Synthesis, Spectroscopic Characterization and Thermal Studies of Polymer-Metal Complexes Derived from Modified Poly Styrene-Alt-(Maleic Anhydride) as a Prospects for Biomedical Applications
Abstract
1. Introduction
2. Experimental
Materials and Measurements
- Preparation of coordination polymers Ligand (PSMAP)
- Preparation of coordination polymers Ligand (PSMAM)
- Synthesis of [M(PSMAP)2(Cl)2](H2O)n (M = Mn2+, Ni2+, Co2+ and Cu2+) complexes
- Synthesis of [M(PSMAM)2(Cl)2](H2O)n (M = Mn2+, Ni2+, Co2+ and Cu2+) complexes
- [Mn(PSMAP)2Cl2]2H2O (1)
- [Mn(PSMAM)2Cl2]2H2O (2)
- [Ni(PSMAP)2Cl2]2H2O (3)
- [Ni(PSMAM)2Cl2]2H2O (4)
- [Co(PSMAP)2Cl2]H2O (5)
- [Co(PSMAM)2Cl2]2H2O (6)
- [Cu(PSMAP)2Cl2]H2O (7)
- [Cu(PSMAM)2Cl2]2H2O (8)
3. Results and Discussion
3.1. Elemental and Conductance Data
3.2. FT-IR Analysis
3.3. Electronic Spectra and Magnetic Investigation of Metal Polymer Complexes
3.4. Thermal Studies
3.5. SEM and TEM Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Q.; Xu, Y.; Yan, X.; Jiang, T.; Jiang, Y. Synthetic approaches to metal-coordination-directed macrocyclic complexes. Front. Chem. 2022, 10, 1078432. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; Meraz, M.M.; Yang, W.; Zhang, Q.; Sage, D.D.; Sun, W.H. Catalytic Performance of Cobalt(II) Polyethylene Catalysts with Sterically Hindered. Dibenzopyranyl Substituents Studied by Experimental and MLR Methods. Molecules 2022, 27, 5455. [Google Scholar] [CrossRef] [PubMed]
- Sassone, D.; Zeng, J.; Fontana, M.; Sacco, A.; Farkhondehfal, M.A.; Periolatto, M.; Pirri, C.F.; Bocchini, S. Polymer-metal complexes as emerging catalysts for electrochemical reduction of carbon dioxide. J. Appl. Electrochem. 2021, 51, 1301–1311. [Google Scholar] [CrossRef]
- Anderegg, G.; Arnaud-Neu, F.; Delgado, R.; Felcman, J.; Popov, K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1445–1495. [Google Scholar] [CrossRef]
- Wei, Z.; Duan, H.; Weng, G.; He, J. Metals in polymers: Hybridization enables new functions. J. Mater. Chem. C 2020, 8, 15956–15980. [Google Scholar] [CrossRef]
- Téllez, F.; Peña-Hueso, A.; Barba-Behrens, N.; Contreras, R.; Flores-Parra, A. Coordination compounds in a pentacyclic aromatic system from 2-aminobenzothiazole derivatives and transition metal ions. Polyhedron 2006, 25, 2363–2374. [Google Scholar] [CrossRef]
- Yousif, E.; Farina, Y.; Kasar, K.; Graisa, A.; Ayid, K. Complexes of 2-thioacetic acid benzothiazole with some metal ions. Am. J. Appl. Sci. 2009, 6, 582–585. [Google Scholar] [CrossRef]
- Mbaba, M.; Golding, T.M.; Smith, G.S. Recent advances in the biological investigation of organometallic platinum-group metal (Ir, Ru, Rh, Os, Pd, Pt) complexes as antimalarial agents. Molecules 2020, 25, 5276. [Google Scholar] [CrossRef]
