Abstract
The structure, elastic properties and electronic structure of Ti-Al intermetallics including Ti3Al (space group P63/mmc), TiAl (space group I4/mmm) and TiAl3 (space group P4/mmm) are systematically studied by first-principles calculations. The results show that Ti-Al intermetallics can exist stably whether Cr replaces Ti or Al. The ductility of the alloy cannot be improved when Ti is replaced in Cr-doped TiAl and TiAl3. However, when it replaces Al, the alloy has better ductility. In Ti3Al, the ductility can be improved regardless of whether Cr replaces Ti or Al, and the effect is better when it replaces Al. The bond in Ti-Al intermetallics is mainly a Ti-Ti metal bond. The metal bond between Ti-Ti is strengthened and a solid metal bond is formed between Cr and Ti, inducing a better ductility of the material, after Cr replaces Al in Ti-Al intermetallics.
1. Introduction
As with Ni-based superalloys, Ti-Al intermetallics have good elastic modulus, excellent oxidation resistance and high specific strength. However, their density is only half that of Ni-based superalloys. Therefore, Ti-Al alloys are the most ideal substitutes for Ni-based superalloys. In addition, Ti-Al intermetallics have good flame retardancy and can replace expensive Ti-based components. Ti-Al intermetallics have become a new generation of key materials in aerospace, automotive and other industries [1]. After extensive theoretical and experimental research, researchers have found many ways to improve most mechanical properties of Ti-Al intermetallics. However, their room temperature brittleness still greatly limits their wide application. Song et al. [2] highlighted that TiAl3 has the highest hardness and brittleness among the three alloys. Jian et al. [3] recently evaluated the effect of Al concentration in Ti-Al-based alloys on the mechanical properties. They found that as Al increased, the hardness of the materials increased and their ductility decreased. To improve the ductility of TiAl-based alloys, researchers found that ternary or more complex alloy additives can have a good effect. By changing the electronic structure of alloy materials and improving the type and strength of electronic bonding bonds, the improvement in the ductility and toughness of materials can be achieved. These additives exist in interstitial or alternative forms [4,5], and the most commonly used alloying elements are Cr, V, Nb, La, Ta and Cd.
In order to improve the properties of Ti-Al intermetallics, researchers have conducted a terrific amount of theoretical and experimental research. The position of alloying elements on different sublattices in Ti-Al-based alloys has a great influence on their mechanical properties. Therefore, it is necessary to discuss the occupation of alloying atoms in Ti-Al-based alloys. Zhang et al. [6] observed that Mn tends to replace Al in TiAl. Song et al. [7] pointed out that in TiAl, Y, Zr, Nb, Mo and Sb preferentially replace Ti atoms, while Ga and In preferentially replace Al atoms. The occupying position of V, Mn, Co and Ge depends on the concentration ratio of Ti and Al in Ti-Al intermetallics.
In recent years, the method of improving material properties by doping alloying elements has attracted more attention [8,9,10,11]. Fan et al. [8] found that La, Ce, Pr, Nd and Sm can be used as strengthening phases to improve the rigidity and tensile strength of Al alloys, which is significantly helpful as a way of enhancing the mechanical properties and thermodynamic stability of Al alloys. Qi et al. [10] detected that the increase in Zr content will result in the aggravation of element segregation in a CoCrFeNiZrx alloy, forming a Zr-rich intermetallic compound. The compressive yield strength and fracture strain of the alloy are also improved. Trong et al. [11] found that the concentration of Au in a NiAu alloy can change the microstructure of materials. Related studies have reported that W and Y can improve the oxidation resistance [12,13] and C and Si can enhance the creep property of Ti-Al alloys [14,15]. Liu et al. [16] considered the influence of Ru on the microstructure change and mechanical properties of TiAl through thermal compression and the three point bend test. The experimental results displayed that Ru can increase the strength of TiAl and improve its ductility. Tetsui [17] studied the effect of Nb concentration in the Ti-50Al-xNb alloy on the malleability and high temperature strength of the material, and found that there was a suitable composition near Ti-46Al-7.5Nb (at. %) in the casting material. Liu et al. [18] discussed the influence of Nb content on the microstructure and yield strength of a high-Nb-containing TiAl-based alloy, and found that the Nb content remarkably improved the high-temperature strength. Duan et al. [19] investigated the mechanical properties of Ti-16Al-8Nb and Ti-16Al-8Nb-1Sn through experiments, and pointed out that the high temperature ductility of Ti-16Al-8Nb was significantly improved after adding 1% Sn. Music et al. [20] investigated the influence of some transition metals on the elastic constants of γ-TiAl and α2-Ti3Al using ab initio calculations and found that alloying could improve their bulk modulus B. Yuan et al. [21] found that Ta particles can enhance the thermal deformation ability of TiAl through experimental research. Therefore, adding alloys is significant as a way of reducing the brittleness of Ti-Al intermetallics.
As a commonly used alloying element, Cr has a good effect on improving the properties of Ti-Al alloys. Xiong [22] and Ye [23] found that adding Cr into a TiAl alloy can make the β phase more stable and effectively inhibit ω0 phase precipitation. Tetsui [24] found that a TiAl alloy has better impact resistance after adding Cr. Moreover, it was found that the Ti-Al-Cr ternary alloy showed higher oxidation resistance [25,26]. However, there are not many researches on the influence of Cr on the mechanical properties of Ti-Al intermetallics. If Cr can significantly improve the ductility of Ti-Al intermetallics, it will supply terrific assistance for the design and manufacture of Ti-Al intermetallics in the future. Therefore, it is necessary to explore the mechanical properties of Ti-Al-Cr ternary alloys. In this study, we calculated the total energy, formation energy, elastic constant and electronic structure of Ti-Al intermetallics containing Cr by using the first-principles method, and the position of Cr in a Ti-Al-based alloy and its effect on the ductility of a Ti-Al-based alloy were discussed.
