Synthesis and Structural Studies of Complexes of Bis(pentafluorophenyl)mercury with Di(phosphane oxide) Ligands
Abstract
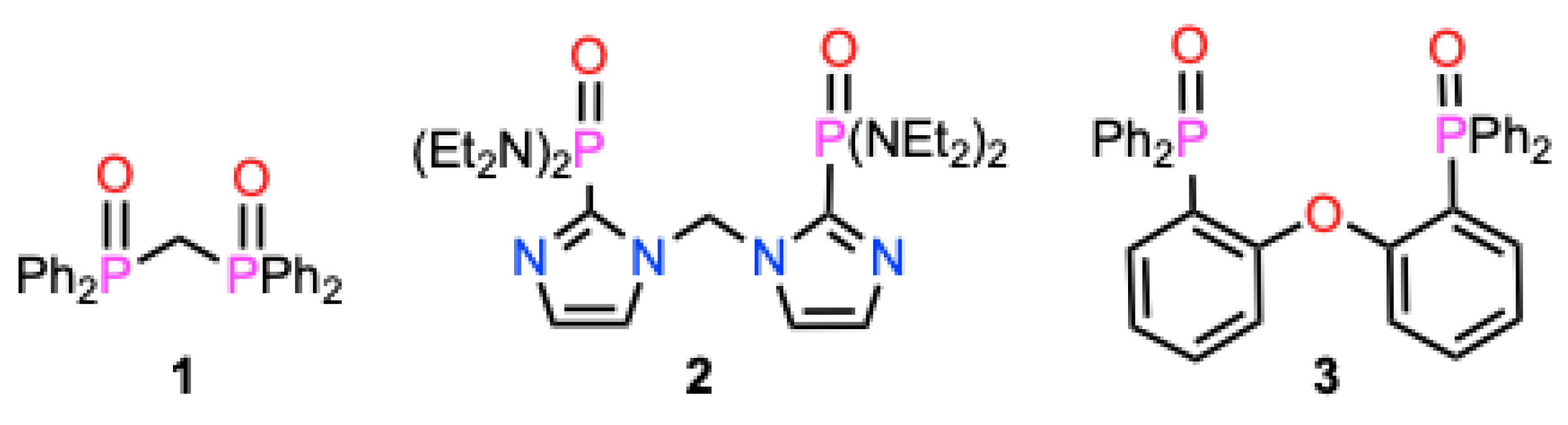
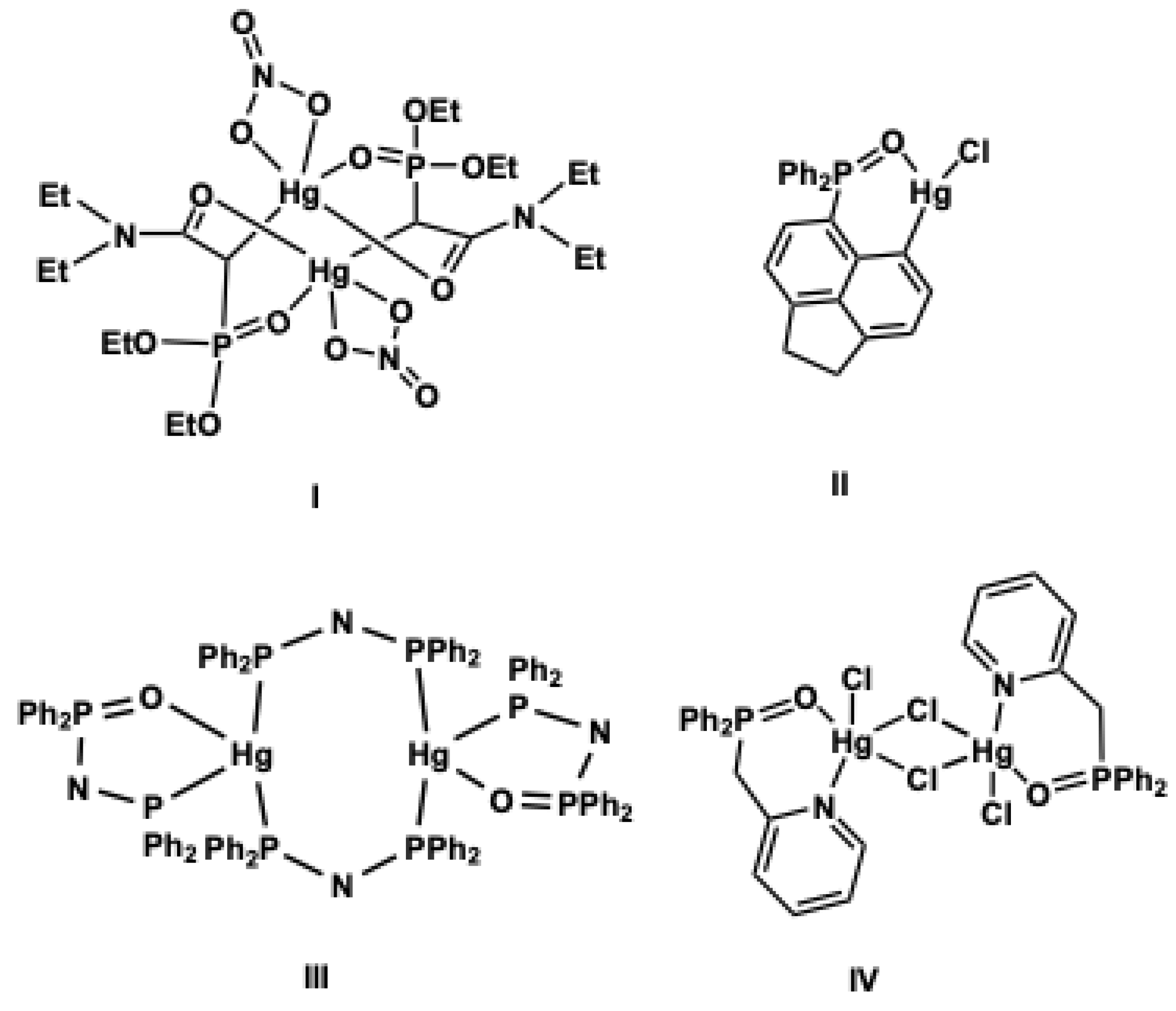
1. Introduction
2. Results and Discussion
2.1. Synthesis of [{Hg(C6F5)2}2{Ph2P(O)}2CH2] (1Hg)
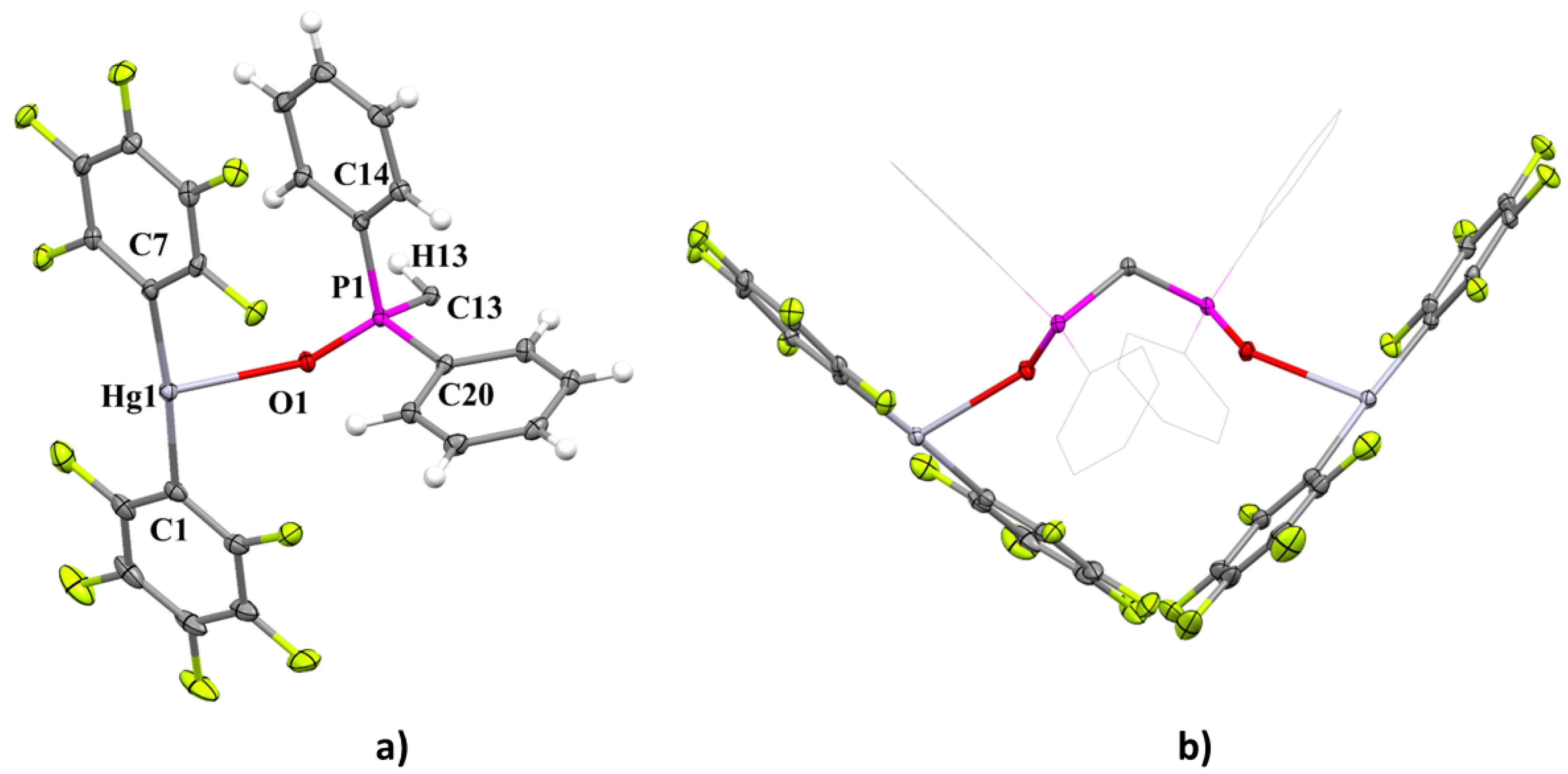
2.2. Molecular Structure of [{Hg(C6F5)2}2{Ph2P(O)}2CH2] (1Hg)
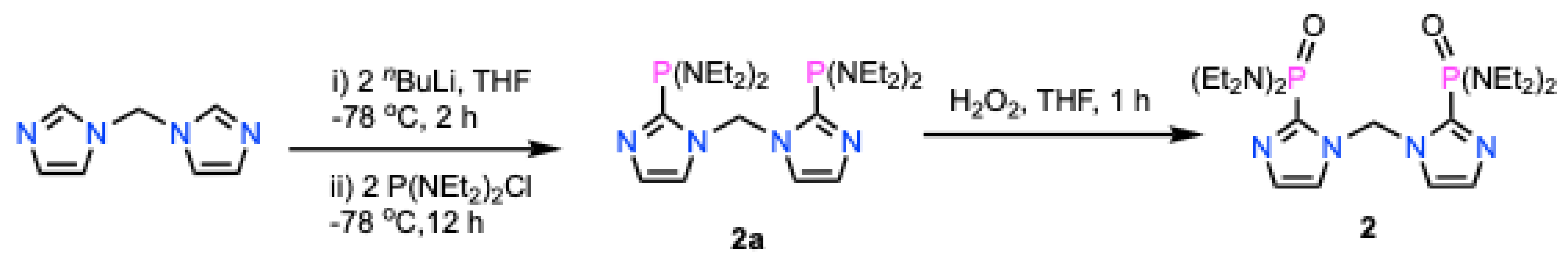
2.3. Synthesis of {2-PO(NEt2)2C3N2H2}2CH2 (2)
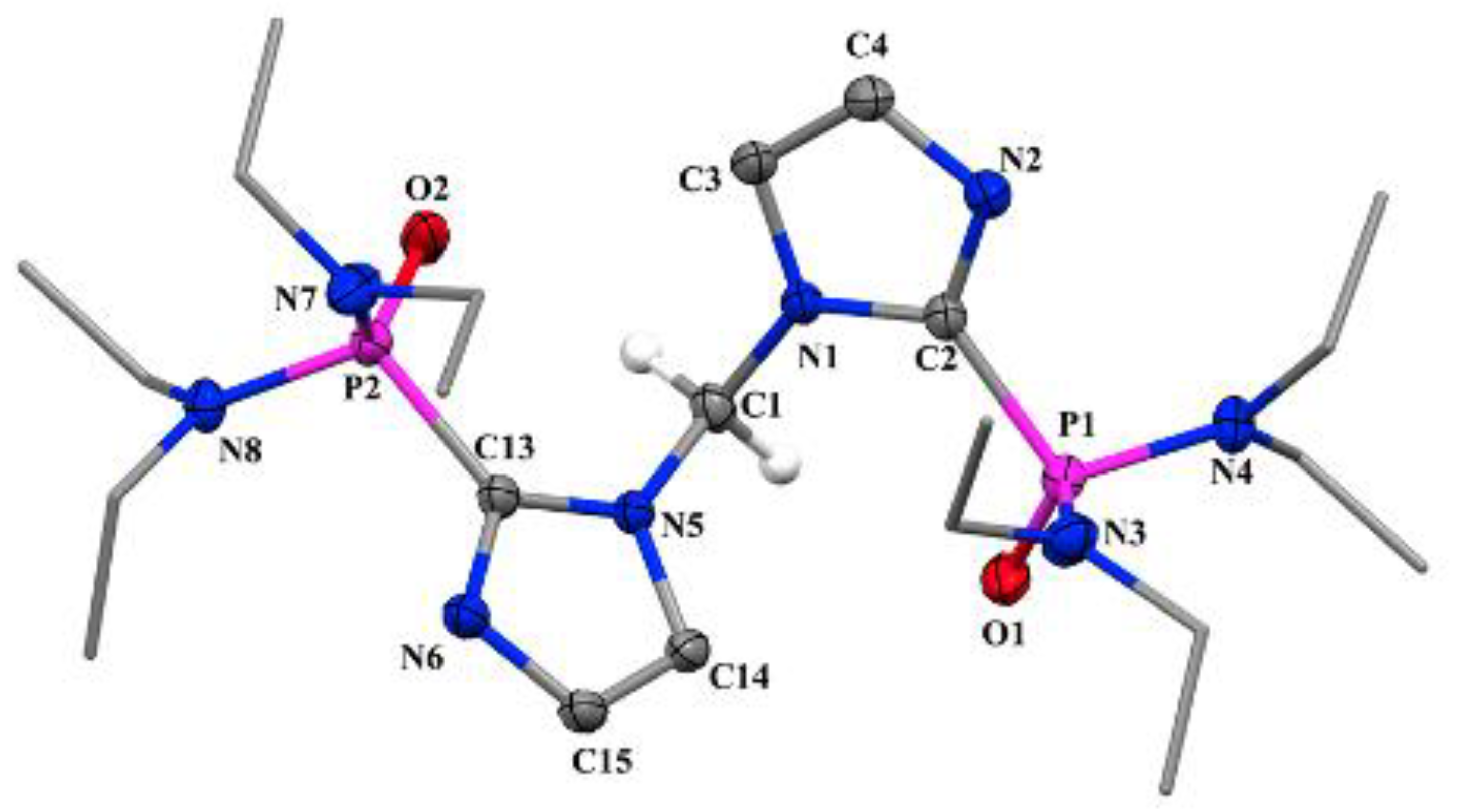
2.4. Molecular Structure of {2-PO(NEt2)2C3N2H2}2CH2 (2)
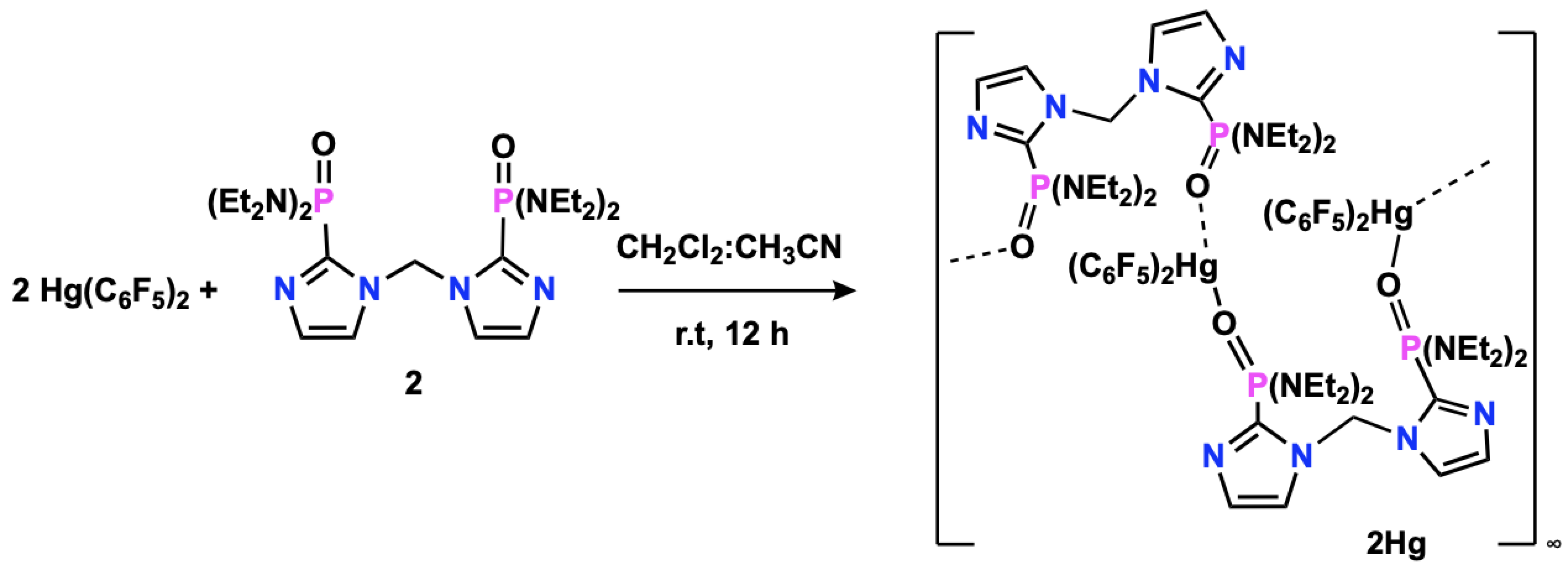
2.5. Synthesis of [Hg(C6F5)2{2-PO(NEt2)2C3N2H2}2CH2]n (2Hg)
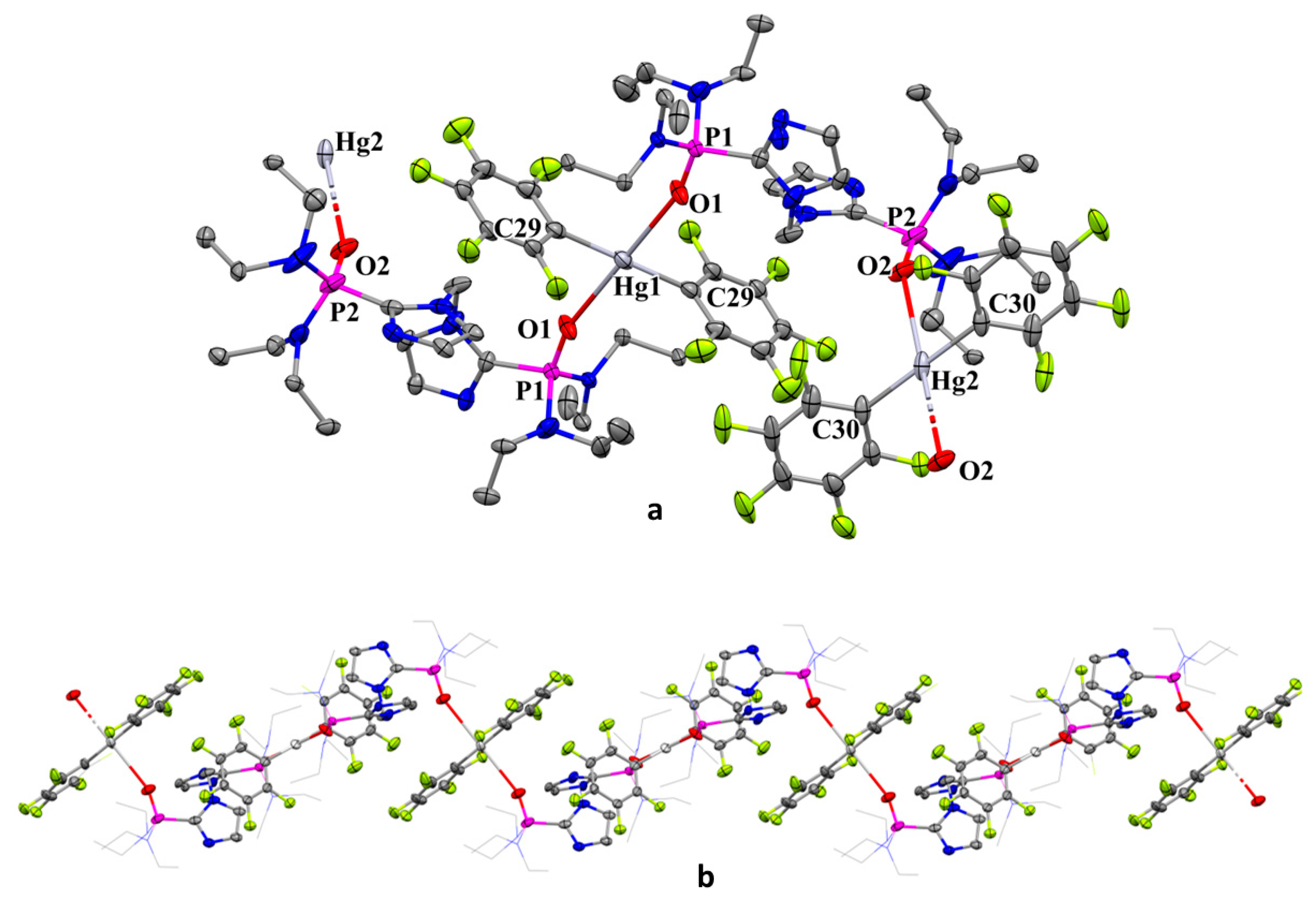
2.6. Structure of [Hg(C6F5)2{2-PO(NEt2)2C3N2H2}2CH2]n (2Hg)
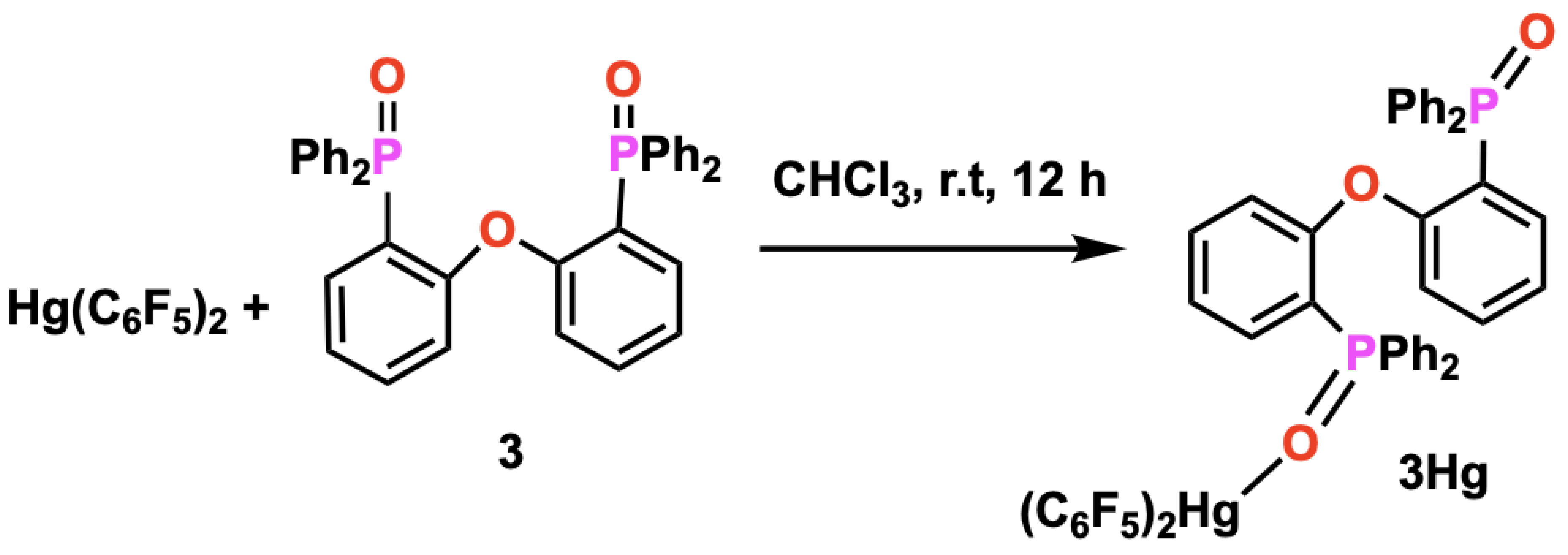
2.7. Synthesis of [Hg(C6F5)2{2-PPh2(O)C6H4}2O] (3Hg)
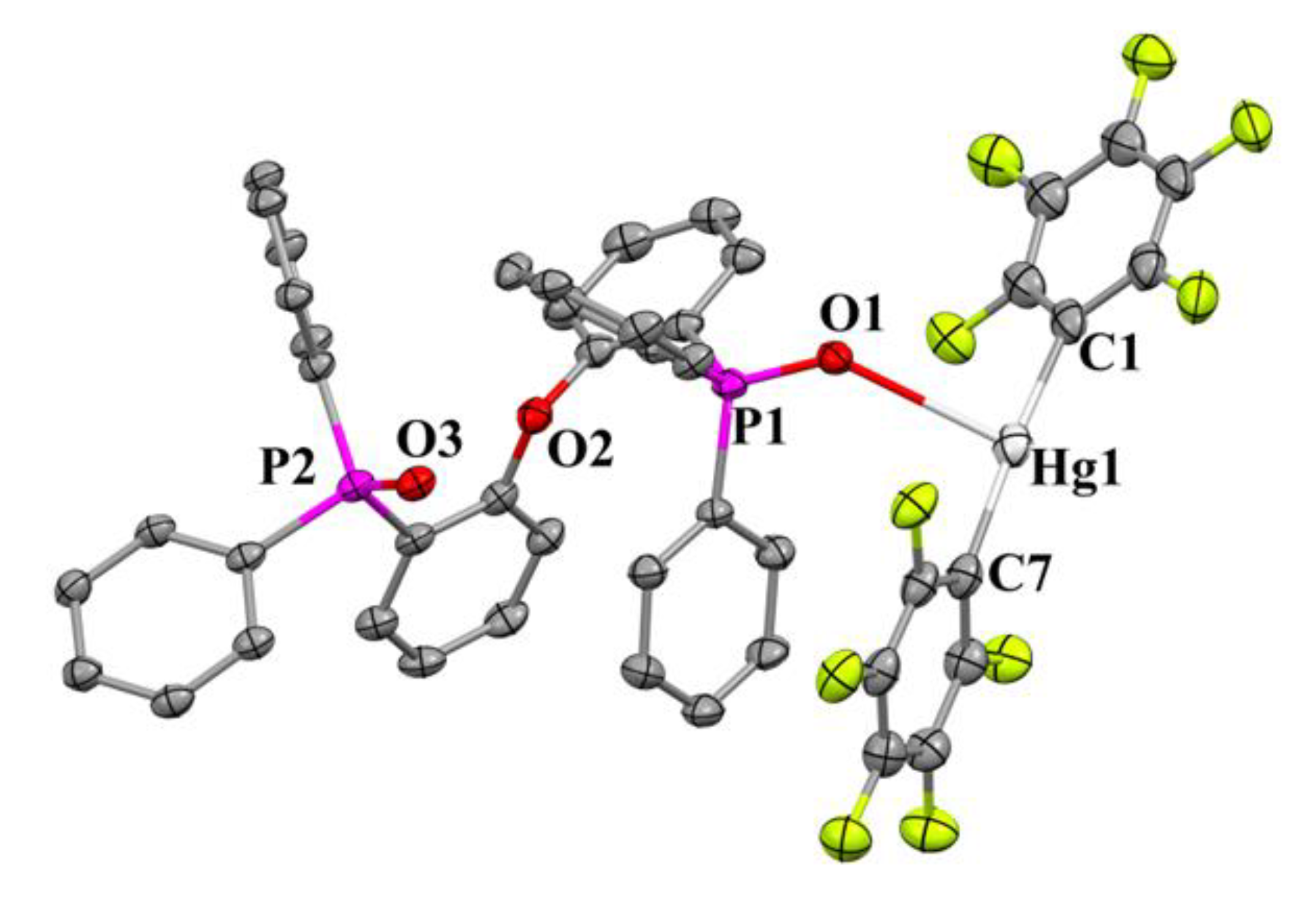
2.8. Molecular Structure of [Hg(C6F5)2{2-PPh2(O)C6H4}2O] (3Hg)
3. Conclusions
4. Materials and Methods
4.1. General Considerations
4.2. Single Crystal X-ray Structure Determination
4.3. Experimental Section
4.3.1. Synthesis of {PO(NEt2)2C3N2H2}2CH2 (2)
4.3.2. Synthesis of [{Hg(C6F5)2}2(Ph2P(O))2CH2] (1Hg)
4.3.3. Synthesis of [Hg(C6F5)2{2-PO(NEt2)2C3N2H2}2CH2]n (2Hg)
4.3.4. Synthesis of [Hg(C6F5)2{PPh2(O)C6H4}2O] (3Hg)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambers, R.D.; Coates, G.E.; Livingstone, J.G.; Musgrave, W.K.R. 847. The preparation and study of some pentafluorophenylmercury compounds. J. Chem. Soc. 1962, 4367–4371. [Google Scholar] [CrossRef]
- Lin, T.-P.; Nelson, R.C.; Wu, T.; Miller, J.T.; Gabbaï, F.P. Lewis acid enhancement by juxtaposition with an onium ion: The case of a mercury stibonium complex. Chem. Sci. 2012, 3, 1128–1136. [Google Scholar] [CrossRef]