- Li, J.; Li, J. A luminescent porous metal–organic framework with Lewis basic pyridyl sites as a fluorescent chemosensor for TNP detection. Inorg. Chem. Commun. 2018, 89, 51–54. [Google Scholar] [CrossRef]
- Mathew, B.; Madhusudanan, P.M.; Pillai, V.R. Effect of the nature of crosslinking agents on the thermal decomposition of metal complexes of crosslinked polyacrylamide-supported dithiocarbamates. Thermochim. Acta 1992, 207, 265–277. [Google Scholar] [CrossRef]
- Jose, L.; Pillai, V.N.R. Catalase-like activity of divinylbenzene (DVB)-crosslinked polyacrylamide supported amino metal complexes. Eur. Polym. J. 1966, 32, 1431–1435. [Google Scholar] [CrossRef]
- El-Sonbati, A.Z.; Diab, M.A.; El-Bindary, A.A. Stoichiometry of Polymer Complexes. Polym. Deg. Stab. 1990, 29, 271–277. [Google Scholar]
- Yasuyoshi, M.; Hiroshi, Y.; Yutaka, F. Structural Investigation of Iron(III) and Copper(II) Complexes with Poly (vinyl alcohol) by NMR Techniques. Polym. J. 1995, 27, 271–279. [Google Scholar]
- Rogers, D.R.; Bond, A.H.; Aguinaga, S.; Reyers, A. Polyethylene glycol complexation of Cd2+. Structures of triethylene glycol complexes of CdCl2, CdBr2 and CdI2. Inorg. Chim. Acta 1993, 212, 225–231. [Google Scholar] [CrossRef]
- An, Y.; Ushida, T.; Suzuki, M.; Koyama, T.; Hanabusa, K.; Shirai, H. Complex formation of partially phosphorylated poly (vinyl alcohol), with metal ions in aqueous solution. Polymer 1996, 37, 3097–3100. [Google Scholar] [CrossRef]
- Amiri, N.; Hajji, M.; Roisnel, T.; Simonneaux, G.; Nasri, H. Synthesis, molecular structure, photophysical properties and spectroscopic characterization of new 1D-magnesium(II) porphyrin-based coordination polymer. Res. Chem. Intermed. 2018, 44, 5583–5595. [Google Scholar]
- Li, J.; Wang, X.; Li, R.; Zhang, W.; Bai, H.; Liu, Y.; Liu, Z.; Yu, T.; Liu, Z.; Yang, Y.; et al. Twelve cadmium(II) coordination frameworks with asymmetric pyridinyl triazole carboxylate: Syntheses, structures, and fluorescence properties. Cryst. Growth Des. 2019, 19, 3785–3806. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.; Zava, O.; Deschenaux, R.; Therrien, B. Encapsulation of pyrene-functionalized poly (benzyl ether) dendrons into a water-soluble organometallic cage. Chem. Asian J. 2011, 6, 1595–1603. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Tang, G.; Chu, P.K.; Bai, H. A zipped-up tunable metal coordinated cationic polymer for nanomedicine. J. Mater. Chem. B 2020, 8, 1350–1358. [Google Scholar] [CrossRef]
- Qi, X.; Li, J.; Wei, W.; Zuo, G.; Su, T.; Pan, X.; Zhang, J.; Dong, W. Cationic Salecan-based hydrogels for release of 5-fluorouracil. RSC Adv. 2017, 7, 14337–14347. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Zuo, G.; Hu, X.; Zhang, J.; Dong, W. Development of novel hydrogels based on Salecan and poly (N-isopropylacrylamide-co-methacrylic acid) for controlled doxorubicin release. RSC Adv. 2016, 6, 69869–69881. