Determination of Nicotine in Human Saliva Using Electrochemical Sensor Modified with Green Synthesized Silver Nanoparticles Using Phyllanthus reticulatus Fruit Extract
Abstract
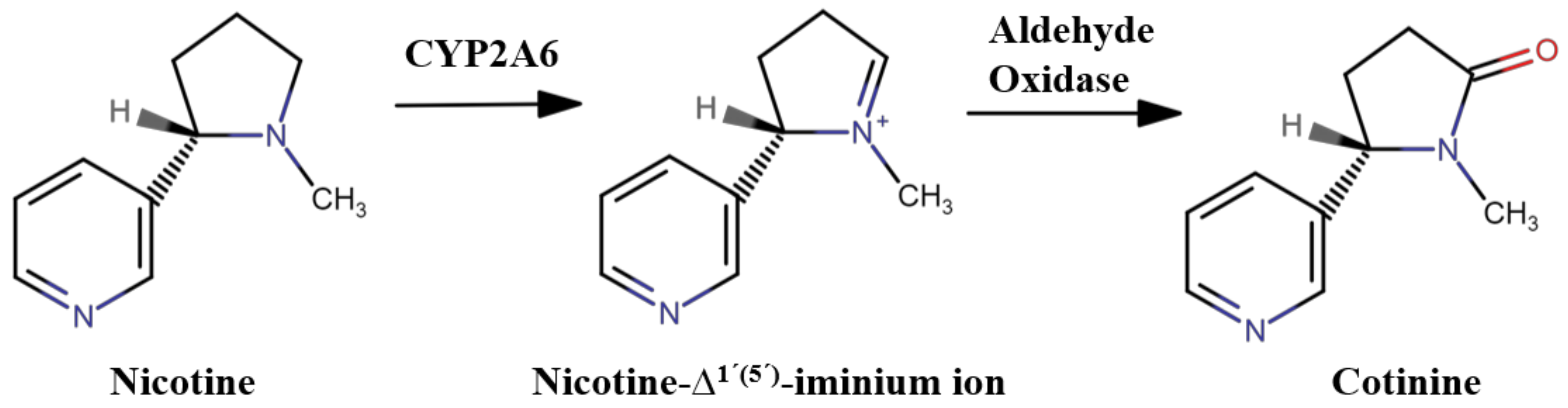
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Silver Nanoparticles (Ag-NPs)
2.3. Characterization
2.4. Electrochemical Measurements and Sensor Preparation
2.5. Nicotine Standard Sample Preparation
3. Results and Discussion
3.1. X-ray Diffraction Analysis
3.2. UV Visible (UV-Vis) Spectroscopy
3.3. Surface Morphology Studies by FE-SEM
3.4. Electrochemical Impedance Spectroscopy (EIS) Studies
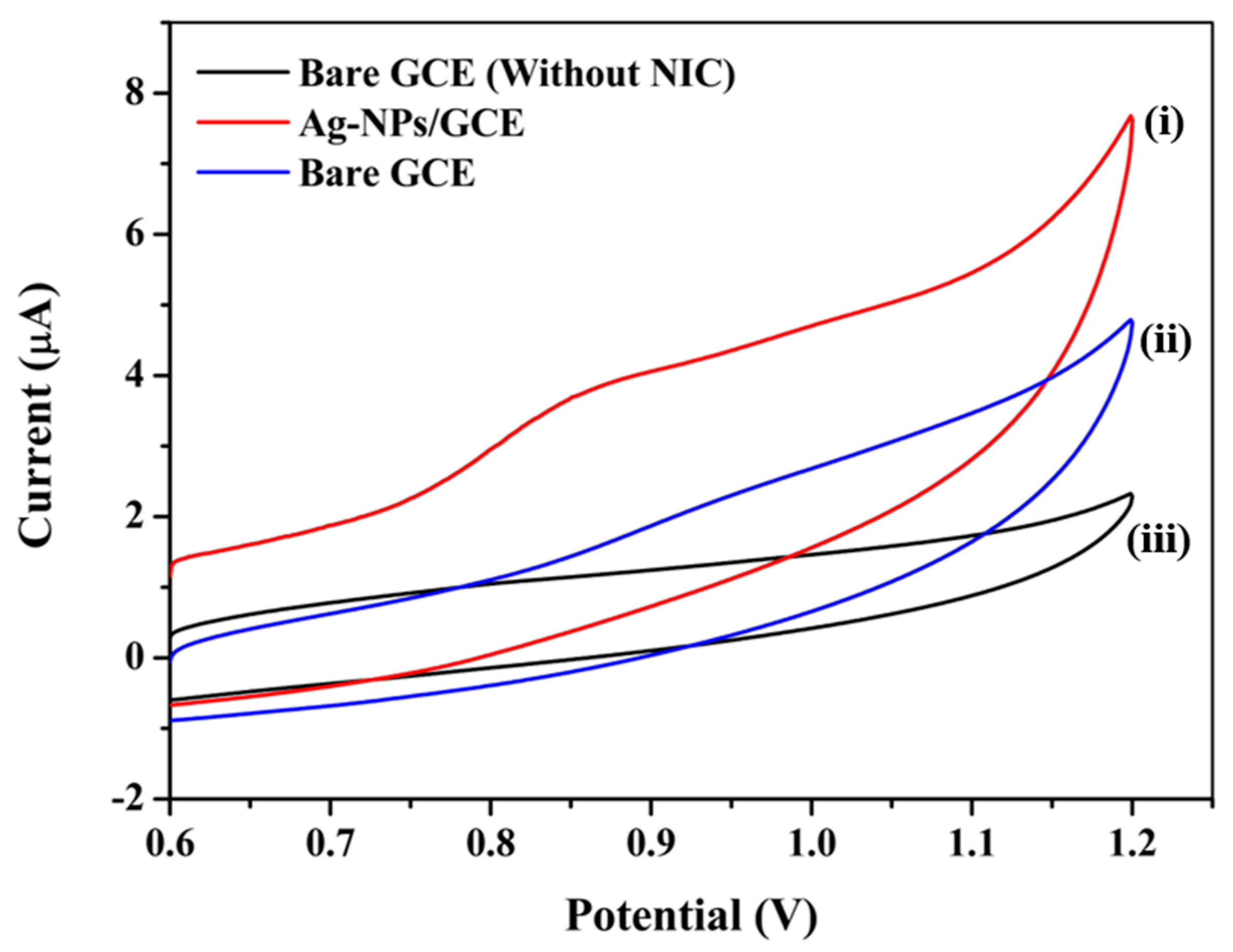
