Enhancing ORR Catalytic Activity and Electrochemical Investigation of La1−2xBaxBixFeO3 Cathode for Low-Temperature Solid Oxide Fuel Cell
Abstract
1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Fuel Cell Fabrication
2.3. Characterization of Materials and Electrochemical Measurements
3. Results and Discussion
3.1. Crystalline Structure and Surface Morphology Analysis
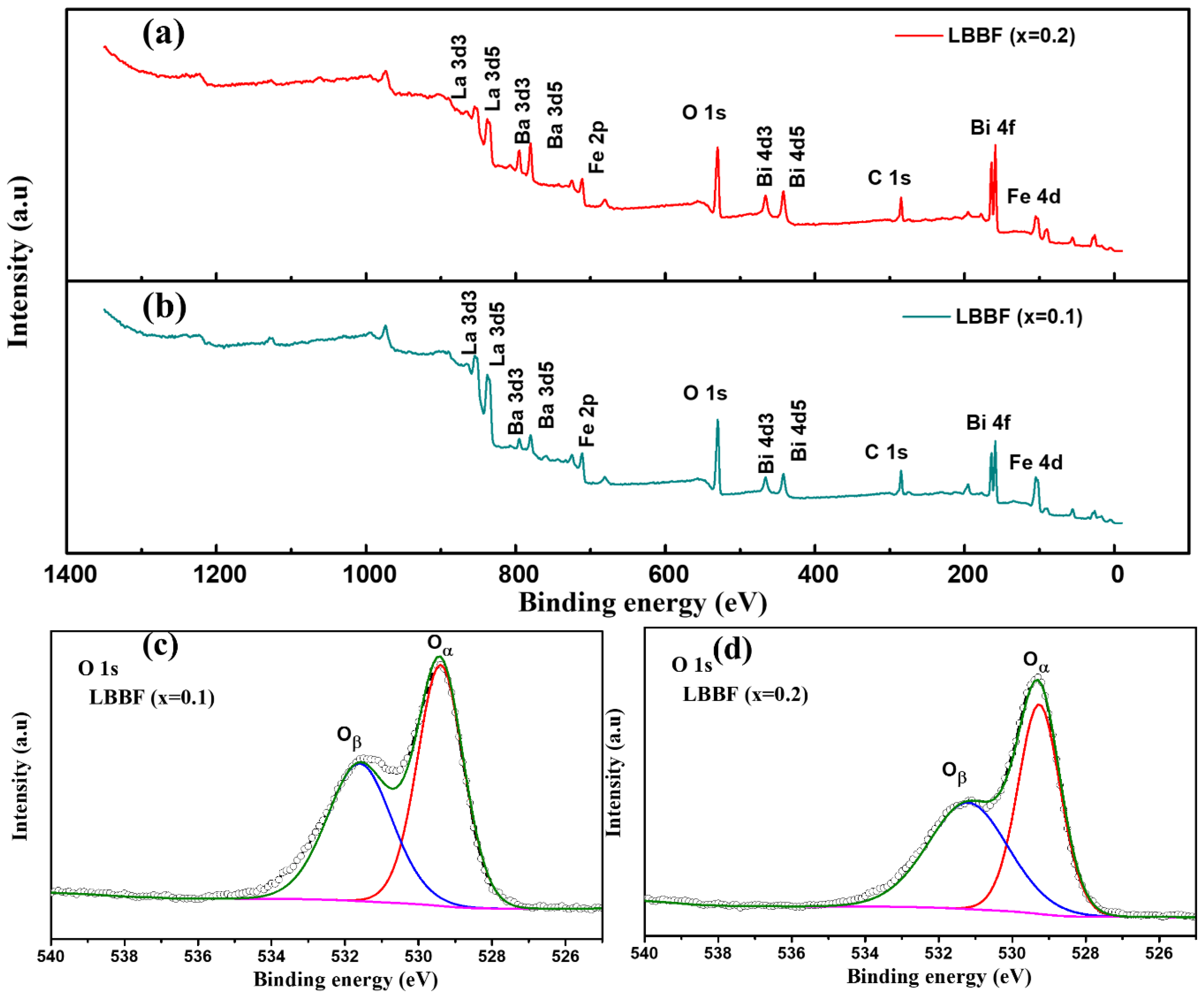
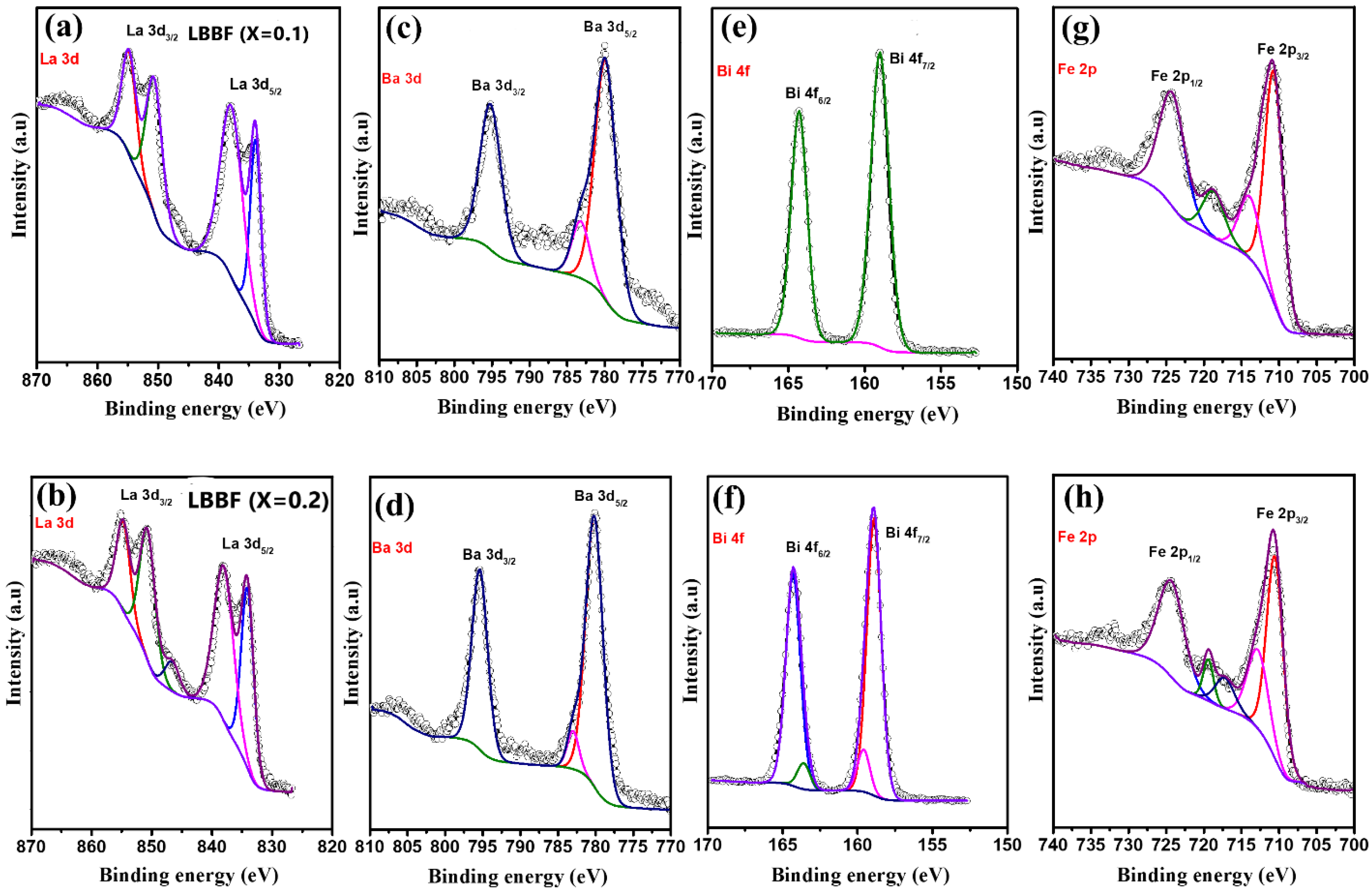
3.2. XPS Analysis of the LBBF Cathode
3.3. Electrochemical Performances
3.4. Electrochemical Impedance Spectra Analysis
Future Outlook
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, J.; Huang, J.; Wu, X.; Xu, Y.; Chen, H.; Li, X. Solid Oxide Fuel Cell (SOFC) Performance Evaluation, Fault Diagnosis and Health Control: A Review. J. Power Sources 2021, 505, 230058. [Google Scholar] [CrossRef]
- Al-Khori, K.; Bicer, Y.; Koc, M. Integration of Solid Oxide Fuel Cells into Oil and Gas Operations: Needs, Opportunities, and Challenges. J. Clean. Prod. 2020, 245, 118924. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Yan, M. A Review on the Preparation of Thin-Film YSZ Electrolyte of SOFCs by Magnetron Sputtering Technology. Sep. Purif. Technol. 2022, 298, 121627. [Google Scholar] [CrossRef]
- Duan, C.; Hook, D.; Chen, Y.; Tong, J.; O’Hayre, R. Zr and Y Co-Doped Perovskite as a Stable, High Performance Cathode for Solid Oxide Fuel Cells Operating below 500 C. Energy Environ. Sci. 2017, 10, 176–182. [Google Scholar] [CrossRef]
- Rehman, A.U.; Li, M.; Knibbe, R.; Khan, M.S.; Peterson, V.K.; Brand, H.E.A.; Li, Z.; Zhou, W.; Zhu, Z. Enhancing Oxygen Reduction Reaction Activity and CO2 Tolerance of Cathode for Low-Temperature Solid Oxide Fuel Cells by in Situ Formation of Carbonates. ACS Appl. Mater. Interfaces 2019, 11, 26909–26919. [Google Scholar] [CrossRef]
- Fleig, J. Solid Oxide Fuel Cell Cathodes: Polarization Mechanisms and Modeling of the Electrochemical Performance. Annu. Rev. Mater. Res. 2003, 33, 361–382. [Google Scholar] [CrossRef]