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Li, J.; Zuo, G.; Sheng, W.; Zhang, J.; Dong, W. Smart macroporous salecan/poly (N, N-diethylacrylamide) semi-IPN hydrogel for anti-inflammatory drug delivery. ACS Biomater. Sci. Eng. 2016, 2, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhao, W.; Wu, L.; Dong, W.; Qi, X. Facile fabrication of functional hydrogels consisting of pullulan and polydopamine fibers for drug delivery. Int. J. Biol. Macromol. 2020, 163, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Xu, F.; Wang, C.; Li, Y.; Chen, Y.; Li, X.; Lu, Z.; Wang, D. A multifunctional metal-biopolymer coordinated double network hydrogel combined with multi-stimulus responsiveness, self-healing, shape memory and antibacterial properties. Biomater. Sci. 2020, 8, 3193–3201. [Google Scholar] [CrossRef]
- Wei, Z.; Thanneeru, S.; Rodriguez, E.M.; Weng, G.; He, J. Adaptable Eu-containing polymeric films with dynamic control of mechanical properties in response to moisture. Soft Matter 2020, 16, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Sun, S.; Sun, R.; Cui, G.; Hong, L.; Rao, B.; Li, A.; Yu, Z.; Kan, Q.; Mao, Z. A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy. Adv. Mater. 2020, 32, 1906024. [Google Scholar] [CrossRef]
- Tao, B.; Yin, Z. Redox-responsive coordination polymers of dopamine-modified hyaluronic acid with copper and 6-mercaptopurine for targeted drug delivery and improvement of anticancer activity against cancer cells. Polymers 2020, 12, 1132. [Google Scholar] [CrossRef]
- Kondo, A.; Noro, S.I.; Kajiro, H.; Kanoh, H. Structure- and phase-transformable coordination polymers/metal complexes with fluorinated anions. Coord. Chem. Rev. 2022, 471, 214728. [Google Scholar] [CrossRef]
- Amiri, N.; Taheur, F.B.; Chevreux, S.; Wenger, E.; Lemercier, G.; Nasri, H. Synthesis, crystal structure and spectroscopic characterizations of porphyrin-based Mg(II) complexes—Potential application as antibacterial agent. Tetrahedron 2017, 73, 7011–7016. [Google Scholar] [CrossRef]
- Maksimov, A.; Vagapova, A.; Kutyreva, M.; Kutyrev, G. Polymer metal-organic clusters based on hyperbranched polyester polybenzoylthiocarbamate and Cu(II) and Co(II) ions. J. Mol. Struct. 2022, 1258, 132575. [Google Scholar] [CrossRef]
- Rashid, S.; Shen, C.; Yang, J.; Liu, J.; Li, J. Preparation and properties of chitosan–metal complex: Some factors influencing the adsorption capacity for dyes in aqueous solution. J. Environ. Sci. 2018, 66, 301–309. [Google Scholar] [CrossRef]
