Recent Progress of Film Fabrication Process for Carbon-Based All-Inorganic Perovskite Solar Cells
Abstract
1. Introduction
2. Primary Challenges with Film Fabrication Process for C-IPSCs
2.1. Energy Level Mismatch
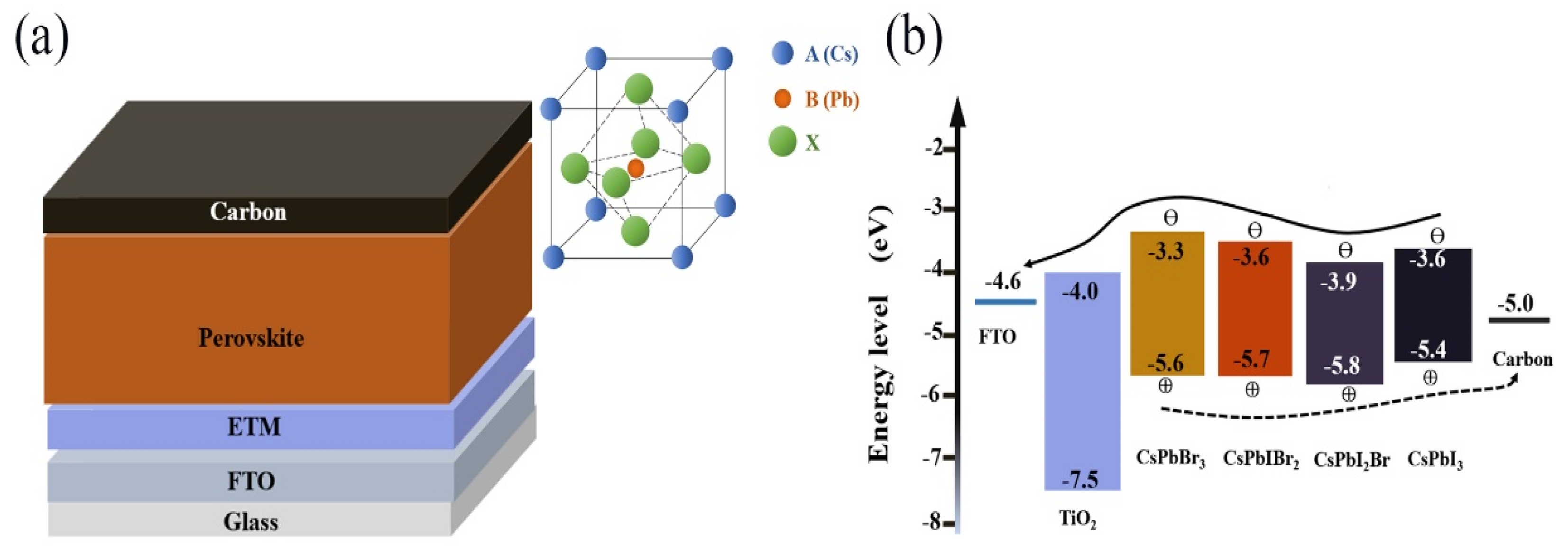
2.2. Phase Stability
2.3. Exceeding the Solubility Limit of Cs Salts
3. Strategies of Film Fabrication Process for C-IPSCs
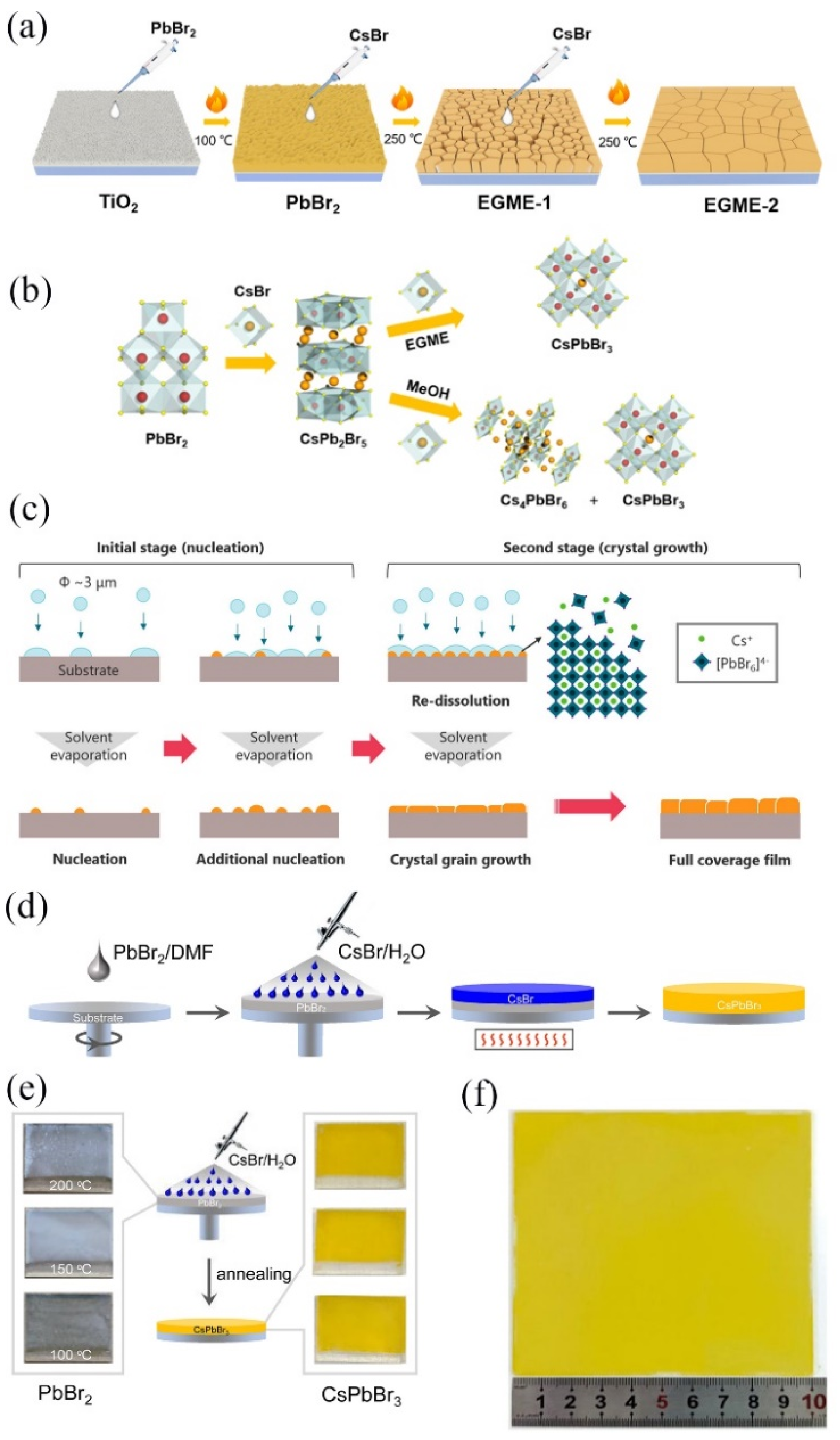
3.1. CsPbBr3
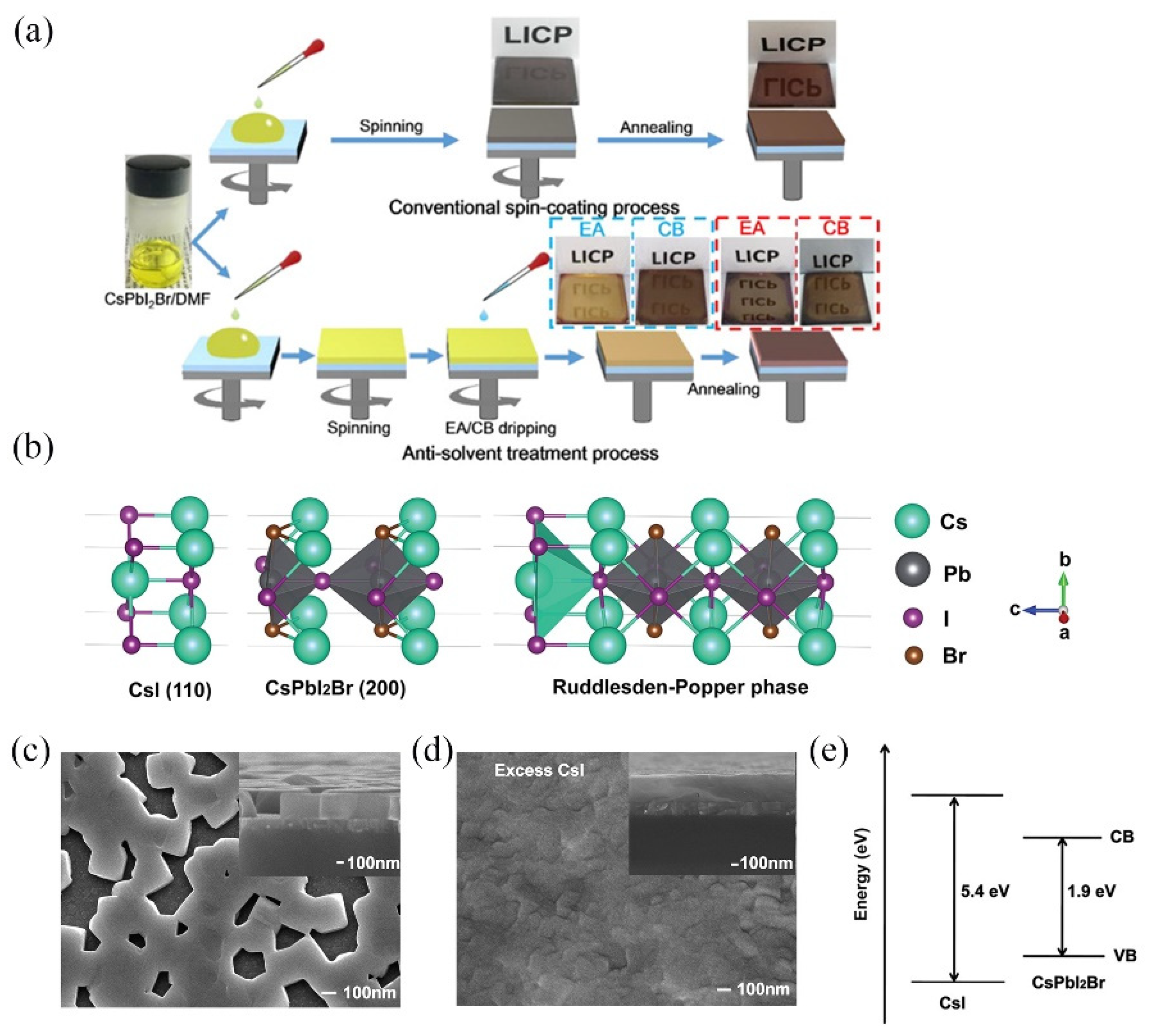
3.2. CsPbIBr2
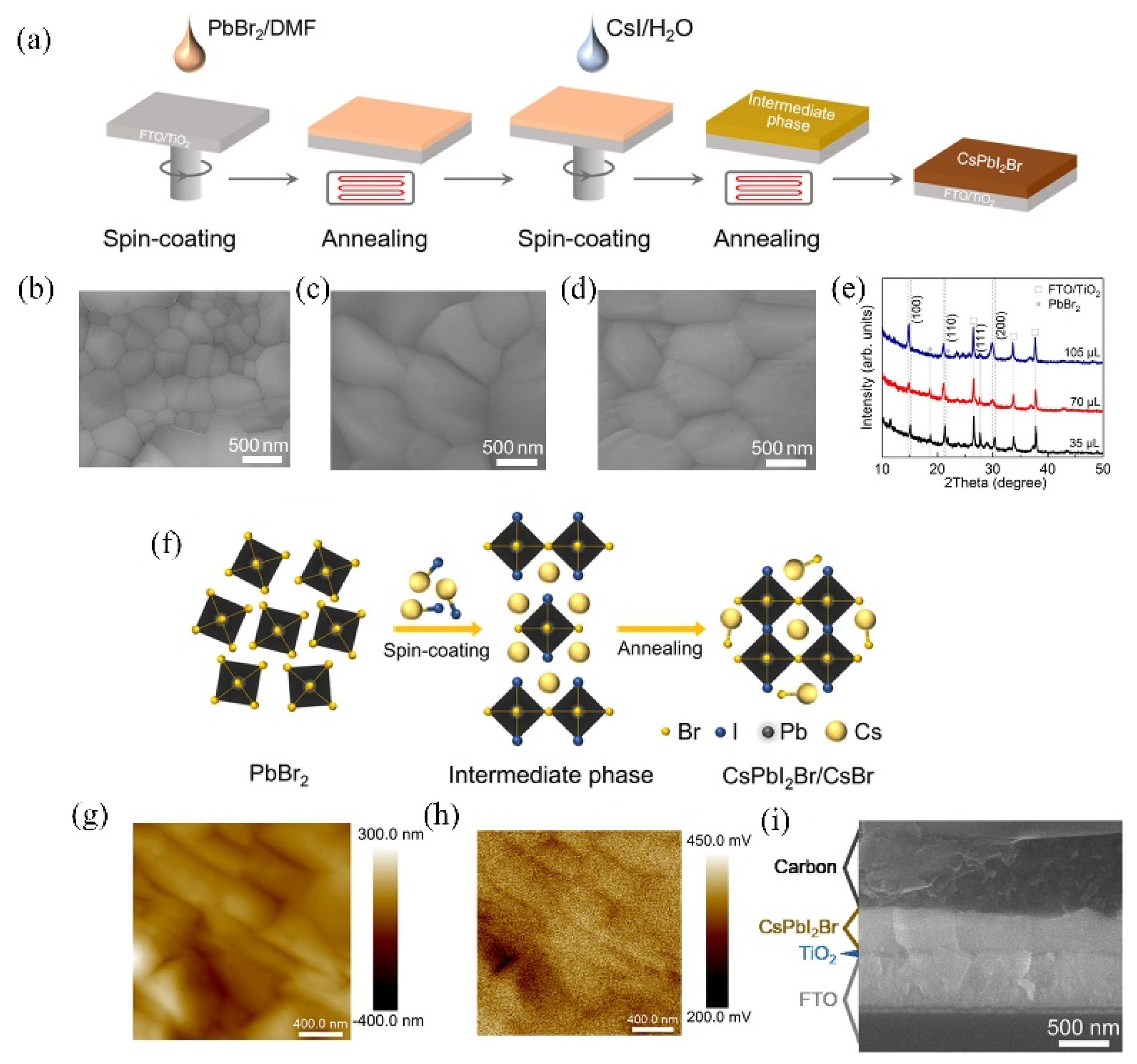
3.3. CsPbI2Br
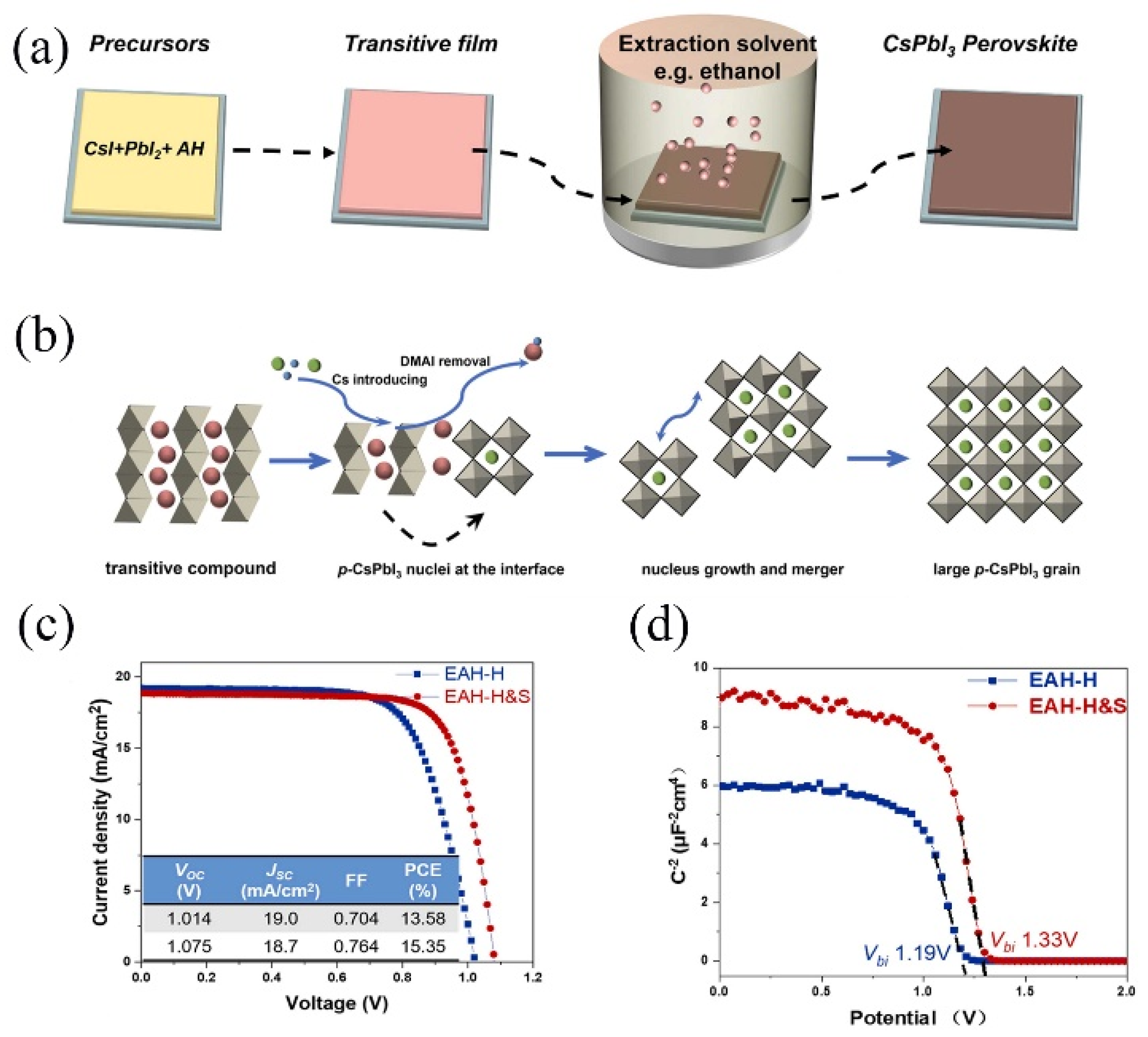
3.4. CsPbI3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho−Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photon. 