3.5. Cyclic Voltammetry Response of the Sensor
3.5.1. Effect of Scan Rate on NIC
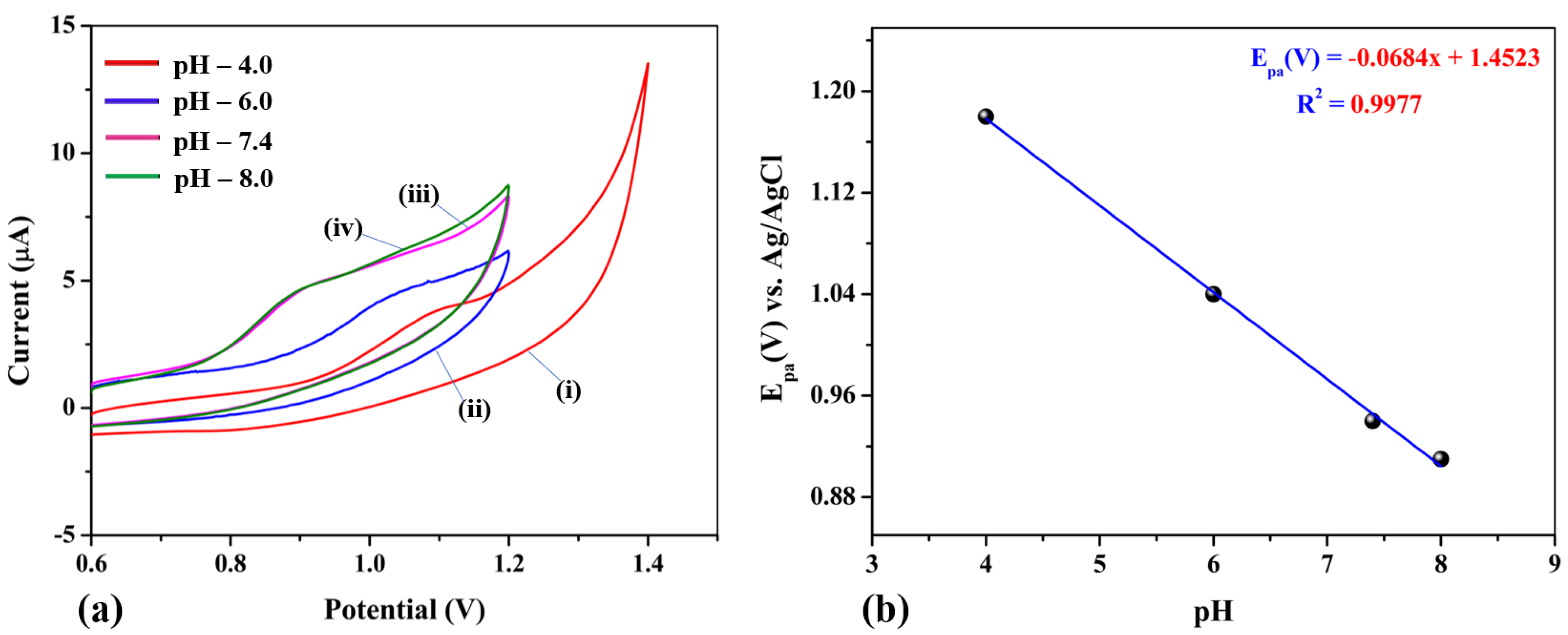
3.5.2. Effect of pH on NIC Oxidation
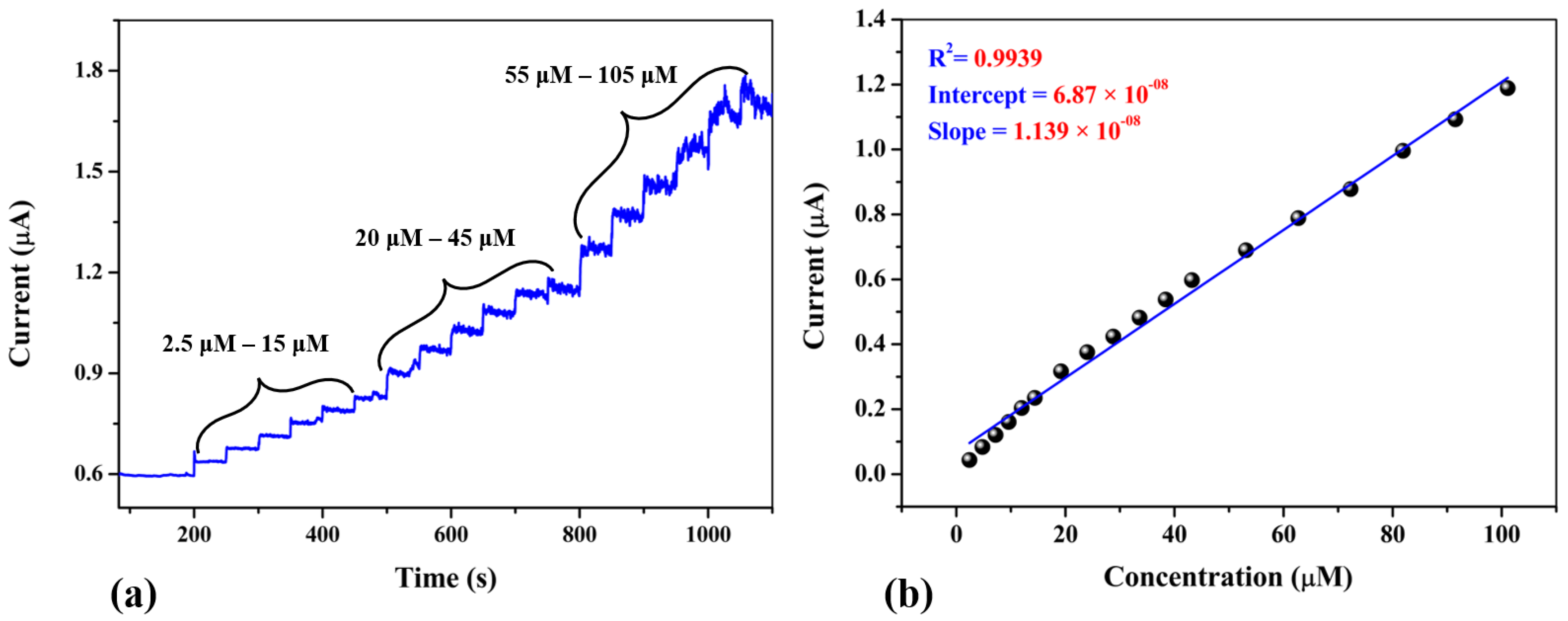
3.6. Amperometric Study
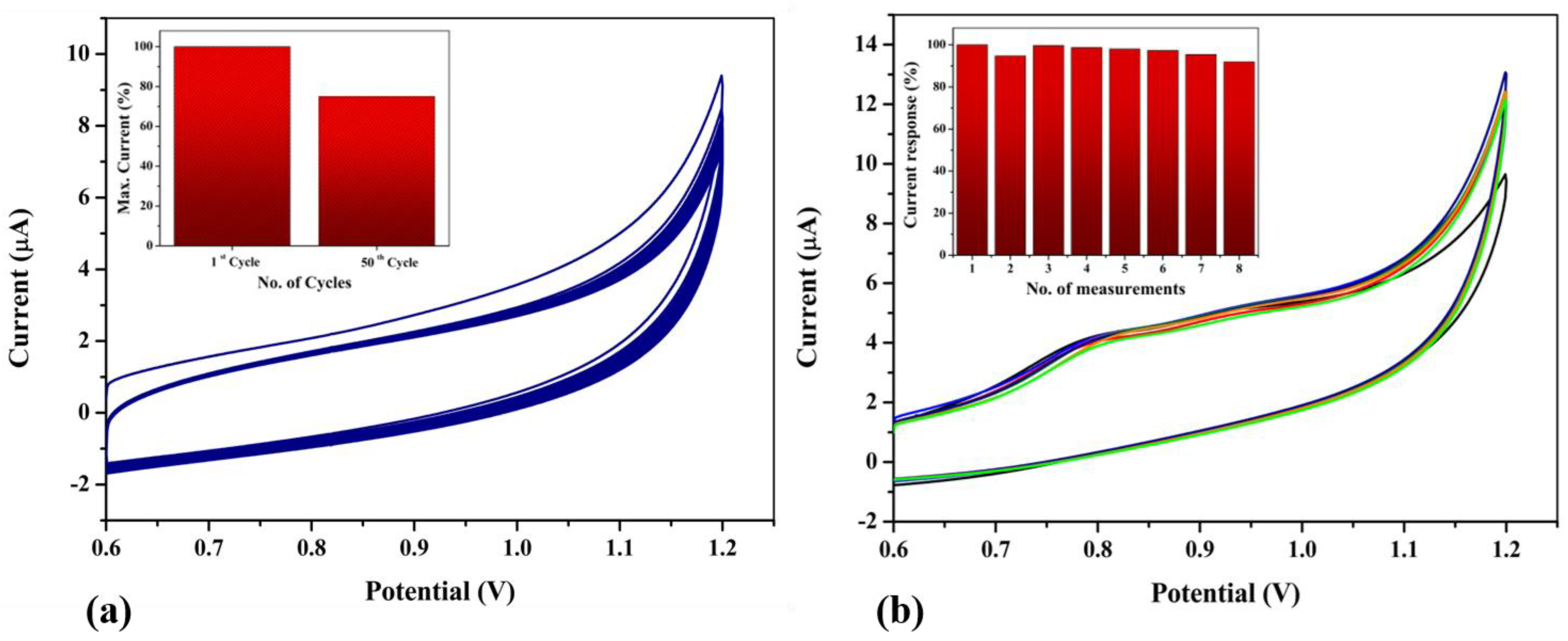
3.7. Stability, Repeatability and Interference Studies
3.8. NIC Detection in Tobacco and Saliva Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 27 January 2023).
- Cinková, K.; Dianová, L.; Vojs, M.; Marton, M.; Švorc, Ľ. Rapid Electrochemical Platform for Nicotine Sensing in Cigarettes and Chewing Gums. Acta Chim. Slovaca 2015, 8, 166–171. [Google Scholar] [CrossRef]