- Murali, A.; Sarswat, P.K.; Benedict, J.; Plummer, M.J.; Shine, A.E.; Free, M.L. Determination of metallic and polymeric contents in electronic waste materials and evaluation of their hydrometallurgical recovery potential. Int. J. Environ. Sci. Technol. 2022, 19, 2295–2308. [Google Scholar]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Tu, L.; Fan, Z.; Zhu, F.; Zhang, Q.; Zeng, S.; Chen, Z.; Ren, L.; Hou, Z.; Ye, S.; Li, Y. Self-recognizing and stimulus-responsive carrier free metal-coordinated nanotheranostics for magnetic resonance/photoacoustic/fluorescence imaging-guided synergistic photochemotherapy. J. Mater. Chem. B 2020, 8, 5667–5681. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.Q.; Rodrigues, C.F.; Fernandes, N.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Metal-Polymer Nanoconjugates Application in Cancer Imaging and Therapy. Nanomaterials 2022, 12, 3166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Shi, R.; Yao, J.F.; Sheng, C.F.; Li, H. Supramolecular self-assembly of nucleotide–metal coordination complexes: From simple molecules to nanomaterials. Coord. Chem. Rev. 2015, 292, 107–143. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, B.; Bishnoi, S.; Mishra, S.K.; Nayak, D.; Kumar, A.; Sarma, T.K. Multifunctional inosine monophosphate coordinated metal-organic hydrogel: Multistimuli responsiveness, self-healing properties, and separation of water from organic solvents. ACS Sustain. Chem. Eng. 2018, 6, 8659–8671. [Google Scholar] [CrossRef]
- Ezzayani, K.; Khelifa, A.B.; Taheur, F.B.; Guergueb, M.; Mansour, A.; Daran, J.C.; Nasri, H. Building-up novel coordination polymer with magnesium porphyrin: Synthesis, molecular structure, photophysical properties and spectroscopic characterization. Potential application as antibacterial agent. Inorg. Chim. Acta 2021, 514, 119960. [Google Scholar] [CrossRef]
- Tabasi, H.; Babaei, M.; Abnous, K.; Taghdisi, S.M.; Saljooghi, A.S.; Ramezani, M.; Alibolandi, M. Metal–polymer-coordinated complexes as potential nanovehicles for drug delivery. J. Nanostructure Chem. 2021, 11, 501–526. [Google Scholar]
- Kaliyappan, T.; Swaminathan, C.S.; Kannan, P. Synthesis and characterization of a new metal chelating polymer and derived Ni(II) and Cu(II) polymer complexes. Polymer 1996, 37, 2865–2869. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Ren, L.; Tang, C. Metal-containing and Related Polymers for Biomedical Applications. Chem. Soc. Rev. 2016, 45, 5232–5263. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A. Metal Binding Proteins. Encyclopedia 2021, 1, 261–292. [Google Scholar] [CrossRef]
- Gucwa, M.; Lenkiewicz, J.; Zheng, H.; Cymborowski, M.; Cooper, D.R.; Murzyn, K.; Minor, W. CMM—An enhanced platform for interactive validation of metal binding sites. Protein Sci. 2023, 32, e4525. [Google Scholar] [CrossRef]
- Williams, C.K.; Nozaki, K. Metal Complexes for Catalytic Polymerizations. Inorg. Chem. 2020, 59, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 27, 81–2804. [Google Scholar] [CrossRef]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Magnetic nanoporous coordination polymers. J. Mater. Chem. 2004, 14, 2713–2723. [Google Scholar] [CrossRef]