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Maes, J.; Balcaen, L.; Drijvers, E.; Zhao, Q.; De Roo, J.; Vantomme, A.; Vanhaecke, F.; Geiregat, P.; Hens, Z. Light Absorption Coefficient of CsPbBr3 Perovskite Nanocrystals. J. Phys. Chem. Lett. 2018, 9, 3093–3097. [Google Scholar] [CrossRef] [PubMed]
- Weidman, M.C.; Seitz, M.; Stranks, S.D.; Tisdale, W.A. Highly Tunable Colloidal Perovskite Nanoplatelets through Variable Cation, Metal, and Halide Composition. ACS Nano 2016, 10, 7830–7839. [Google Scholar] [CrossRef]
- Leng, K.; Abdelwahab, I.; Verzhbitskiy, I.; Telychko, M.; Chu, L.; Fu, W.; Chi, X.; Guo, N.; Chen, Z.; Chen, Z.; et al. Molecularly thin two-dimensional hybrid perovskites with tunable optoelectronic properties due to reversible surface relaxation. Nat. Mater. 2018, 17, 908–914. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Li, Z.; Crisp, R.; Zhu, K.; Beard, M.C. Comparison of Recombination Dynamics in CH3NH3PbBr3 and CH3NH3PbI3 Perovskite Films: Influence of Exciton Binding Energy. J. Phys. Chem. Lett. 2015, 6, 4688–4692. [Google Scholar] [CrossRef]
- Leijtens, T.; Stranks, S.D.; Eperon, G.E.; Lindblad, R.; Johansson, E.M.J.; McPherson, I.J.; Rensmo, H.; Ball, J.M.; Lee, M.M.; Snaith, H.J. Electronic Properties of Meso−Superstructured and Planar Organometal Halide Perovskite Films: Charge Trapping, Photodoping, and Carrier Mobility. ACS Nano 2014, 8, 7147–7155. [Google Scholar] [CrossRef]
- Oga, H.; Saeki, A.; Ogomi, Y.; Hayase, S.; Seki, S. Improved Understanding of the Electronic and Energetic Landscapes of Perovskite Solar Cells: High Local Charge Carrier Mobility, Reduced Recombination, and Extremely Shallow Traps. J. Am. Chem. Soc. 2014, 136, 13818–13825. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 5 February 2023).