2. Calculation Method
Our calculations were based on the framework of density functional theory (DFT) [27,28] that is implemented in the Vienna ab initio Simulation Package (VASP) [29,30]. The VASP can calculate the formation enthalpy, binding energy, dissolution enthalpy and elasticity of the crystal to judge its structural stability. It can also calculate the electronic structure in the crystal, predict the bonding situation and explain the macroscopic properties. The projection enhancement wave (PAW) is used to describe the interaction between atomic nucleus and valence electron. All calculations were performed using the projector augmented wave (PAW) [31,32] potentials and the generalized gradient approximation (GGA) in the exchange correlation function of Perdew–Burke–Ernzerhof (PBE) [33] was used for all calculations. The plane wave cutoff energy was 450 eV, which has been found to be sufficient for all the elements considered in this work to obtain precise energetics. The supercells of Ti3Al (2 × 1 × 1), TiAl (4 × 2 × 2) and TiAl3 (2 × 2 × 1) were commonly arranged with 32 atoms. The k-point meshes of these three structures were selected as 4 × 4 × 10, 4 × 8 × 6 and 6 × 6 × 5, respectively. In order to make the results more accurate, the conjugate gradient minimization method was used for structural optimization to allow the relaxation of crystal cells and internal atoms. The convergence criteria for energy and force were 1 × 10−5 eV and 0.02 eV/Å, respectively. The PAW pseudopotentials of Ni, Ti_sv and Cr_sv were used to simulate the real pseudopotentials. The calculation of elastic constant adopted energy-strain method, which is realized in VASPKIT code. After obtaining the stable structure of each system, the total energy, elastic constant, density of states and other physical properties of each system were calculated.
3. Results and Discussion
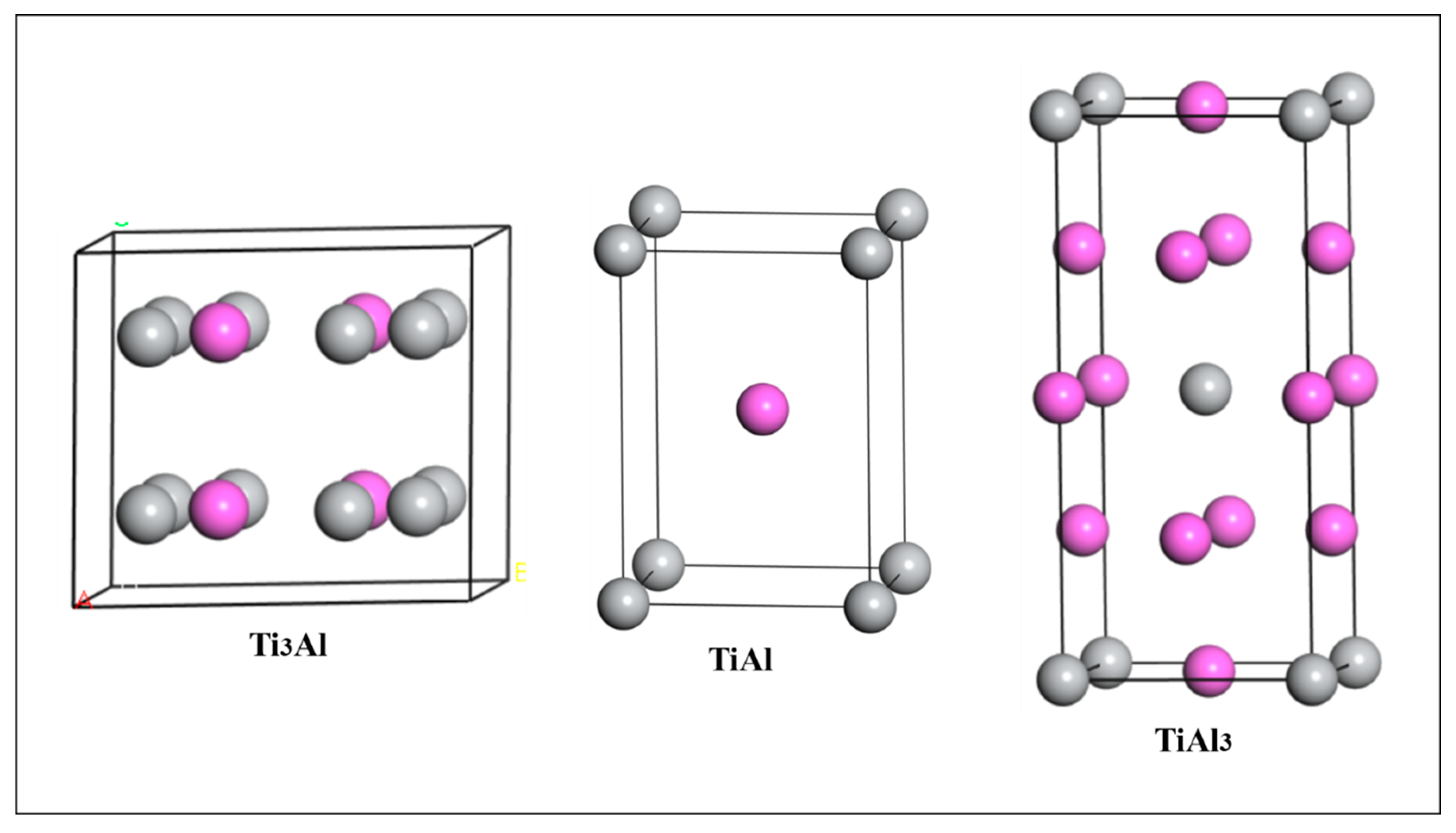
It is well known that Ti-Al binary compounds usually have several different crystal structures. There are three main kinds: Ti3Al, TiAl and TiAl3. The structures of Ti3Al, TiAl and TiAl3 belong to space groups of P63/mmc (Ti3Al), P4/mmm (TiAl) and I4/mmm (TiAl3), respectively. The lattice structures of these crystals are shown in Figure 1, where the purple balls represent Al, and the gray balls represent Ti.
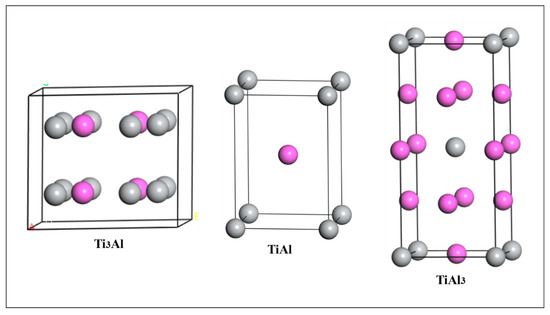
Figure 1.