- Kukla, S.; Cimrova, V.; Vyprachticky, D. Terbium binding in highly luminescent polymer complexes. Collect. Czech. Chem. Commun. 2006, 71, 1332–1349. [Google Scholar] [CrossRef]
- Jima’a, R.B.; Shaalan, N.D. Synthesis, Characterization, and Biological Activity of New Metal Ion Complexes with Schiff Base (Z)-3((E)-2-Hydroxybenzylidene) hydrazineylidene) indolin-2-one. J. Med. Chem. Sci. 2023, 6, 1660–1674. [Google Scholar]
- Soto-Acosta, S.; Campos-Gaxiola, J.J.; Reynoso-Soto, E.A.; Cruz-Enríquez, A.; Baldenebro-López, J.; Höpfl, H.; García, J.J.; Flores-Álamo, M.; Miranda-Soto, V.; Glossman-Mitnik, D. Synthesis, Crystal Structure, DFT Studies and Optical/Electrochemical Properties of Two Novel Heteroleptic Copper(I) Complexes and Application in DSSC. Crystals 2022, 12, 1240. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Kim, H.J.; Tewolde, H.; Zhang, H. Fourier transform infrared spectral features of plant biomass components during cotton organ development and their biological implications. J. Cotton Res. 2022, 5, 11. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Altalhi, T.; Bakare, S.B.; Al-Hazmi, G.H.; Alam, K. New Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Zn(II), Cd(II), and Hg(II) Gibberellate Complexes: Synthesis, Structure, and Inhibitory Activity Against COVID-19 Protease. Russ. J. Gen. Chem. 2021, 91, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Misawa-Suzuki, T.; Ikeda, R.; Komatsu, R.; Toriba, R.; Miyamoto, R.; Nagao, H. Geometry and electronic structures of cobalt(II) and iron(III) complexes bearing bis(2-pyridylmethyl)ether or alkylbis(2-pyridylmethyl)amine. Polyhedron 2022, 218, 115735. [Google Scholar] [CrossRef]
- Nabil, N.; Adly, O.M.; Shebl, M.; Taha, A.; Samy, F. NiII and CoII binary and ternary complexes of 3-formylchromone: Spectroscopic characterization, antimicrobial activities, docking and modeling studies. RSC Adv. 2022, 12, 29939–29958. [Google Scholar] [CrossRef] [PubMed]
- Aljuhani, E.; Aljohani, M.M.; Alsoliemy, A.; Shah, R.; Abumelha, H.M.; Saad, F.A.; Hossan, A.; Al-Ahmed, Z.A.; Alharbi, A.; El-Metwaly, N.M. Synthesis and characterization of Cu(II)-pyrazole complexes for possible anticancer agents; conformational studies as well as compatible in-silico and in-vitro assays. Heliyon 2021, 7, e08485. [Google Scholar] [CrossRef]
- Drzewiecka-Antonik, A.; Ferenc, W.; Mirosław, B.; Osypiuk, D.; Sarzyński, J. Structure, thermal stability and magnetic behavior of Mn(II) complexes with phenoxyacetic acid herbicides. Polyhedron 2021, 207, 115370. [Google Scholar] [CrossRef]
- Yallur, B.C.; Krishna, P.M.; Challa, M. Bivalent Ni(II), Co(II) and Cu(II) complexes of [(E)-[(2-methyl-1,3-thiazol-5-yl)methylidene]amino]thiourea: Synthesis, spectral characterization, DNA and in-vitro anti-bacterial studies. Heliyon 2021, 7, e06838. [Google Scholar] [CrossRef]
