Glycine Dissolution Behavior under Forced Convection
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
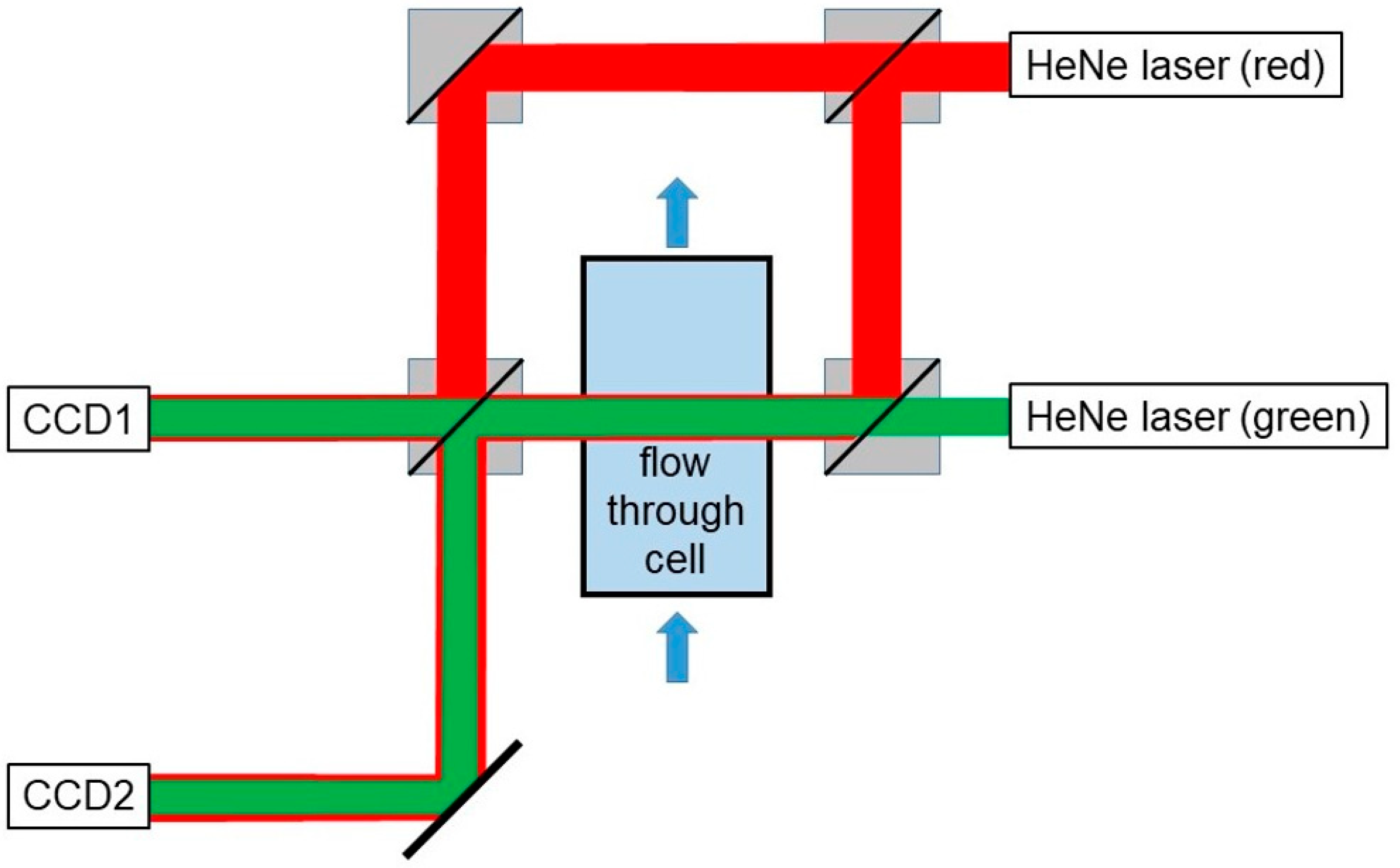
2.1.1. Optical Set-Up
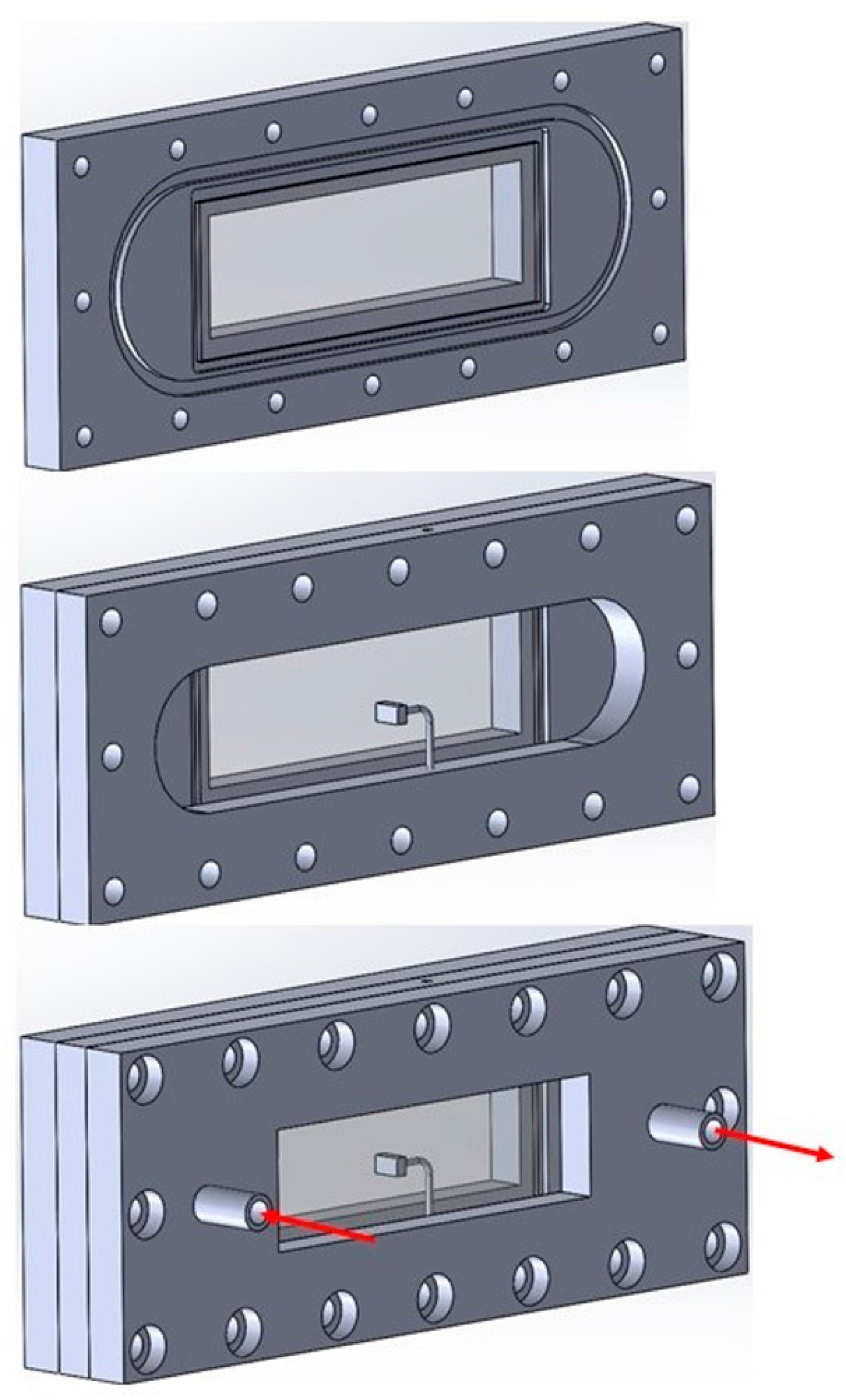
2.1.2. Fluidmechanical Set-Up
2.2. Crystals and Solvents
2.3. Image Analysis
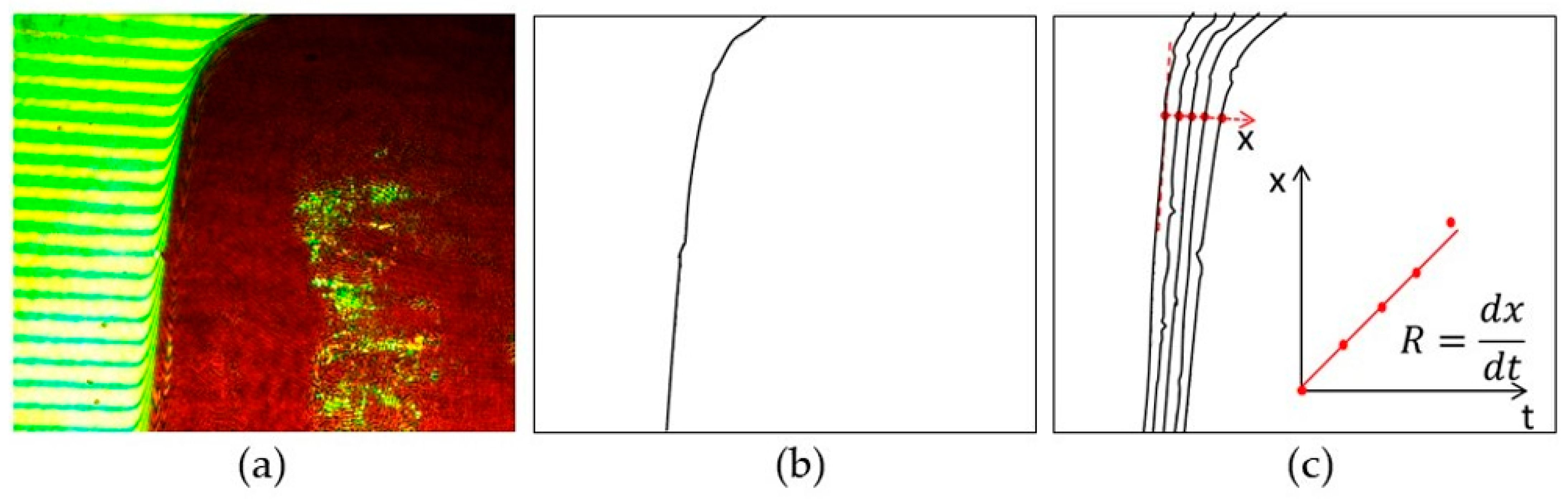
2.3.1. Extraction of Crystal Outline and Face Displacement Velocity
2.3.2. Extraction of the Concentration Field
3. Results and Discussion
3.1. Face Displacement Velocity in Stagnant Solvents

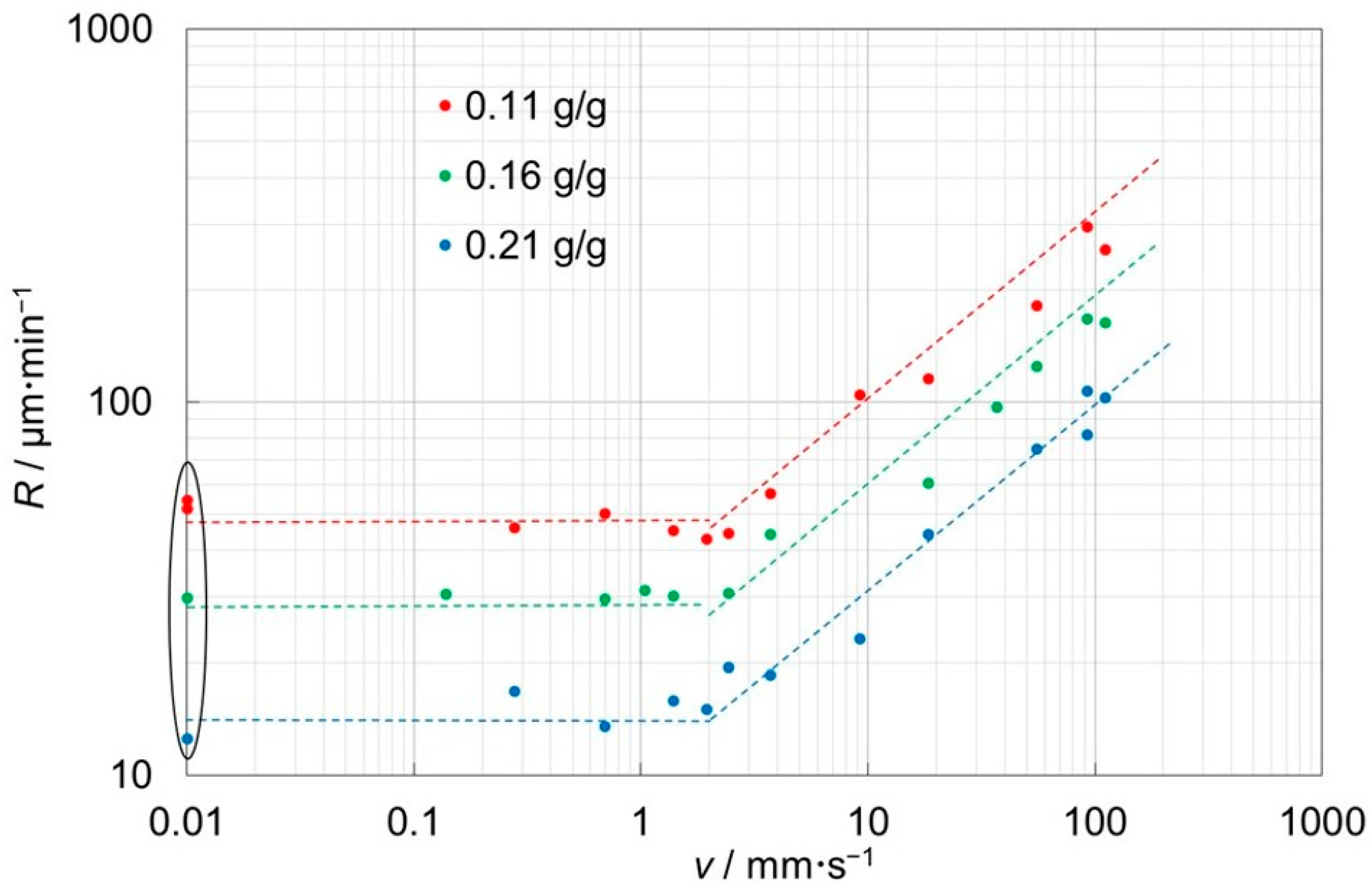
3.2. Face Displacement Velocities under Forced Convection
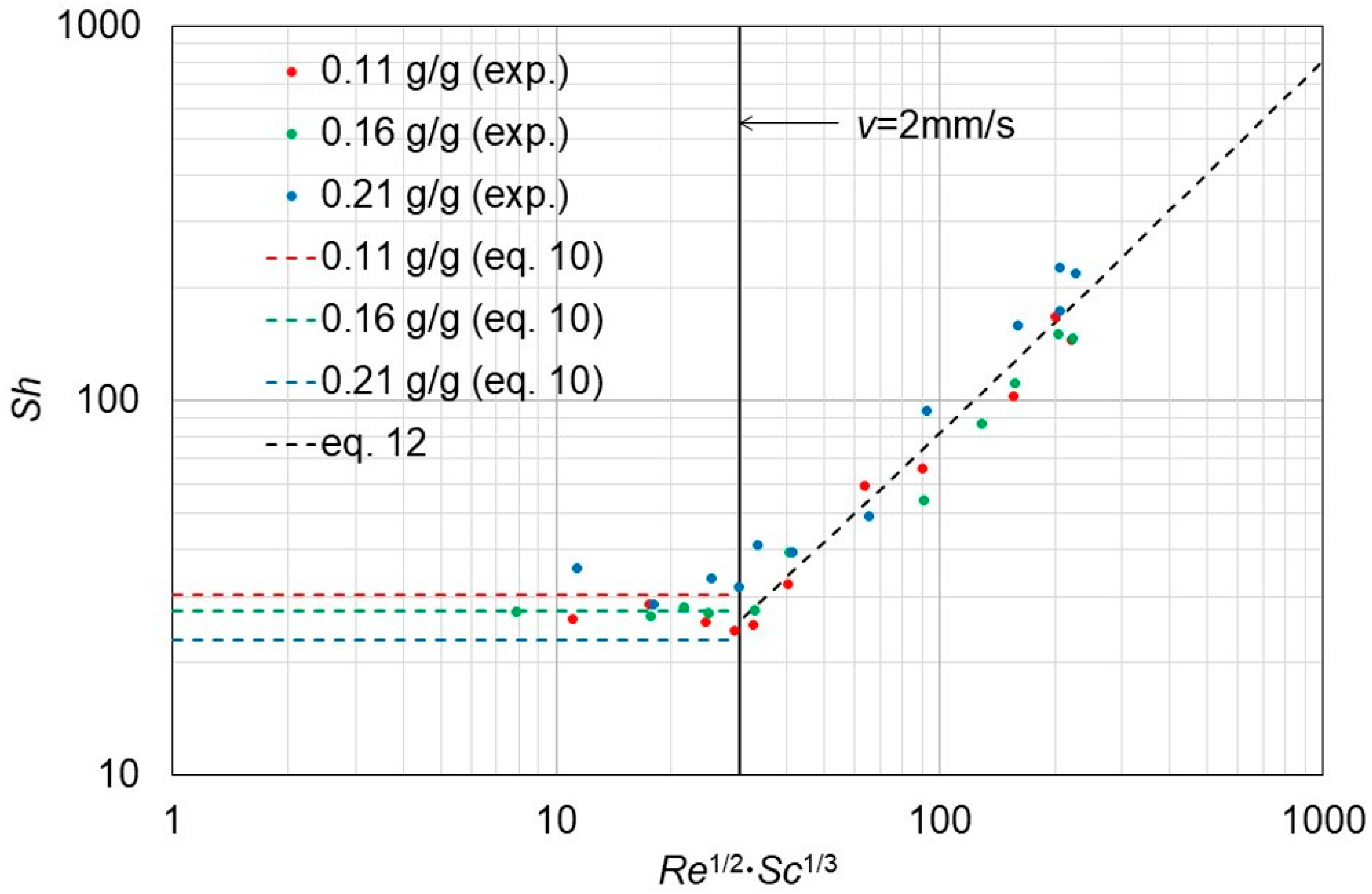
3.3. Description in Terms of Dimensionless Numbers
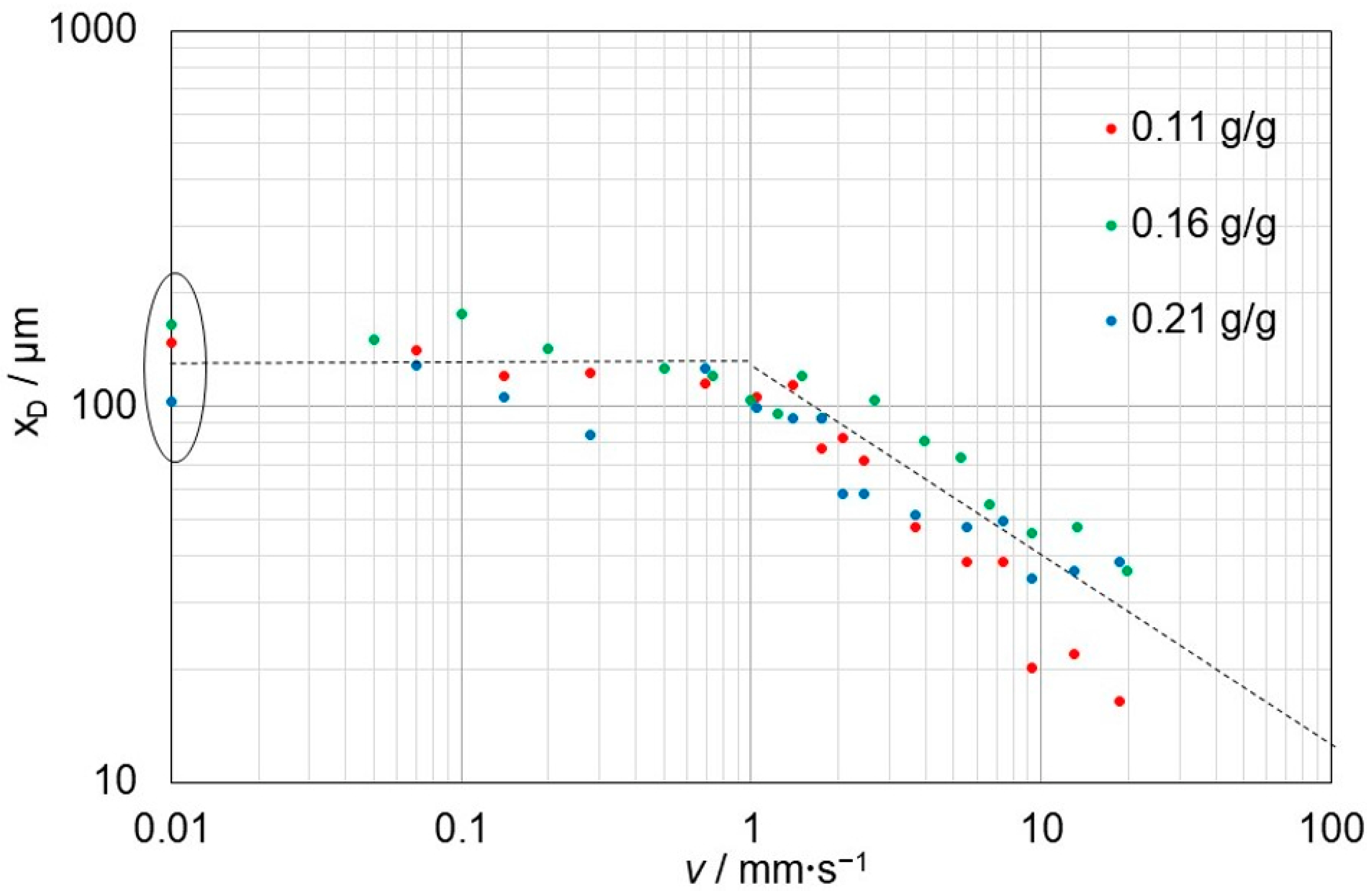
3.4. Thickness of the Diffusive Layer
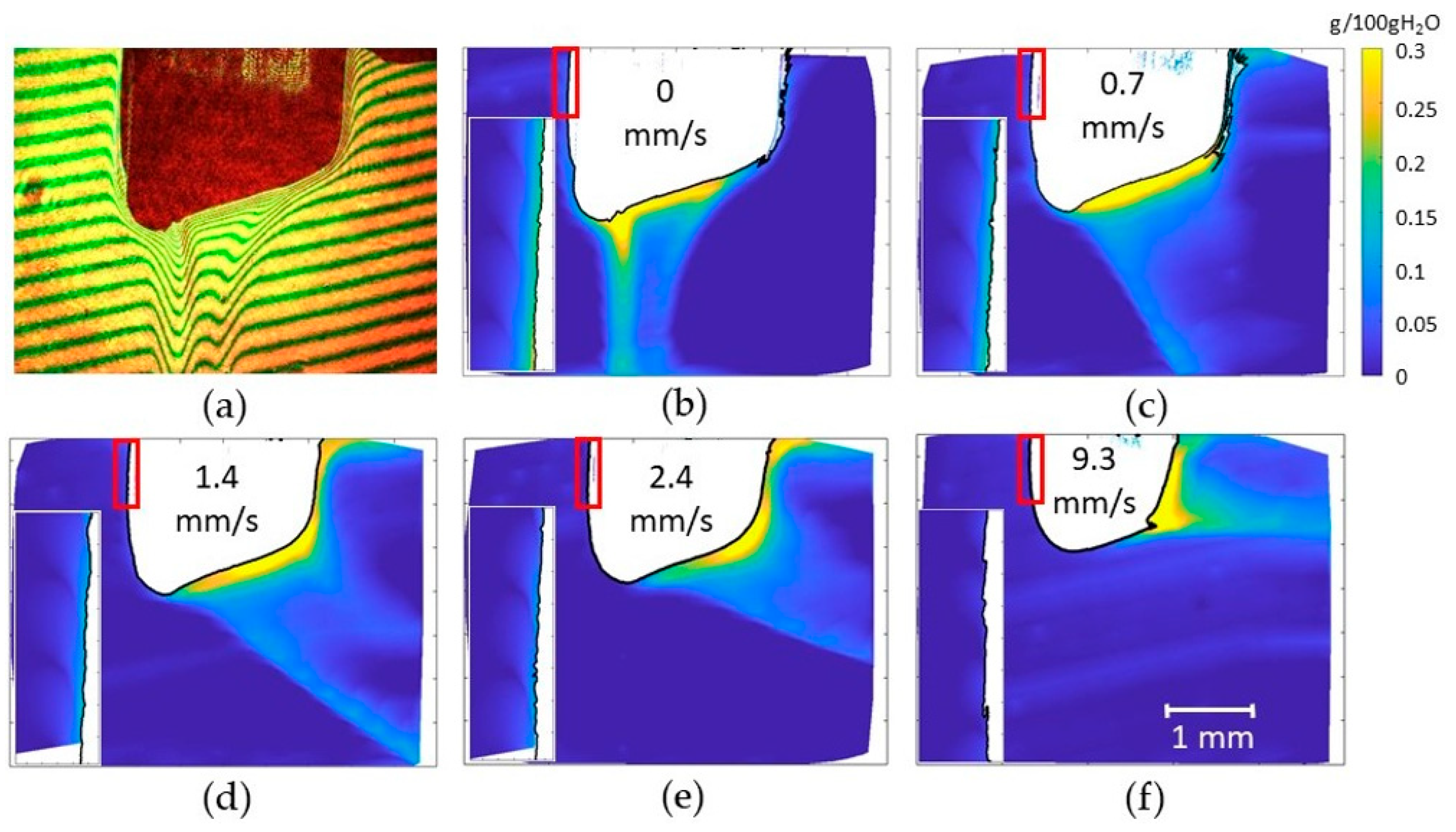
3.5. Visualisation of Concentration Fields
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Mach–Zehnder Interferometer: Visualization of Concentration Field | |
| light source | laser head HeNe 633-15 P (Qioptiq Photonics GmbH & Co. KG, Königsallee 23, 37081 Göttingen, Germany) |
| beam expander | beamexpander 10x (Qioptiq Photonics GmbH & Co. KG, Königsallee 23, 37081 Göttingen, Germany) |
| beam splitters | beamsplitter cube VIS; N-BK7; L = 16 (Qioptiq Photonics GmbH & Co. KG, Königsallee 23, 37081 Göttingen, Germany) |
| Crystal Imaging | |
| light source | Laser Head HeNe 543-2 (Qioptiq Photonics GmbH & Co. KG, Königsallee 23, 37081 Göttingen, Germany) |
| beam expander | beamexpander 10x (Qioptiq Photonics GmbH & Co. KG, Königsallee 23, 37081 Göttingen, Germany) |
| Image Acquisition | |
| CCD1 | U3-3560XCP-C-HQ (IDS Imaging Development Systems GmbH, Dimbacher Strasse 10, 74182 Obersulm, Germany) |
| lens of CCD1 | Canon Macro Lens FL 1:3.5/50 mm (Canon Deutschland GmbH, Europark Fichtenhain A10, 47807 Krefeld, Germany) with adapter Fotodiox-Canon FD lens to C-mount (Vision Dimension GmbH, Am Kiel-Kanal 1, 24106 Kiel, Germany) |
| CCD2 | UI-2280SE-C (IDS Imaging Development Systems GmbH, Dimbacher Strasse 10, 74182 Obersulm, Germany) |
| lens of CCD2 | Optem Zoom 70XL (0.75×–5×), selected magnification 2.0 (Polytec GmbH, Polytec-Platz 1-7, 76337 Waldbronn, Germany) |