- Kassa, H.; Geto, A.; Admassie, S. Voltammetric Determination of Nicotine in Cigarette Tobacco at Electrochemically Activated Glassy Carbon Electrode. Bull. Chem. Soc. Ethiop. 2013, 27, 321–328. [Google Scholar] [CrossRef]
- Kowalcze, M.; Prof, M.J. Diamond & Related Materials Voltammetric Determination of Nicotine in Electronic Cigarette Liquids Using a Boron-Doped Diamond Electrode (BDDE). Diam. Relat. Mater. 2020, 103, 107710. [Google Scholar] [CrossRef]
- Stolerman, I.; Rose, J. Nicotine Psychopharmacology. J. Psychopharmacol. 1992, 6, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Yamagishi, S.I.; Yamamoto, H.; Yamamoto, T.; Kuroiwa, Y.; Yokoi, T. Deficient Cotinine Formation from Nicotine Is Attributed to the Whole Deletion of the CYP2A6 Gene in Humans. Clin. Pharm. Ther. 2000, 67, 57–69. [Google Scholar] [CrossRef]
- Sridharan, G.; Ganapathy, D.; Ramadoss, R.; Atchudan, R.; Arya, S.; Sundramoorthy, A.K. Biosensors for Rapid and Accurate Determination of Oral Cancer. Oral Oncol. Rep. 2023, 5, 100021. [Google Scholar] [CrossRef]
- Nicotine Cotinine (Urine)-Health Encyclopedia-University of Rochester Medical Center. Available online: https://www.urmc.rochester.edu/encyclopedia/content.aspx?contentid=nicotine_cotinine&contenttypeid=167 (accessed on 27 January 2023).