- Chen, J.; Park, N.-G. Materials and Methods for Interface Engineering toward Stable and Efficient Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2742–2786. [Google Scholar] [CrossRef]
- Brown, A.A.M.; Damodaran, B.; Jiang, L.; Tey, J.N.; Pu, S.H.; Mathews, N.; Mhaisalkar, S.G. Lead Halide Perovskite Nanocrystals: Room Temperature Syntheses toward Commercial Viability. Adv. Energy Mater. 2020, 10, 2001349. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W. Perovskite Tandem Solar Cells: From Fundamentals to Commercial Deployment. Chem. Rev. 2020, 120, 9835–9950. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, F.; Gong, J.; Ma, Y.; Gu, J.; Liu, X.; Chen, S.; Liu, M. Pre-buried Additive for Cross−Layer Modification in Flexible Perovskite Solar Cells with Efficiency Exceeding 22%. Adv. Mater. 2022, 34, 2109879. [Google Scholar] [CrossRef]
- Ru, P.; Bi, E.; Zhang, Y.; Wang, Y.; Kong, W.; Sha, Y.; Tang, W.; Zhang, P.; Wu, Y.; Chen, W.; et al. High Electron Affinity Enables Fast Hole Extraction for Efficient Flexible Inverted Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1903487. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, S.; Li, N.; Huang, B.; Niu, X.; Li, L.; Sun, M.; Zhang, Y.; Zhang, X.; Zhu, C.; et al. Self-Elimination of Intrinsic Defects Improves the Low−Temperature Performance of Perovskite Photovoltaics. Joule 2020, 4, 1961–1976. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Accelerated degradation of methylammonium lead iodide perovskites induced by exposure to iodine vapour. Nat. Energy 2016, 2, 16195. [Google Scholar] [CrossRef]
- Bisquert, J.; Juarez-Perez, E.J. The Causes of Degradation of Perovskite Solar Cells. J. Phys. Chem. Lett. 2019, 10, 5889–5891. [Google Scholar] [CrossRef] [PubMed]
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; van Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From Defects to Degradation: A Mechanistic Understanding of Degradation in Perovskite Solar Cell Devices and Modules. Adv. Energy Mater. 2020, 10, 1904054. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Zhang, C.; Chang, J.; Lin, Z.; Chen, D.; Xi, H.; Hao, Y. Effects of Annealing Conditions on Mixed Lead Halide Perovskite Solar Cells and Their Thermal Stability Investigation. Materials 2017, 10, 837. [Google Scholar] [CrossRef]
- Reese, M.O.; Gevorgyan, S.A.; Jørgensen, M.; Bundgaard, E.; Kurtz, S.R.; Ginley, D.S.; Olson, D.C.; Lloyd, M.T.; Morvillo, P.; Katz, E.A.; et al. Consensus stability testing protocols for organic photovoltaic materials and devices. Sol. Energy Mater. Sol. Cells 2011, 95, 1253–1267. [Google Scholar] [CrossRef]
- Kulbak, M.; Cahen, D.; Hodes, G. How Important Is the Organic Part of Lead Halide Perovskite Photovoltaic Cells? Efficient CsPbBr3 Cells. J. Phys. Chem. Lett. 2015, 6, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hodes, G.; Jin, Z.; Liu, S. All-inorganic CsPbX3 Perovskite Solar Cells: Progress and Prospects. Angew. Chem. Int. Ed. 2019, 58, 15596–15618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, F.; Zhang, M.; Yang, Z. Recent Progress on Boosting the Perovskite Film Quality of All-Inorganic Perovskite Solar Cells. Coatings 2023, 13, 281. [Google Scholar] [CrossRef]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Hörantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T.; et al. Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Guo, Z.; Jena, A.K.; Takei, I.; Kim, G.M.; Kamarudin, M.A.; Sanehira, Y.; Ishii, A.; Numata, Y.; Hayase, S.; Miyasaka, T. VOC Over 1.4 V for Amorphous Tin-Oxide-Based Dopant-Free CsPbI2Br Perovskite Solar Cells. J. Am. Chem. Soc. 2020, 142, 9725–9734. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Wu, C.; Wang, K.; Feng, X.; Jiang, Y.; Yang, D.; Hou, Y.; Yan, Y.; Sanghadasa, M.; Priya, S. Ultrahigh Durability Perovskite Solar Cells. Nano Lett. 2019, 19, 1251–1259. [Google Scholar] [CrossRef]
- Schloemer, T.H.; Christians, J.A.; Luther, J.M.; Sellinger, A. Doping strategies for small molecule organic hole-transport materials: Impacts on perovskite solar cell performance and stability. Chem. Sci. 2019, 10, 1904–1935. [Google Scholar] [CrossRef]
- Liang, L.; Cai, Y.; Li, X.; Nazeeruddin, M.K.; Gao, P. All that glitters is not gold: Recent progress of alternative counter electrodes for perovskite solar cells. Nano Energy 2018, 52, 211–238. [Google Scholar] [CrossRef]
- Dong, C.; Xu, B.; Liu, D.; Moloney, E.G.; Tan, F.; Yue, G.; Liu, R.; Zhang, D.; Zhang, W.; Saidaminov, M.I. Carbon-based all-inorganic perovskite solar cells: Progress, challenges and strategies toward 20% efficiency. Mater. Today 2021, 50, 239–258. [Google Scholar] [CrossRef]
- Li, W.; Rothmann, M.U.; Liu, A.; Wang, Z.; Zhang, Y.; Pascoe, A.R.; Lu, J.; Jiang, L.; Chen, Y.; Huang, F.; et al. Phase Segregation Enhanced Ion Movement in Efficient Inorganic CsPbIBr2 Solar Cells. Adv. Energy Mater. 2017, 7, 1700946. [Google Scholar] [CrossRef]
- Meng, F.; Liu, A.; Gao, L.; Cao, J.; Yan, Y.; Wang, N.; Fan, M.; Wei, G.; Ma, T. Current progress in interfacial engineering of carbon-based perovskite solar cells. J. Mater. Chem. A 2019, 7, 8690–8699. [Google Scholar] [CrossRef]
- Tress, W. Maximum Efficiency and Open−Circuit Voltage of Perovskite Solar Cells. In Organic-Inorganic Halide Perovskite Photovoltaics: From Fundamentals to Device Architectures; Park, N.−G., Grätzel, M., Miyasaka, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–77. [Google Scholar]
- Wang, H.; Liu, H.; Dong, Z.; Li, W.; Zhu, L.; Chen, H. Composition manipulation boosts the efficiency of carbon-based CsPbI3 perovskite solar cells to beyond 14%. Nano Energy 2021, 84, 105881. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Dong, Z.; Song, T.; Li, W.; Zhu, L.; Bai, Y.; Chen, H. Size mismatch induces cation segregation in CsPbI3: Forming energy level gradient and 3D/2D heterojunction promotes the efficiency of carbon-based perovskite solar cells to over 15%. Nano Energy 2021, 89, 106411. [Google Scholar] [CrossRef]
- Singh, A.; Satapathi, S. Advances in Processing Kinetics for All-inorganic Halide Perovskite: Towards Efficient and Thermodynamic Stable Solar Cells. Adv. Mater. Interfaces 2022, 9, 2200847. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Liu, F.; Kitabatake, Y.; Hayase, S.; Toyoda, T.; Yoshino, K.; Minemoto, T.; Katayama, K.; Shen, Q. Effect of the conduction band offset on interfacial recombination behavior of the planar perovskite solar cells. Nano Energy 2018, 53, 17–26. [Google Scholar] [CrossRef]