- Ioannidou, E.; Neofytidis, C.; Sygellou, L.; Niakolas, D.K. Au-Doped Ni/GDC as an Improved Cathode Electrocatalyst for H2O Electrolysis in SOECs. Appl. Catal. B Environ. 2018, 236, 253–264. [Google Scholar] [CrossRef]
- Aliotta, C.; Liotta, L.; Deganello, F.; La Parola, V.; Martorana, A. Direct Methane Oxidation as Potential Anode for Intermediate Temperature Solid Oxide Fuel Cells. Appl. Catal. B Environ. 2016, 180, 424–433. [Google Scholar] [CrossRef]
- Chiara, A.; Giannici, F.; Pipitone, C.; Longo, A.; Aliotta, C.; Gambino, M.; Martorana, A. Solid–Solid Interfaces in Protonic Ceramic Devices: A Critical Review. ACS Appl. Mater. Interfaces 2020, 12, 55537–55553. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Belotti, A.; Ciucci, F. P-Substituted Ba0. 95La0. 05FeO3− δ as a Cathode Material for SOFCs. ACS Appl. Energy Mater. 2019, 2, 5472–5480. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, W.; Ding, D.; Liu, M.; Ciucci, F.; Tade, M.; Shao, Z. Advances in Cathode Materials for Solid Oxide Fuel Cells: Complex Oxides without Alkaline Earth Metal Elements. Adv. Energy Mater. 2015, 5, 1500537. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Perovskite as a Cathode Material: A Review of Its Role in Solid-oxide Fuel Cell Technology. ChemElectroChem 2016, 3, 511–530. [Google Scholar]
- Wu, M.; Cai, H.; Jin, F.; Sun, N.; Xu, J.; Zhang, L.; Han, X.; Wang, S.; Su, X.; Long, W. Assessment of Cobalt–Free Ferrite–Based Perovskite Ln0.5Sr0. 5Fe0.9Mo0.1O3–δ (Ln= Lanthanide) as Cathodes for IT-SOFCs. J. Eur. Ceram. Soc. 2021, 41, 2682–2690. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Wang, Y.; Chen, F.; Xia, C. Bismuth Doped Lanthanum Ferrite Perovskites as Novel Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 11286–11294. [Google Scholar] [CrossRef] [PubMed]
- dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Recent Progress in Nanostructured Electrodes for Solid Oxide Fuel Cells Deposited by Spray Pyrolysis. J. Power Sources 2021, 507, 230277. [Google Scholar] [CrossRef]