Crystal structures of three kinds of Ti-Al intermetallics.
In this paper, lattice constants of Ti24Al8, Ti16Al16 and Ti8Al24 are optimized, and the equilibrium lattice constants at the minimum energy of the crystal are obtained. Table 1 summarizes the equilibrium lattice parameters calculated in this paper and the data calculated by other researchers. The difference between the calculated results and the data available from previous studies is within 1%, which indicates the good accuracy of this work. Therefore, it can be considered that the equilibrium lattice constant is suitable for the subsequent calculation.

Table 1.
Equilibrium lattice constants of Ti-Al intermetallics.
Alloying elements occupy different sublattice positions in Ti-Al intermetallics, which seriously affects the lattice constants, electronic structures and elastic constants of their solid solutions. Studies have shown that the occupancy of some alloy atoms depends on the ratio of Ti and Al. Thus, it is necessary to identify the site preference of Cr first. In this paper, the formation energies of Cr occupying Ti and Al sites in Ti-Al intermetallics are calculated, and the occupation preference of alloy atoms is described according to the formation energy.
The structural stability of the crystal is closely related to the formation energy. The smaller the negative value of crystal formation energy, the more stable the structure of the formed compound is. For Cr-doped Ti-Al-based alloys, the formation energy can be expressed as:
where Etotal is the total energy of the crystal; NAl, NTi and NCr are the number of Al, Ti and Cr atoms in the crystal, respectively; and EsolidAl, EsolidTi and EsolidCr are the average energy per atom in elemental Al, Ti and Cr, respectively, which are −3.742, −7.762 and −9.486 eV, respectively. The calculation results of the total energy and formation energy of each intermetallic compound are listed in Table 2.

Table 2.
The Etotal and Ef of each system.
As shown in Table 2, the formation energies of Ti7Al24Cr, Ti15Al16Cr and Ti23Al8Cr are lower than those of Ti8Al23Cr, Ti16Al15Cr and Ti23Al8Cr, respectively, indicating that the Cr atom is more likely to replace the Ti atom in Ti-Al intermetallics. Nevertheless, the formation energy of all alloys is negative, indicating that these ternary alloys can exist stably. In other words, Cr can exist stably in Ti3Al, TiAl and TiAl3 alloys regardless of whether Cr replaces Ti or Al.
The low ductility and toughness of Ti-Al intermetallics at room temperature limit their applications. In order to improve their ductility, adding alloying elements to Ti-Al intermetallics is an effective way. Elastic constant is one of the important parameters that characterizes the mechanical properties of materials, and can calculate the mechanical stability and stress resistance of materials. The periodic arrangement of crystal atoms makes its structure symmetrical. The Ti3Al, TiAl and TiAl3 intermetallics studied in this paper are tetragonal systems. Doped alloy atoms cause appropriate lattice distortion, making a tetragonal system into an orthorhombic system. The orthogonal system has nine independent elastic constants (C11, C22, C33, C12, C13, C23, C44, C55 and C66). We calculated the elastic constants of Cr substituting Ti and Al in the intermetallics to determine the mechanical stability and mechanical properties of ternary alloys, and the calculation results are shown in Table 3.

Table 3.
The elastic constants of doped Ti-Al intermetallics.
According to Born’s criterion of elastic stability, the elastic constant of a tetragonal crystal must simultaneously satisfy Equations (2)–(4) so that the crystal can exist stably:
By substituting the calculated elastic constants of Ti7Al24Cr, Ti8Al23Cr, Ti15Al16Cr, Ti16Al15Cr, Ti23Al8Cr and Ti24Al7Cr into Equations (2)–(4), it is found that they all meet these necessary conditions, indicating that they can exist stably.
Based on the elastic constants, the bulk modulus B, Young’s modulus E, shear modulus G and Poisson’s ratio v can be further calculated. Since the compression resistance of materials is positively correlated with the bulk modulus B, the bulk modulus B is usually used to describe the compression resistance of materials. Shear modulus G and Young’s modulus E can be used to characterize the strength and wear resistance of materials. Large shear modulus G and Young’s modulus E indicate high strength and wear resistance of the material. The Pugh’s B/G ratio can be used to evaluate the ductility of materials, and the critical value of the B/G ratio is 1.75 [37]. If the material’s B/G ratio is greater than 1.75, the material is considered to have ductility, and the larger the ratio, the better the ductility of the material. If the material B/G ratio is less than 1.75, the material is considered brittle, and the smaller the ratio, the more brittle the material. In general, shear modulus and Young’s modulus E can be used to characterize the strength and wear resistance of materials. A large shear modulus and Young’s modulus E indicates high strength and wear resistance of materials. The B/G ratio can be used to evaluate the ductility of materials. A small B/G ratio denotes a good ductility of materials. Poisson’s ratio v refers to the ratio of transverse normal strain to axial normal strain of a material under uniaxial tension or compression, which reflects the transverse deformation of the material. The shear stability of the material can be quantified by calculating the Poisson’s ratio v of the material. Generally speaking, when Poisson’s ratio v is in the range of 0.1~0.3, the material is hard. When Poisson’s ratio v is between 0.3 and 0.4, the material has proper plasticity and low hardness. Bulk modulus B, shear modulus G and Poisson’s ratio v are calculated using Equations (5), (6) and (7), respectively:
The calculated values of bulk modulus B, shear modulus G and elastic modulus ratio G/B of Ti8Al24, Ti7Al24Cr, Ti8Al23Cr, Ti16Al16, Ti15Al16Cr, Ti16Al15Cr, Ti24Al8, Ti23Al8Cr and Ti24Al7Cr alloys are summarized in Table 4.

Table 4.
The bulk modulus B (GPa), shear modulus G (GPa), the B/G ratio and Poisson’s ratio v of doped Ti-Al intermetallics.
