Calculated Outstanding Energy-Storage Media by Aluminum-Decorated Carbon Nitride (g-C3N4): Elucidating the Synergistic Effects of Electronic Structure Tuning and Localized Electron Redistribution
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
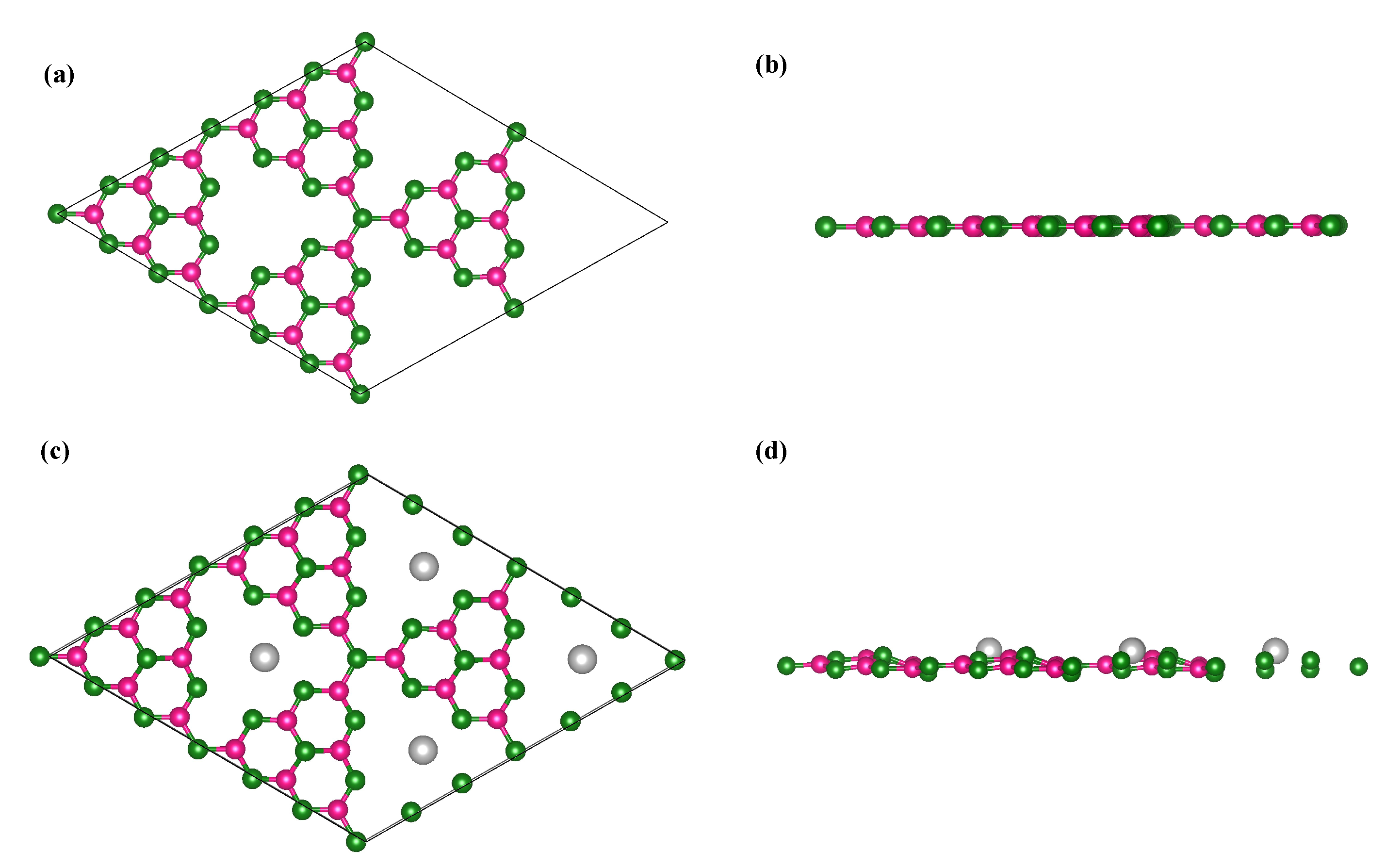
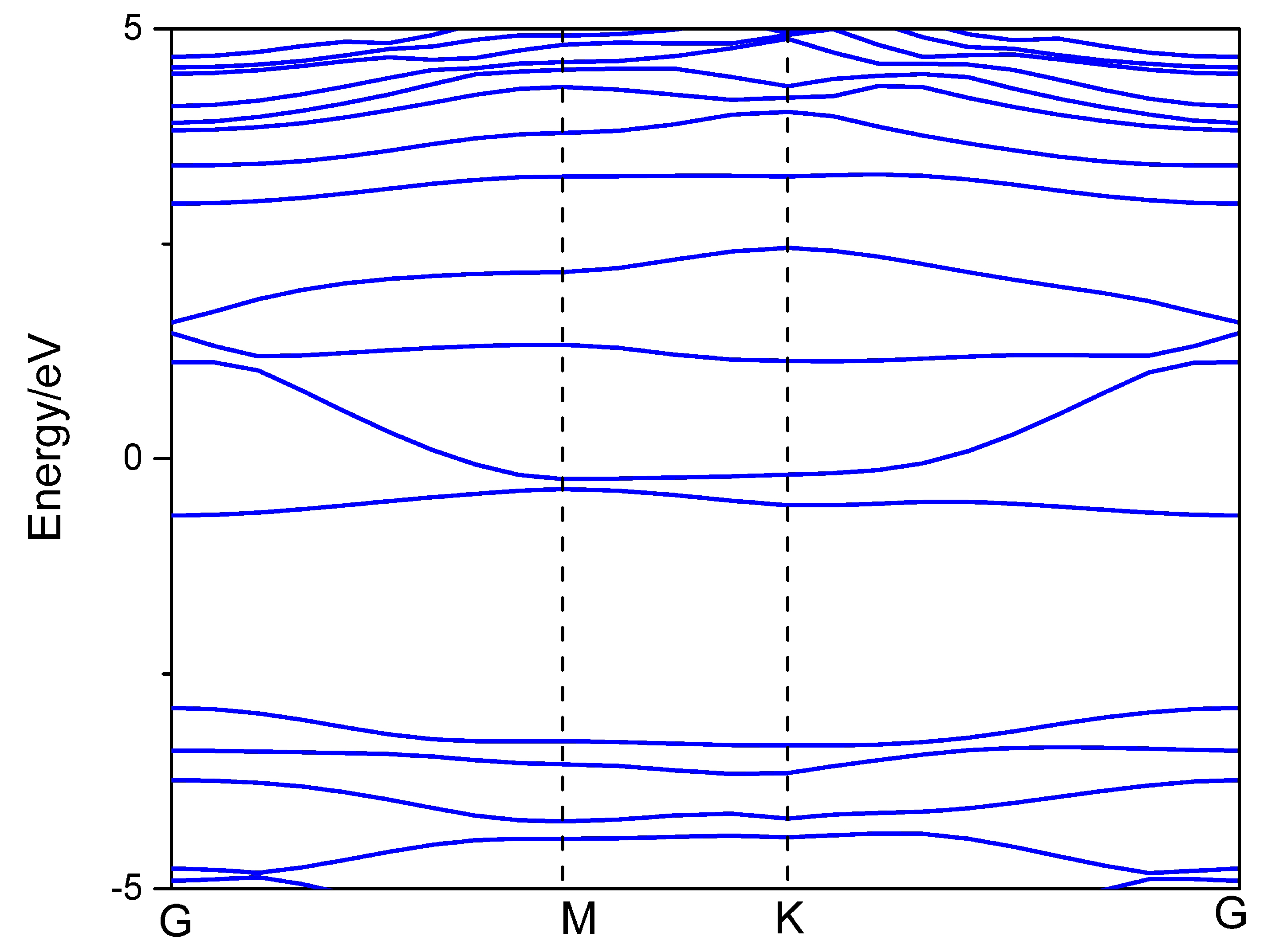
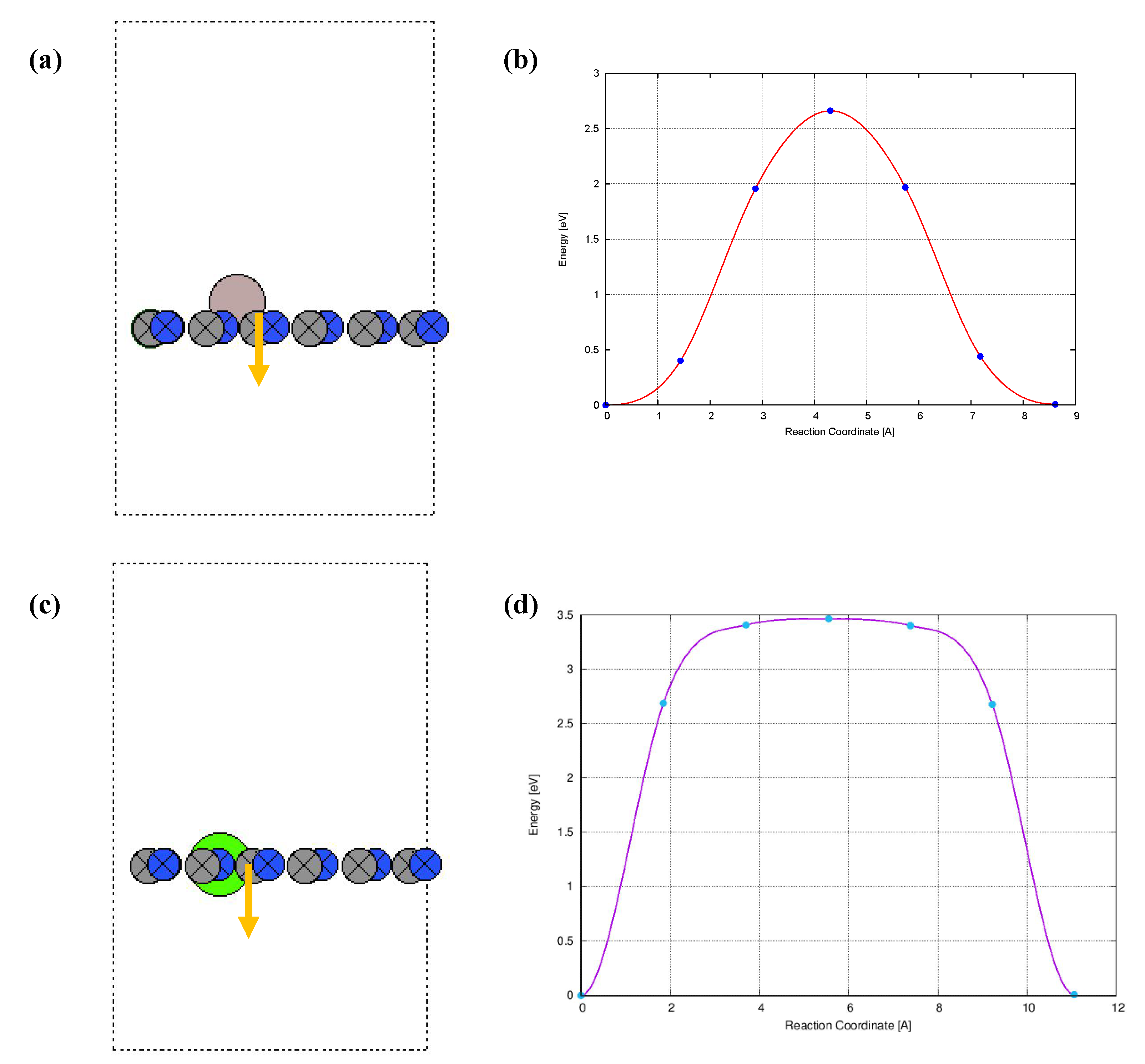
3.1. Structural Properties and Al Binding
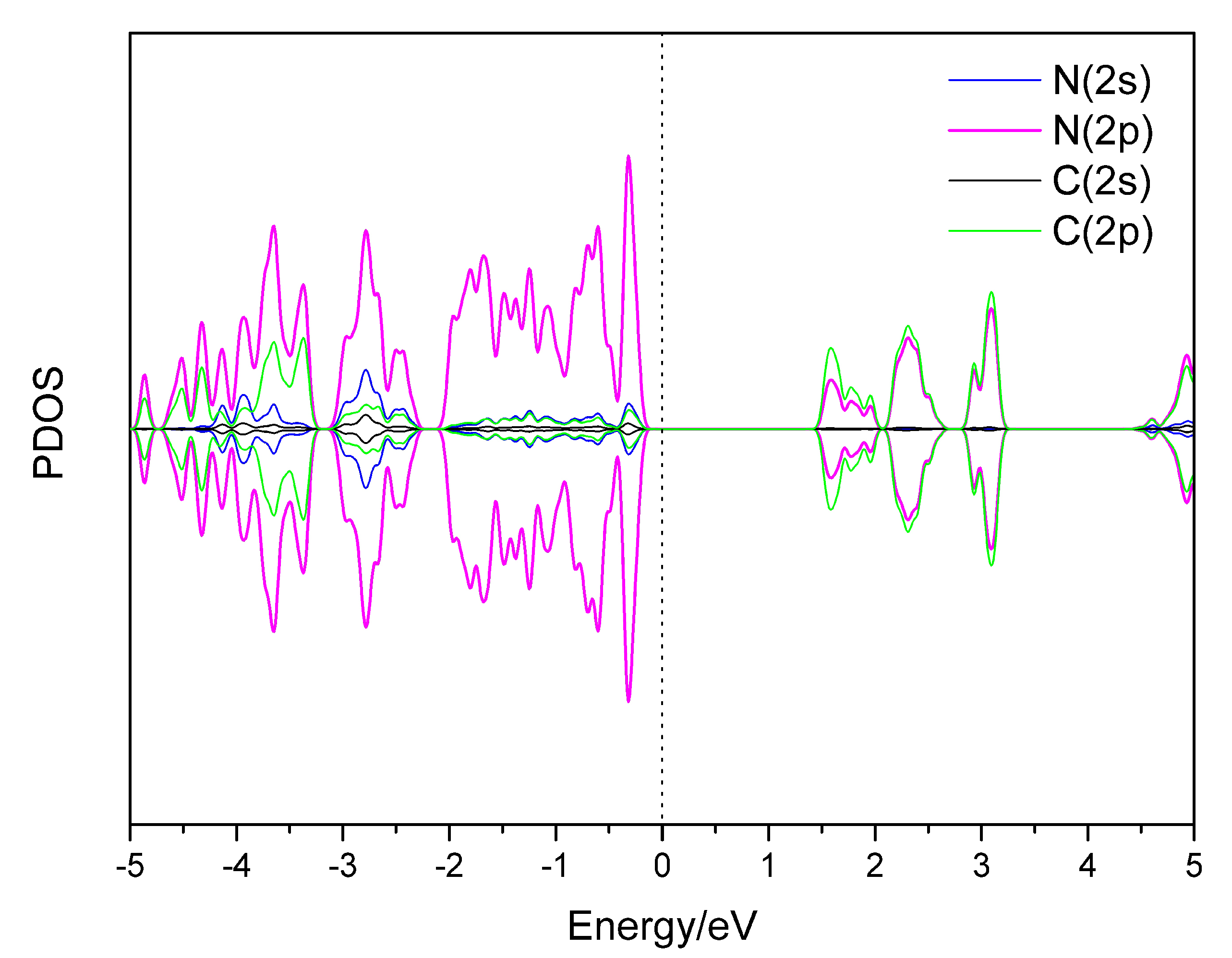
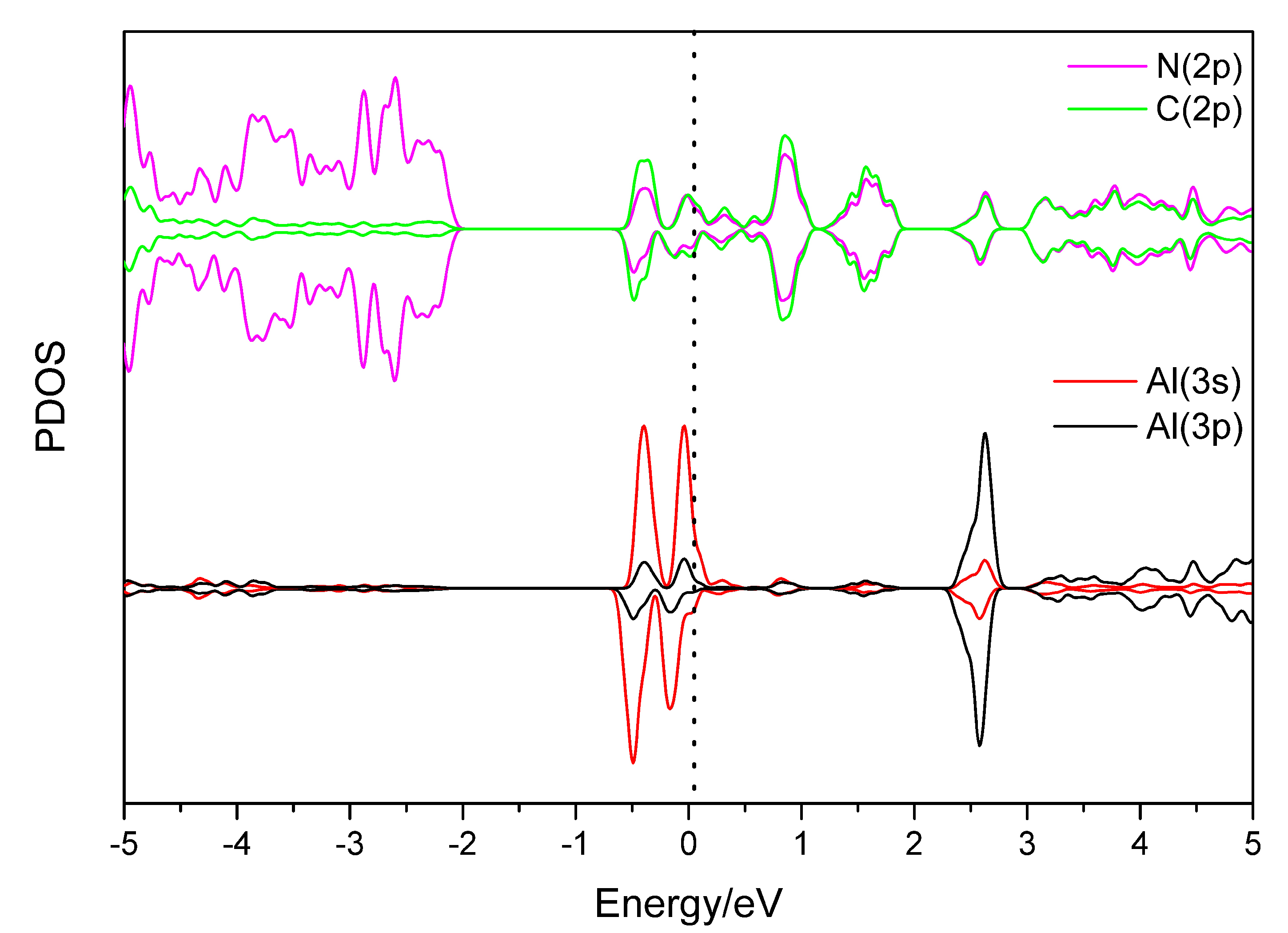
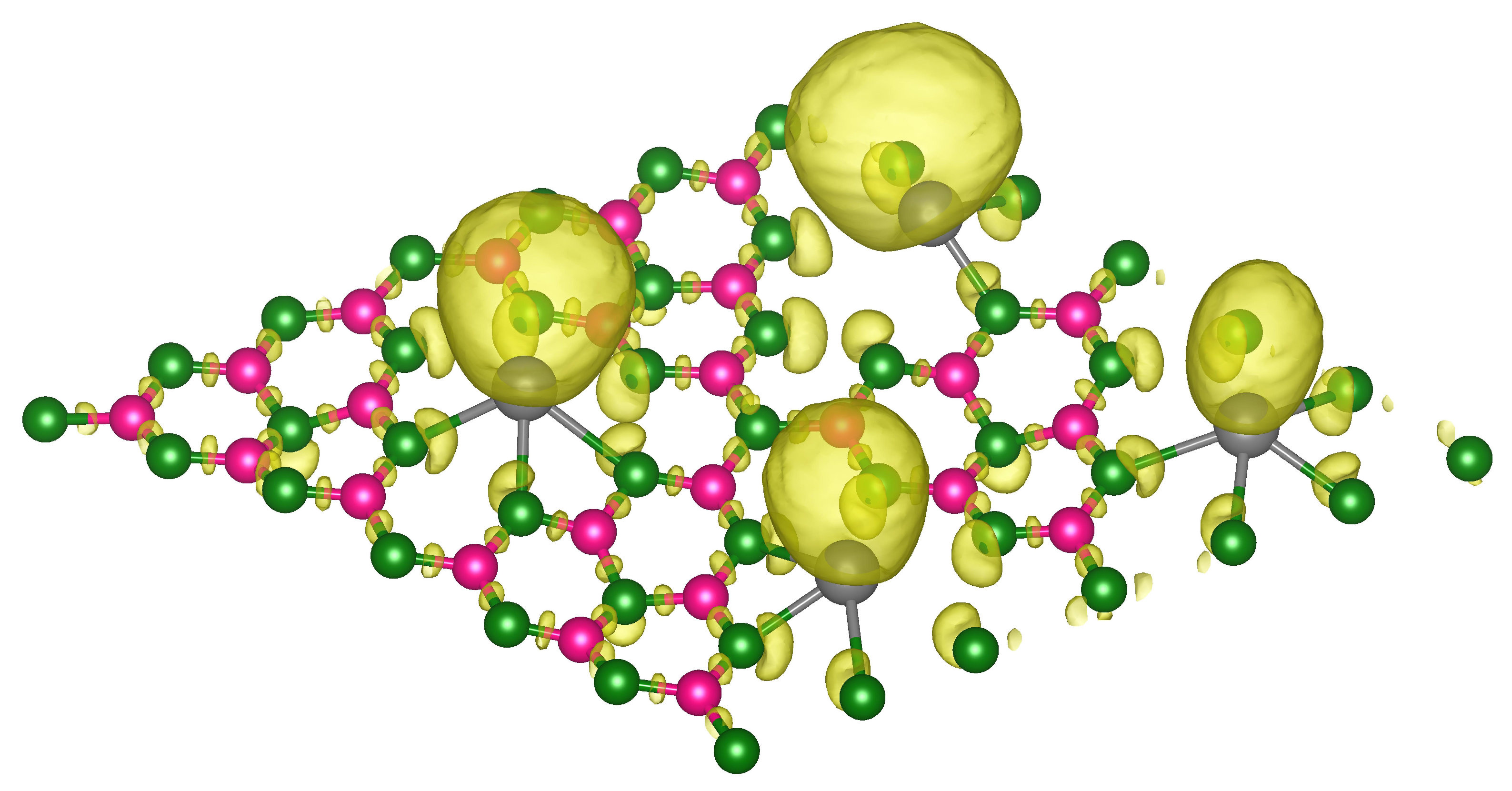
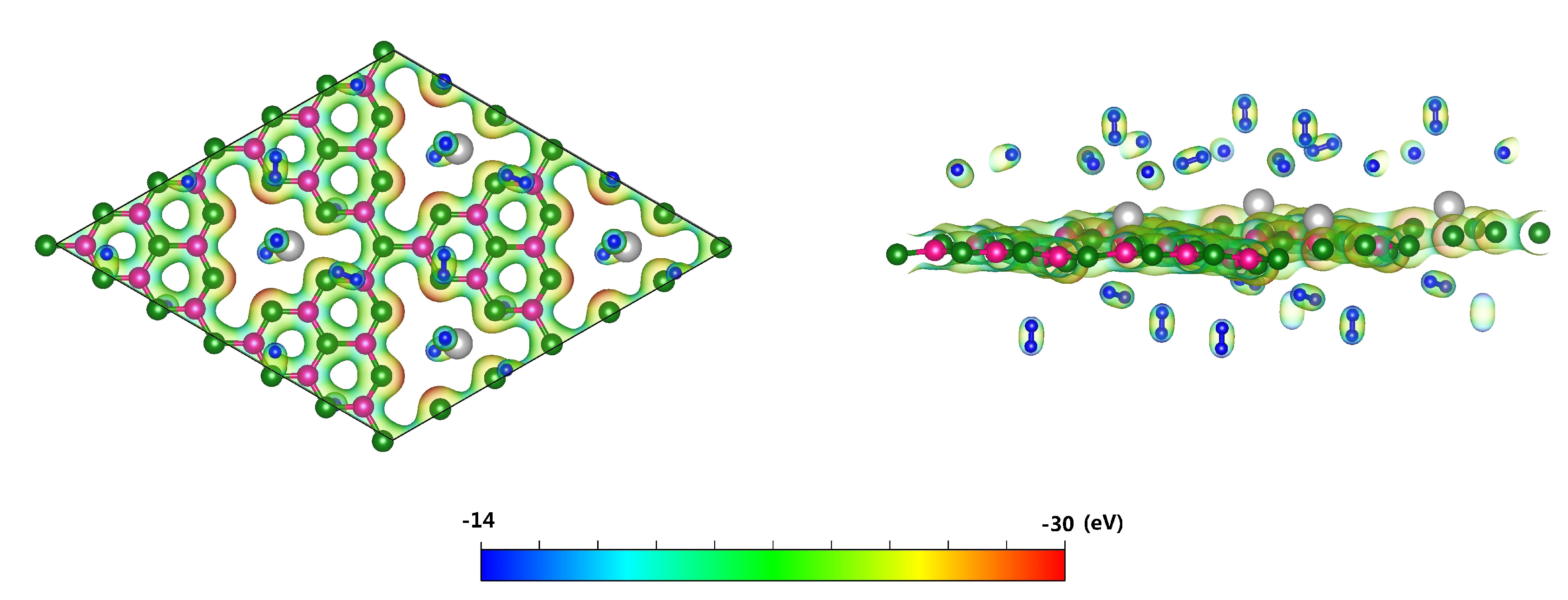
3.2. Hydrogen Storage by Al-Decorated g-C3N4
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Hulvey, Z.; Gennett, T.; Ahmed, A.; Autrey, T.; Camp, J.; Seon Cho, E.; Furukawa, H.; Haranczyk, M.; Head-Gordon, M.; et al. An assessment of strategies for the development of solid-state adsorbents for vehicular hydrogen storage. Energy Environ. Sci. 2018, 11, 2784–2812. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Fuel Cell Technologies Office Multi-Year Research, Development and Demonstration Plan; U.S. Department of Energy: Washington, DC, USA, 2020.
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Huang, Z.; Autrey, T. Boron–nitrogen–hydrogen (BNH) compounds: Recent developments in hydrogen storage, applications in hydrogenation and catalysis, and new syntheses. Energy Environ. Sci. 2012, 5, 9257–9268. [Google Scholar] [CrossRef]