- Xu, M.; Yu, J.; Song, Y.; Ran, R.; Wang, W.; Shao, Z. Advances in Ceramic Thin Films Fabricated by Pulsed Laser Deposition for Intermediate-Temperature Solid Oxide Fuel Cells. Energy Fuels 2020, 34, 10568–10582. [Google Scholar] [CrossRef]
- Ralph, J.M.; Rossignol, C.; Kumar, R. Cathode Materials for Reduced-Temperature SOFCs. J. Electrochem. Soc. 2003, 150, A1518. [Google Scholar] [CrossRef]
- Lee, S.J.; Yong, S.-M.; Kim, D.S.; Kim, D.K. Cobalt-Free Composite Cathode for SOFCs: Brownmillerite-Type Calcium Ferrite and Gadolinium-Doped Ceria. Int. J. Hydrog. Energy 2012, 37, 17217–17224. [Google Scholar] [CrossRef]
- Gao, J.; Li, Q.; Zhang, Z.; Lü, Z.; Wei, B. A Cobalt-Free Bismuth Ferrite-Based Cathode for Intermediate Temperature Solid Oxide Fuel Cells. Electrochem. Commun. 2021, 125, 106978. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kim-Lohsoontorn, P.; Bae, J. Effect of Unsintered Gadolinium-Doped Ceria Buffer Layer on Performance of Metal-Supported Solid Oxide Fuel Cells Using Unsintered Barium Strontium Cobalt Ferrite Cathode. J. Power Sources 2010, 195, 6420–6427. [Google Scholar] [CrossRef]
- Simner, S.; Anderson, M.; Bonnett, J.; Stevenson, J. Enhanced Low Temperature Sintering of (Sr, Cu)-Doped Lanthanum Ferrite SOFC Cathodes. Solid State Ionics 2004, 175, 79–81. [Google Scholar] [CrossRef]
- Wang, W.G.; Mogensen, M. High-Performance Lanthanum-Ferrite-Based Cathode for SOFC. Solid State Ionics 2005, 176, 457–462. [Google Scholar] [CrossRef]
- Ma, J.; Tao, Z.; Kou, H.; Fronzi, M.; Bi, L. Evaluating the Effect of Pr-Doping on the Performance of Strontium-Doped Lanthanum Ferrite Cathodes for Protonic SOFCs. Ceram. Int. 2020, 46, 4000–4005. [Google Scholar] [CrossRef]
- Hou, J.; Bi, L.; Qian, J.; Zhu, Z.; Zhang, J.; Liu, W. High Performance Ceria–Bismuth Bilayer Electrolyte Low Temperature Solid Oxide Fuel Cells (LT-SOFCs) Fabricated by Combining Co-Pressing with Drop-Coating. J. Mater. Chem. A 2015, 3, 10219–10224. [Google Scholar] [CrossRef]