- Su, G.; He, B.; Gong, Z.; Ding, Y.; Duan, J.; Zhao, Y.; Chen, H.; Tang, Q. Enhanced charge extraction in carbon-based all-inorganic CsPbBr3 perovskite solar cells by dual-function interface engineering. Electrochim. Acta 2019, 328, 135102. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z.; Chai, W.; Zhang, Q.; Chen, D.; Lin, Z.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Band Alignment Engineering Towards High Efficiency Carbon-based Inorganic Planar CsPbIBr2 Perovskite Solar Cells. ChemSusChem 2019, 12, 2318–2325. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, S.; Liu, A.; Kamata, Y.; Teo, S.; Yang, S.; Xu, Z.; Hayase, S.; Ma, T. Niobium Incorporation into CsPbI2Br for Stable and Efficient All-inorganic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 19994–20003. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Li, R.; Sun, W.; Wu, J.; Lan, Z. High−Efficiency Carbon-based CsPbIBr2 Solar Cells with Interfacial Energy Loss Suppressed by a Thin Bulk-Heterojunction Layer. Sol. RRL 2021, 5, 2100375. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Fu, H.; Gong, L.; He, H.; Fang, Z.; Zhou, C.; Chen, J.; Chao, Z.; Fan, J. Low-temperature preparation achieving 10.95%-efficiency of hole-free and carbon-based all-inorganic CsPbI3 perovskite solar cells. J. Alloy. Compd. 2021, 862, 158454. [Google Scholar] [CrossRef]
- Deng, F.; Li, X.; Lv, X.; Zhou, J.; Chen, Y.; Sun, X.; Zheng, Y.-Z.; Tao, X.; Chen, J.-F. Low-Temperature Processing All-inorganic Carbon-based Perovskite Solar Cells up to 11.78% Efficiency via Alkali Hydroxides Interfacial Engineering. ACS Appl. Energy Mater. 2020, 3, 401–410. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, M.; He, B.; Zhang, W.; Li, X.; Gong, Z.; Chen, H.; Duan, Y.; Tang, Q. Improved charge extraction through interface engineering for 10.12% efficiency and stable CsPbBr3 perovskite solar cells. J. Mater. Chem. A 2020, 8, 20987–20997. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Xiang, S.; Wang, H.; Liu, H.; Li, W.; Chen, H. Cs−Doped TiO2 Nanorod Array Enhances Electron Injection and Transport in Carbon-based CsPbI3 Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 16927–16932. [Google Scholar] [CrossRef]
- Mi, L.; Zhang, Y.; Chen, T.; Xu, E.; Jiang, Y. Carbon electrode engineering for high efficiency all-inorganic perovskite solar cells. RSC Adv. 2020, 10, 12298–12303. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Liu, Z.; Tan, X.; Sun, B.; Xi, S.; Shi, T.; Tang, Z.; Liao, G. Vapor−assisted deposition of CsPbIBr2 films for highly efficient and stable carbon-based planar perovskite solar cells with superior Voc. Electrochim. Acta 2020, 330, 135266. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Q.; Chen, D.; Zhang, Z.; Lin, Z.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Intermolecular Exchange Boosts Efficiency of Air-Stable, Carbon-based All-inorganic Planar CsPbIBr2 Perovskite Solar Cells to Over 9%. Adv. Energy Mater. 2018, 8, 1802080. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, F.; Wang, X.; Tao, J.; Zheng, D.; Jiang, J.; Hu, Z.; Chu, J. Multi−source cation/anion doping towards efficient carbon-based CsPbIBr2 solar cells with superior open voltage up to 1.37 V. Sol. Energy Mater. Sol. Cells 2021, 221, 110918. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Krystallbau und chemische Zusammensetzung. Ber. Der Dtsch. Chem. Ges. A B Ser. 1927, 60, 1263–1296. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Khan, J.; Niu, G.; Tang, J. Inorganic CsPbI3 Perovskite−Based Solar Cells: A Choice for a Tandem Device. Sol. RRL 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near−Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Lu, X.; Gao, X.; Gao, J.; Shui, L.; Wu, S.; Liu, J.-M. Promoting the Hole Extraction with Co3O4 Nanomaterials for Efficient Carbon-based CsPbI2Br Perovskite Solar Cells. Sol. RRL 2019, 3, 1800315. [Google Scholar] [CrossRef]
- Ding, J.; Duan, J.; Guo, C.; Tang, Q. Toward charge extraction in all-inorganic perovskite solar cells by interfacial engineering. J. Mater. Chem. A 2018, 6, 21999–22004. [Google Scholar] [CrossRef]
- Liang, J.; Han, X.; Yang, J. −H.; Zhang, B.; Fang, Q.; Zhang, J.; Ai, Q.; Ogle, M.M.; Terlier, T.; Martí, A.A.; et al. Defect−Engineering-Enabled High-Efficiency All-inorganic Perovskite Solar Cells. Adv. Mater. 2019, 31, 1903448. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; Yang, X.; Wang, Y.; He, B.; Tang, Q. Lanthanide Ions Doped CsPbBr3 Halides for HTM-Free 10.14%-Efficiency Inorganic Perovskite Solar Cell with an Ultrahigh Open-Circuit Voltage of 1.594 V. Adv. Energy Mater. 2018, 8, 1802346. [Google Scholar] [CrossRef]
- Xiang, S.; Li, W.; Wei, Y.; Liu, J.; Liu, H.; Zhu, L.; Yang, S.; Chen, H. Natrium Doping Pushes the Efficiency of Carbon-based CsPbI3 Perovskite Solar Cells to 10.7%. iScience 2019, 15, 156–164. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; Qian, W.; Zhu, Z.; Zhang, T.; Bai, Y.; Hu, C.; Xiao, S.; Yang, Y.; Yang, S. Excess Cesium Iodide Induces Spinodal Decomposition of CsPbI2Br Perovskite Films. J. Phys. Chem. Lett. 2019, 10, 194–199. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Y.; He, B.; Zhang, W.; Cui, L.; Wang, S.; Chen, H.; Duan, Y.; Tang, Q. Efficient interface engineering of N, N’−Dicyclohexylcarbodiimide for stable HTMs−free CsPbBr3 perovskite solar cells with 10.16%-efficiency. Chem. Eng. J. 2022, 428, 131950. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, G.; Zhang, J.; Pan, Z.; Yang, S.; Liu, B.; Rao, H.; Zhong, X. Synergistic passivation by alkali metal and halogenoid ions for high efficiency HTM-free carbon-based CsPbI2Br solar cells. Chem. Eng. J. 2022, 430, 133083. [Google Scholar] [CrossRef]