- Canty, A.; Gatehouse, B. The crystal structures of disordered crystals of adducts of diphenylmercury with bidentate ligands. Acta Crystallogr. B 1972, 28, 1872–1888. [Google Scholar] [CrossRef]
- Korpar-Cǒlig, B.; Popović, Z.; Bruvo, M.; Vicković, I. Addition compounds of bis(trifluoromethyl)mercury with tetraphenylphosphonium and tetraphenylarsonium halides and thiocyanates. Inorg. Chim. Acta 1988, 150, 113–118. [Google Scholar] [CrossRef]
- Connett, J.; Davies, A.G.; Deacon, G.; Green, J. Organomercury compounds. Part I. Mercury pentafluorobenzoates and their decarboxylation to pentafluorophenylmercury compounds. J. Chem. Soc. C 1966, 106–111. [Google Scholar] [CrossRef]
- Canty, A.; Deacon, G. Organomercury compounds. XI. Some coordination derivatives of bispentafluorophenylmercury. Aust. J. Chem. 1971, 24, 489–499. [Google Scholar] [CrossRef]
- Canty, A.J.; Gatehouse, B.M. Crystal structure of tetrakispentafluorophenyl-µ-bisdiphenylarsinomethane-dimercury(II), a three-co-ordinate mercury complex. J. Chem. Soc. D 1971, 443–444. [Google Scholar] [CrossRef]
- Canty, A.J.; Deacon, G.B. The van der Waals radius of mercury. Inorg. Chim. Acta 1980, 45, L225–L227. [Google Scholar] [CrossRef]
- Schulz, F.; Pantenburg, I.; Naumann, D. Synthesen und Strukturen von Perfluororganohalogenomercuraten [Hg(Rf)2X]—(Rf = CF3, C6F5; X = Br, I). Z. Anorg. Allg. Chem. 2003, 629, 2312–2316. [Google Scholar] [CrossRef]
- Naumann, D.; Schulz, F. Strukturen von neuen Bis(pentafluorophenyl)halogenomercuraten [{Hg(C6F5)2}3(μ-X)]—(X = Cl, Br, I). Z. Anorg. Allg. Chem. 2005, 631, 715–718. [Google Scholar] [CrossRef]