| mirror | plano mirror Pl. Mirror RAL; D = 22.4 × 31.5 oval; d = 3.5; L/2 (Qioptic Photonics GmbH & Co. KG, Königsallee 23, 37081 Göttingen, Germany) |
| Flow-Through Cell | |
| channel dimension | 110 mm × 24 mm × 5 mm |
| windows | soda lime glass, 76 mm × 26 mm × 1 mm (Menzel-Gläser, VWR International GmbH, Fraunhoferstr. 11, 85737 Ismaning, Germany), optical accessible: 70 mm × 22 mm |
| tube connections | inner diameter: 4 mm |
| glue | dual component epoxy adhesive (UHU plus endfest, |
| Pumps | |
| syringe pump 0.5 to 17.5 mL/min | Atlas Syringe Pump (Syrris Ltd., Unit 3, Anglian Business Park, Royston, Herts, SG8 5TW, UK) 2.5 mL/l syringe, continuous mode |
| gear pump 27 to 800 mL/min | Verdergear VGS04027 with built-in frequency converter (Verder Deutschland GmbH & Co. KG, Retsch-Allee 1-5, 42781 Haan, Germany) |
| Tubing | |
| silicone tubes | Rotilabo silicone standard design 5.0 mm, 8.0 mm (Carl Roth GmbH + Co. KG, Schoemperlenstr. 3-5, 76185 Karlsruhe, Germany) |
| Temperature Control | |
| water bath | Thermo Electron Haake Phönix II Type P1 (Thermo Haake, Dieselstr. 4, 76227 Karlsruhe, Germany) |
| heat exchanger | aluminum block, contact areas: 40 cm2 to solvent, 200 cm2 to bathwater |
| temperature measurement | Greisinger GMH 3230 with thermocouple GTF 3000 (GHM Messtechnik GmbH, Hans-Sachs-Str. 26, 93128 Regenstauf, Germany) |
Appendix B
| Step | Action | MATLAB Command |
|---|---|---|
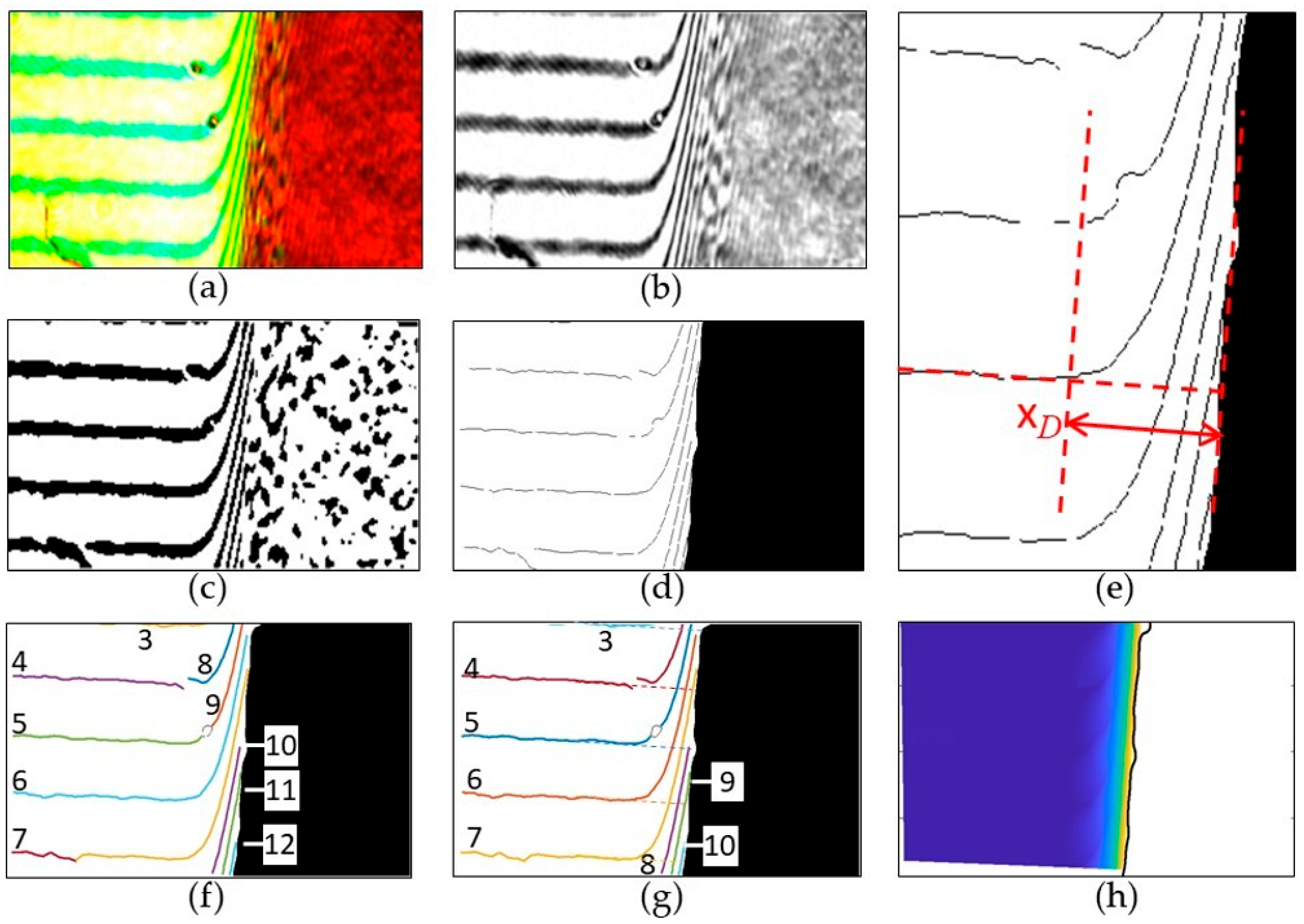
| 1 | select a region of interest (ROI) see Figure A1a | |
| 2 | restrict to green channel see Figure A1b | ROI = ROI(:,:,2) |
| 3 | smooth to reduce image noise | ROI = imgaussfilt(ROI,3) |
| 4 | determinate global image threshold | T = graythresh(ROI) |
| 5 | binarize to identify the crystal see Figure A1c | ROI = imbinarize(ROI,T) |
| 6 | morphological opening to remove small dropouts and smooth the crystal outline | ROI = imopen(ROI,strel(‘disk’,20) |
| 7 | remove artifacts outside the crystal, see Figure A1d | ROI = bwselect(~ROI,x,y) (x,y: coordinates of a pixel inside the crystal) |
| 8 | create the crystal outline see Figure A1e | OUTLINE = bwmorph(ROI,‘remove’) |
