Investigation of the Effect of Aluminum Powder on the Combustion Rate of the Composite
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aknazarov, S.K.; Mutushev, A.Z.; Gonzalez-Leal, J.M.; Bairakova, O.S.; Golovchenko, O.Y.; Golovchenko, N.Y.; Ponomareva, E.A. Kinetics of the Synthesis of Aluminum Boride by the Self-Propagating High-Temperature Synthesis Method. Ceramics 2022, 5, 435–446. [Google Scholar] [CrossRef]
- Aknazarov, S.K.; Mutushev, A.Z.; Gonzalez-Leal, J.M.; Bairakova, O.S.; Golovchenko, O.Y.; Golovchenko, N.Y.; Ponomareva, E.A. Optimization of Aluminum Boride Synthesis in the Self-Propagating High-Temperature Synthesis Mode to Create Waste-Free Technology. Ceramics 2022, 5, 1286–1299. [Google Scholar] [CrossRef]
- Aknazarov, S.K.; Seisenova, A.B.; Golovchenko, O.Y.; Golovchenko, N.Y.; Gonzalez-Leal, J.M. Determination of Thermodynamic Characteristics of Phase-Stabilized Ammonium Nitrate-Based High-Energy Solid Combustible Materials. Combust. Sci. Technol. 2022, 4, 768–784. [Google Scholar] [CrossRef]
- Zhukov, B.P. Brief Encyclopedic Dictionary’—Energy Condensed Systems; IAnus-K: Moscow, Russia, 2000; p. 5, (In Russian). Available online: https://studizba.com/files/show/pdf/15500-1-zhukov-b-p--energeticheskie.html (accessed on 1 April 2023).
- Gavrilova, L.A. Brief Encyclopedic Dictionary’. Applications of Solid Fuels in the National Economy—Energy Condensed Systems; IAnus-K: Moscow, Russia, 2000; pp. 241–242. (In Russian) [Google Scholar]
- Prokhorov, A.M. Great Soviet Encyclopedia; T26: Moscow, Russia, 1978; p. S-398. (In Russian) [Google Scholar]
- Paushkina, I.M.; Chulkova, A.Z. Rocket Fuels; Mir.: Moscow, Russia, 1975; p. S.189. (In Russian) [Google Scholar]
- Glushko, V.P. Thermodynamic and Thermophysical Properties of Combustion Products; VINITI: Moscow, Russia, 1971; p. 265s. (In Russian) [Google Scholar]
- Egorychev, V.S.; Konurusov, V.S. Fuel Chemical Rocket Engines; SGAU: Samara, Russia, 2007; p. 72s. (In Russian) [Google Scholar]
- Tsutsuran, V.I.; Petrukhin, N.V.; Gusev, A. Military-Technical Analysis of the State and Prospects for the Development of Rocket Fuels. 1989, p. 323. (In Russian). Available online: https://f.eruditor.one/file/429585/ (accessed on 1 April 2023).
- Smirnov, L.A. Creation of Mixed Solid Fuels; MGAKHM: Moscow, Russia, 1997; p. ch1. (In Russian) [Google Scholar]
- Kharitonov, V.A. Introduction to Energy Materials Technology; Altai State Technical University: Biysk, Russia, 2009; p. 254s. (In Russian) [Google Scholar]
- Soten, V.F. Analysis of the trend of scientific and technical development of leading foreign countries in the field of technology and synthesis of promising components of aircraft compositions. In Proceedings of the Modern Problems of Technical Chemistry: Mat. Reports of the All-Russian Scientific and Technical Conference, Kazan, Russia, 28 May 2003; pp. 40–45. (In Russian). [Google Scholar]
- Luk’ianov, O.A. ANDA. In Brief Encyclopedic Dictionary Energy Condensed Systems; IAnus-K: Moscow, Russia, 2000; pp. 351–353. (In Russian) [Google Scholar]
- Gan’kin, I.U.A. Oktogen. In Brief Encyclopedic Dictionary’ Condensed Systems; IAnus-K: Moscow, Russia, 2000; pp. 131–133. (In Russian) [Google Scholar]
- Gan’kin, I.U.A. Geksogen. In Brief Encyclopedic Dictionary’ Condensed Systems; IAnus-K: Moscow, Russia, 2000; pp. 334–335. (In Russian) [Google Scholar]
- Fioshina, M.A.; Rusin, D.L. Fundamentals of Chemistry and Technology of Powders and Solid Rocket Fuels. 2001. (In Russian). Available online: https://search.rsl.ru/ru/record/01002454918 (accessed on 1 April 2023).