- Burress, C.N.; Bodine, M.I.; Elbjeirami, O.; Reibenspies, J.H.; Omary, M.A.; Gabbaï, F.P. Enhancement of external spin−orbit coupling effects caused by metal−metal cooperativity. Inorg. Chem. 2007, 46, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Forsyth, C.M.; Junk, P.C.; Ness, T.J.; Izgorodina, E.; Baldamus, J.; Meyer, G.; Pantenburg, I.; Hitzbleck, J.; Ruhlandt-Senge, K. The Supramolecular Architecture of Arene Complexes of Bis(polyfluorophenyl)mercurials. Eur. J. Inorg. Chem. 2008, 2008, 4770–4780. [Google Scholar] [CrossRef]
- Kim, M.; Taylor, T.J.; Gabbaï, F.P. Hg(II)···Pd(II) metallophilic interactions. J. Am. Chem. Soc. 2008, 130, 6332–6333. [Google Scholar] [CrossRef]
- Tsunoda, M.; Fleischmann, M.; Jones, J.S.; Bhuvanesh, N.; Scheer, M.; Gabbaï, F.P. Supramolecular aggregation of Ni(salen) with (C6F5)2Hg and [o-C6F4Hg]3. Dalton Trans. 2016, 45, 5045–5051. [Google Scholar] [CrossRef]
- López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Pascual, D.; Lasanta, T. Amalgamating at the molecular level. A study of the strong closed-shell Au(i)⋯Hg(ii) interaction. Chem. Commun. 2011, 47, 6795–6797. [Google Scholar] [CrossRef] [PubMed]
- Lasanta, T.; López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Pascual, D. Experimental and Theoretical Evidence of the Existence of Gold(I)⋅⋅⋅Mercury(II) Interactions in Solution through Fluorescence-Quenching Measurements. Chem. Eur. J. 2013, 19, 4754–4766. [Google Scholar] [CrossRef]
- Rosario-Amorin, D.; Dehaudt, J.P.; Caudle, L.J.; Dickie, D.A.; Paine, R.T. Synthesis and molecular structures of mercury(II) complexes of carbamoylmethylphosphoryl ligands. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 520–526. [Google Scholar] [CrossRef]