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials1: Invited paper presented at the International Symposium on Metal-Hydrogen Systems. J. Alloys Comp. 1997, 253–254, 1–9. [Google Scholar]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Teichmann, D.; Stark, K.; Müller, K.; Zöttl, G.; Wasserscheid, P.; Arlt, W. Energy storage in residential and commercial buildings via Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2012, 5, 9044–9054. [Google Scholar] [CrossRef]
- Gao, P.; Huang, Z.; Yu, H. Exploration of the Dehydrogenation Pathways of Ammonia Diborane and Diammoniate of Diborane by Molecular Dynamics Simulations Using Reactive Force Fields. J. Phys. Chem. A 2020, 124, 1698–1704. [Google Scholar] [CrossRef]
- Luo, W.; Zakharov, L.N.; Liu, S.Y. 1,2-BN Cyclohexane: Synthesis, Structure, Dynamics, and Reactivity. J. Am. Chem. Soc. 2011, 133, 13006–13009. [Google Scholar] [CrossRef]
- Luo, W.; Campbell, P.G.; Zakharov, L.N.; Liu, S.Y. A Single-Component Liquid-Phase Hydrogen Storage Material. J. Am. Chem. Soc. 2011, 133, 19326–19329. [Google Scholar] [CrossRef]
- Campbell, P.G.; Zakharov, L.N.; Grant, D.J.; Dixon, D.A.; Liu, S.Y. Hydrogen Storage by Boron-Nitrogen Heterocycles: A Simple Route for Spent Fuel Regeneration. J. Am. Chem. Soc. 2010, 132, 3289–3291. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, J. Understanding the Dehydrogenation Pathways of Ammonium Octahydrotriborate (NH4B3H8) by Molecular Dynamics Simulations with the Reactive Force Field (ReaxFF). Adv. Theory Simul. 2020, 3, 2000139. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, J. Understanding the Intra-Molecular Proton Transfer of Octahydrotriborate and Exploring the Dehydrogenation Pathways of NH4B3H8 by DFT Calculations. Adv. Theory Simul. 2021, 4, 2000287. [Google Scholar] [CrossRef]
- Holst, J.R.; Gillan, E.G. From Triazines to Heptazines: Deciphering the Local Structure of Amorphous Nitrogen-Rich Carbon Nitride Materials. J. Am. Chem. Soc. 2008, 130, 7373–7379. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Wei, W.; Jacob, T. Strong excitonic effects in the optical properties of graphitic carbon nitride g-C3N4 from first principles. Phys. Rev. B 2013, 87, 085202. [Google Scholar] [CrossRef]
- Liu, G.; Xue, M.; Liu, Q.; Yang, H.; Zhou, Y. Facile synthesis of C-doped hollow spherical g-C3N4 from supramolecular self-assembly for enhanced photoredox water splitting. Int. J. Hydrogen Energy 2019, 44, 25671–25679. [Google Scholar] [CrossRef]