| Step | Action | MATLAB Command |
|---|---|---|
| 1 | Select a region of interest (ROI), see Figure A2a | |
| 2 | Restrict to red channel see Figure A2b | ROI = ROI(:,:,1) |
| 3 | Smooth to reduce image noise | ROI = imgaussfilt(ROI,3) |
| 5 | Binarize with adaptive threshold, see Figure A2c | ROI = imbinarize(ROI,‘adaptive’,… ‘ForegroundPolarity’,‘dark’,‘Sensitivity’,0.5); |
| 6 | Mask with segmented crystal see | ROI = or(ROI, MASK) |
| 7 | Shrink the fringes to lines see Figure A2d | FRINGES = bwskel(~ROI,‘MinBranchLength’,40) |
| 8 | Determine the thickness of the diffusion layer according to the method shown in Figure A2e | |
| 9 | Remove branchpoints to get clear fringes | POINT = bwmorph(FRINGES,‘Branchpoints’); FRINGES = and(FRINGES,~POINT); |
| 10 | Remove line segments shorter than 5% of the longest (index IX) | LINES = bwconncomp(FRINGES,8); STATS = regionprops(LINES,… ‘MajorAxisLength’); LONG = max([STATS.MajorAxisLength]); IX = find([STATS.MajorAxisLength] >… 0.05×LONG); |
| 11 | The remaining lines are numbered (Figure A2f). Manual renumbering assigns the same number to all line segments belonging to one fringe | |
| 12 | Sort the lines in ascending order (Figure A2g). The assigned number indicates the local phase shift between test and reference beam as a multiple of 2π | |
| 13 | Generate a phase shift field in the entire unmasked region by interpolation | F0 = scatteredInterpolant(X,Y,M,… ’natural’,’none’); |
| 14 | Select a region unaffected by the crystal | |
| 15 | Fit linear functions to the fringes in the unaffected region; extrapolate to the rest of the unmasked area keeping the assigned number, see Figure A2g, dashed lines | polyfit(X,Y,1) |
| 16 | Generate the hypothetical unaffected phase shift field | F1 = scatteredInterpolant(X,Y,M,… ’natural’,’none’); |
| 17 | Calculate the concentration field (Figure A2h) using Equations (2) and (3) | |

References
- Farid, S.S.; Baron, M.; Stamatis, C.; Nie, W.; Coffman, J. Benchmarking biopharmaceutical process development and manufacturing cost contributions to R&D. MAbs 2020, 12, 1754999. [Google Scholar] [PubMed]