- Mersal, G.A.M.; Mostafa, N.Y.; Omar, A.E.H. Hydrothermal Synthesis and Processing of Hydrogen Titanate Nanotubes for Nicotine Electrochemical Sensing. Mater. Res. Express 2017, 4, 085031. [Google Scholar] [CrossRef]
- Sebokolodi, T.I.; Sipuka, D.S.; Muzenda, C.; Nkwachukwu, O.V.; Nkosi, D.; Arotiba, O.A. Electrochemical Detection of Nicotine at a Carbon Nanofiber-Poly(Amidoamine) Dendrimer Modified Glassy Carbon Electrode. Chemosphere 2022, 303, 134961. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Wu, S.M. High-Performance Liquid Chromatography with Electrochemical Detection for Analysis of Gliclazide in Plasma. J. Chromatogr. A 2005, 1088, 131–135. [Google Scholar] [CrossRef]
- Bezuneh, T.T.; Fereja, T.H.; Kitte, S.A.; Li, H.; Jin, Y. Gold Nanoparticle-Based Signal Amplified Electrochemiluminescence for Biosensing Applications. Talanta 2022, 248, 123611. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Bakirhan, N.K.; Ozkan, S.A. Novel Advances in Nanomaterial-Based Electrochemical Sensing of the Biomarker. Detect. Biomarkers 2022, 209–224. [Google Scholar] [CrossRef]
- Jerome, R.; Sundramoorthy, A.K. Preparation of Hexagonal Boron Nitride Doped Graphene Film Modified Sensor for Selective Electrochemical Detection of Nicotine in Tobacco Sample. Anal. Chim. Acta 2020, 1132, 110–120. [Google Scholar] [CrossRef]
- Molaakbari, E.; Mostafavi, A.; Beitollahi, H. First Electrochemical Report for Simultaneous Determination of Norepinephrine, Tyrosine and Nicotine Using a Nanostructure Based Sensor. Electroanalysis 2014, 26, 2252–2260. [Google Scholar] [CrossRef]
- Fekry, A.M.; Azab, S.M.; Shehata, M.; Ameer, M.A. A Novel Electrochemical Nicotine Sensor Based on Cerium Nanoparticles with Anionic Surfactant. RSC Adv. 2015, 5, 51662–51671. [Google Scholar] [CrossRef]
- Preethika, M.; Sundramoorthy, A.K. Humic Acid/Halloysite Nanotube/Flavin Adenine Dinucleotide Nanocomposite Based Selective Electrochemical Biosensor for Hydrogen Peroxide. Appl. Surf Sci. 2019, 488, 503–511. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-Titanium Carbide (MXene) Based Selective Electrochemical Sensor for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Nagarajan, R.D.; Murugan, P.; Palaniyandi, K.; Atchudan, R.; Sundramoorthy, A.K. Biocompatible MXene (Ti3C2Tx) Immobilized with Flavin Adenine Dinucleotide as an Electrochemical Transducer for Hydrogen Peroxide Detection in Ovarian Cancer Cell Lines. Micromachines 2021, 12, 862. [Google Scholar] [CrossRef]
- Murugan, P.; Sundramoorthy, A.K.; Ganapathy, D.; Atchudan, R.; Nallaswamy, D.; Khosla, A. Electrochemical Detection of H2O2 Using an Activated Glassy Carbon Electrode. ECS Sens. Plus 2022, 1, 034401. [Google Scholar] [CrossRef]
- Murugan, P.; Nagarajan, R.D.; Shetty, B.H.; Govindasamy, M.; Sundramoorthy, A.K. Recent Trends in the Applications of Thermally Expanded Graphite for Energy Storage and Sensors–A Review. Nanoscale Adv. 2021, 3, 6294–6309. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Annamalai, J.; Atchudan, R.; Govindasamy, M.; Nallaswamy, D.; Ganapathy, D.; Reshetilov, A.; Sundramoorthy, A.K. Electrochemical Sensing of Glucose Using Glucose Oxidase/PEDOT: 4-Sulfocalix [4] Arene/MXene Composite Modified Electrode. Micromachines 2022, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, J.; Kannan, T.S.; Dhanasekaran, L.S.; Murugan, P.; Atchudan, R.; ALOthman, Z.A.; Ouladsmane, M.; Sundramoorthy, A.K. Preparation of 2D Graphene/MXene Nanocomposite for the Electrochemical Determination of Hazardous Bisphenol A in Plastic Products. Chemosphere 2022, 287, 132106. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.G.E.; Kamel, A.H.; Almehizia, A.A.; Sayed, A.Y.A.; Elsayed, E.A.; Abd-Rabboh, H.S.M. Paper-Based Potentiometric Sensors for Nicotine Determination in Smokers’ Sweat. ACS Omega 2021, 6, 11340–11347. [Google Scholar] [CrossRef] [PubMed]