- Sarat, S.; Sammes, N.; Smirnova, A. Bismuth Oxide Doped Scandia-Stabilized Zirconia Electrolyte for the Intermediate Temperature Solid Oxide Fuel Cells. J. Power Sources 2006, 160, 892–896. [Google Scholar] [CrossRef]
- Jung, D.W.; Lee, K.T.; Wachsman, E.D. Dysprosium and Gadolinium Double Doped Bismuth Oxide Electrolytes for Low Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2016, 163, F411. [Google Scholar] [CrossRef]
- Lee, K.T.; Jung, D.W.; Yoon, H.S.; Lidie, A.A.; Camaratta, M.A.; Wachsman, E.D. Interfacial Modification of La0.80Sr0.20MnO3−δ–Er0.4Bi0.63 Cathodes for High Performance Lower Temperature Solid Oxide Fuel Cells. J. Power Sources 2012, 220, 324–330. [Google Scholar] [CrossRef]
- Afzal, M.; Raza, R.; Du, S.; Lima, R.B.; Zhu, B. Synthesis of Ba0.3Ca0.7Co0.8Fe0.2O3-δ Composite Material as Novel Catalytic Cathode for Ceria-Carbonate Electrolyte Fuel Cells. Electrochim. Acta 2015, 178, 385–391. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, J.; Fan, Y.; Xi, X.; Li, J.; Lu, Y.; Huo, G.; Shao, L.; Fu, X.-Z.; Luo, J.-L. Pr 2 BaNiMnO 7− δ Double-Layered Ruddlesden–Popper Perovskite Oxides as Efficient Cathode Electrocatalysts for Low Temperature Proton Conducting Solid Oxide Fuel Cells. J. Mater. Chem. A 2020, 8, 7704–7712. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, Z.; Tu, B.; Cheng, M. Reaction Tuned Formation of Hierarchical BaCo0.4Fe0.4Zr0.1Y0.1O3-δ Cathode. J. Power Sources 2020, 455, 227971. [Google Scholar] [CrossRef]
- Yan, A.; Yang, M.; Hou, Z.; Dong, Y.; Cheng, M. Investigation of Ba1−XSrxCo0.8Fe0. 2O3−δ as Cathodes for Low-Temperature Solid Oxide Fuel Cells Both in the Absence and Presence of CO2. J. Power Sources 2008, 185, 76–84. [Google Scholar] [CrossRef]
- Kumar, R.V.; Khandale, A.P. A Review on Recent Progress and Selection of Cobalt-Based Cathode Materials for Low Temperature-Solid Oxide Fuel Cells. Renew. Sustain. Energy Rev. 2022, 156, 111985. [Google Scholar] [CrossRef]