- Rakita, Y.; Kedem, N.; Gupta, S.; Sadhanala, A.; Kalchenko, V.; Böhm, M.L.; Kulbak, M.; Friend, R.H.; Cahen, D.; Hodes, G. Low-Temperature Solution-Grown CsPbBr3 Single Crystals and Their Characterization. Cryst. Growth Des. 2016, 16, 5717–5725. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Luo, Y.; Brenner, T.M.; Snaider, J.; Sun, S.; Li, X.; Jensen, M.A.; Hartono, N.T.P.; Nienhaus, L.; Wieghold, S.; et al. Homogenized halides and alkali cation segregation in alloyed organic-inorganic perovskites. Science 2019, 363, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, S.; Chen, W.; Zhu, H.; Xiong, Z.; Yang, Z.; Chen, C.; Chen, R.; Han, L.; Chen, W. Solvent engineering for efficient inverted perovskite solar cells based on inorganic CsPbI2Br light absorber. Mater. Today Energy 2018, 8, 125–133. [Google Scholar] [CrossRef]
- Rao, H.; Ye, S.; Gu, F.; Zhao, Z.; Liu, Z.; Bian, Z.; Huang, C. Morphology Controlling of All-inorganic Perovskite at Low Temperature for Efficient Rigid and Flexible Solar Cells. Adv. Energy Mater. 2018, 8, 1800758. [Google Scholar] [CrossRef]
- Chen, H.; Yang, S. Methods and strategies for achieving high-performance carbon-based perovskite solar cells without hole transport materials. J. Mater. Chem. A 2019, 7, 15476–15490. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, Q.; Liu, Y.; Bao, C.; Fang, Y.; Lin, Y.; Tang, S.; Wang, Q.; Xiao, X.; Bai, Y.; et al. Thin single crystal perovskite solar cells to harvest below-bandgap light absorption. Nat. Commun. 2017, 8, 1890. [Google Scholar] [CrossRef]
- Chen, H.; Yang, S. Carbon-based Perovskite Solar Cells without Hole Transport Materials: The Front Runner to the Market? Adv. Mater. 2017, 29, 1603994. [Google Scholar] [CrossRef]
- Zhang, G.; Xie, P.; Huang, Z.; Yang, Z.; Pan, Z.; Fang, Y.; Rao, H.; Zhong, X. Modification of Energy Level Alignment for Boosting Carbon-based CsPbI2Br Solar Cells with 14% Certified Efficiency. Adv. Funct. Mater. 2021, 31, 2011187. [Google Scholar] [CrossRef]
- Ullah, S.; Liu, L.; Yang, S.-E.; Liu, P.; Guo, H.; Chen, Y. A modified hybrid chemical vapor deposition method for the fabrication of efficient CsPbBr3 perovskite solar cells. J. Phys. D Appl. Phys. 2021, 55, 064001. [Google Scholar] [CrossRef]
- Luo, P.; Zhou, Y.; Zhou, S.; Lu, Y.; Xu, C.; Xia, W.; Sun, L. Fast anion-exchange from CsPbI3 to CsPbBr3 via Br2-vapor-assisted deposition for air-stable all-inorganic perovskite solar cells. Chem. Eng. J. 2018, 343, 146–154. [Google Scholar] [CrossRef]
- Bidikoudi, M.; Simal, C.; Stathatos, E. Exploring the Effect of Lewis−Base Additives on the Performance and Stability of Mesoscopic Carbon-Electrode Perovskite Solar Cells. ACS Appl. Energy Mater. 2021, 4, 8810–8823. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Lei, M.; Zhang, W.; Zhu, G. Optimizing the substrate pre-heating and post-annealing temperatures for fabricating high-performance carbon-based CsPbIBr2 inorganic perovskite solar cells. Electrochim. Acta 2020, 349, 136354. [Google Scholar] [CrossRef]
- Yan, J.; Lin, S.; Qiu, X.; Chen, H.; Li, K.; Yuan, Y.; Long, M.; Yang, B.; Gao, Y.; Zhou, C. Accelerated hole-extraction in carbon-electrode based planar perovskite solar cells by moisture-assisted post-annealing. Appl. Phys. Lett. 2019, 114, 103503. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.-E.; Liu, P.; Chen, Y. High-quality and full-coverage CsPbBr3 thin films via electron beam evaporation with post−annealing treatment for all-inorganic perovskite solar cells. Sol. Energy 2022, 232, 320–327. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Zeng, J.; Jiang, Z.; Ai, Q.; Zhang, X.; Hu, B.; Wang, X.; Yang, S.; Xu, B. Inkjet-Printing Controlled Phase Evolution Boosts the Efficiency of Hole Transport Material Free and Carbon-based CsPbBr3 Perovskite Solar Cells Exceeding 9%. Energy Environ. Mater. 2022, e12543. [Google Scholar] [CrossRef]
- Haruta, Y.; Ikenoue, T.; Miyake, M.; Hirato, T. One-Step Coating of Full-Coverage CsPbBr3 Thin Films via Mist Deposition for All-inorganic Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 11523–11528. [Google Scholar] [CrossRef]
- Zhang, Z.; Ba, Y.; Chen, D.; Ma, J.; Zhu, W.; Xi, H.; Chen, D.; Zhang, J.; Zhang, C.; Hao, Y. Generic water-based spray-assisted growth for scalable high-efficiency carbon-electrode all-inorganic perovskite solar cells. iScience 2021, 24, 103365. [Google Scholar] [CrossRef]
- Mastrikov, Y.A.; Chuklina, N.G.; Sokolov, M.N.; Popov, A.I.; Gryaznov, D.V.; Kotomin, E.A.; Maier, J. Small radius electron and hole polarons in PbX2 (X = F, Cl, Br) crystals: A computational study. J. Mater. Chem. C 2021, 9, 16536–16544. [Google Scholar] [CrossRef]
- Popov, A.I.; Kotomin, E.A.; Maier, J. Analysis of self-trapped hole mobility in alkali halides and metal halides. Solid State Ion. 2017, 302, 3–6. [Google Scholar] [CrossRef]
- Slotcavage, D.J.; Karunadasa, H.I.; McGehee, M.D. Light-Induced Phase Segregation in Halide−Perovskite Absorbers. ACS Energy Lett. 2016, 1, 1199–1205. [Google Scholar] [CrossRef]
- Ball, J.M.; Petrozza, A. Defects in perovskite-halides and their effects in solar cells. Nat. Energy 2016, 1, 16149. [Google Scholar] [CrossRef]
- Wang, Y.; Dar, M.I.; Ono, L.K.; Zhang, T.; Kan, M.; Li, Y.; Zhang, L.; Wang, X.; Yang, Y.; Gao, X.; et al. Thermodynamically stabilized β-CsPbI3-based perovskite solar cells with efficiencies >18%. Science 2019, 365, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Han, X.; Zhao, Y.; Li, J.; Chang, L.; Zhao, W. A Green Anti−Solvent Process for High Performance Carbon-based CsPbI2Br All-inorganic Perovskite Solar Cell. Sol. RRL 2018, 2, 1800139. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, H.; Han, Y.; Duan, C.; Liu, Z.; Liu, S. Europium and Acetate Co−doping Strategy for Developing Stable and Efficient CsPbI2Br Perovskite Solar Cells. Small 2019, 15, 1904387. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, C.; Wang, Y.; Xu, Z.; Lu, Z.; Ma, Y.; Zhu, H.; Hu, Y.; Xiao, C.; Yi, X.; et al. All-inorganic Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 15829–15832. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-Purity Inorganic Perovskite Films for Solar Cells with 9.72% Efficiency. Angew. Chem. Int. Ed. 2018, 57, 3787–3791. [Google Scholar] [CrossRef]
- Bu, F.; He, B.; Ding, Y.; Li, X.; Sun, X.; Duan, J.; Zhao, Y.; Chen, H.; Tang, Q. Enhanced energy level alignment and hole extraction of carbon electrode for air-stable hole-transporting material-free CsPbBr3 perovskite solar cells. Sol. Energy Mater. Sol. Cells 2020, 205, 110267. [Google Scholar] [CrossRef]