- Rauwel, P.; Küünal, S.; Ferdov, S. A Review on Green Synthesis of Silver. Adv. Mater. Sci. Eng. 2014, 2015, 682749. [Google Scholar]
- Al-Ghamdi, A.Y. Antimicrobial and Catalytic Activities of Green Synthesized Silver Nanoparticles Using Bay Laurel (Laurus Nobilis) Leaves Extract. J. Biomater. Nanobiotechnol. 2019, 10, 26–39. [Google Scholar] [CrossRef]
- Nagarajan, R.D.; Sundramoorthy, A.K. One-Pot Electrosynthesis of Silver Nanorods/Graphene Nanocomposite Using 4-Sulphocalix[4]Arene for Selective Detection of Oxalic Acid. Sens. Actuators B Chem. 2019, 301, 127132. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green Synthesis of Silver Nanoparticles: Biomolecule-Nanoparticle Organizations Targeting Antimicrobial Activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Savar Dashtaki, A.; Babapoor, A.; Arjmand, O. Green Synthesis of Silver Nanoparticles toward Bio and Medical Applications: Review Study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef]
- Jing, Y.; Ning, S.; Guan, Y.; Cao, M.; Li, J.; Zhu, L.; Zhang, Q.; Cheng, C.; Deng, Y. Electrochemical Determination of Nicotine in Tobacco Products Based on Biosynthesized Gold Nanoparticles. Front. Chem. 2020, 8, 922. [Google Scholar] [CrossRef]
- Debnath, B.; Das, R. Controlled Synthesis of Saponin-Capped Silver Nanotriangles and Their Optical Properties. Plasmonics 2019, 14, 1365–1375. [Google Scholar] [CrossRef]
- Birusanti, A.B.; Mallavarapu, U.; Nayakanti, D.; Espenti, C.S.; Mala, S. Sustainable Green Synthesis of Silver Nanoparticles by Using Rangoon Creeper Leaves Extract and Their Spectral Analysis and Anti-Bacterial Studies. IET Nanobiotechnol. 2019, 13, 71–76. [Google Scholar] [CrossRef]
- Matter, I.A.; Darwesh, O.M.; Matter, H.A.B. Nanomaterials for Soil Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 221–237. [Google Scholar]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N.; Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Light Induced Green Synthesis of Silver Nanoparticles Using Aqueous Extract of Prunus Amygdalus. Green Sustain. Chem. 2016, 6, 26–33. [Google Scholar] [CrossRef]
- Mandal, S.; Marpu, S.B.; Hughes, R.; Omary, M.A.; Shi, S.Q. Green Synthesis of Silver Nanoparticles Using Cannabis Sativa Extracts and Their Anti-Bacterial Activity. Green Sustain. Chem. 2021, 11, 28–38. [Google Scholar] [CrossRef]
- Thi Lan Huong, V.; Nguyen, N.T. Green Synthesis, Characterization and Antibacterial Activity of Silver Nanoparticles Using Sapindus Mukorossi Fruit Pericarp Extract. Mater. Today Proc. 2019, 42, 88–93. [Google Scholar] [CrossRef]
- Widdatallah, M.O.; Mohamed, A.A.; Alrasheid, A.A.; Widatallah, H.A.; Yassin, L.F.; Eltilib, S.H.; Ahmed, S.A.R. Green Synthesis of Silver Nanoparticles Using Nigella Sativa Seeds and Evaluation of Their Antibacterial Activity. Adv. Nanopart. 2020, 9, 41–48. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green Synthesis of Silver Nanoparticles from Tectona Grandis Seeds Extract: Characterization and Mechanism of Antimicrobial Action on Different Microorganisms. J. Anal. Sci. Technol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall Analysis in Estimation of Lattice Strain in Nanometer-Sized ZnO Particles. J. Theor. Appl. Phys. 2012, 6, 2–9. [Google Scholar] [CrossRef]