- Arab Ahmadi, R.; Hasanvand, F.; Bruno, G.; Amiri Rudbari, H.; Amani, S. Synthesis, Spectroscopy, and Magnetic Characterization of Copper(II) and Cobalt(II) Complexes with 2-Amino-5-bromopyridine as Ligand. ISRN Inorg. Chem. 2013, 7. [Google Scholar] [CrossRef]
- Comarmond, J.; Plumere, P.; Lehn, J.M.; Agnus, Y.; Louis, R.; Weiss, R.; Kahn, O.; Morgenstern-Badarau, I. Dinuclear copper(II) cryptates of macrocyclic ligands: Synthesis, crystal structure, and magnetic properties. Mechanism of the exchange interaction through bridging azido ligands. J. Am. Chem. Soc. 1982, 23, 6330–6340. [Google Scholar] [CrossRef]
- Hasanvand, F.; Hoseinzadeh, A.; Zolgharnein, J.; Amani, S. Synthesis and characterization of two acetato-bridged dinuclear copper(II) complexes with 4-bromo-2-((4 or 6-methylpyridin-2-ylimino)methyl)phenol as ligand. J. Coord. Chem. 2010, 63, 346–352. [Google Scholar] [CrossRef]
- Komaei, S.A.; Albada, G.A.; Reedijk, J. Synthesis, spectroscopic and magnetic properties of methoxo-bridged copper(II) complexes with 2-amino-4-methylpyridine as the ligand. Transit. Met. Chem. 1999, 24, 104–107. [Google Scholar] [CrossRef]
- Rodríguez, L.; Labisbal, E.; Sousa-Pedrares, A.; García-Vázquez, J.A.; Romero, J.; Durán, M.L.; Real, J.A.; Sousa, A. Coordination chemistry of amine bis(phenolate) cobalt(II), nickel(II), and copper(II) complexes. Inorg. Chem. 2006, 45, 7903–7914. [Google Scholar] [CrossRef]
- Chandra, S.; Gupta, L.K.; Agrawal, S. Synthesis spectroscopic and biological approach in the characterization of novel [N4] macrocyclic ligand and its transition metal complexes. Transit. Met. Chem. 2007, 32, 558–563. [Google Scholar] [CrossRef]
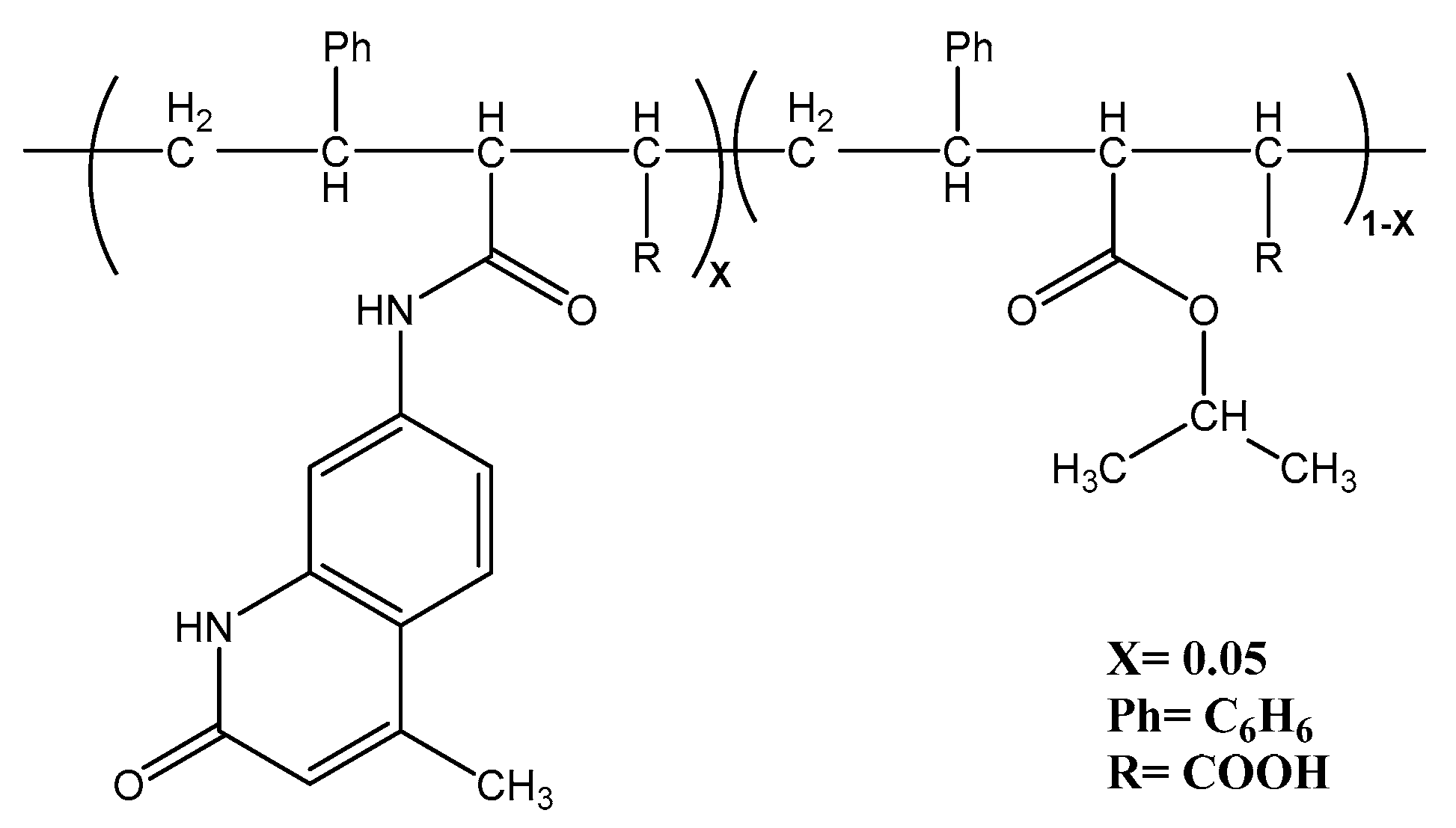
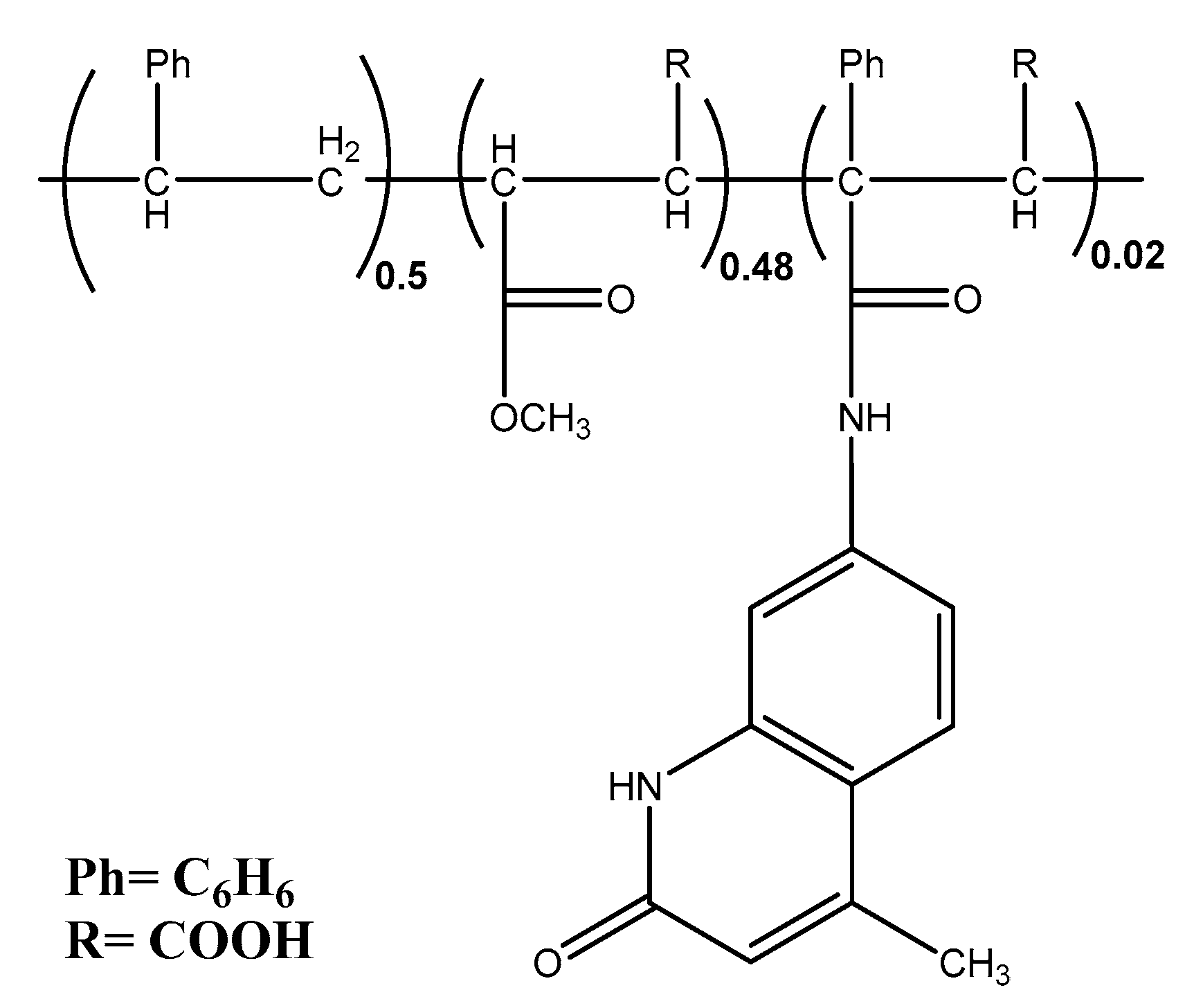
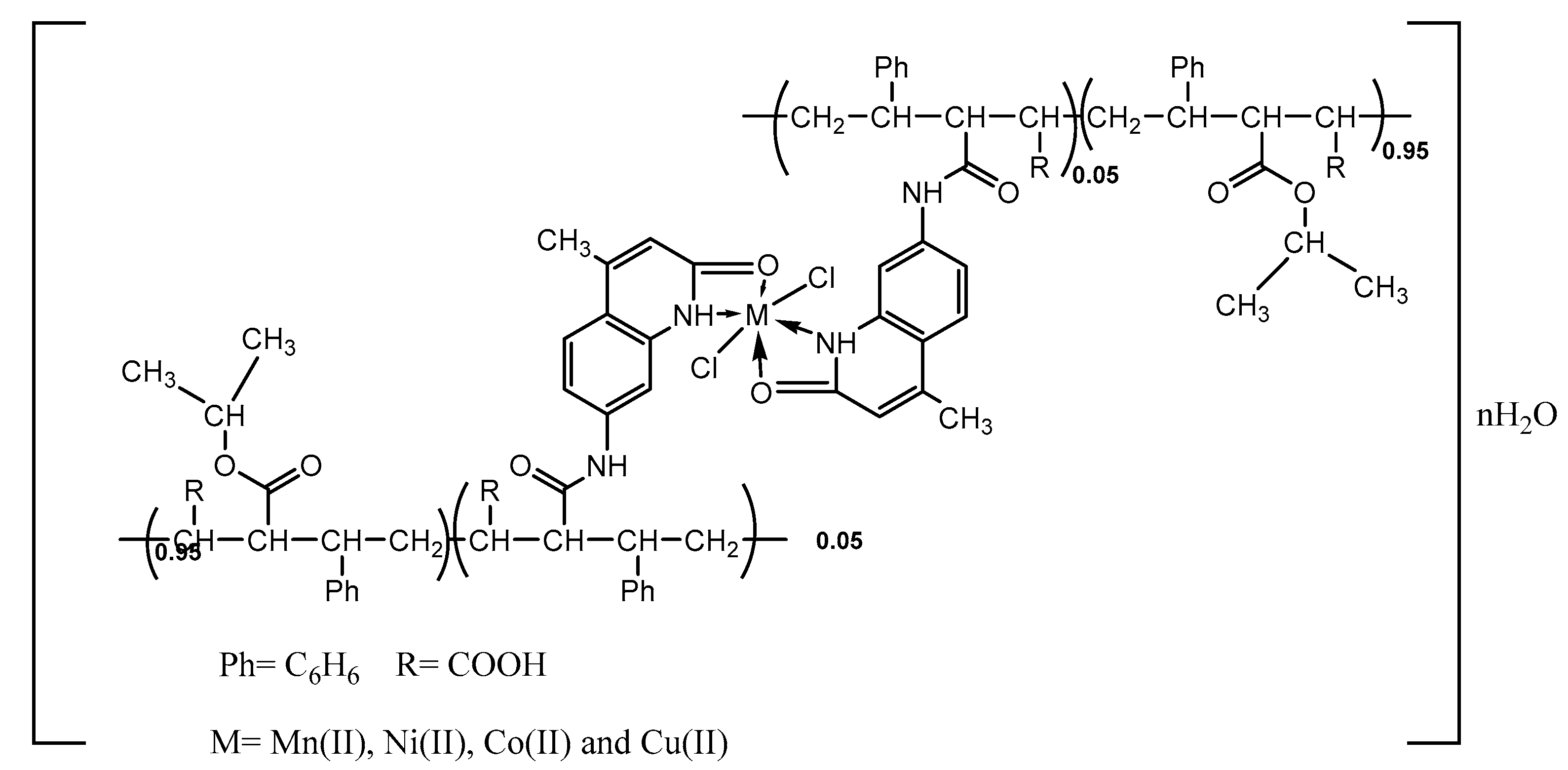

| No | Compound | Yield% | Color and m.p (°C) | Elemental Analysis Found (calcd.) % | |||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| C51H85N2O8 (PSMAP) | 79 | - | White ˃ 300 °C | 71.61 (71.62) | 9.83 (9.98) | 3.18 (3.21) | |
| C42H72N2O8 (PSMAM) | 64 | - | White ˃ 300 °C | 66.85 (66.82) | 9.70 (9.85) | 3.62 (3.75) | |
| (1) | [Mn(PSMAP)2Cl2]·2H2O | 78 | 7 | Light brown ˃ 300 °C | 65.47 (65.57) | 9.12 (9.28) | 3.08 (3.00) |
| (2) | [Mn(PSMAM)2Cl2]·2H2O | 64 | 13 | Light brown ˃ 300 °C | 61.33 (61.97) | 9.14 (9.16) | 3.38 (3.44) |
| (3) | [Ni(PSMAP)2Cl2]·2H2O | 63 | 10 | Green ˃ 300 °C | 66.53 (66.63) | 9.40 (9.54) | 3.17 (3.19) |
| (4) | [Ni(PSMAM)2Cl2]·2H2O | 77 | 8 | Green ˃ 300 °C | 61.17 (61.83) | 9.22 (9.14) | 3.57 (3.60) |
| (5) | [Co(PSMAP)2Cl2]·H2O | 75 | 10 | Purple ˃ 300 °C | 66.12 (66.00) | 9.30 (9.34) | 3.03 (3.02) |
| (6) | [Co(PSMAM)2Cl2]·2H2O | 73 | 12 | Purple ˃ 300 °C | 61.76 (61.82) | 9.12 (9.14) | 3.47 (3.43) |
| (7) | [Cu(PSMAP)2Cl2]·H2O | 71 | 12 | Blue ˃ 300 °C | 65.70 (65.83) | 9.30 (9.32) | 3.00 (3.01) |