- Algara-Siller, G.; Severin, N.; Chong, S.Y.; Björkman, T.; Palgrave, R.G.; Laybourn, A.; Antonietti, M.; Khimyak, Y.Z.; Krasheninnikov, A.V.; Rabe, J.P.; et al. Triazine-Based Graphitic Carbon Nitride: A Two-Dimensional Semiconductor. Angew. Chem. Int. Ed. Engl. 2014, 53, 7450–7455. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, B.; Irran, E.; Senker, J.; Kroll, P.; Müller, H.; Schnick, W. Melem (2,5,8-Triamino-tri-s-triazine), an Important Intermediate during Condensation of Melamine Rings to Graphitic Carbon Nitride: Synthesis, Structure Determination by X-ray Powder Diffractometry, Solid-State NMR, and Theoretical Studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, F.; Yuan, B.; Xiao, S.; Kuang, A.; Zhong, M.; Dang, S.; Long, X.; Zhang, W. Strain-Tunable Visible-Light-Responsive Photocatalytic Properties of Two-Dimensional CdS/g-C3N4: A Hybrid Density Functional Study. Nanomaterials 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural Investigation of Graphitic Carbon Nitride via XRD and Neutron Diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Hussain, T.; Hankel, M.; Searles, D.J. Computational Evaluation of Lithium-Functionalized Carbon Nitride (g-C6N5) Monolayer as an Efficient Hydrogen Storage Material. J. Phys. Chem. C. 2016, 120, 25180–25188. [Google Scholar] [CrossRef]