- Simoens, S.; Huys, I. R&D costs of new medicines: A landscape analysis. Front. Med. 2021, 8, 1891. [Google Scholar]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Domingos, S.; André, V.; Quaresma, S.; Martins, I.C.; Minas da Piedade, M.F.; Duarte, M.T. New forms of old drugs: Improving without changing. J. Pharm. Pharmacol. 2015, 67, 830–846. [Google Scholar] [CrossRef]
- Uddin, R.; Saffoon, N.; Sutradhar, K.B. Dissolution and dissolution apparatus: A review. Int. J. Curr. Biomed. Pharm. Res. 2011, 1, 201–207. [Google Scholar]
- Mann, J.; Dressman, J.; Rosenblatt, K.; Ashworth, L.; Muenster, U.; Frank, K.; Hutchins, P.; Williams, J.; Klumpp, L.; Wielockx, K.; et al. Validation of dissolution testing with biorelevant media: An OrBiTo study. Mol. Pharm. 2017, 14, 4192–4201. [Google Scholar] [CrossRef]
- Berben, P.; Ashworth, L.; Beato, S.; Bevernage, J.; Bruel, J.-L.; Butler, J.; Dressman, J.; Schäfer, K.; Hutchins, P.; Klumpp, L.; et al. Biorelevant dissolution testing of a weak base: Interlaboratory reproducibility and investigation of parameters controlling in vitro precipitation. Eur. J. Pharm. Biopharm. 2019, 140, 141–148. [Google Scholar] [CrossRef]
- Kulkarni, A.; Bachhav, R.; Hol, V.; Shete, S. Co-crystals of active pharmaceutical ingredient-ibuprofen lysine. Int. J. Appl. Pharm. 2020, 12, 22–32. [Google Scholar] [CrossRef]
- Ambasana, M.A.; Kapuriya, N.P.; Ambasana, P.A.; Bhalodia, J.J.; Bapodra, A.H. Biorelevant dissolution studies of platelet aggregation inhibitor: Ticagrelor hydrochloride and its co-crystal with sodium salt of Aspirin. Indian J. Chem. Technol. 2022, 29, 99–103. [Google Scholar] [CrossRef]
- Cammarn, S.R.; Sakr, A. Predicting dissolution via hydrodynamics: Salicylic acid tablets in flow through cell dissolution. Int. J. Pharm. 2000, 201, 199–209. [Google Scholar] [CrossRef]
- Emara, L.H.; Emam, M.F.; Taha, N.F.; El-ashmawy, A.A.; Mursi, N.M. In-vitro dissolution study of meloxicam immediate release products using flow through cell (USP apparatus 4) under different operational conditions. Int. J. Pharm. Pharm. Sci. 2014, 6, 254–260. [Google Scholar]