| (8) | [Cu(PSMAM)2Cl2]·2H2O | 75 | 8 | Blue ˃ 300 °C | 61.66 (61.65) | 9.15 (9.12) | 3.36 (3.42) |
| Polymer | O-H Stretching | C-H Stretching | C=O Stretching | Amide Bond | C-C Ring Stretching | C-O-H Bending | C-O-C Anti Sys Stretching | C-H Out-of Plane, Ring Bending | M-O | M-N |
|---|---|---|---|---|---|---|---|---|---|---|
| PSMAP | ــــــ | 3029, 2926 | 1718 | 1653 | 1496 | 1455 | 1400 | 1200, 1171 | ــــــ | ــــــ |
| PSMAM | ــــــ | 3029, 2926 | 1718 | 1653 | 1495 | 1455 | 1405 | 1200, 1171 | ــــــ | ــــــ |
| (1) | 3361 | 3038, 2946 | 1724 | 1563 | 1494 | 1442 | 1402 | 1212, 1166 | 699 | 521 |
| (2) | 3345 | 3021, 2944 | 1703 | 1562 | 1497 | 1447 | 1405 | 1210, 1167 | 705 | 537 |
| (3) | 3371 | 3028, 2947 | 1710 | 1556 | 1488 | 1430 | 1401 | 1212, 1173 | 704 | 532 |
| (4) | 3360 | 3028, 2944 | 1710 | 1556 | 1488 | 1430 | 1401 | 1213, 1173 | 704 | 532 |
| (5) | 3381 | 3018, 2943 | 1700 | 1553 | 1494 | 1436 | 1408 | 1217, 1166 | 693 | 538 |
| (6) | 3366 | 3035, 2944 | 1717 | 1561 | 1490 | 1450 | 1401 | 1210, 1161 | 703 | 515 |
| (7) | 3377 | 3028, 2953 | 1707 | 1565 | 1490 | 1440 | 1402 | 1210, 1172 | 705 | 524 |
| (8) | 3372 | 3024, 2935 | 1707 | 1563 | 1493 | 1415 | 1404 | 1213, 1168 | 705 | 534 |
| Compounds | Spectral Data | Electronic Transition | Assignments | |
|---|---|---|---|---|
| PSMAP-Mn(II) (1) | 16,233 27,397 36,764 | 6A1g → 4T1g (G) 6A1g → 4T2g (G) 6A1g → 4A1g (G), 4Eg (G) | 5.40 | Octahedral |
| PSMAM-Mn(II) (2) | 16,666 31,347 38,461 | 6A1g → 4T1g (G) 6A1g → 4T2g (G) 6A1g → 4A1g (G), 4Eg (G) | 5.78 | Octahedral |
| PSMAP-Ni(II) (3) | 16,474 21,929 28,985 | 3A2g (F) → 3T1g (P) 3A2g (F) → 3T1g (F) 3A2g (F) → 3T2g (F) | 3.07 | Octahedral |
| PSMAM-Ni(II) (4) | 17,241 22,026 31,250 | 3A2g (F) → 3T1g (P) 3A2g (F) → 3T1g (F) 3A2g (F) → 3T2g (F) | 3.18 | Octahedral |
| PSMAP-Co(II) (5) | 18,181 14,492 | 4T1g (F) → 4T1g (P) 4T1g (F) → 4A2g (F) | 4.98 | Octahedral |
| PSMAM-Co(II) (6) | 17,605 14,749 | 4T1g (F) → 4T1g (P) 4T1g (F) → 4A2g (F) | 4.86 | Octahedral |
| PSMAP-Cu(II) (7) | 24.376 17.322 14.556 | charge transfer band d-d transitions 2Eg → 2T2g | 2.00 | Octahedral |
| PSMAM-Cu(II) (8) | 24.376 17.322 14.556 | charge transfer band d-d transitions 2Eg → 2T2g | 1.58 | Octahedral |
| No | Compounds | Weight Loss (%) | Lost Species | ||
|---|---|---|---|---|---|
| TGA Range (°C) | Found | Calc. | |||
| C51H85N2O8 | 30–100 100–330 330–450 | 18.14 26.18 55.68 | 18.26 26.22 55.72 | 6C2H2 8CO C31H73N2 | |
| C42H72N2O8 | 30–100 100–320 320–450 | 17.69 30.58 51.39 | 17.73 30.56 51.57 | 5C2H2 8CO C24H62N2 | |
| (1) | C102H174Cl2MnN4O18 | 30–115 115–384 384–450 450–800 | 1.89 20.51 71.41 6.19 | 1.92 20.53 71.98 5.57 | 2H2O 2Cl + 4NO2 + 5C2H2 C92H160O7 MnO↓ + 2C↓ |
| (2) | C84H148Cl2MnN4O18 | 20–120 120–370 370–450 45–800 | 2.18 20.39 72.10 5.33 | 2.21 20.43 72.02 5.34 | 2H2O 2Cl + 3C2H2 + 4NO2 C78H138O7 MnO↓ + C↓ |