- Tikhonova, I.A.; Dolgushin, F.M.; Tugashov, K.I.; Petrovskii, P.V.; Furin, G.G.; Shur, V.B. Coordination chemistry of polymercuramacrocycles. Complexation of cyclic trimeric perfluoro-o-phenylenemercury with neutral oxygeneous Lewis bases. J. Organomet. Chem. 2002, 654, 123–131. [Google Scholar] [CrossRef]
- Olaru, M.; Krupke, S.; Lork, E.; Mebs, S.; Beckmann, J. Transmetallation of bis(6-diphenylphosphinoxy-acenapth-5-yl)mercury with tin tetrachloride, antimony trichloride and bismuth trichloride. Dalton Trans. 2019, 48, 5585–5594. [Google Scholar] [CrossRef]
- Cole, M.L.; Deacon, G.B.; Junk, P.C.; Konstas, K.; Roesky, P.W. Novel Mercury Phosphanylamides. Eur. J. Inorg. Chem. 2005, 2005, 1090–1098. [Google Scholar] [CrossRef]
- Padron, D.A.; Klausmeyer, K.K. Syntheses and coordination studies of 2-(diphenylphosphinomethyl)pyridine and its oxide towards mercury(II). Polyhedron 2012, 34, 215–220. [Google Scholar] [CrossRef]
- Aslandukov, A.N.; Utochnikova, V.V.; Goriachiy, D.O.; Vashchenko, A.A.; Tsymbarenko, D.M.; Hoffmann, M.; Pietraszkiewicz, M.; Kuzmina, N.P. The development of a new approach toward lanthanide-based OLED fabrication: New host materials for Tb-based emitters. Dalton Trans. 2018, 47, 16350–16357. [Google Scholar] [CrossRef]
- Deacon, G.B.; Cosgriff, J.E.; Lawrenz, E.T.; Forsyth, C.M.; Wilkinson, D.L. Section 2.3: Organolanthanide(II) Complexes in Lanthanides and Actinides. In Synthetic Methods of Organometallic and Inorganic Chemistry; Edelmann, F.T., Herrmann, W.A., Eds.; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 1997; Volume 6, p. 48. [Google Scholar]
- Rangarajan, S.; Beaumont, O.A.; Balakrishna, M.S.; Deacon, G.B.; Blair, V.L. Synthesis and Characterisation of Novel Bis(diphenylphosphane oxide)methanidoytterbium(III) Complexes. Molecules 2022, 27, 7704. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Green, J.H.S. Vibrational spectra of ligands and complexes—VI: Infrared spectra of triphenylarsine, triphenylarsine oxide, and triphenylphosphine oxide complexes. Spectrochim. Acta A Mol. Spectrosc. 1969, 25, 355–364. [Google Scholar] [CrossRef]