- Medina-López, J.R.; Sánchez-Badajos, S.; Carreto-Jiménez, R.M.; García-Hernández, P.; Contreras-Jiménez, J.M. Comparative in vitro dissolution studies of fixed-dose combination formulations: Analgesic tablets of acetaminophen/caffeine. Int. J. Res. Pharm. Sci. 2021, 12, 1063–1073. Available online: https://ijrps.com/index.php/home/article/view/79/ (accessed on 29 January 2023).
- Greiner, M.; Choscz, C.; Eder, C.; Elts, E.; Briesen, H. Multiscale modeling of aspirin dissolution: From molecular resolution to experimental scales of time and size. Crystengcomm 2016, 18, 5302–5312. [Google Scholar] [CrossRef]
- Shaw, L.R.; Irwin, W.J.; Grattan, T.J.; Conway, B.R. The effect of selected water-soluble excipients on the dissolution of paracetamol and ibuprofen. Drug Dev. Ind. Pharm. 2005, 31, 515–525. [Google Scholar] [CrossRef]
- Sugano, K. A simulation of oral absorption using classical nucleation theory. Int. J. Pharm. 2009, 378, 142–145. [Google Scholar] [CrossRef]
- Kostewicz, E.S.; Wunderlich, M.; Brauns, U.; Becker, R.; Bock, T.; Dressman, J.B. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J. Pharm. Pharmacol. 2004, 56, 43–51. [Google Scholar] [CrossRef]
- Elts, E.; Greiner, M.; Briesen, H. In Silico Prediction of Growth and Dissolution Rates for Organic Molecular Crystals: A Multiscale Approach. Crystals 2017, 7, 288. [Google Scholar] [CrossRef]
- Dogan, B.; Schneider, J.; Reuter, K. In silico dissolution rates of pharmaceutical ingredients. Chem. Phys. Lett. 2016, 662, 52–55. [Google Scholar] [CrossRef]
- Offiler, C.A.; Cruz-Cabeza, A.J.; Davey, R.J.; Vetter, T. Crystal Growth Cell Incorporating Automated Image Analysis Enabling Measurement of Facet Specific Crystal Growth Rates. Cryst. Growth Des. 2021, 22, 2837–2848. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; Grant, D.J. In situ optical interferometric studies of the growth and dissolution behavior of paracetamol (acetaminophen). 1. Growth kinetics. J. Phys. Chem. B 1997, 101, 3973–3979. [Google Scholar] [CrossRef]
- Goudarzi, N.M.; Samaro, A.; Vervaet, C.; Boone, M.N. Development of Flow-Through Cell Dissolution Method for In Situ Visualization of Dissolution Processes in Solid Dosage Forms Using X-ray μCT. Pharmaceutics 2022, 14, 2475. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, J.; Lenke, J.; Jensen, S.S.; Sun, Y.; Ye, F. UV imaging for in vitro dissolution and release studies: Initial experiences. Dissolution Technol. 2014, 21, 27–38. [Google Scholar] [CrossRef]
