Successive Photocatalytic Degradation of Methylene Blue by ZnO, CuO and ZnO/CuO Synthesized from Coriandrum sativum Plant Extract via Green Synthesis Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO
2.3. Synthesis of CuO
2.4. Synthesis of ZnO/CuO Nanocomposite
2.5. Photocatalytic Activity
2.6. Characterization of ZnO, CuO and ZnO/CuO Nanocomposite
3. Results and Discussion
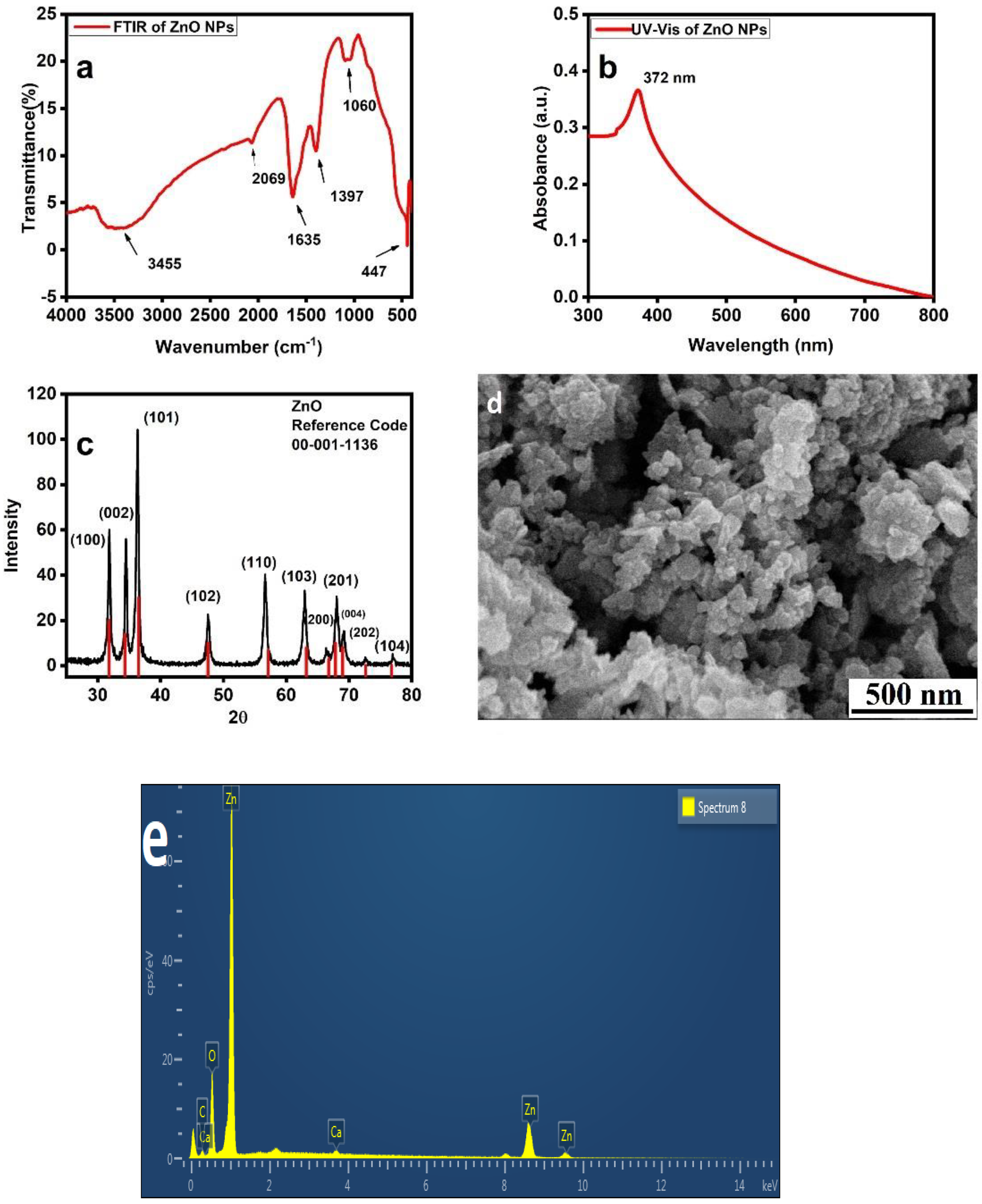
3.1. ZnO NPs
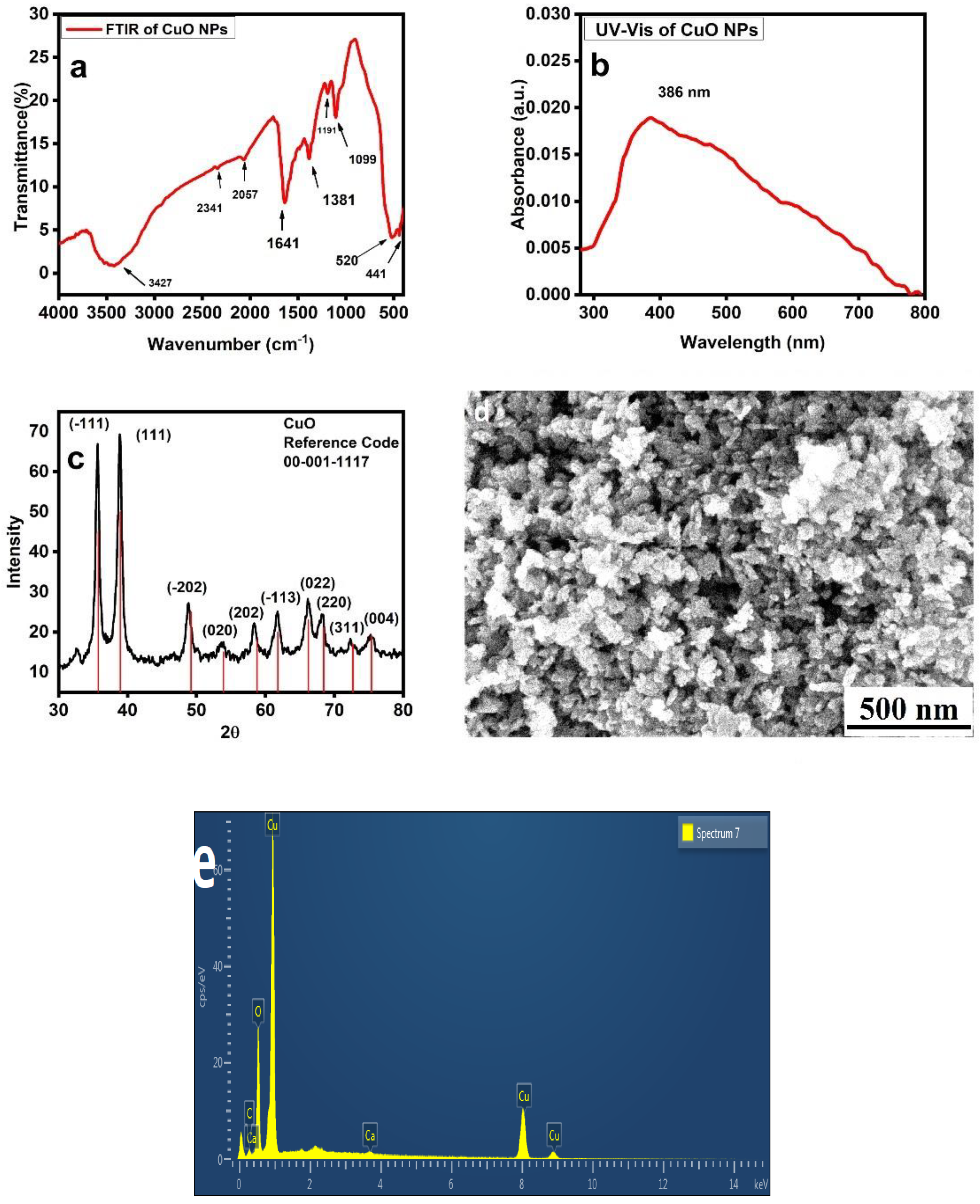
3.2. CuO NPs
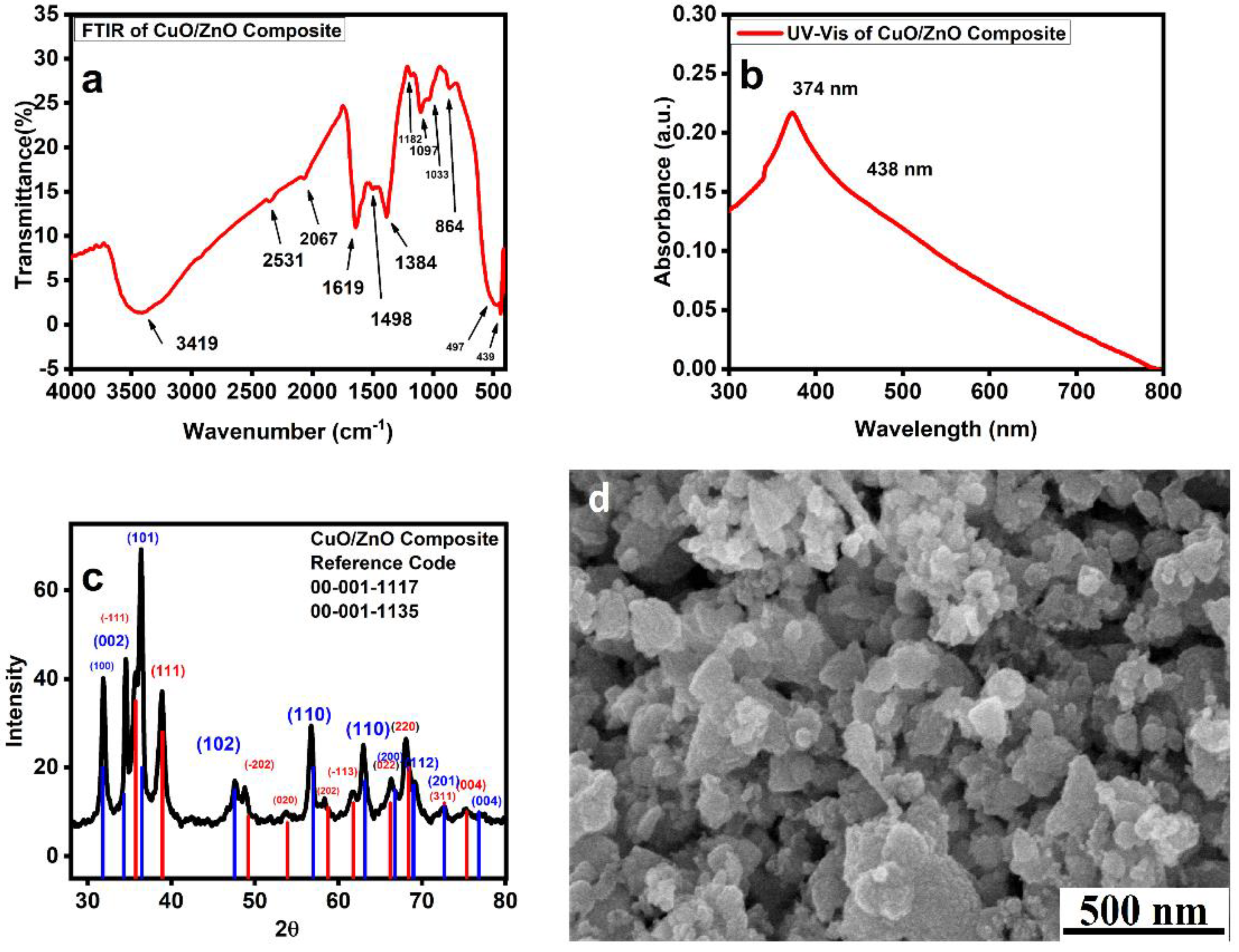
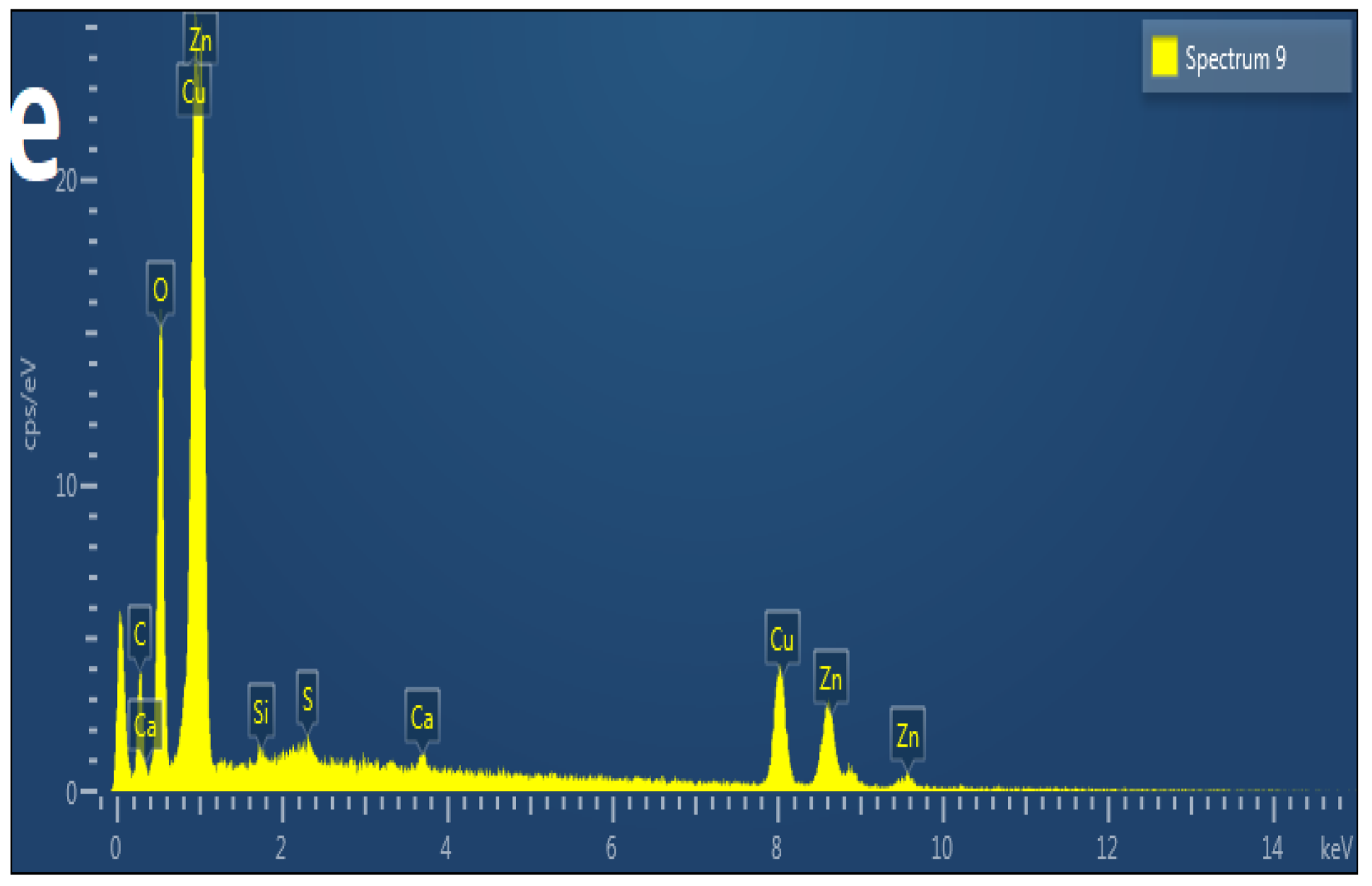
3.3. ZnO/CuO Nanocomposites
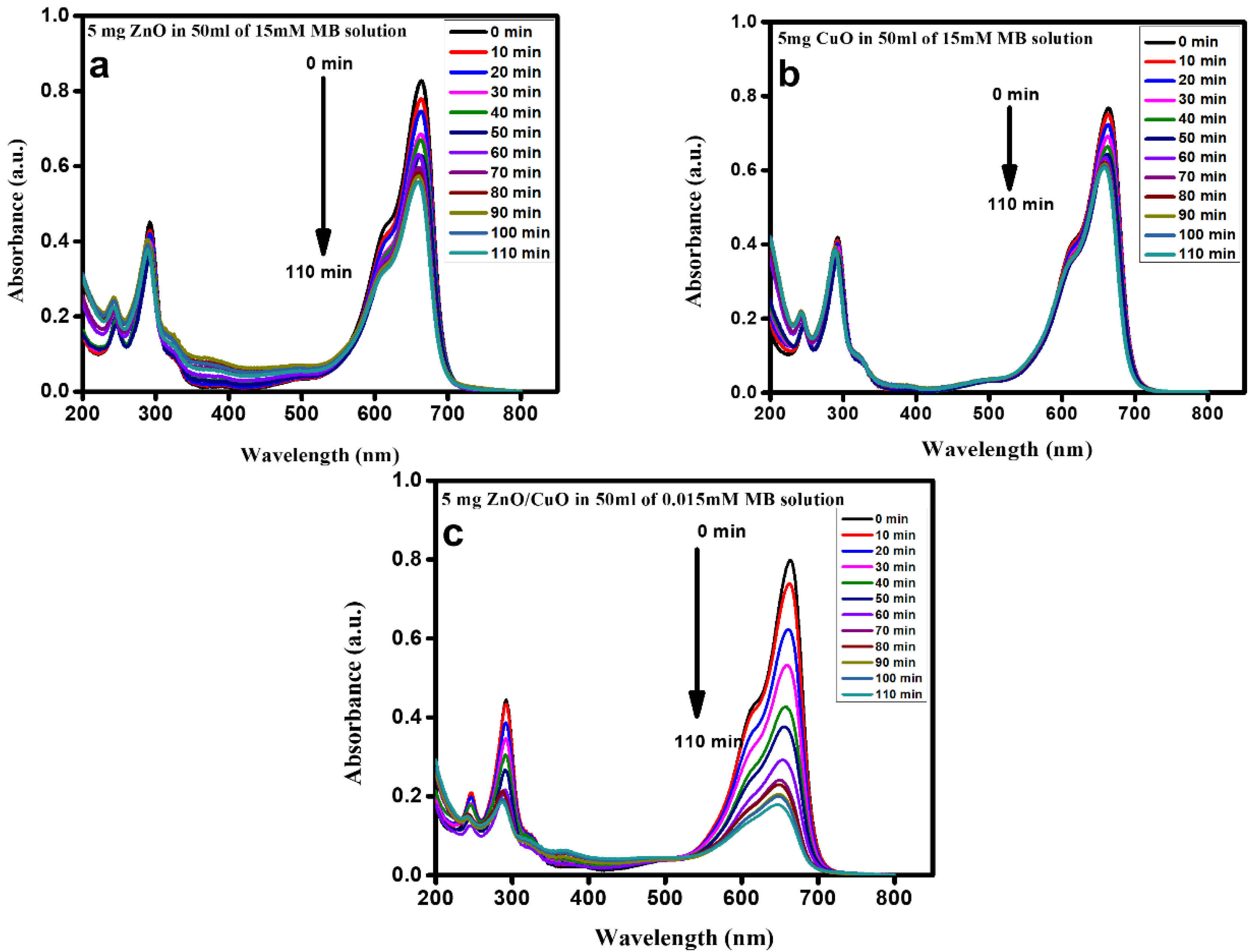
3.4. Photocatalytic Activity
3.5. Comparison of Photocatalytic Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palmero, P. Structural ceramic nanocomposites: A review of properties and powders’ synthesis methods. Nanomaterials 2015, 5, 656–696. [Google Scholar] [CrossRef]