- Meva, F.E.; Mbeng, J.O.A.; Ebongue, C.O.; Schlüsener, C.; Kökçam-Demir, Ü.; Ntoumba, A.A.; Kedi, P.B.E.; Elanga, E.; Loudang, E.-R.N.; Nko’o, M.H.J.; et al. Stachytarpheta Cayennensis Aqueous Extract, a New Bioreactor towards Silver Nanoparticles for Biomedical Applications. J. Biomater. Nanobiotechnol. 2019, 10, 102–119. [Google Scholar] [CrossRef]
- Dhar, S.A.; Chowdhury, R.A.; Das, S.; Nahian, M.K.; Islam, D.; Gafur, M.A. Plant-Mediated Green Synthesis and Characterization of Silver Nanoparticles Using Phyllanthus Emblica Fruit Extract. Mater. Today Proc. 2021, 42, 1867–1871. [Google Scholar] [CrossRef]
- Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green Synthesis of Silver Nanoparticles, Their Characterization, Application and Antibacterial Activity. Int. J. Environ. Res. Public Health 2013, 10, 5221–5238. [Google Scholar] [CrossRef]
- Elemike, E.E.; Dare, E.O.; Samuel, I.D.; Onwuka, J.C. 2-Imino-(3,4-Dimethoxybenzyl) Ethanesulfonic Acid Schiff Base Anchored Silver Nanocomplex Mediated by Sugarcane Juice and Their Antibacterial Activities. J. Appl. Res. Technol. JART 2016, 14, 38–46. [Google Scholar] [CrossRef]
- Osonga, F.J.; Le, P.; Luther, D.; Sakhaee, L.; Sadik, O.A. Water-Based Synthesis of Gold and Silver Nanoparticles with Cuboidal and Spherical Shapes Using Luteolin Tetraphosphate at Room Temperature. Environ. Sci. Nano 2018, 5, 917–932. [Google Scholar] [CrossRef]
- Nycz, M.; Arkusz, K.; Pijanowska, D.G. Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials 2019, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Sundramoorthy, A.K.; Chen, S.-L.; Chen, S.-M. Disposable Redox Polymer Coated Screen-Printed Carbon Electrode for NADH Sensing. Micro Nanosyst. 2012, 4, 172–179. [Google Scholar] [CrossRef]
- Kumar, T.H.V.; Sundramoorthy, A.K. Electrochemical Biosensor for Methyl Parathion Based on Single-Walled Carbon Nanotube/Glutaraldehyde Crosslinked Acetylcholinesterase-Wrapped Bovine Serum Albumin Nanocomposites. Anal. Chim. Acta 2019, 1074, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Yu, B.; Li, P.; Xiong, B.; Cheng, Y.; Li, Y.; Li, C.; Xiao, X.; Chen, M.; Chen, L.; et al. Synthesis of Graphene/DPA Composite for Determination of Nicotine in Tobacco Products. Sci. Rep. 2017, 7, 14332. [Google Scholar] [CrossRef]
- Magesh, V.; Sundramoorthy, A.K.; Ganapathy, D.; Atchudan, R.; Arya, S.; Alshgari, R.A.; Aljuwayid, A.M. Palladium Hydroxide (Pearlman’s Catalyst) Doped MXene (Ti3C2Tx) Composite Modified Electrode for Selective Detection of Nicotine in Human Sweat. Biosensors 2022, 13, 54. [Google Scholar] [CrossRef]
- Takeda, N.; Hirata, K.; Tsuruta, K.; Santis, G.D.; Xantheas, S.S.; Ishiuchi, S.I.; Fujii, M. Gas Phase Protonated Nicotine Is a Mixture of Pyridine- and Pyrrolidine-Protonated Conformers: Implications for Its Native Structure in the Nicotinic Acetylcholine Receptor. Phys. Chem. Chem. Phys. 2022, 24, 5786–5793. [Google Scholar] [CrossRef]
- Ameer, M.A.; Fekry, A.M.; Azab, S.M.; Shehata, M. Synthesis of a Simply Modified Electrochemical Nicotine Sensor Based on Silver Nanoparticles. Can. J. Chem. 2018, 96, 821–827. [Google Scholar] [CrossRef]