- Adobes-Vidal, M.; Shtukenberg, A.G.; Ward, M.D.; Unwin, P.R. Multiscale visualization and quantitative analysis of L-cystine crystal dissolution. Cryst. Growth Des. 2017, 17, 1766–1774. [Google Scholar] [CrossRef]
- Verma, S.; Shlichta, P.J. Imaging techniques for mapping solution parameters, growth rate, and surface features during the growth of crystals from solution. Prog. Cryst. Growth Charact. Mater. 2008, 54, 1–120. [Google Scholar] [CrossRef]
- Srivastava, A.; Muralidhar, K.; Panigrahi, P. Optical imaging and three dimensional reconstruction of the concentration field around a crystal growing from an aqueous solution: A review. Prog. Cryst. Growth Charact. Mater. 2012, 58, 209–278. [Google Scholar] [CrossRef]
- Eder, C.; Briesen, H. Interferometric Probing of Physical and Chemical Properties of Solutions: Noncontact Investigation of Liquids. Annu. Rev. Chem. Biomol. Eng. 2022, 13, 99–121. [Google Scholar] [CrossRef]
- Otálora, F.; Novella, M.L.; Gavira, J.A.; Thomas, B.R.; Ruiz, J.M.G. Experimental evidence for the stability of the depletion zone around a growing protein crystal under microgravity. Acta Crystallogr. Sect. D Biol. Crystallogr. 2001, 57, 412–417. [Google Scholar] [CrossRef]
- Yin, D.; Inatomi, Y.; Kuribayashi, K. Study of lysozyme crystal growth under a strong magnetic field using a Mach–Zehnder interferometer. J. Cryst. Growth 2001, 226, 534–542. [Google Scholar] [CrossRef]
- Zhao, J.; Miao, H.; Duan, L.; Kang, Q.; He, L. The mass transfer process and the growth rate of NaCl crystal growth by evaporation based on temporal phase evaluation. Opt. Lasers Eng. 2012, 50, 540–546. [Google Scholar] [CrossRef]
- Eder, C.; Choscz, C.; Müller, V.; Briesen, H. Jamin-interferometer-setup for the determination of concentration and temperature dependent face-specific crystal growth rates from a single experiment. J. Cryst. Growth 2015, 426, 255–264. [Google Scholar] [CrossRef]
- Adawy, A.; Marks, K.; de Grip, W.J.; van Enckevort, W.J.P.; Vlieg, E. The development of the depletion zone during ceiling crystallization: Phase shifting interferometry and simulation results. Crystengcomm 2013, 15, 2275–2286. [Google Scholar] [CrossRef]