- Gao, P.; Li, J.; Wang, G. Computational evaluation of superalkali-decorated graphene nanoribbon as advanced hydrogen storage materials. Int. J. Hydrogen Energy 2021, 46, 24510–24516. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of New Low Compressibility Solids. Science 1989, 245, 841–842. [Google Scholar] [CrossRef]
- Zhu, G.; Lü, K.; Sun, Q.; Kawazoe, Y.; Jena, P. Lithium-doped triazine-based graphitic C3N4 sheet for hydrogen storage at ambient temperature. Comput. Mater. Sci. 2014, 81, 275–279. [Google Scholar] [CrossRef]
- Nair, A.A.; Sundara, R.; Anitha, N. Hydrogen storage performance of palladium nanoparticles decorated graphitic carbon nitride. Int. J. Hydrogen Energy 2015, 40, 3259–3267. [Google Scholar] [CrossRef]
- Panigrahi, P.; Kumar, A.; Karton, A.; Ahuja, R.; Hussain, T. Remarkable improvement in hydrogen storage capacities of two-dimensional carbon nitride (g-C3N4) nanosheets under selected transition metal doping. Int. J. Hydrogen Energy 2020, 45, 3035–3045. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Chen, C. New template for metal decoration and hydrogen adsorption on graphene-like C3N4. Phys. Lett. A 2009, 373, 2778–2781. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Sun, Q.; Jena, P. Functionalized Graphitic Carbon Nitride for Efficient Energy Storage. J. Phys. Chem. C 2013, 117, 6055–6059. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, L.; Wang, L.; Huang, J.; She, H.; Wang, Q. Construction of heterostructured g-C3N4@TiATA/Pt composites for efficacious photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 24407–24417. [Google Scholar] [CrossRef]
