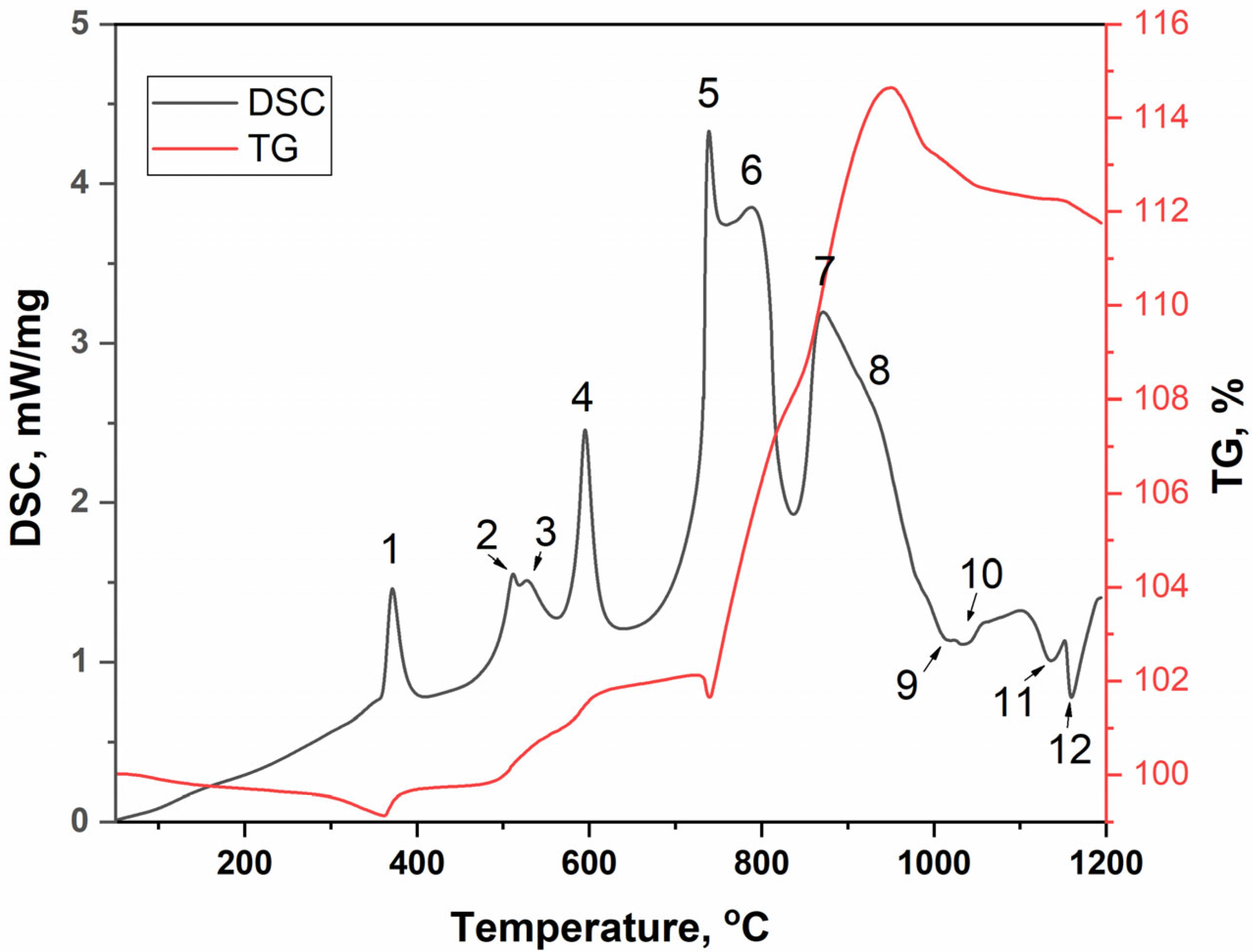
| Heating Stop Temperature, °C | Peak Number | Phase Composition | Note |
|---|---|---|---|
| 380 | 1 | BaSO4 + CuLaS2 + BaLaCuS3 | halo at 20° 2θ |
| 518 | 2 | BaSO4 + CuLaS2 + BaLaCuS3 | halo increase |
| 560 | 3 | BaSO4 + CuLaS2 + BaLaCuS3 | halo increase |
| 630 | 4 | BaSO4 + CuLaS2 + BaLaCuS3 | halo increase |
| 760 | 5 | BaSO4 + LaCuOS + BaLaCuS3 | lack of halo |
| 790 | 6 | BaSO4 + LaCuOS | |
| 872 | 7 | BaSO4 + LaCuOS | |
| 940 | 8 | BaSO4 + LaCuOS + La2O2SO4 | formation Cu2O |
| 1020 | 9 | BaSO4 + La2O2SO4 + Cu2O + CuO | increase in Cu2O |
| 1045 | 10 | BaSO4 + La2O2SO4 + Cu2O + CuO | decrease in Cu2O |
| 1137 | 11 | BaSO4 + La2O2SO4 + CuO | halo at 20° 2θ |
| 1165 | 12 | BaSO4 + La2O2SO4 + CuO + La2CuO4 |
| Temperature, °C | Chemical Process |
|---|---|
| 380–739 | BaLaCuS3 + 2O2 = BaSO4 + CuLaS2 |
| 788–871 | CuLaS2 + 1.5O2 = LaCuOS + SO2 |
| 938 | 6LaCuOS + 11O2 = 3La2O2SO4 + 2CuO/Cu2O + 3SO2 |
| 938–1032 | Cu2O + 0.5O2 = 2CuO |
| from 1135 | La2O2SO4 = La2O3 + SO2 + 0.5O2 |
| from 1159 | La2O3+ CuO = La2CuO4 |
| BET, m2·g−1 | Micropore Volume, cm3·g−1 | BJH, cm3·g−1 | D *, μm |
|---|---|---|---|
| 0.2698 | 0.000143 | 0.001681 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azarapin, N.O.; Khritokhin, N.A.; Atuchin, V.V.; Gubin, A.A.; Molokeev, M.S.; Mukherjee, S.; Andreev, O.V. Kinetics and Mechanism of BaLaCuS3 Oxidation. Crystals 2023, 13, 903. https://doi.org/10.3390/cryst13060903
Azarapin NO, Khritokhin NA, Atuchin VV, Gubin AA, Molokeev MS, Mukherjee S, Andreev OV. Kinetics and Mechanism of BaLaCuS3 Oxidation. Crystals. 2023; 13(6):903. https://doi.org/10.3390/cryst13060903
Chicago/Turabian StyleAzarapin, Nikita O., Nikolay A. Khritokhin, Victor V. Atuchin, Alexey A. Gubin, Maxim S. Molokeev, Shaibal Mukherjee, and Oleg V. Andreev. 2023. "Kinetics and Mechanism of BaLaCuS3 Oxidation" Crystals 13, no. 6: 903. https://doi.org/10.3390/cryst13060903
APA StyleAzarapin, N. O., Khritokhin, N. A., Atuchin, V. V., Gubin, A. A., Molokeev, M. S., Mukherjee, S., & Andreev, O. V. (2023). Kinetics and Mechanism of BaLaCuS3 Oxidation. Crystals, 13(6), 903. https://doi.org/10.3390/cryst13060903