- Hou, W.; Kudryavtsev, A.; Bray, T.; DeLucas, L.; Wilson, W. Real time evolution of concentration distribution around tetragonal lysozyme crystal: Case study in gel and free solution. J. Cryst. Growth 2001, 232, 265–272. [Google Scholar] [CrossRef]
- Fukui, K.; Nakajima, M. Diffusion processes near the crystal surface in growth and dissolution. J. Chem. Eng. Jpn. 1985, 18, 27–32. [Google Scholar] [CrossRef]
- Divya, H.; Reddy, G.R.; Sobhan, C.B.P. Digital Interferometric Measurement of Forced Convection Fields in Compact Channels. Int. J. Optomechatron. 2015, 9, 9–34. [Google Scholar] [CrossRef]
- Van Dam, J.; Mischgofsky, F. The application of a new, simple interference technique to the determination of growth concentration gradients of the layer perovskite NH3(CH2)3NH3CdCl4. J. Cryst. Growth 1987, 84, 539–551. [Google Scholar] [CrossRef]
- Han, G.; Chow, P.S.; Tan, R.B.H. Salt-dependent growth kinetics in glycine polymorphic crystallization. Crystengcomm 2016, 18, 462–470. [Google Scholar] [CrossRef]
- Marsh, R.E. A refinement of the crystal structure of glycine. Acta Crystallogr. 1958, 11, 654–663. [Google Scholar] [CrossRef]
- Kovacevic, T.; Reinhold, A.; Briesen, H. Identifying faceted crystal shape from three-dimensional tomography data. Cryst. Growth Des. 2014, 14, 1666–1675. [Google Scholar] [CrossRef]
- Nernst, W. Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Z. Phys. Chem. 1904, 47, 52–55. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Onuma, K.; Tsukamoto, K.; Sunagawa, I. Role of buoyancy driven convection in aqueous solution growth; A case study of Ba(NO3)2 crystal. J. Cryst. Growth 1988, 89, 177–188. [Google Scholar] [CrossRef]
- Han, G.; Chow, P.S.; Tan, R. Understanding the Salt-Dependent Outcome of Glycine Polymorphic Nucleation. Pharmaceutics 2021, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, S.K. A Design Procedure and Predictive Models for Solution Crystallisation Processes; Delft University of Technology, TU Delft: Delft, The Netherlands, 2003. [Google Scholar]