Comparing the elastic constants of Ti8Al24, Ti16Al16 and Ti24Al8, it can be seen that the B/G ratio of Ti8Al24 and Ti16Al16 are less than 1.75, which indicates that they are brittle materials. The B/G ratio of Ti24Al8 is more than 1.75, which indicates that it is a ductile material. With the increase in Ti content, the bulk modulus B, B/G ratio and Poisson’s ratio v of the material increase gradually, and the compression resistance and ductility of the three Ti-Al-based alloys are gradually improved. Comparing the B/G ratio of Ti8Al24, Ti7Al24Cr and Ti8Al23Cr, it can be noted that the B/G ratio of Ti7Al24Cr is 1.149, which is smaller than that of Ti8Al24. This indicates that when Cr replaces the Ti atom in Ti8Al24, its ductility cannot be improved. In addition, the bulk modulus B decreases, and the anti-compression performance of the material becomes worse. However, when Cr replaces the Al atom in Ti8Al24, the B/G ratio of Ti8Al23Cr is 1.250. Although it is still a brittle material, it shows that this substitution method improved the ductility. The v of Ti7Al24Cr is 0.163 (which is 0.008 smaller than that of Ti8Al24), while the v of Ti8Al23Cr is 0.184 (which is 0.013 larger than that of Ti8Al24). It shows that Cr can increase the anisotropy by replacing the Al atom in Ti8Al24, rather than replacing the Ti atoms.
Comparing the B/G ratios of Ti16Al16, Ti15Al16Cr and Ti16Al15Cr, it is found that the B/G ratio of Ti15Al16Cr is the smallest, which indicates that when Cr replaces Ti atoms in Ti16Al16, it cannot improve the compression and ductility properties of the material. When Cr replaces the Al atom in Ti16Al16, the bulk modulus B and B/G ratio of Ti16Al15Cr increase. Moreover, the B/G ratio of Ti16Al15Cr is 1.768; this value is larger than 1.75, indicating that the material exhibits ductility. It