- Xiong, H.; Zhao, Y.; Liu, P.; Zhang, X.; Wang, S. Electrochemical Properties and the Determination of Nicotine at a Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode. Microchim. Acta 2010, 168, 31–36. [Google Scholar] [CrossRef]
- Stočes, M.; Švancara, I. Electrochemical Behavior of Nicotine at Unmodified Carbon Paste Electrode and Its Determination in a Set of Refilling Liquids for Electronic Cigarettes. Electroanalysis 2014, 26, 2655–2663. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Shi, L.; Zhu, X.; Lan, M.; Zhang, Q.; Hugh Fan, Z. Electrochemical Sensing of Nicotine Using Screen-Printed Carbon Electrodes Modified with Nitrogen-Doped Graphene Sheets. J. Electroanal. Chem. 2017, 784, 77–84. [Google Scholar] [CrossRef]
- Liang, J.; Han, F.; Chen, Y. An Electrochemical Method for high Sensitive Detection of Nicotine and Its Interaction with Bovine Serum Albumin. Electrochem. Commun. 2012, 24, 93–96. [Google Scholar] [CrossRef]












| 2θ | θ (Rad) | Sin2 θ | Ratio 1 | Ratio 3 | h2 + k2 + l2 | h k l | Lattice Parameter ‘a’ (Å) |
|---|---|---|---|---|---|---|---|
| 38.15 | 0.33292 | 0.10680187 | 1.04676 | 3.14028 | 3 | 111 | 4.0825 |
| 44.31 | 0.38668 | 0.14221459 | 1.39384 | 4.18152 | 4 | 200 | 4.0852 |
| 64.49 | 0.56278 | 0.28466569 | 2.79000 | 8.36999 | 8 | 220 | 4.0835 |
| 77.4 | 0.67544 | 0.39092838 | 3.83147 | 11.4944 | 11 | 311 | 4.0847 |
| Electrode | Linear Range | LOD | Reference |
|---|---|---|---|
| BDE | 9.9 to 170 μM | 0.3 μM | [2] |
| AGCE | 1200 μM | 0.7 μM | [3] |
| BN doped graphene | 1 to 1000 μM | 0.42 μM | [14] |
| bAuNPs/SPE | 10–2000 μM | 2.33 μM | [30] |
| RGO/DPA/PGE | 131–1,900 µM | 7.60 µM | [48] |
| MWCNT | 31–220 μM | 7.6 μM | [52] |
| Carbon paste | 50–500 μM | 6.1 μM | [53] |
| NDG/GCE | 0–200 µM | 0.27 µM | [54] |
| PoPD/GCE | 0.000183 to 1.01 µM | 55.00 pM | [55] |
| Green synthesized Ag-NPs/GCE | 2.5 to 105 μM | 0.135 μM | This work |
| S.No. | Samples | Added (μM) | Found * (μM) | RSD % | Recovery % |
|---|---|---|---|---|---|
| 1. | Saliva | - | 0.494 | - | - |
| 2. | Std. sample | 0.5 | 0.984 | 0.0314 | 98 |
| 3. | Std. sample | 1 | 1.514 | 0.0097 | 102 |
| 4. | Std. sample | 2 | 2.474 | 0.0391 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridharan, G.; Babu, K.L.; Ganapathy, D.; Atchudan, R.; Arya, S.; Sundramoorthy, A.K. Determination of Nicotine in Human Saliva Using Electrochemical Sensor Modified with Green Synthesized Silver Nanoparticles Using Phyllanthus reticulatus Fruit Extract. Crystals 2023, 13, 589. https://doi.org/10.3390/cryst13040589
Sridharan G, Babu KL, Ganapathy D, Atchudan R, Arya S, Sundramoorthy AK. Determination of Nicotine in Human Saliva Using Electrochemical Sensor Modified with Green Synthesized Silver Nanoparticles Using Phyllanthus reticulatus Fruit Extract. Crystals. 2023; 13(4):589. https://doi.org/10.3390/cryst13040589
Chicago/Turabian StyleSridharan, Gokul, Kaushik L. Babu, Dhanraj Ganapathy, Raji Atchudan, Sandeep Arya, and Ashok K. Sundramoorthy. 2023. "Determination of Nicotine in Human Saliva Using Electrochemical Sensor Modified with Green Synthesized Silver Nanoparticles Using Phyllanthus reticulatus Fruit Extract" Crystals 13, no. 4: 589. https://doi.org/10.3390/cryst13040589
APA StyleSridharan, G., Babu, K. L., Ganapathy, D., Atchudan, R., Arya, S., & Sundramoorthy, A. K. (2023). Determination of Nicotine in Human Saliva Using Electrochemical Sensor Modified with Green Synthesized Silver Nanoparticles Using Phyllanthus reticulatus Fruit Extract. Crystals, 13(4), 589. https://doi.org/10.3390/cryst13040589