- Guo, R.; Zhao, Y.; Deng, Q.; Zhang, Y.; Wu, Z.; Duan, Y.; Zhang, W.; Shen, Y.; Shao, G. Energy level matching between transparent conducting electrodes and the electronic transport layer to enhance performance of all-inorganic CsPbBr3 solar cells. Vacuum 2022, 200, 111028. [Google Scholar] [CrossRef]
- Yao, X.; He, B.; Zhu, J.; Ti, J.; Cui, L.; Tui, R.; Wei, M.; Chen, H.; Duan, J.; Duan, Y.; et al. Tailoring type-II all-in-one buried interface for 1.635V-voltage, all-inorganic CsPbBr3 perovskite solar cells. Nano Energy 2022, 96, 107138. [Google Scholar] [CrossRef]
- Wang, S.; Cao, F.; Sun, W.; Wang, C.; Yan, Z.; Wang, N.; Lan, Z.; Wu, J. A green Bi-Solvent system for processing high-quality CsPbBr3 films in efficient all-inorganic perovskite solar cells. Mater. Today Phys. 2022, 22, 100614. [Google Scholar] [CrossRef]
- Teng, P.; Han, X.; Li, J.; Xu, Y.; Kang, L.; Wang, Y.; Yang, Y.; Yu, T. Elegant Face−Down Liquid-Space-Restricted Deposition of CsPbBr3 Films for Efficient Carbon-based All-inorganic Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, W.; Du, Z.; Li, G.; Sun, W.; Wu, J.; Lan, Z. Highly Efficient CsPbBr3 Planar Perovskite Solar Cells via Additive Engineering with NH4SCN. ACS Appl. Mater. Interfaces 2020, 12, 10579–10587. [Google Scholar] [CrossRef]
- Liu, X.; Tan, X.; Liu, Z.; Ye, H.; Sun, B.; Shi, T.; Tang, Z.; Liao, G. Boosting the efficiency of carbon-based planar CsPbBr3 perovskite solar cells by a modified multistep spin−coating technique and interface engineering. Nano Energy 2019, 56, 184–195. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Z.; Zhang, J.; Bai, D.; Bian, H.; Wang, K.; Sun, J.; Wang, Q.; Liu, S.F. All-Ambient Processed Binary CsPbBr3-CsPb2Br5 Perovskites with Synergistic Enhancement for High-Efficiency Cs-Pb-Br-Based Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 7145–7154. [Google Scholar] [CrossRef]
- Tong, G.; Chen, T.; Li, H.; Qiu, L.; Liu, Z.; Dang, Y.; Song, W.; Ono, L.K.; Jiang, Y.; Qi, Y. Phase transition induced recrystallization and low surface potential barrier leading to 10.91%-efficient CsPbBr3 perovskite solar cells. Nano Energy 2019, 65, 104015. [Google Scholar] [CrossRef]
- Jiang, L.; Peng, Y.; Xiang, T.; Liu, Y.; Xu, M.; Wang, J.; Tremblay, P.-L.; Zhang, T. A counter electrode modified with renewable carbonized biomass for an all-inorganic CsPbBr3 perovskite solar cell. J. Alloy. Compd. 2022, 902, 163725. [Google Scholar] [CrossRef]
- Liu, J.; He, Q.; Bi, J.; Lei, M.; Zhang, W.; Wang, G. Remarkable quality improvement of CsPbIBr2 perovskite film by cellulose acetate addition for efficient and stable carbon-based inorganic perovskite solar cells. Chem. Eng. J. 2021, 424, 130324. [Google Scholar] [CrossRef]
- Liu, J.; Lei, M.; Zhang, W.; Wang, G. A multifunctional CuSCN interlayer in carbon electrode-based CsPbIBr2 all-inorganic perovskite solar cells for boosting the efficiency and stability. Sustain. Energy Fuels 2020, 4, 4249–4256. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Gao, L.; Cao, J.; Han, Q.; Yu, F.; Kamata, Y.; Zhang, C.; Fan, M.; Wei, G.; et al. Excellent Moisture Stability and Efficiency of Inverted All-inorganic CsPbIBr2 Perovskite Solar Cells through Molecule Interface Engineering. ACS Appl. Mater. Interfaces 2020, 12, 13931–13940. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, P.; Wang, C.; Wang, Y.; Hu, Y.; Zhu, G.; Ma, L.; Liu, J.; Jin, Z. CsPb0.9Sn0.1IBr2 Based All-inorganic Perovskite Solar Cells with Exceptional Efficiency and Stability. J. Am. Chem. Soc. 2017, 139, 14009–14012. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tang, S.; Yuan, G.; Zhu, W.; Huang, Y.; Li, S.; Lin, M. Tailored graphene quantum dots to passivate defects and accelerate charge extraction for all-inorganic CsPbIBr2 perovskite solar cells. J. Alloy. Compd. 2022, 895, 162529. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Gu, Y.; Xu, C.; Lu, Y.; Hu, J.; Chen, T.; Zhu, C.; Luo, P. Solvent-engineering-processed CsPbIBr2 inorganic perovskite solar cells with efficiency of ∼11%. Sol. Energy Mater. Sol. Cells 2022, 238, 111640. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, S.; Wen, X.; Green, M.A.; Ho−Baillie, A.W.Y. Hole Transport Layer Free Inorganic CsPbIBr2 Perovskite Solar Cell by Dual Source Thermal Evaporation. Adv. Energy Mater. 2016, 6, 1502202. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Deng, X.; Ma, Q.; Zheng, J.; Yun, J.S.; Green, M.A.; Huang, S.; Ho−Baillie, A.W.Y. CsPbIBr2 Perovskite Solar Cell by Spray-Assisted Deposition. ACS Energy Lett. 2016, 1, 573–577. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, X.; Liu, J.; Wen, S.; Wu, Y.; Que, W. Inorganic CsPbIBr2-Based Perovskite Solar Cells: Fabrication Technique Modification and Efficiency Improvement. Sol. RRL 2019, 3, 1900135. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, W.; Chen, D.; Zhang, Z.; Lin, Z.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Light Processing Enables Efficient Carbon-based, All-inorganic Planar CsPbIBr2 Solar Cells with High Photovoltages. ACS Appl. Mater. Interfaces 2019, 11, 2997–3005. [Google Scholar] [CrossRef]
- Zhu, W.; Chai, W.; Deng, M.; Chen, D.; Chen, D.; Zhang, J.; Zhang, C.; Hao, Y. Flux−mediated growth strategy enables low−temperature fabrication of high−efficiency all-inorganic CsPbIBr2 perovskite solar cells. Electrochim. Acta 2020, 330, 135325. [Google Scholar] [CrossRef]
- Zhu, W.; Chai, W.; Chen, D.; Xi, H.; Chen, D.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Recycling of FTO/TiO2 Substrates: Route toward Simultaneously High-performance and Cost−Efficient Carbon-based, All-inorganic CsPbIBr2 Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 4549–4557. [Google Scholar] [CrossRef]
- Nam, J.K.; Jung, M.S.; Chai, S.U.; Choi, Y.J.; Kim, D.; Park, J.H. Unveiling the Crystal Formation of Cesium Lead Mixed-Halide Perovskites for Efficient and Stable Solar Cells. J. Phys. Chem. Lett. 2017, 8, 2936–2940. [Google Scholar] [CrossRef]