- Su, C. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef]
- Mohammadi-Aloucheh, R.; Habibi-Yangjeh, A.; Bayrami, A.; Latifi-Navid, S.; Asadi, A. Green synthesis of ZnO and ZnO/CuO nanocomposites in Mentha longifolia leaf extract: Characterization and their application as anti-bacterial agents. J. Mater. Sci. Mater. Electron. 2018, 29, 13596–13605. [Google Scholar] [CrossRef]
- Vibitha, B.V.; Anitha, B.; Tharayil, N.J. Green synthesis of ZnO: CuO nanocomposites by Aloe Barbadansis leaf extract: Structure and photo catalytic properties. In Proceedings of the AIP Conference Proceedings, AIP Publishing LLC, 2020; p. 020031. [Google Scholar]
- Parashar, U.K.; Saxena, P.S.; Srivastava, A. Bioinspired synthesis of silver nanoparticles. Dig. J. Nanomater. Biostructures (DJNB) 2009, 4, 159–166. [Google Scholar]
- Khalid, A.; Ahmad, P.; Khan, A.; Muhammad, S.; Khandaker, M.U.; Alam, M.; Asim, M.; Din, I.U.; Chaudhary, R.G.; Kumar, D.J.B.C.; et al. Effect of Cu Doping on ZnO Nanoparticles as a Photocatalyst for the Removal of Organic Wastewater. Bioinorg. Chem. Appl. 2022, 2022, 9459886. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.A.; Mondal, S.; Basu, S.; Laskar, R.A.; Mandal, D. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of Black Tea leaf extracts. Colloids Surf. B Biointerfaces 2009, 71, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Wang, X.; Hanson, J.C.; Liu, G.; Iglesias-Juez, A.; Fernández-Garcı́a, M. The behavior of mixed-metal oxides: Structural and electronic properties of Ce1−xCaxO2 and Ce1−xCaxO2−x. J. Chem. Phys. 2003, 119, 5659–5669. [Google Scholar] [CrossRef]
- Ahmed, S.; Jiang, X.; Wang, C.; Kalsoom, U.e.; Wang, B.; Khan, J.; Muhammad, Y.; Duan, Y.; Zhu, H.; Ren, X.; et al. An Insightful Picture of Nonlinear Photonics in 2D Materials and their Applications: Recent Advances and Future Prospects. Adv. Opt. Mater. 2021, 9, 2001671. [Google Scholar] [CrossRef]