- Li, L.; Qin, H.; Shi, C.; Zhang, L.; Chen, Y.; Hu, J. CO 2 Sensing Properties of La1−xBaxFeO3 Thick Film and Packed Powder Sensors. RSC Adv. 2015, 5, 103073–103081. [Google Scholar] [CrossRef]
- Yao, C.; Meng, J.; Liu, X.; Zhang, X.; Meng, F.; Wu, X.; Meng, J. Effects of Bi Doping on the Microstructure, Electrical and Electrochemical Properties of La2-XBixCu0.5Mn1.5O6 (X= 0, 0.1 and 0.2) Perovskites as Novel Cathodes for Solid Oxide Fuel Cells. Electrochim. Acta 2017, 229, 429–437. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Akbar, M.; Jin, B.; Tu, Z.; Mushtaq, N.; Wang, B.; Qu, X.; Xia, C.; Huang, Y. A Bulk-Heterostructure Nanocomposite Electrolyte of Ce0.8Sm0.2O2-δ–SrTiO3 for Low-Temperature Solid Oxide Fuel Cells. Nano-Micro Lett. 2021, 13, 46. [Google Scholar] [CrossRef]
- Lin, B.; Chen, J.; Ling, Y.; Zhang, X.; Jiang, Y.; Zhao, L.; Liu, X.; Meng, G. Low-Temperature Solid Oxide Fuel Cells with Novel La0. 6Sr0. 4Co0. 8Cu0. 2O3− δ Perovskite Cathode and Functional Graded Anode. J. Power Sources 2010, 195, 1624–1629. [Google Scholar] [CrossRef]
- Lu, Y.; Akbar, M.; Xia, C.; Mi, Y.; Ma, L.; Wang, B.; Zhu, B. Catalytic Membrane with High Ion–Electron Conduction Made of Strongly Correlated Perovskite LaNiO3 and Ce0.8Sm0.2O2-δfor Fuel Cells. J. Catal. 2020, 386, 117–125. [Google Scholar] [CrossRef]
- Akbar, M.; Qu, G.; Yang, W.; Gao, J.; Yousaf, M.; Mushtaq, N.; Wang, X.; Dong, W.; Wang, B.; Xia, C. Fast Ionic Conduction and Rectification Effect of NaCo0.5Fe0.5O2-CeO2 Nanoscale Heterostructure for LT-SOFC Electrolyte Application. J. Alloys Compd. 2022, 924, 166565. [Google Scholar] [CrossRef]
- Marinha, D.; Hayd, J.; Dessemond, L.; Ivers-Tiffée, E.; Djurado, E. Performance of (La, Sr)(Co, Fe) O3−x Double-Layer Cathode Films for Intermediate Temperature Solid Oxide Fuel Cell. J. Power Sources 2011, 196, 5084–5090. [Google Scholar] [CrossRef]