- Rogov, N.G.; Ishchenko, M.A. Mixed Solid Rocket Propellants, Components, Requirements, Properties; OOO IPK Biont: Saint Petersburg, Russia, 2005; p. 195s. (In Russian) [Google Scholar]
- Bestuzheva, V.V.; Dushenko, S.A.; Ishchenko, M.A.; Karkulin, I.V.; Sirotinkin, N.V.; Vasil’ev, A.V. Polymer binders for energy condensed systems. In Technology of Macromolecular Compounds; St. Petersburg State Technological Institute: Saint Petersburg, Russia, 2013; pp. 93–101. (In Russian) [Google Scholar]
- Robert, C.; Gill, G.; Nauflett, W. Process for the Preparation of 2,4-dinitro-2,4diazapentane. 1982. Available online: https://patentimages.storage.googleapis.com/52/04/d1/b1235cfb7597aa/US4469888.pdf (accessed on 1 April 2023).
- Tartakovskii, V.A.; Ermakov, A.S.; Koroban, V.A. Obtaining H1H-dinalkylmethylene-Bisnitramines. 1990. Available online: https://yandex.ru/patents/doc/SU1616905A1_19901230 (accessed on 1 April 2023).
- Denisyu, A.P.; Shepelev, Y.G.; Yudaev, S.V.; Kalashnikov, I.A. Patterns of combustion of systems containing linear nitramines. Phys. Combust. Explos. 2005, 41, 98–106. (In Russian) [Google Scholar]
- Kustaval, V.; Kirpichev, E.P.; Rubstov, Y.J. Standart enthalpies of formulation of certain nitro compounds. Bulletin of the Academy of Sciences of the USSR. Div. Chem. Sci. 1981, 30, 1830–1836. [Google Scholar]
- Simmons, R.L. Thermochemistry of NENA plasticizers. In Energetic materials. In Proceedings of the 25 International Annual Conference of ICT, Karlsruhe, Germany, 28 June–1 July 1994. (In Russian). [Google Scholar]
- Spravochnik; Zinov’ev, V.M. Vysokoenergeticheskie Plastifikatory Smesevykh i Ballisticheskikh Tverdykh Raketnykh Topliv; Izd. PGU: Perm, Russia, 2011; p. 253s. (In Russian) [Google Scholar]
- Maltsev, V.M.; Maltsev, M.I.; Kashkorov, L.Y. The main characteristics of combustion. Chemistry. Referat. ZHurnal KHimiia 1999, 11, 69. [Google Scholar]