- Guo, Z.; Huo, R.; Tan, Y.Q.; Flosbach, N.T.; Wang, N.; Leonhardt, C.; Urbatsch, A.; Deacon, G.B.; Junk, P.C.; Izgorodina, E.I.; et al. A new twist on an old molecule: A rotameric isomer of bis(pentafluorophenyl)mercury. J. Coord. Chem. 2021, 74, 2947–2958. [Google Scholar] [CrossRef]
- Nyburg, S.C.; Faerman, C.H. A revision of van der Waals atomic radii for molecular crystals: N, O, F, S, Cl, Se, Br and I bonded to carbon. Acta Crystallogr. B Struct. 1985, 41, 274–279. [Google Scholar] [CrossRef]
- Grachova, E.; Linti, G.; Vologzhanina, A. CCDC 877465: EBIXEV Experimental Crystal Structure Determination. In CSD Communications; University of Cambridge: Cambridge, UK, 2016. [Google Scholar]
- Dunitz, J.D.; Taylor, R. Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. Chem. Eur. J. 1997, 3, 89–98. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Antiñolo, A.; Carrillo-Hermosilla, F.; Díez-Barra, E.; Fernández-Baeza, J.; Fernández-López, M.; Lara-Sánchez, A.; Moreno, A.; Otero, A.; Maria Rodriguez, A.; Tejeda, J. New functionalized bis(pyrazol-1-yl)methane ligands. Synthesis, spectroscopic characterization of early and late transition metal complexes containing a functionalized N,N or P,P-chelate bis(5-diphenylphosphinopyrazol-1-yl)methane ligand. J. Chem. Soc. Dalton Trans. 1998, 3737–3744. [Google Scholar] [CrossRef]