shows that when Cr replaces the Al atom, the ductility and compression resistance of the material significantly improves. The Poisson’s ratio v of Ti15Al16Cr is 0.229, which is 0.004 less than that of Ti8Al24. It can be predicted that the plasticity of Ti15Al16Cr is basically unchanged compared with Ti8Al24. However, the Poisson’s ratio v of Ti8Al23Cr is 0.262, which is 0.029 higher than that of Ti8Al24, indicating that the substitution of Cr for Al in Ti8Al24 not only preserves the high hardness of the material but also significantly increases its plasticity.
Comparing the bulk modulus B and B/G ratios of Ti24Al8, Ti23Al8Cr and Ti24Al7Cr, it is found that their B/G ratios are more than 1.75 and they are ductile materials. The bulk modulus B and B/G ratios of Ti23Al8Cr and Ti24Al7Cr are higher than those of Ti24Al8, which indicates that Cr can improve the ductility and compression resistance of Ti24Al8 whether it replaces Ti or Al atoms. However, the bulk modulus B and B/G ratio of Ti24Al7Cr are the largest. The replacement of Al by Cr in Ti24Al8 can better improve its mechanical properties. In conclusion, the substitution of Al atoms with Cr in Ti8Al24, Ti16Al16 and Ti24Al8 alloys improves the ductility of the material. The Poisson ratio of Ti24Al8 is 0.286, which belongs to a high hardness alloy, and compared with Ti24Al8, the Poisson’s ratios v of Ti23Al8Cr and Ti24Al7Cr increased by 0.032 and 0.049, respectively. It shows that, regardless of whether Cr replaces the Al atom or Ti atom in Ti8Al24, it will increase the plasticity of the material. However, the Poisson’s ratios v of Ti23Al8Cr and Ti24Al7Cr are between 0.3–0.4, and the hardness of the materials is low.