- Bovington, C.H.; Jones, A.L. Tracer study of the kinetics of dissolution of barium sulphate. Trans. Faraday Soc. 1970, 66, 764–768. [Google Scholar] [CrossRef]
- Mullin, J.W.; Garside, J. Crystallization of aluminium potassium sulphate: A study in the assessment of crystallizer design data. Part I: Single crystal growth rates. Trans. Inst. Chem. Eng. 1967, 45, T285–T290. [Google Scholar]
- Bomio, P.; Bourne, J.; Davey, R. The growth and dissolution of hexamethylene tetramine in aqueous solution. J. Cryst. Growth 1975, 30, 77–85. [Google Scholar] [CrossRef]
- Pohar, A.; Likozar, B. Dissolution, nucleation, crystal growth, crystal aggregation, and particle breakage of amlodipine salts: Modeling crystallization kinetics and thermodynamic equilibrium, scale-up, and optimization. Ind. Eng. Chem. Res. 2014, 53, 10762–10774. [Google Scholar] [CrossRef]
- Ninni, L.; Meirelles, A.J.A. Water activity, pH and density of aqueous amino acids solutions. Biotechnol. Prog. 2001, 17, 703–711. [Google Scholar] [CrossRef]
- Devine, W.; Lowe, B.M. Viscosity B-coefficients at 15 and 25 °C for glycine, β-alanine, 4-amino-n-butyric acid, and 6-amino-n-hexanoic acid in aqueous solution. J. Chem. Soc. A Inorg. Phys. Theor. 1971, 2113–2116. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, C.; Ma, P.; Yu, K.T. Studies on the diffusion coefficients of amino acids in aqueous solutions. J. Chem. Eng. Data 2005, 50, 1192–1196. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eder, C.; Schiele, S.A.; Luxenburger, F.; Briesen, H. Glycine Dissolution Behavior under Forced Convection. Crystals 2023, 13, 315. https://doi.org/10.3390/cryst13020315
Eder C, Schiele SA, Luxenburger F, Briesen H. Glycine Dissolution Behavior under Forced Convection. Crystals. 2023; 13(2):315. https://doi.org/10.3390/cryst13020315
Chicago/Turabian StyleEder, Cornelia, Simon A. Schiele, Frederik Luxenburger, and Heiko Briesen. 2023. "Glycine Dissolution Behavior under Forced Convection" Crystals 13, no. 2: 315. https://doi.org/10.3390/cryst13020315
APA StyleEder, C., Schiele, S. A., Luxenburger, F., & Briesen, H. (2023). Glycine Dissolution Behavior under Forced Convection. Crystals, 13(2), 315. https://doi.org/10.3390/cryst13020315