- Bhat, S.A.; Mague, J.T.; Balakrishna, M.S. Gold(i) complexes of bisphosphines with bis(azol-1-yl)methane backbone: Structure of a rare dinuclear gold(i) complex [(Au2Cl){CH2(1,2-C3H2N2PPh2)2}3Cl]. Dalton Trans. 2015, 44, 17696–17703. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sonawane, S.C.; Mague, J.T.; Balakrishna, M.S. Synthesis and characterization of Mo(0) and W(0) complexes of bis(azol-1-yl)methane based bisphosphines. J. Coord. Chem. 2021, 74, 2253–2262. [Google Scholar] [CrossRef]
- Geiger, D.K.; DeStefano, M.R. Conformational differences and intermolecular C-H...N interactions in three polymorphs of a bis(pyridinyl)-substituted benzimidazole. Acta Cryst. C 2016, 72, 867–874. [Google Scholar] [CrossRef]
- Pietraszkiewicz, M.; Mal, S.; Pietraszkiewicz, O.; Górski, K.; Chelwani, N. Photoluminescent Tetrazolate-based Eu(III) Complexes: An Outstanding Impact of Aromatic Phosphine Oxide Co-ligands on the Photoluminescence Quantum Yields. Z Naturforsch. 2014, 69, 239–247. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.-Q.; Das, S.; Pisiewicz, S.; Junge, K.; Beller, M. Highly Chemoselective Metal-Free Reduction of Phosphine Oxides to Phosphines. J. Am. Chem. Soc. 2012, 134, 18325–18329. [Google Scholar] [CrossRef] [PubMed]
- Díez-Barra, E.; de la Hoz, A.; Sánchez-Migallón, A.; Juan, T. Phase Transfer Catalysis without Solvent. Synthesis of Bisazolylalkanes. Heterocycles 1992, 34, 1365–1373. [Google Scholar] [CrossRef]
- Qin, L.; Ren, X.; Lu, Y.; Li, Y.; Zhou, J. Intermolecular Mizoroki–Heck Reaction of Aliphatic Olefins with High Selectivity for Substitution at the Internal Position. Angew. Chem. Int. Ed. 2012, 51, 5915–5919. [Google Scholar] [CrossRef]
- Agilent Technologies Ltd. CrysAlisPRO, Version 39; Agilent Technologies Ltd.: Yarnton, UK, 2021. [Google Scholar]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| D–H‧‧‧A | D–H | H‧‧‧A | D‧‧‧A | D–H‧‧‧A |
|---|---|---|---|---|
| C15–H15‧‧‧F6 [i] | 0.96 | 2.496 | 3.164 | 127 |