The properties of materials are closely related to their internal chemical bonds. The density of states indicates the number of electrons in the unit energy range. It can be used as an intuitive result of the energy band structure, which can reflect the distribution of electrons in various orbits, thus reflecting the interaction between atoms. In order to further clarify the reason why the elastic property of Ti-Al intermetallics is affected by the substitution of Ti and Al by Cr, we discuss the electronic structure of these Ti-Al intermetallics.
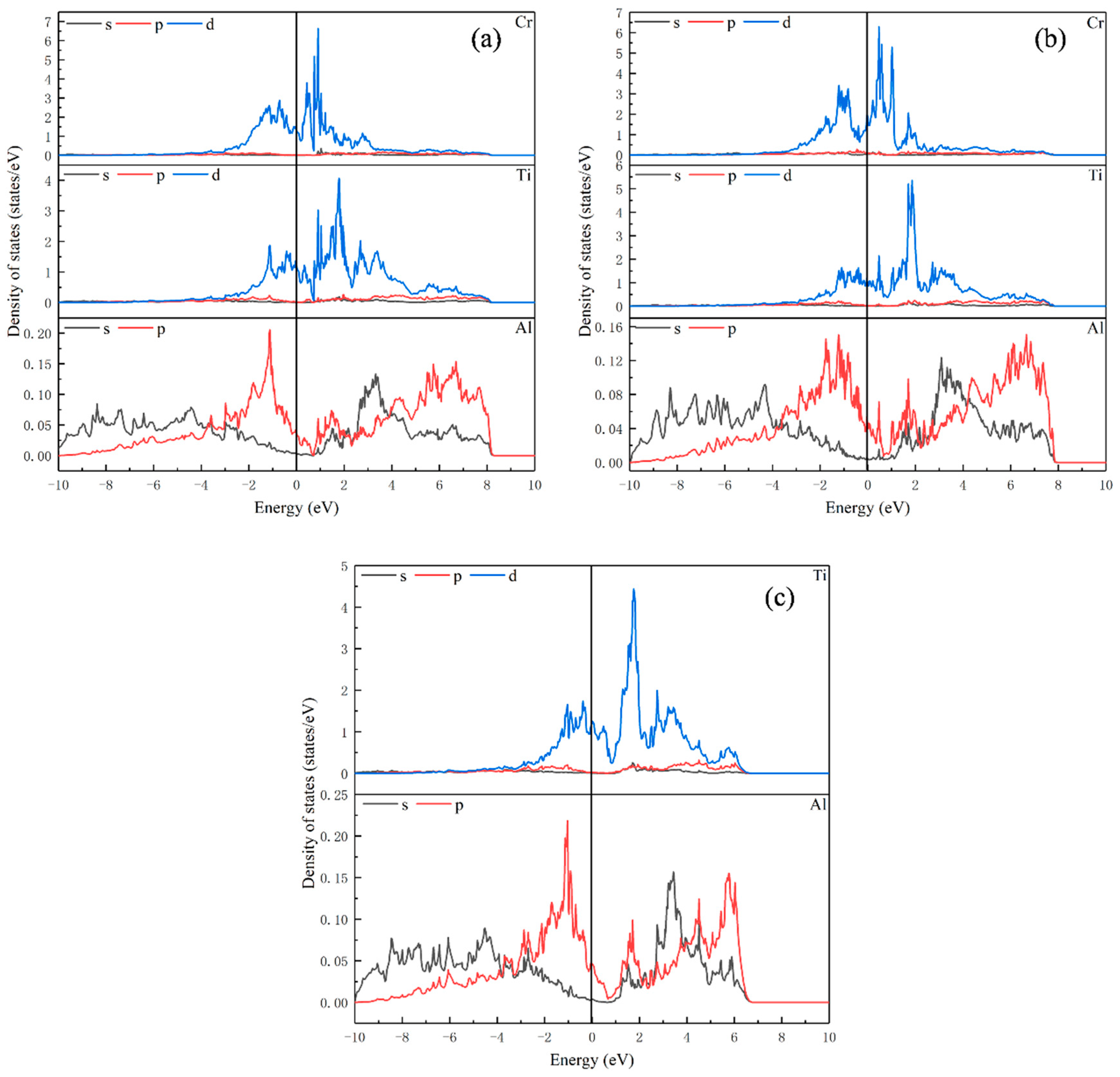
Figure 2, Figure 3 and Figure 4 show the partial density of states data of elements in Ti8Al24, Ti7Al24Cr, Ti8Al23Cr, Ti16Al16, Ti15Al16Cr, Ti16Al15Cr, Ti24Al8, Ti23Al8Cr and Ti24Al7Cr. It can be seen from these figures that the Ti-d-Ti-d interaction is dominant in the Ti-Al intermetallics, and the bonding characteristics are metallic. From Figure 2c, it can be noted that for Ti8Al24, there is an overlap between Al s, Al p and Ti d orbitals from about −2 eV to 5 eV, and there is a low energy weak spd hybrid band. However, the energy is so small that the covalent bonds formed by hybridization between Al and Ti elements are almost negligible. When Cr replaces Ti, the peaks of state density of Ti and Al decrease, and the interactions between Ti-Ti, Ti-Al and Al-Al become weaker. When Cr replaces Al, the peak of the density of states of Ti increases, while Al decreases. The interaction between Ti-Ti is strengthened, and the interaction between Al-Al is weakened.
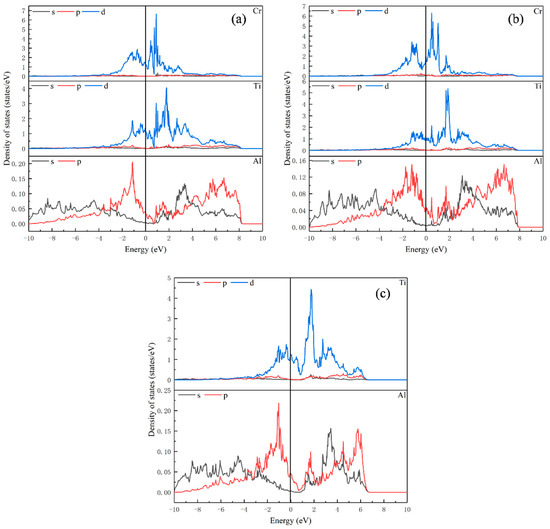
Figure 2.
Partial density of states for (a) Ti7Al24Cr; (b) Ti8Al23Cr; (c) Ti8Al24.
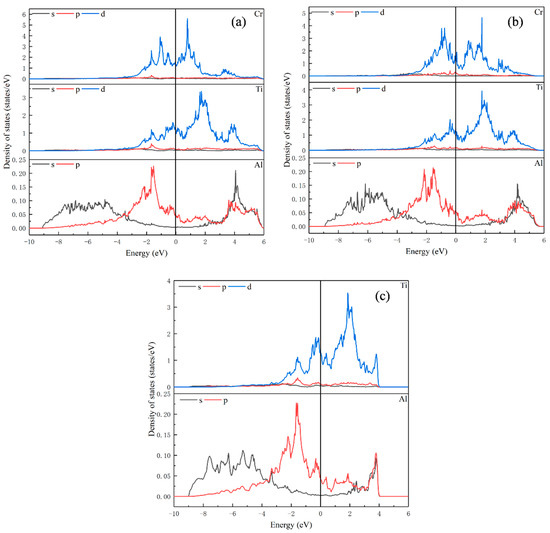
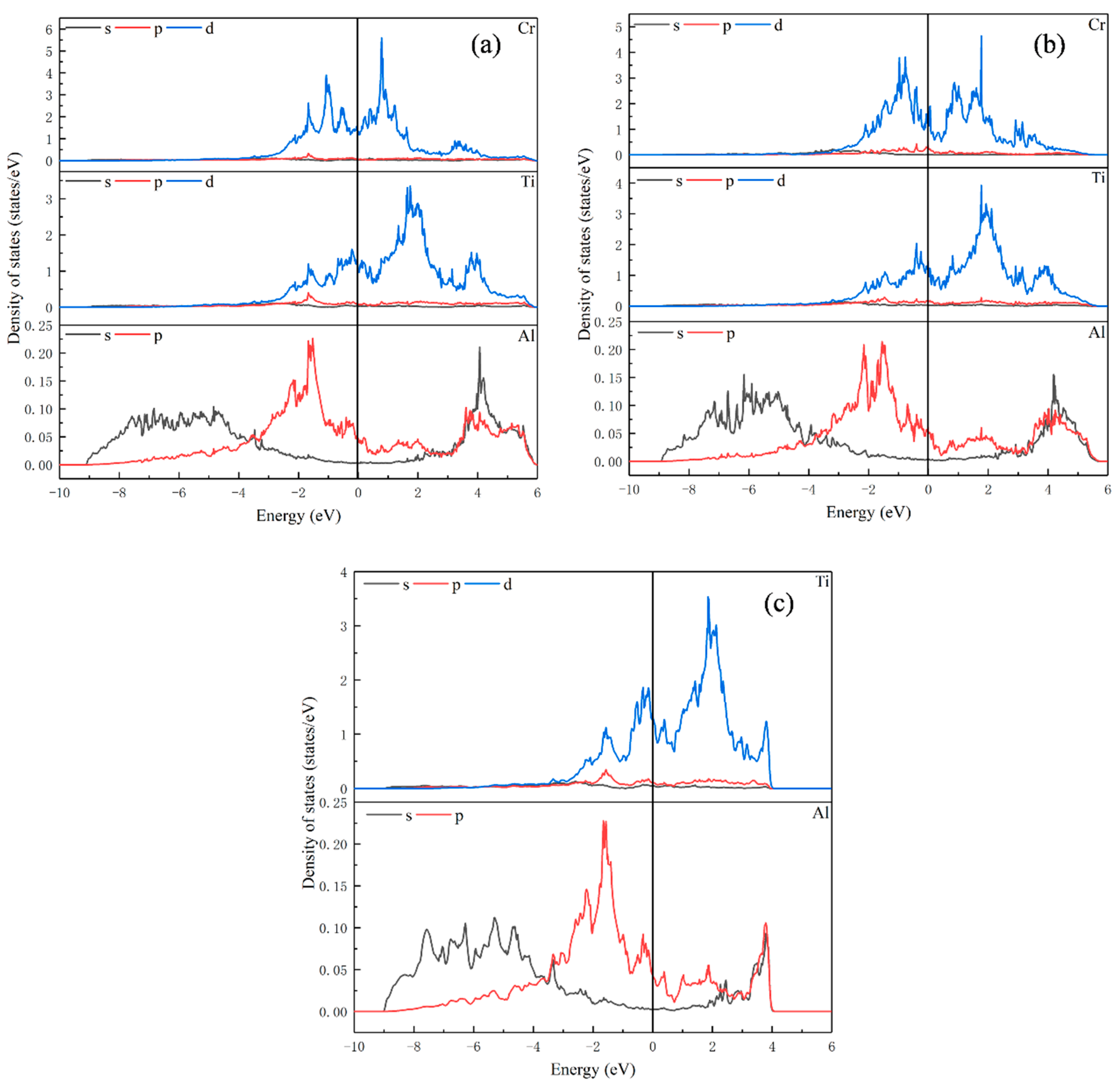
Figure 3.
Partial density of states for (a) Ti15Al16Cr; (b) Ti16Al15Cr; (c) Ti16Al16.
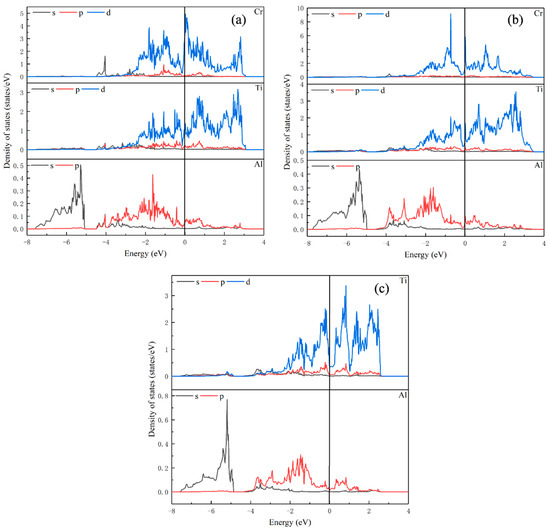
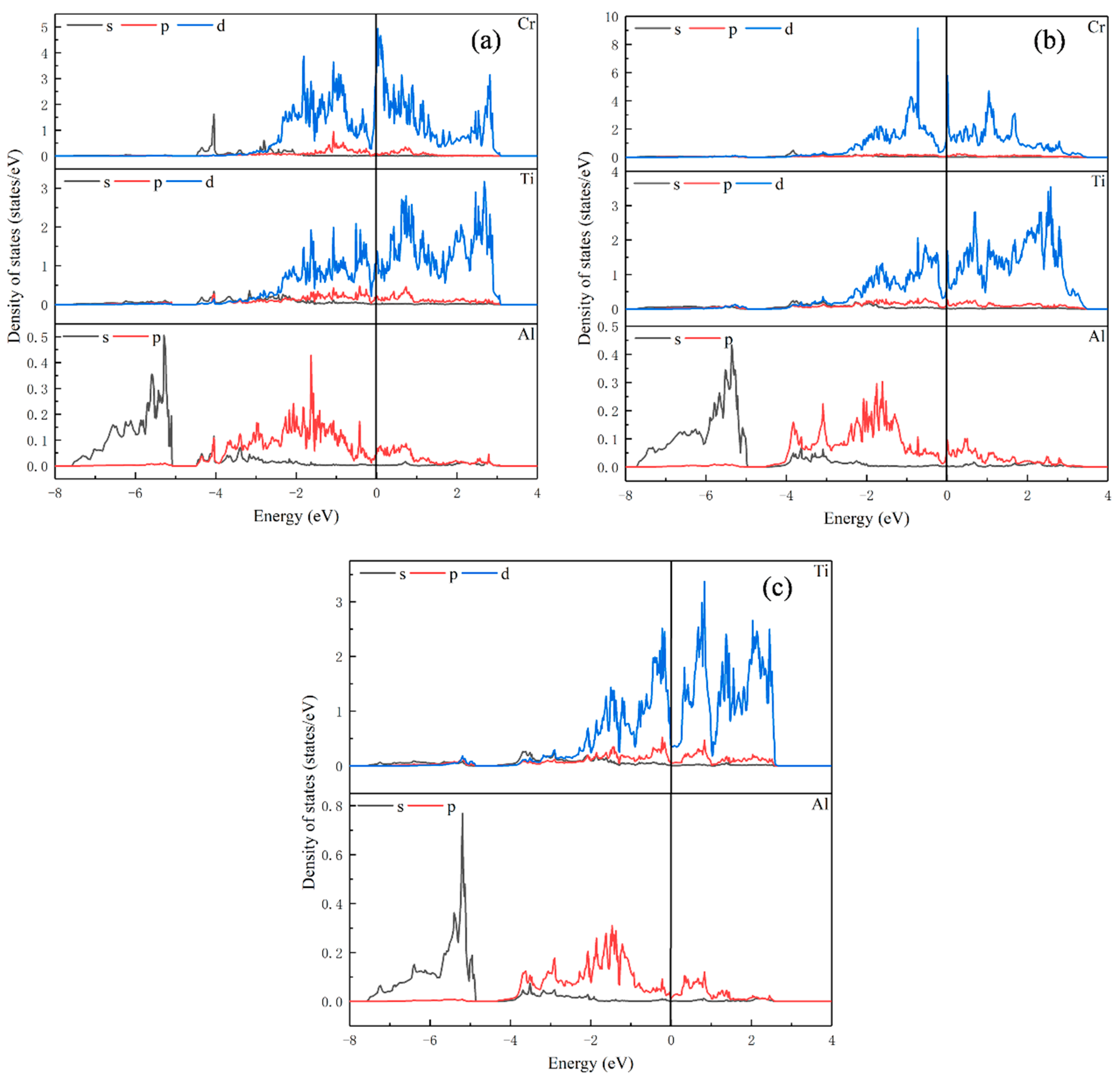
Figure 4.