- Arkhipov, V.A.; Gorbenko, T.I.; Bespalov, I.S.; Savel’eva, L.A. Combustion of condensed systems with catalysts. Phys. Combust. Explos. 2009, 52, 3–6. (In Russian) [Google Scholar]
- Pokhiia, P.F.; Beliaev, A.F.; Frolov, I.U.V.; Logachev, V.S.; Korotkov, A.I. Combustion of Powdered Metals in Active Media; Nauka: Moscow, Russia, 1972; p. 294s. (In Russian) [Google Scholar]
- Mandakov, A.V.; Komarov, V.F.; Materialy, K.H.K. H Influence of the nature of dispersed metals on the patterns of combustion of condensed systems. In Proceedings of the XX Russian Conference on Synchrotron Radiation, New Siberia, Russia, 21–25 September 2015; pp. 19–26. (In Russian). [Google Scholar]
- Olevskogo, V.M. Ammonium Nitrate Technology; Chemistry: Moscow, Russia, 1978; p. 184s. (In Russian) [Google Scholar]
- Lur’e, B.A. Sodium Nitrate. In Great Soviet Encyclopedia; Sovetskaya Entsiklopediya: Moscow, Russia, 1964; p. S.521. [Google Scholar]
- Big Encyclopedia of Oil and Gas; Nedra: Moscow, Russia, 1970; p. 576s. (In Russian)
- Glazkova, A.P. Explosive Combustion Catalysis; Science: Moscow, Russia, 1977; pp. 323–324. (In Russian) [Google Scholar]

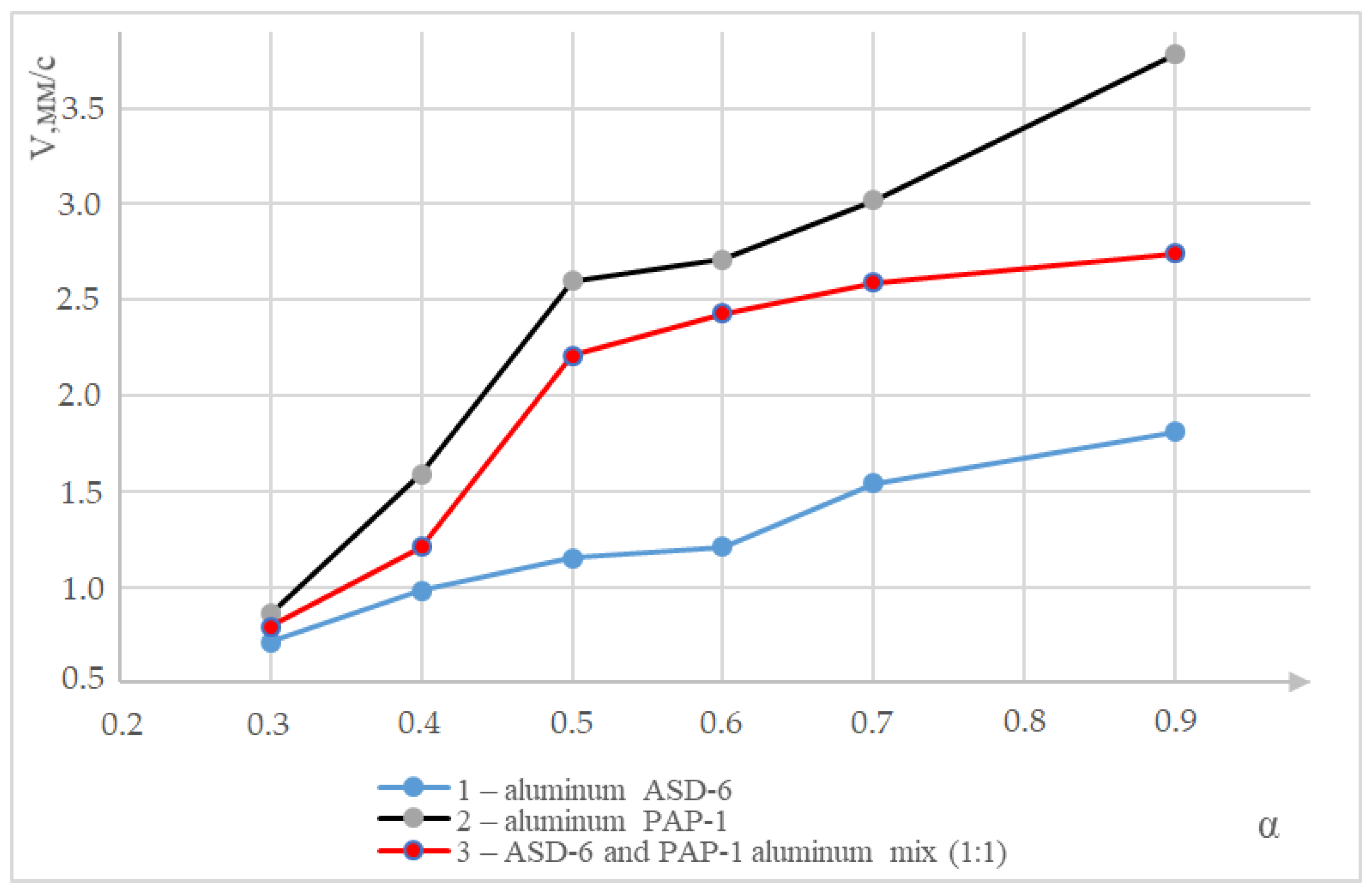
| High Energy Condensed Systems | α | Content of Components, wt % | ||||
|---|---|---|---|---|---|---|
| Ammonium Nitrate | Potassium Nitrate | Fuel Binding | ASD-6 | PAP-1 | ||
| 1a | 0.4 | 64 | - | 21 | 15 | - |
| 2b | 0.4 | 32 | 32 | 21 | - | 15 |
| 3c | 0.5 | 72 | - | 13 | 7.5 | 7.5 |
| 4b | 0.5 | 36 | 36 | 13 | - | 15 |
| Dispersity, microns | 100–125 | 90–100 | - | 10–20 | - | |
| Pressure, MPa | 1a | 1b | 2a | 2b | 3c | 3b | 4a | 4b |
|---|---|---|---|---|---|---|---|---|
| atm. | 52 | 23.07 | 51.4 | 22.8 | 50 | 21 | 51.8 | 22.0 |
| 0.2 | 47 | 17.4 | 46.3 | 16.5 | 46.0 | 18 | 45.2 | 16.8 |
| 0.4 | 41.2 | 15.7 | 39 | 14 | 36.4 | 9.4 | 43.4 | 10.9 |