| C13-H13···F3 [ii] | 0.95 | 2.471 | 3.398 | 176 |
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| C22–H22B‧‧‧C3 [i] | 0.97 | 2.789 | 3.651 | 148 |
| C1-H1B···O2 [ii] | 0.97 | 2.642 | 3.412 | 137 |
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| C43–H43A‧‧‧F1 [i] | 0.96 | 2.438 | 3.365 | 162 |
| C23-H23B···F2 [ii] | 0.96 | 2.373 | 3.261 | 154 |
| C10-H10B···Cg1 [iii] | 0.97 | 3.043 | 3.831 | 139.3 |
| C22A-H22A···N6 [iv] | 0.97 | 2.693 | 3.492 | 140 |
| D–H‧‧‧A | D–H | H‧‧‧A | D‧‧‧A | D–H‧‧‧A |
|---|---|---|---|---|
| C24–H24‧‧‧F4 [i] | 0.93 | 2.483 | 3.232 | 138 |
| C17-H17···F10 [ii] | 0.93 | 2.593 | 3.423 | 149 |
| C35-H35···F9 [iii] | 0.93 | 2.407 | 3.283 | 157 |
| C45-H45···F9 [iv] | 0.93 | 2.561 | 3.151 | 122 |
| Compound | 2 | 1Hg | 2Hg | 3Hg |
|---|---|---|---|---|
| Empirical formula | C23H46N8O2P2 | [C49H22F20Hg2O2P2] | [C35H46F10HgN8O2P2] | [C48H28F10HgO3P2] |
| Formula weight | 528.62 | 1485.78 | 1063.33 | 1105.23 |
| Temperature/K | 150 | 123 | 100 | 100 |
| Crystal system | Monoclinic | Monoclinic | Triclinic | Triclinic |
| Space group | P21/n | C2/c | P-1 | P-1 |
| a/Å | 9.3556(3) | 14.0695(2) | 11.480(2) | 8.510(17) |
| b/Å | 12.0193(4) | 17.0277(3) | 12.900(3) | 11.680(2) |
| c/Å | 25.8957(8) | 19.1180(3) | 15.330(3) | 21.020(4) |
| α/° | 90 | 90 | 110.03(3) | 84.47(3) |
| β/° | 94.663(3) | 92.6470(10) | 97.81(3) | 85.62(3) |
| γ/° | 90 | 90 | 95.36(3) | 83.13(3) |
| Volume/Å3 | 2902.28(16) | 4575.24(13) | 2089.2(8) | 2060.2(7) |
| Z | 4 | 4 | 2 | 2 |
| ρcalcg/cm3 | 1.210 | 2.157 | 1.690 | 1.782 |
| μ/mm−1 | 0.184 | 6.897 | 3.847 | 3.903 |
| F(000) | 1144.0 | 2808.0 | 1056.0 | 1080.0 |
| Mo Kα radiation/Synchrotron, λ/Å | Mo Kα (λ = 0.71073) | Mo Kα (λ = 0.71073) | Synchrotron(λ = 0.71073) | Mo Kα (λ = 0.71073) |