- Ruan, L.; Xu, G.; Gu, L.; Li, C.; Zhu, Y.; Lu, Y. The physical properties of Li-doped g-C3N4 monolayer sheet investigated by the first-principles. Mater. Res. Bull. 2015, 66, 156–162. [Google Scholar] [CrossRef]
- Wei, J.; Huang, C.; Wu, H.; Kan, E. High-capacity hydrogen storage in Li-adsorbed g-C3N4. Mater. Chem. Phys. 2016, 180, 440–444. [Google Scholar] [CrossRef]
- Gao, P.; Li, J.; Zhang, J.; Wang, G. Computational exploration of magnesium-decorated carbon nitride (g-C3N4) monolayer as advanced energy storage materials. Int. J. Hydrogen Energy 2021, 46, 21739–21747. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.-w.; Dou, X.; Gao, P. Computational evaluation of Mg-decorated g-CN as clean energy gas storage media. Int. J. Hydrogen Energy 2021, 46, 35130–35136. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Phys. Rev. B 1992, 46, 12947–12954. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Chadi, D.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1977, 16, 1746–1747. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Bader, R. Atoms in Molecules—A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Gao, P.; Liu, Z.; Zhang, F. Computational Evaluation of Li-doped g-C2N Monolayer as Advanced Hydrogen Storage Media. Int. J. Hydrogen Energy 2022, 47, 3625–3632. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Long time scale kinetic Monte Carlo simulations without lattice approximation and predefined event table. J. Chem. Phys. 2001, 115, 9657–9666. [Google Scholar] [CrossRef]
- Xu, L.; Henkelman, G. Adaptive kinetic Monte Carlo for first-principles accelerated dynamics. J. Chem. Phys. 2008, 129, 114104. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]


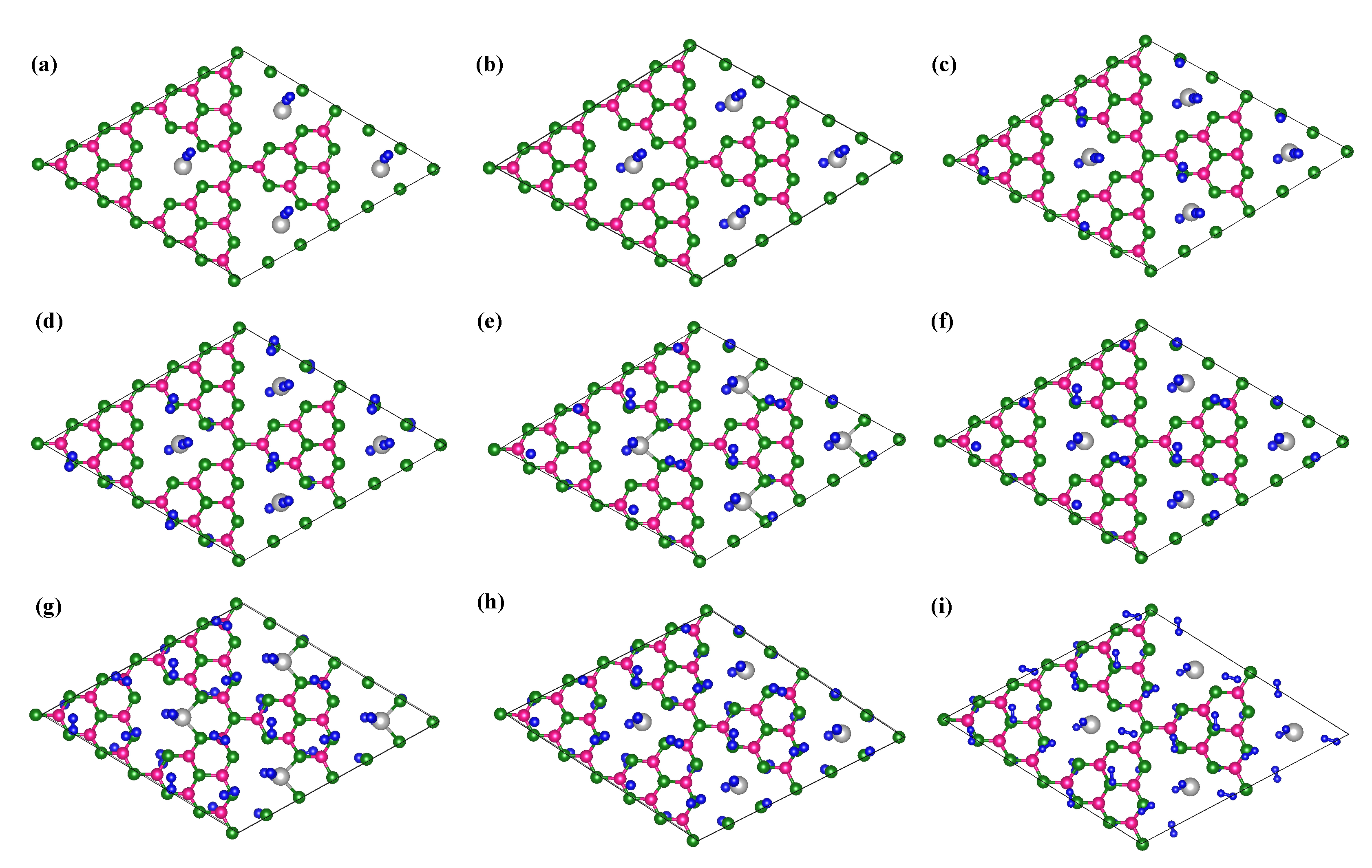
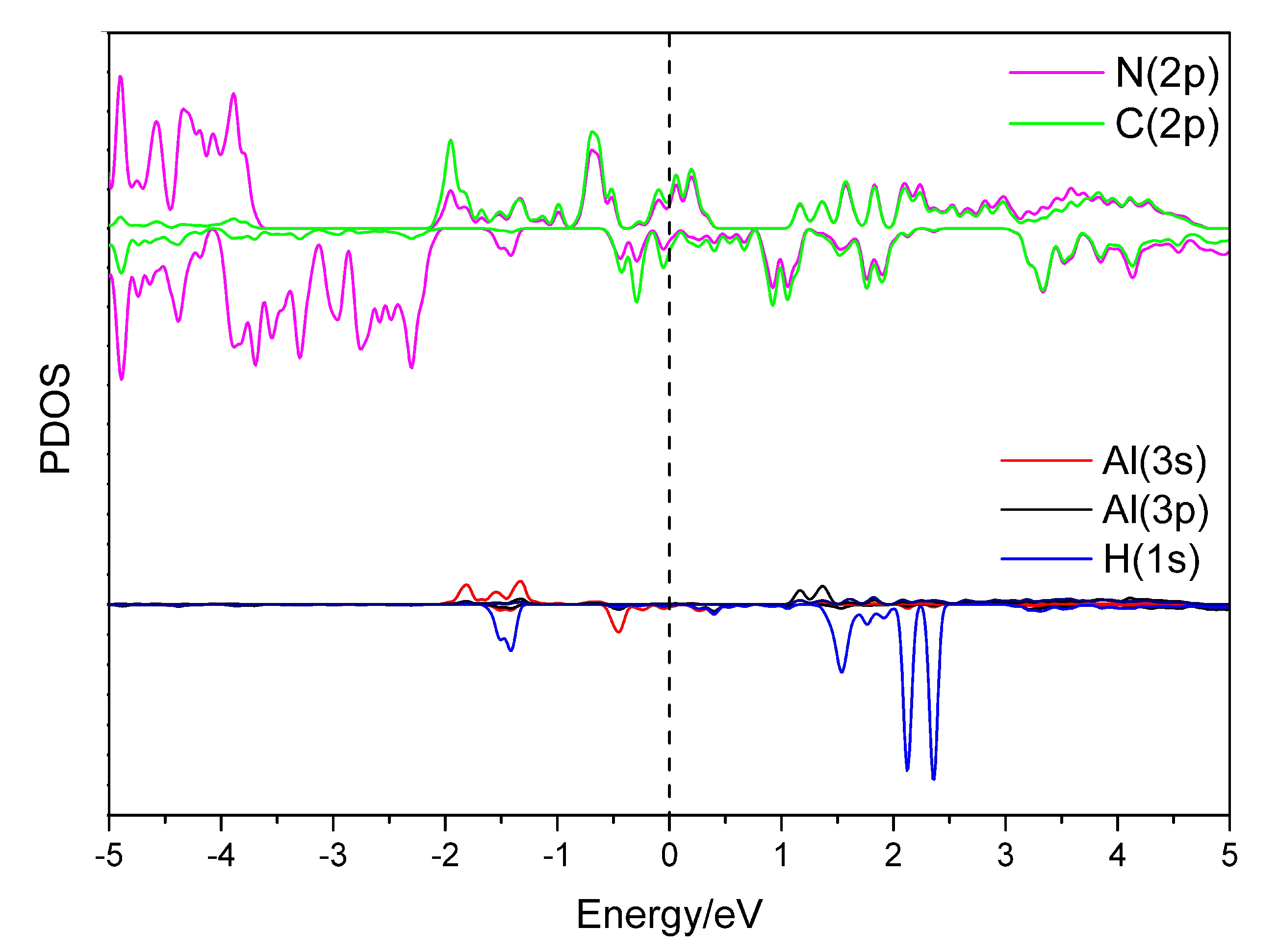
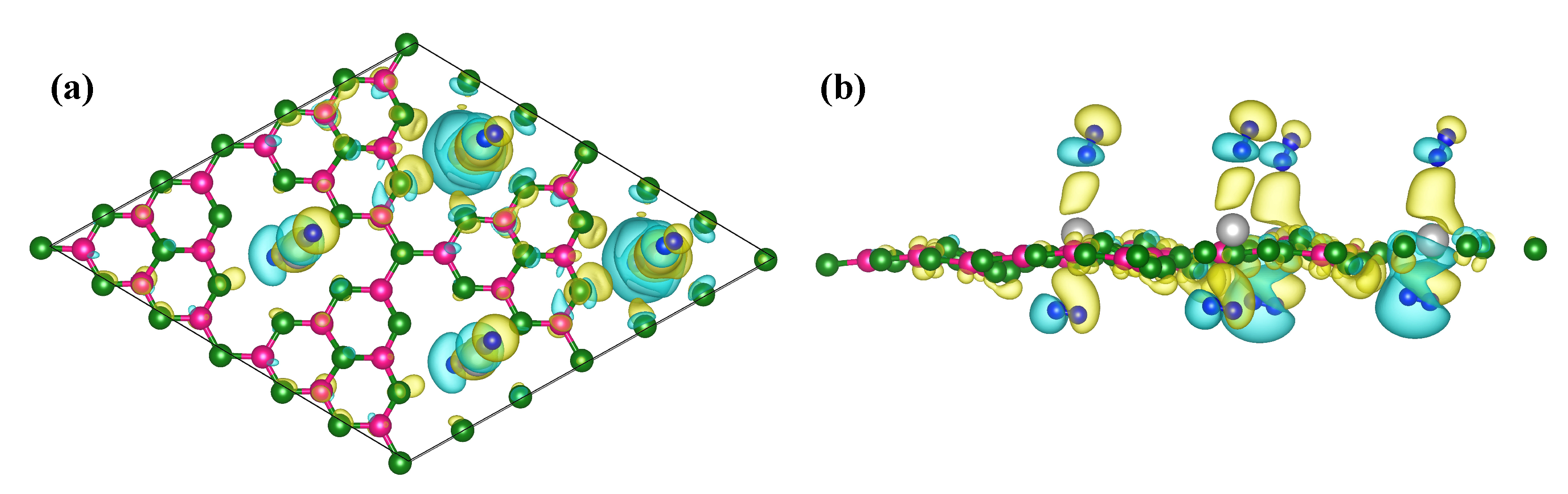

| System | Adsorption E | H-H Bond | Capacity | Desorption T |
|---|---|---|---|---|
| Al4C24N32 + 4H2 | −0.26 | 0.760 | 0.94 | 329 |
| Al4C24N32 + 8H2 | −0.14 | 0.758 | 1.86 | 182 |
| Al4C24N32 + 12H2 | −0.13 | 0.756 | 2.76 | 169 |
| Al4C24N32 + 16H2 | −0.13 | 0.756 | 3.65 | 169 |
| Al4C24N32 + 20H2 | −0.11 | 0.754 | 4.52 | 146 |
| Al4C24N32 + 24H2 | −0.11 | 0.754 | 5.38 | 141 |
| Al4C24N32 + 28H2 | −0.11 | 0.753 | 6.22 | 146 |
| Al4C24N32 + 32H2 | −0.10 | 0.753 | 7.05 | 124 |