- Xiong, C.; Taillon, J.A.; Pellegrinelli, C.; Huang, Y.-L.; Salamanca-Riba, L.G.; Chi, B.; Jian, L.; Pu, J.; Wachsman, E.D. Long-Term Cr Poisoning Effect on LSCF-GDC Composite Cathodes Sintered at Different Temperatures. J. Electrochem. Soc. 2016, 163, F1091. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.; Lai, S.Y.; Gerdes, K.; Liu, M. Enhancing SOFC Cathode Performance by Surface Modification through Infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Tekale, S.U.; Pagore, V.P.; Kauthale, S.S.; Pawar, R.P. La2O3/TFE: An Efficient System for Room Temperature Synthesis of Hantzsch Polyhydroquinolines. Chinese Chem. Lett. 2014, 25, 1149–1152. [Google Scholar] [CrossRef]
- Akbar, M.; Jin, B.; Tu, Z.; Gao, J.; Yousaf, M.; Mushtaq, N.; Wang, X.; Dong, W.; Wang, B.; Cai, Y. High-Performing and Stable Non-Doped Ceria Electrolyte with Amorphous Carbonate Coating Layer for Low-Temperature Solid Oxide Fuel Cells. Electrochim. Acta 2021, 393, 139067. [Google Scholar] [CrossRef]
- Yousaf, M.; Akbar, M.; Shah, M.A.K.Y.; Noor, A.; Lu, Y.; Akhtar, M.N.; Mushtaq, N.; Hu, E.; Yan, S.; Zhu, B. Enhanced ORR Catalytic Activity of Rare Earth-Doped Gd Oxide Ions in a CoFe2O4 Cathode for Low-Temperature Solid Oxide Fuel Cells (LT-SOFCs). Ceram. Int. 2022, 48, 28142–28153. [Google Scholar] [CrossRef]
- Tu, Z.; Tian, Y.; Liu, M.; Jin, B.; Akbar, M.; Mushtaq, N.; Wang, X.; Dong, W.; Wang, B.; Xia, C. Remarkable Ionic Conductivity in a LZO-SDC Composite for Low-Temperature Solid Oxide Fuel Cells. Nanomaterials 2021, 11, 2277. [Google Scholar] [CrossRef]
- Khaled, A.; Pireaux, J.-J.; Khelili, S. Synthesis and Characterization of Ca and Ba Doped LAMOX Materials and Surface Study by X-Ray Photoelectron Spectroscopy. Acta Chim. Slov 2012, 59, 766–778. [Google Scholar]
- Craciun, V.; Singh, R.K. Characteristics of the Surface Layer of Barium Strontium Titanate Thin Films Deposited by Laser Ablation. Appl. Phys. Lett. 2000, 76, 1932–1934. [Google Scholar] [CrossRef]
- Rioult, M. Hematite-Based Epitaxial Thin Films as Photoanodes for Solar Water Splitting. Theses. Hal. Sci. 2015, 3709, 2240. [Google Scholar]