| 0.8 | 30.4 | 12.1 | 30.3 | 12.54 | 30.8 | 9 | 42 | 10.4 |
| N | Composition of Oxidizers, % | |
|---|---|---|
| NH4NO3 | KNO3 | |
| 1 | 95 | 5 |
| 2 | 90 | 10 |
| 3 | 85 | 15 |
| 4 | 100 | |
| 5 | 100 | |
| N | Component Content, wt % | |||
|---|---|---|---|---|
| KNO3 | NH4NO3 | SKDM-80 | Al | |
| 1 | 2 | 3 | 4 | 5 |
| 1 | - | 80.68 | 19.32 | - |
| 2 | - | 64 | 21 | 15 |
| 3 | - | - | 23.2 | 15 |
| 4 | - | - | 23.2 | - |
| 5 | - | 7.2 | 13 | 15 |
| 6 | - | 36.2 | 13 | 15 |
| 7 | - | 36.2 | 27.6 | - |
| 8 | - | 7.2 | 13 | - |
| 9 | - | - | 18.8 | - |
| 10 | - | - | 18.8 | 15 |
| 11 | 36.2 | 36.2 | 13 | 15 |
| 12 | 80.64 | - | 21 | - |
| 13 | 64 | - | 21 | 15 |
| № | Component Name | Compound, % | Mass, g | Height, mm | ρ, g/cm3 | α | Burning Speed, mm/s |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 1 | NH4NO3 | 80 | 4.2 | 3.02 | 1.75 | 0.5 | 0.6 |
| SKDM-80 | 20 | ||||||
| 2 | NH4NO3 | 64 | 4.3 | 3.06 | 1.78 | 0.5 | 1.15 ± 0.15 |
| SKDM-80 | 21 | ||||||
| ASD-6 | 15 | ||||||
| 3 | NH4NO3 | 64 | 4.5 | 3.20 | 1.78 | 0.5 | 2.01 ± 0.57 |
| SKDM-80 | 21 | ||||||
| ASD-6 | 7.5 | ||||||
| PAP-1 | 7.5 | ||||||
| 4 | NH4NO3 | 32 | 4.4 | 3.12 | 1.79 | 0.5 | 2.16 ± 0.5 |
| SKDM-80 | 21 | ||||||
| ASD-6 | 7.5 | ||||||
| PAP-1 | 7.5 | ||||||
| KNO3 | 32 | ||||||
| 5 | NH4NO3 | 64 | 4.8 | 3.40 | 1.8 | 0.5 | 2.6 ± 0.3 |
| SKDM-80 | 21 | ||||||
| PAP-1 | 15 | ||||||
| 6 | KNO3 | 32 | 4.5 | 3.19 | 1.72 | 0.5 | 2.8 ± 0.61 |
| NH4NO3 | 32 | ||||||
| SKDM-80 | 21 | ||||||
| PAP-1 | 15 | ||||||
| 7 | KNO3 | 64 | 4.5 | 3.1 | 1.79 | 0.5 | 3.0 ± 0.5 |
| SKDM-80 | 21 | ||||||
| PAP-1 | 15 | ||||||
| 8 | NH4NO3 | 87 | 4.7 | 3.31 | 1.79 | 0.5 | 0.8 |
| SKDM-80 | 13 | ||||||
| 9 | NH4NO3 | 72 | 4.7 | 3.30 | 1.81 | 0.5 | 1.67 |
| SKDM-80 | 13 | ||||||
| ASD-6 | 15 | ||||||
| 10 | NH4NO3 | 72 | 4.6 | 3.2 | 1.80 | 0.5 | 3.78 ± 0.28 |