| (3) | C102H174ClN4NiO18 | 30–120 120–300 300–420 420–800 | 1.85 23.10 70.16 4.89 | 1.96 23.26 70.15 4.63 | 2.5H2O 2Cl + 3NO2 + 8C2H2 C86H154NO9 NiO↓ + 0.5C↓ |
| (4) | C84H148Cl2N4NiO18 | 30–130 130–300 300–400 400–700 | 2.15 25.16 67.20 5.49 | 2.21 25.13 67.28 5.38 | 2H2O 2Cl + 4NO2 + 6C2H2 C72H132O7 NiO↓ + C↓ |
| (5) | C102H172Cl2CoN4O17 | 30–130 130–350 350–450 450–780 | 0.92 26.32 67.90 4.86 | 0.97 26.28 67.88 4.87 | H2O 2Cl + 4NO2 + 9C2H2 C84H154O6 CoO↓ + C↓ |
| (6) | C84H148Cl2CoN4O18 Mw = 1631.93 | 30–150 150–300 300–430 430–780 | 2.11 25.19 64.05 8.65 | 2.21 25.12 66.24 6.43 | 2H2O 2Cl + 4NO2 + 6C2H2 C72H136O5 CoO↓ + 2.5 C↓ |
| (7) | C102H172Cl2CuN4O17 | 25–200 200–300 300–450 450–800 | 0.91 23.35 70.50 5.24 | 0.97 23.43 70.51 5.09 | H2O 2Cl + 4NO2 + 7C2H2 C88H158O6 CuO↓ + C↓ |
| (8) | C84H148Cl2CuN4O18 | 30–120 120–220 220–450 450–790 | 2.21 29.82 61.25 6.72 | 2.20 29.86 61.38 6.56 | 2H2O 2Cl + 4NO2 + 12C2H2 C66H130O5 CuO↓ + 2C↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almehizia, A.A.; Alkahtani, H.M.; Al-Omar, M.A.; Obaidullah, A.J.; Bhat, M.A.; Alrasheed, L.S.; Naglah, A.M.; Younes, A.A.O.; Alsuhaibani, A.M.; Refat, M.S.; et al. Synthesis, Spectroscopic Characterization and Thermal Studies of Polymer-Metal Complexes Derived from Modified Poly Styrene-Alt-(Maleic Anhydride) as a Prospects for Biomedical Applications. Crystals 2023, 13, 728. https://doi.org/10.3390/cryst13050728
Almehizia AA, Alkahtani HM, Al-Omar MA, Obaidullah AJ, Bhat MA, Alrasheed LS, Naglah AM, Younes AAO, Alsuhaibani AM, Refat MS, et al. Synthesis, Spectroscopic Characterization and Thermal Studies of Polymer-Metal Complexes Derived from Modified Poly Styrene-Alt-(Maleic Anhydride) as a Prospects for Biomedical Applications. Crystals. 2023; 13(5):728. https://doi.org/10.3390/cryst13050728
Chicago/Turabian StyleAlmehizia, Abdulrahman A., Hamad M. Alkahtani, Mohamed A. Al-Omar, Ahmad J. Obaidullah, Mashooq A. Bhat, Lamees S. Alrasheed, Ahmed M. Naglah, Ayman A. O. Younes, Amnah Mohammed Alsuhaibani, Moamen S. Refat, and et al. 2023. "Synthesis, Spectroscopic Characterization and Thermal Studies of Polymer-Metal Complexes Derived from Modified Poly Styrene-Alt-(Maleic Anhydride) as a Prospects for Biomedical Applications" Crystals 13, no. 5: 728. https://doi.org/10.3390/cryst13050728
APA StyleAlmehizia, A. A., Alkahtani, H. M., Al-Omar, M. A., Obaidullah, A. J., Bhat, M. A., Alrasheed, L. S., Naglah, A. M., Younes, A. A. O., Alsuhaibani, A. M., Refat, M. S., Adam, A. M. A., El-Sayed, M. Y., & Asla, K. A. (2023). Synthesis, Spectroscopic Characterization and Thermal Studies of Polymer-Metal Complexes Derived from Modified Poly Styrene-Alt-(Maleic Anhydride) as a Prospects for Biomedical Applications. Crystals, 13(5), 728. https://doi.org/10.3390/cryst13050728