- Zhang, W.H.; Chen, L.; Tao, Y.T.; Chen, J.; Zhang, J.X. Raman Study of Barium Titanate with Oxygen Vacancies. Phys. B Condens. Matter 2011, 406, 4630–4633. [Google Scholar] [CrossRef]
- Felhi, H.; Smari, M.; Bajorek, A.; Nouri, K.; Dhahri, E.; Bessais, L. Controllable Synthesis, XPS Investigation and Magnetic Property of Multiferroic BiMn2O5 System: The Role of Neodyme Doping. Prog. Nat. Sci. Mater. Int. 2019, 29, 198–209. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; He, Q.; He, T. Double-Perovskites A2FeMoO6−δ (A = Ca, Sr, Ba) as Anodes for Solid Oxide Fuel Cells. J. Power Sources 2010, 195, 6356–6366. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; He, T.; Wang, J.; Su, W. The Effect of Pr Co-Dopant on the Performance of Solid Oxide Fuel Cells with Sm-Doped Ceria Electrolyte. J. Alloys Compd. 2005, 389, 317–322. [Google Scholar] [CrossRef]
- Liu, M.; Ding, D.; Bai, Y.; He, T.; Liu, M. An Efficient SOFC Based on Samaria-Doped Ceria (SDC) Electrolyte. J. Electrochem. Soc. 2012, 159, B661. [Google Scholar] [CrossRef]
- Saebea, D.; Authayanun, S.; Patcharavorachot, Y.; Chatrattanawet, N.; Arpornwichanop, A. Electrochemical Performance Assessment of Low-Temperature Solid Oxide Fuel Cell with YSZ-Based and SDC-Based Electrolytes. Int. J. Hydrogen Energy 2018, 43, 921–931. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Nikumb, S.; Decès-Petit, C.; Hui, R.; Maric, R.; Ghosh, D. Low Temperature Solid Oxide Fuel Cells with Pulsed Laser Deposited Bi-Layer Electrolyte. J. Power Sources 2007, 164, 182–188. [Google Scholar] [CrossRef]
- Xia, C.; Chen, F.; Liu, M. Reduced-Temperature Solid Oxide Fuel Cells Fabricated by Screen Printing. Electrochem. Solid-State Lett. 2001, 4, A52. [Google Scholar] [CrossRef]
- Akbar, M.; Tu, Z.; Jin, B.; Mushtaq, N.; He, Z.; Dong, W.; Wang, B.; Wang, X.; Xia, C. Demonstrating the Dual Functionalities of CeO2–CuO Composites in Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 9938–9947. [Google Scholar] [CrossRef]