| Crystal size/mm3 | 0.258 × 0.115 × 0.085 | 0.076 × 0.064 ×0.03 | 0.089 × 0.068 × 0.056 | 0.1 × 0.1 × 0.08 |
| 2θ range for datacollection/° | 4.764 to 67.282 | 7.038 to 63.782 | 2.874 to 50.696 | 1.95 to 58.188 |
| Reflections collected | 32458 | 29404 | 37445 | 41307 |
| Independent reflections | 9353 | 6548 | 7572 | 8939 |
| Data/restraints/parameters | 9353/27/382 | 6548/0/383 | 7572/228/711 | 8942/0/577 |
| Goodness-of-fit on F2 | 1.018 | 1.035 | 1.068 | 1.060 |
| R1 [a] | 0.0481 | 0.0193 | 0.0372 | 0.0376 |
| wR2 [b] | 0.1287 | 0.0434 | 0.0965 | 0.1078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangarajan, S.; Beaumont, O.A.; Guo, Z.; Balakrishna, M.S.; Deacon, G.B.; Blair, V.L. Synthesis and Structural Studies of Complexes of Bis(pentafluorophenyl)mercury with Di(phosphane oxide) Ligands. Crystals 2023, 13, 530. https://doi.org/10.3390/cryst13030530
Rangarajan S, Beaumont OA, Guo Z, Balakrishna MS, Deacon GB, Blair VL. Synthesis and Structural Studies of Complexes of Bis(pentafluorophenyl)mercury with Di(phosphane oxide) Ligands. Crystals. 2023; 13(3):530. https://doi.org/10.3390/cryst13030530
Chicago/Turabian StyleRangarajan, Shalini, Owen A. Beaumont, Zhifang Guo, Maravanji S. Balakrishna, Glen B. Deacon, and Victoria L. Blair. 2023. "Synthesis and Structural Studies of Complexes of Bis(pentafluorophenyl)mercury with Di(phosphane oxide) Ligands" Crystals 13, no. 3: 530. https://doi.org/10.3390/cryst13030530
APA StyleRangarajan, S., Beaumont, O. A., Guo, Z., Balakrishna, M. S., Deacon, G. B., & Blair, V. L. (2023). Synthesis and Structural Studies of Complexes of Bis(pentafluorophenyl)mercury with Di(phosphane oxide) Ligands. Crystals, 13(3), 530. https://doi.org/10.3390/cryst13030530