- Lin, J.; Lai, M.; Dou, L.; Kley, C.S.; Chen, H.; Peng, F.; Sun, J.; Lu, D.; Hawks, S.A.; Xie, C.; et al. Thermochromic halide perovskite solar cells. Nat. Mater. 2018, 17, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Lin, H.-Y.; Chiang, K.-M.; Tsai, W.-L.; Huang, Y.-C.; Tsao, C.-S.; Lin, H.-W. All-Vacuum-Deposited Stoichiometrically Balanced Inorganic Cesium Lead Halide Perovskite Solar Cells with Stabilized Efficiency Exceeding 11%. Adv. Mater. 2017, 29, 1605290. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Ki, M.J.; Lee, H.J.; Park, J.K.; Hong, S.Y.; Kim, B.W.; Heo, J.H.; Im, S.H. Fully Scalable and Stable CsPbI2Br Solar Cells Realized by an All-Spray-Coating Process. ACS Appl. Mater. Interfaces 2022, 14, 7926–7935. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, W.; Li, W.; Yu, J.; Zhang, M.; Han, X.; Tanaka, T. Defect healing via a gradient cooling strategy for efficient all-inorganic perovskite solar cells. J. Mater. Chem. C 2022, 10, 4276–4285. [Google Scholar] [CrossRef]
- Fatima, K.; Haider, M.I.; Bashir, A.; Qamar, S.; Qureshi, A.A.; Akhter, Z.; Sultan, M. Surface modification of CsPbI2Br for improved performance of inorganic perovskite solar cells. Phys. E Low Dimens. Syst. Nanostructures 2022, 142, 115265. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Liu, C.; Wu, M.; Wu, Y.; Wang, D.; Zhang, T. Efficient all-inorganic CsPbI2Br perovskite solar cell with carbon electrode by revealing crystallization kinetics and improving crystal quality. J. Power Sour. 2020, 447, 227389. [Google Scholar] [CrossRef]
- Xu, X.; Qin, W.; Liu, S.; Xing, C.; Ge, G.; Wang, D.; Zhang, T. Low temperature-processed stable and high-efficiency carbon-based CsPbI2Br perovskite solar cells by additive strategy. Org. Electron. 2022, 103, 106463. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, J.; Chai, W.; Han, T.; Chen, D.; Xie, X.; Liu, G.; Dong, P.; Xi, H.; Chen, D.; et al. Intermediate Phase-Assisted Sequential Deposition toward 15.24%-Efficiency Carbon-Electrode Cspbi2br Perovskite Solar Cells. Solar RRL 2022, 6, 2200020. [Google Scholar] [CrossRef]
- Tan, S.; Shi, J.; Yu, B.; Zhao, W.; Li, Y.; Li, Y.; Wu, H.; Luo, Y.; Li, D.; Meng, Q. Inorganic Ammonium Halide Additive Strategy for Highly Efficient and Stable CsPbI3 Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2010813. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, X.; Miao, Y.; Zhao, Y. The Chemical Design in High-performance Lead Halide Perovskite: Additive vs Dopant? J. Phys. Chem. Lett. 2021, 12, 11636–11644. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Paternò, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, X.; Deng, Y.; Zhao, J.; Chen, Z.; Huang, J. Stabilizing the α−Phase of CsPbI3 Perovskite by Sulfobetaine Zwitterions in One-Step Spin-Coating Films. Joule 2017, 1, 371–382. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhou, Y.; Jiang, Q.; Ye, Q.; Chu, Z.; Li, X.; Yang, X.; Yin, Z.; You, J. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells. Nat. Commun. 2018, 9, 2225. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Fu, Z.; Li, W.; Wei, Y.; Liu, J.; Liu, H.; Zhu, L.; Zhang, R.; Chen, H. Highly Air−Stable Carbon-based α-CsPbI3 Perovskite Solar Cells with a Broadened Optical Spectrum. ACS Energy Lett. 2018, 3, 1824–1831. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Dong, Z.; Wei, X.; Song, Y.; Li, W.; Zhu, L.; Bai, Y.; Chen, H. Extracting ammonium halides by solvent from the hybrid perovskites with various dimensions to promote the crystallization of CsPbI3 perovskite. Nano Energy 2022, 94, 106925. [Google Scholar] [CrossRef]
- Yonezawa, K.; Yamamoto, K.; Shahiduzzaman, M.; Furumoto, Y.; Hamada, K.; Ripolles, T.S.; Karakawa, M.; Kuwabara, T.; Takahashi, K.; Hayase, S.; et al. Annealing effects on CsPbI3-based planar heterojunction perovskite solar cells formed by vacuum deposition method. Jpn. J. Appl. Phys. 2017, 56, 04CS11. [Google Scholar] [CrossRef]
- Yuan, J.; Bi, C.; Wang, S.; Guo, R.; Shen, T.; Zhang, L.; Tian, J. Spray-Coated Colloidal Perovskite Quantum Dot Films for Highly Efficient Solar Cells. Adv. Funct. Mater. 2019, 29, 1906615. [Google Scholar] [CrossRef]
- Lin, Z.; Folgueras, M.C.; Le, H.K.D.; Gao, M.; Yang, P. Laser-accelerated phase transformation in cesium lead iodide perovskite. Matter 2022, 5, 1455–1465. [Google Scholar] [CrossRef]
- Gu, X.; Lai, X.; Zhang, Y.; Wang, T.; Tan, W.L.; McNeill, C.R.; Liu, Q.; Sonar, P.; He, F.; Li, W.; et al. Organic Solar Cell with Efficiency Over 20% and VOC Exceeding 2.1 V Enabled by Tandem with All-inorganic Perovskite and Thermal Annealing-Free Process. Adv. Sci. 2022, 9, 2200445. [Google Scholar] [CrossRef]





| Material | STEL | STH | Material | Halogen-Halogen Distance (Å) | Migration Temperatures (K) |
|---|---|---|---|---|---|
| PbCl2 | Pb23+ | Pb3+ | CsCl | 4.123 | 202 |
| PbBr2 | Pb35+ | Pb3+ | CsBr | 4.286 | 122;130 |
| PbI2 | None | None | CsI | 4.567 | 60;85 |
| C-IPSCs | Advantages | Disadvantages |
|---|---|---|
| CsPbBr3 | 1. Excellent stability in ambient air 2. All fabrication in ambient air | 1. Large bandgap (~2.3 eV) 2. Difficult to control film formation 3. Low PCE |
| CsPbIBr2 | 1. Good stability in ambient air 2. All fabrication in ambient air | 1. Higher bandgap (~2.05 eV) 2. Energy level mismatch |
| CsPbI2Br | 1. Proper bandgap (~1.90 eV) 2. Good thermal stability | 1. Humidity instability 2. Inferior phase stability |
| CsPbI3 | 1. Most suitable bandgap (~1.73 eV) 2. Excellent light absorption performance 3. Highest efficiency achieved | 1. Weakest phase stability 2. Worst thermal and moisture stability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, H.; Wang, K.; Liu, D.; Zhao, L.; Chen, D.; Zhu, W.; Zhang, J.; Zhang, C. Recent Progress of Film Fabrication Process for Carbon-Based All-Inorganic Perovskite Solar Cells. Crystals 2023, 13, 679. https://doi.org/10.3390/cryst13040679
Yang H, Wang H, Wang K, Liu D, Zhao L, Chen D, Zhu W, Zhang J, Zhang C. Recent Progress of Film Fabrication Process for Carbon-Based All-Inorganic Perovskite Solar Cells. Crystals. 2023; 13(4):679. https://doi.org/10.3390/cryst13040679
Chicago/Turabian StyleYang, Haifeng, Hui Wang, Ke Wang, Dongqi Liu, Lifang Zhao, Dazheng Chen, Weidong Zhu, Jincheng Zhang, and Chunfu Zhang. 2023. "Recent Progress of Film Fabrication Process for Carbon-Based All-Inorganic Perovskite Solar Cells" Crystals 13, no. 4: 679. https://doi.org/10.3390/cryst13040679
APA StyleYang, H., Wang, H., Wang, K., Liu, D., Zhao, L., Chen, D., Zhu, W., Zhang, J., & Zhang, C. (2023). Recent Progress of Film Fabrication Process for Carbon-Based All-Inorganic Perovskite Solar Cells. Crystals, 13(4), 679. https://doi.org/10.3390/cryst13040679