Partial density of states for (a) Ti23Al8Cr; (b) Ti24Al7Cr; (c) Ti24Al8.
As can be noted from Figure 3c, for Ti16Al16, the orbital overlap between atoms is mainly concentrated at −3 eV to 4 eV. When Cr replaces Ti or Al, the overlap between the atoms gets a little wider. In Figure 3a, when Cr replaces Ti, the peak of density of states of Ti and Al decreases compared with Ti16Al16, and the interaction between Ti-Ti, Ti-Al and Al-Al becomes weaker. In Figure 3b, when Cr replaces Al, the peak of density of states of Cr is somewhat lower than that of Ti15Al16Cr, but it is still larger than that of Ti. There is strong d orbital hybridization with Ti atoms from approximately −3 eV to 4 eV. The Ti-Ti interaction is enhanced by increasing the peak of density of states of Ti.
As shown in Figure 4c, for Ti24Al8, the orbital overlap between atoms is mainly between −3 eV and 3 eV. In Figure 4a, unlike Ti8Al24 and Ti16Al16, the peak of density of states of Ti in Ti23Al8Cr is not significantly reduced after replacing Ti with Cr. However, the peak of density of states of the Cr atom is larger than that of Ti, and there are strong metal bonds between the Cr atom and the surrounding Ti atoms between −3 eV and 3 eV. Thus, the ductility of the alloy is also enhanced after Cr replaces the Al in Ti24Al8.
By analyzing the partial density of states data of elements in nine alloys, including Ti8Al24, Ti7Al24Cr, Ti8Al23Cr, Ti16Al16, Ti15Al16Cr, Ti16Al15Cr, Ti24Al8, Ti23Al8Cr and Ti24Al7Cr, it can be seen that the main reason for the ductility reduction in Ti8Al24 and Ti16Al16 is the weakening of the Ti-Ti metal bond’s strength after replacing Ti with Cr. After replacing Ti atoms with Cr in Ti24Al8, the main reason for the increase in the ductility of the material is that the peak of the density of states of Ti is not significantly reduced, and Cr forms a solid metal bond with the surrounding Ti atoms. After Cr replaces Al in Ti-Al intermetallics, the reason for the increase in material ductility is that Cr and Ti form a solid metal bond, which improves the metal bond strength of the system.
4. Conclusions
- (1)
- The Cr atom prefers to replace Ti atoms in Ti-Al intermetallics. However, according to the formation energy and Born–Huang criterion, whether the Cr atom replaces Ti or Al, the system has energy stability and mechanical stability;
- (2)
- The ductility of TiAl and TiAl3 compounds cannot be improved by the Cr atom replacing the Ti atom but can be improved by the Cr atom replacing the Al atom. In the Ti3Al compound, the Cr atom can improve the ductility no matter whether it replaces Ti or Al, and the effect is better with the latter;
- (3)
- After replacing Al with Cr, the metal bond between Ti-Ti is strengthened, and a strong metal bond is formed between Cr and Ti, thus improving the ductility of Ti-Al intermetallics.
Author Contributions
Methodology, H.W. and F.S.; validation, Z.W.; investigation, H.W.; resources, F.S.; data curation, F.S. and Z.W.; writing—original draft, H.W.; writing—review & editing, H.W. and F.S.; visualization, F.S. and Z.W.; project administration, F.S. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant NO. 51706017).