| SKDM-80 | 13 | ||||||
| PAP-1 | 15 | ||||||
| 11 | NH4NO3 | 72 | 4.7 | 3.3 | 1.79 | 0.5 | 2.74 |
| SKDM-80 | 13 | ||||||
| ASD-6 | 7.5 | ||||||
| PAP-1 | 7.5 | ||||||
| 12 | NH4NO3 | 36 | 4.65 | 3.2 | 1.81 | 0.5 | 2.98 |
| KNO3 | 36 | ||||||
| SKDM-80 | 13 | ||||||
| ASD-6 | 15 | ||||||
| 13 | KNO3 | 72 | 4.7 | 3.28 | 1.79 | 0.5 | 3.7 ± 0.39 |
| SKDM-80 | 13 | ||||||
| PAP-1 | 15 | ||||||
| 14 | KNO3 | 72 | 4.69 | 3.25 | 1.80 | 0.5 | 3.01 |
| SKDM-80 | 13 | ||||||
| ASD-6 | 7.5 | ||||||
| PAP-1 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kydyrali, S.Y.; Aknazarov, S.K.; Mutushev, A.Z.; Gonzalez-Leal, J.M.; Golovchenko, O.Y.; Gabdrashova, S.Y.; Tulepov, M.I.; Seisenova, A.B. Investigation of the Effect of Aluminum Powder on the Combustion Rate of the Composite. Crystals 2023, 13, 867. https://doi.org/10.3390/cryst13060867
Kydyrali SY, Aknazarov SK, Mutushev AZ, Gonzalez-Leal JM, Golovchenko OY, Gabdrashova SY, Tulepov MI, Seisenova AB. Investigation of the Effect of Aluminum Powder on the Combustion Rate of the Composite. Crystals. 2023; 13(6):867. https://doi.org/10.3390/cryst13060867
Chicago/Turabian StyleKydyrali, Sayat Yermekuly, Sestager Khusainovich Aknazarov, Alibek Zhumabekovich Mutushev, Juan Maria Gonzalez-Leal, Olga Yuryevna Golovchenko, Sholpan Yesenzholovna Gabdrashova, Marat Iztleuovich Tulepov, and Aknur Berdibayevna Seisenova. 2023. "Investigation of the Effect of Aluminum Powder on the Combustion Rate of the Composite" Crystals 13, no. 6: 867. https://doi.org/10.3390/cryst13060867
APA StyleKydyrali, S. Y., Aknazarov, S. K., Mutushev, A. Z., Gonzalez-Leal, J. M., Golovchenko, O. Y., Gabdrashova, S. Y., Tulepov, M. I., & Seisenova, A. B. (2023). Investigation of the Effect of Aluminum Powder on the Combustion Rate of the Composite. Crystals, 13(6), 867. https://doi.org/10.3390/cryst13060867