| Al4C24N32 + 36H2 | −0.10 | 0.753 | 7.86 | 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Liu, Z.; Diao, J.; Wang, J.; Li, J.; Tan, Y.; Hai, G.; Henkelman, G. Calculated Outstanding Energy-Storage Media by Aluminum-Decorated Carbon Nitride (g-C3N4): Elucidating the Synergistic Effects of Electronic Structure Tuning and Localized Electron Redistribution. Crystals 2023, 13, 655. https://doi.org/10.3390/cryst13040655
Gao P, Liu Z, Diao J, Wang J, Li J, Tan Y, Hai G, Henkelman G. Calculated Outstanding Energy-Storage Media by Aluminum-Decorated Carbon Nitride (g-C3N4): Elucidating the Synergistic Effects of Electronic Structure Tuning and Localized Electron Redistribution. Crystals. 2023; 13(4):655. https://doi.org/10.3390/cryst13040655
Chicago/Turabian StyleGao, Peng, Zonghang Liu, Jiefeng Diao, Jiaao Wang, Jiwen Li, Yuebin Tan, Guangtong Hai, and Graeme Henkelman. 2023. "Calculated Outstanding Energy-Storage Media by Aluminum-Decorated Carbon Nitride (g-C3N4): Elucidating the Synergistic Effects of Electronic Structure Tuning and Localized Electron Redistribution" Crystals 13, no. 4: 655. https://doi.org/10.3390/cryst13040655
APA StyleGao, P., Liu, Z., Diao, J., Wang, J., Li, J., Tan, Y., Hai, G., & Henkelman, G. (2023). Calculated Outstanding Energy-Storage Media by Aluminum-Decorated Carbon Nitride (g-C3N4): Elucidating the Synergistic Effects of Electronic Structure Tuning and Localized Electron Redistribution. Crystals, 13(4), 655. https://doi.org/10.3390/cryst13040655