Data Availability Statement
The original data can be obtained from the first author of this article.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Zhao, K.; Feng, N.; Wang, Y. Fabrication of Ti-Al intermetallics by a two-stage aluminothermic reduction process using Na2TiF6. Intermetallics 2017, 85, 156–162. [Google Scholar] [CrossRef]
- Song, Y.; Dou, Z.; Zhang, T.; Liu, Y.; Wang, G.C. First-principles calculation on the structural, elastic and thermodynamic properties of Ti-Al intermetallics. Mater. Res. Express 2019, 6, 1065. [Google Scholar] [CrossRef]
- Jian, Y.; Huang, Z.; Xing, J.; Sun, L.; Liu, Y.; Gao, P. Phase stability, mechanical properties and electronic structures of Ti Al binary compounds by first principles calculations. Mater. Chem. Phys. 2019, 221, 311–321. [Google Scholar] [CrossRef]
- Jiang, C. First-principles study of site occupancy of dilute 3d, 4d and 5d transition metal solutes in L10 TiAl. Acta. Mater. 2008, 56, 6224–6231. [Google Scholar] [CrossRef]
- Tang, P.; Tang, B.; Su, X. First-principles studies of typical long-period superstructures Al5Ti3, h-Al2Ti and r-Al2Ti in Al-rich TiAl alloys. Comput. Mater. Sci. 2011, 50, 1467–1476. [Google Scholar] [CrossRef]
- Zhang, L.G.Z.A. Ab initio pseudopotential calculations on the effect of Mn doped on lattice parameters of L10 TiAl. Intermetallics 2000, 8, 637–641. [Google Scholar]
- Song, Y.; Yang, R.; Li, D.; Hu, Z.Q.; Guo, Z.X. A first principles study of the influence of alloying elements on TiAl: Site preference. Intermetallics 2000, 8, 563–568. [Google Scholar] [CrossRef]
- Fan, T.; Lin, L.; Liang, H.; Ma, Y.; Tang, Y.; Hu, T.; Ruan, Z.; Chen, D.; Wu, Y. First-principles study of the structural, mechanical and thermodynamic properties of Al11RE3 in aluminum alloys. Crystals 2023, 13, 347. [Google Scholar] [CrossRef]
- Saraç, U.; Trong, D.N.; Baykul, M.C.; Long, V.C.; Ţălu, Ş. Tuning structural properties, morphology and magnetic characteristics of nanostructured Ni-Co-Fe/ITO ternary alloys by galvanostatic pretreatment process. Microsc. Res. Tech. 2022, 85, 3945–3954. [Google Scholar] [CrossRef]
- Qi, W.; Wang, W.; Yang, X.; Xie, L.; Zhang, J.; Li, D.; Zhang, Y. Effect of Zr on phase separation, mechanical and corrosion behavior of heterogeneous CoCrFeNiZrx high-entropy alloy. J. Mater. Sci. Technol. 2022, 109, 76–85. [Google Scholar] [CrossRef]
- Trong, D.N.; Long, V.C.; Tălu, Ş. The Structure and Crystallizing Process of NiAu Alloy: A Molecular Dynamics Simulation Method. J. Compos. Sci. 2021, 5, 18. [Google Scholar] [CrossRef]
- Couret, A.; Voisin, T.; Thomas, M.; Monchoux, J.P. Development of a TiAl Alloy by Spark Plasma Sintering. JOM 2017, 69, 2576–2582. [Google Scholar] [CrossRef]
- Tang, S.Q.; Qu, S.J.; Feng, A.H.; Shen, J.; Chen, D.L. Core-multishell globular oxidation in a new TiAlNbCr alloy at high temperatures. Sci. Rep. 2017, 7, 3483. [Google Scholar] [CrossRef]
- Kastenhuber, M.; Klein, T.; Clemens, H.; Mayer, S. Tailoring microstructure and chemical composition of advanced γ-TiAl based alloys for improved creep resistance. Intermetallics 2018, 97, 27–33. [Google Scholar] [CrossRef]
- Zhou, C.; Zeng, F.P.; Liu, B.; Zhao, K.; Lu, J.; Qiu, C.; Li, J.; He, Y. Effects of Si on Microstructures and High Temperature Properties of Beta Stabilized TiAl. Alloy. Mater. Trans. 2016, 57, 461–465. [Google Scholar] [CrossRef]
- Liu, Q.; Nash, P. The effect of Ruthenium addition on the microstructure and mechanical properties of TiAl alloys. Intermetallics 2011, 19, 1282–1290. [Google Scholar] [CrossRef]
- Tetsui, T. Effects of high niobium addition on the mechanical properties and high-temperature deformability of gamma TiAl alloy. Intermetallics 2002, 10, 239–245. [Google Scholar] [CrossRef]
- Liu, Z.C.; Lin, J.P.; Li, S.J.; Chen, G.L. Effects of Nb and Al on the microstructures and mechanical properties of high Nb containing TiAl base alloys. Intermetallics 2002, 10, 653–659. [Google Scholar] [CrossRef]
- Duan, Q.; Luan, Q.; Liu, J.; Peng, L. Microstructure and mechanical properties of directionally solidified high-Nb containing Ti–Al alloys. Mater. Des. 2010, 31, 3499–3503. [Google Scholar] [CrossRef]
- Music, D.; Schneider, J. Effect of transition metal additives on electronic structure and elastic properties of TiAl and Ti3Al. Phys. Rev. B 2006, 74, 174110. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B.; Liu, Y.X.; Yong, L.I.U. Processing map and hot deformation behavior of Ta-particle reinforced TiAl composite. T. Nonferr. Met. Soc. 2020, 30, 657–667. [Google Scholar] [CrossRef]
- Xiong, J.; Song, L.; Guo, X.; Liu, X.; Zhang, W.; Zhang, T. Inhibition of ω0 phase precipitation in TNM-based TiAl alloys by Cr and Mn. Intermetallics 2023, 153, 107774. [Google Scholar] [CrossRef]
- Ye, L.; Wang, H.; Zhou, G.; Hu, Q.M.; Yang, R. Phase stability of TiAl-X (X=V, Nb, Ta, Cr, Mo, W, and Mn) alloys. J. Alloys Compd. 2020, 819, 153291. [Google Scholar] [CrossRef]
- Tetsui, T. Impact resistance of commercially applied TiAl alloys and simple-composition TiAl alloys at various temperatures. Metals 2022, 12, 2003. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Weatherly, G.C.; Wilkinson, D.S.; Kovalev, A.I.; Wainstein, D.L. The role of chromium in protective alumina scale formation during the oxidation of ternary TiAlCr alloys in air. Intermetallics 2004, 12, 165–180. [Google Scholar] [CrossRef]
- Brady, M.P.; Wright, I.G.; Gleeson, B. Alloy design strategies for promoting protective oxide-scale formation. JOM 2000, 52, 16–21. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, 1133–1141. [Google Scholar] [CrossRef]
- Kohn, W.; Hohenberg, P. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar]
- Kresse, G. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.H.; Zhu, K.J.; Peng, J.H. First-principles simulation on structure property of Ti-Al intermetallics. J. Comput. Phys. 2017, 34, 365–373. [Google Scholar]
- Hultgren, R.; Desai, P.D.; Hawkins, D.T.; Gleiser, M.; Kelley, K.K. Selected values of the thermodynamic properties of binary alloys. Natl. Stand. Ref. Data Syst. 1973, 58, 1432. [Google Scholar]
- Xie, Y.; Tao, H.; Peng, H.; Li, X.; Liu, X.; Peng, K. Atomic states, potential energies, volumes, stability, and brittleness of ordered FCC TiAl3-type alloys. Phys. B Condens. Matter 2005, 366, 17–37. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, X.; Niu, R.; Zhang, S.; Liao, X.; Moss, S.D.; Finkel, P.; Garbrecht, M.; Ringer, S.P.; Cairney, J.M.; et al. Giant room temperature compression and bending in ferroelectric oxide pillars. Nat. Commun. 2022, 13, 335. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).