- Dai, H.; Da’as, E.H.; Shafi, S.P.; Wang, H.; Bi, L. Tailoring Cathode Composite Boosts the Performance of Proton-Conducting SOFCs Fabricated by a One-Step Co-Firing Method. J. Eur. Ceram. Soc. 2018, 38, 2903–2908. [Google Scholar] [CrossRef]



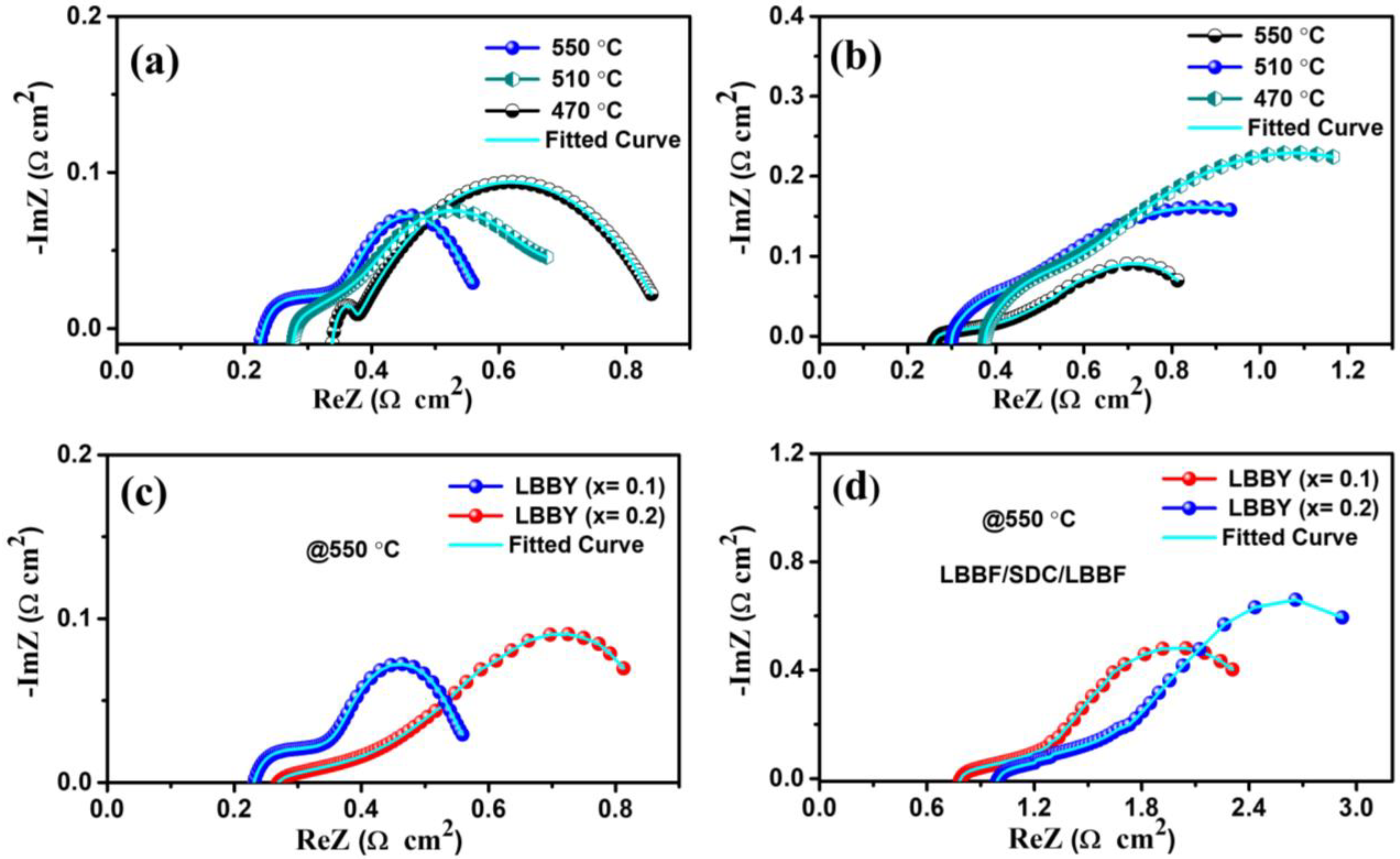
| Fuel Cells | T °C | Ro | Rct | Qct | N1 | C1 | Rmt | Qmt | N2 | C2 | Rp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LBBF (x = 0.1) | 550 | 0.22 | 0.06 | 0.65 | 0.7 | 0.161 | 0.27 | 0.65 | 0.8 | 0.42 | 0.33 |
| 510 | 0.27 | 0.07 | 0.76 | 0.8 | 0.364 | 0.35 | 0.93 | 0.7 | 0.57 | 0.42 | |
| 470 | 0.34 | 0.06 | 0.80 | 1 | 0.800 | 0.45 | 0.88 | 1 | 0.88 | 0.51 | |
| LBBF (x = 0.2) | 550 | 0.25 | 0.11 | 0.81 | 0.7 | 0.287 | 0.46 | 0.75 | 0.8 | 0.57 | 0.57 |
| 510 | 0.30 | 0.13 | 0.66 | 0.9 | 0.502 | 0.51 | 0.77 | 0.9 | 0.60 | 0.64 | |
| 470 | 0.37 | 0.15 | 0.88 | 0.8 | 0.530 | 0.66 | 0.96 | 0.8 | 0.85 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaheen, N.; Chen, Z.; Nong, Y.; Su, T.; Yousaf, M.; Lu, Y.; Li, L. Enhancing ORR Catalytic Activity and Electrochemical Investigation of La1−2xBaxBixFeO3 Cathode for Low-Temperature Solid Oxide Fuel Cell. Crystals 2023, 13, 822. https://doi.org/10.3390/cryst13050822
Shaheen N, Chen Z, Nong Y, Su T, Yousaf M, Lu Y, Li L. Enhancing ORR Catalytic Activity and Electrochemical Investigation of La1−2xBaxBixFeO3 Cathode for Low-Temperature Solid Oxide Fuel Cell. Crystals. 2023; 13(5):822. https://doi.org/10.3390/cryst13050822
Chicago/Turabian StyleShaheen, Nusrat, Zheng Chen, Yumei Nong, Tao Su, Muhammad Yousaf, Yuzheng Lu, and Ling Li. 2023. "Enhancing ORR Catalytic Activity and Electrochemical Investigation of La1−2xBaxBixFeO3 Cathode for Low-Temperature Solid Oxide Fuel Cell" Crystals 13, no. 5: 822. https://doi.org/10.3390/cryst13050822
APA StyleShaheen, N., Chen, Z., Nong, Y., Su, T., Yousaf, M., Lu, Y., & Li, L. (2023). Enhancing ORR Catalytic Activity and Electrochemical Investigation of La1−2xBaxBixFeO3 Cathode for Low-Temperature Solid Oxide Fuel Cell. Crystals, 13(5), 822. https://doi.org/10.3390/cryst13050822