- Abbasi, Z.; Saeed, W.; Shah, S.M.; Shahzad, S.A.; Bilal, M.; Khan, A.F.; Shaikh, A.J. Binding efficiency of functional groups towards noble metal surfaces using graphene oxide–metal nanoparticle hybrids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125858. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, L.; Li, Y.; Tang, Y.; Xie, C. Structural and photoelectrocatalytic characteristic of ZnO/ZnWO4/WO3 nanocomposites with double heterojunctions. Phys. E: Low-Dimens. Syst. Nanostructures 2010, 43, 503–509. [Google Scholar] [CrossRef]
- Arandiyan, H.; Parvari, M. Studies on mixed metal oxides solid solutions as heterogeneous catalysts. Braz. J. Chem. Eng. 2009, 26, 63–74. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloy. Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Hafeez, M.; Arshad, R.; Khan, J.; Akram, B.; Ahmad, M.N.; Hameed, M.U.; Haq, S. Populus ciliata mediated synthesis of copper oxide nanoparticles for potential biological applications. Mater. Res. Express 2019, 6, 055043. [Google Scholar] [CrossRef]
- Ahmad, K.; Khan, B.A.; Akram, B.; Khan, J.; Mahmood, R.; Roy, S.K. Theoretical investigations on copper catalyzed C N cross-coupling reaction between aryl chlorides and amines. Comput. Theor. Chem. 2018, 1134, 1–7. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.; Singh, A.; Singh, H.; Singh, S. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, G.; Rawat, M. A brief review on synthesis and characterization of copper oxide nanoparticles and its applications. J. Bioelectron. Nanotechnol. 2016, 1, 9. [Google Scholar]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-based green synthesis of metallic nanoparticles: Scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol. Environ. Eng. 2016, 1, 1–29. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Boro, B.; Gogoi, B.; Rajbongshi, B.; Ramchiary, A. Nano-structured TiO2/ZnO nanocomposite for dye-sensitized solar cells application: A review. Renew. Sustain. Energy Rev. 2018, 81, 2264–2270. [Google Scholar] [CrossRef]
- Sundar, L.S.; Sharma, K.; Singh, M.K.; Sousa, A. Hybrid nanofluids preparation, thermal properties, heat transfer and friction factor–a review. Renew. Sustain. Energy Rev. 2017, 68, 185–198. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Muhammad, S.; Khan, A.; Khandaker, M.U.; Alam, M.M.; Asim, M.; Din, I.U.; Iqbal, J.; Rehman, I.U. Synthesis of Boron-Doped Zinc Oxide Nanosheets by Using Phyllanthus Emblica Leaf Extract: A Sustainable Environmental Applications. Front. Chem. 2022, 10, 930620. [Google Scholar] [CrossRef]
- Saeed, W.; Abbasi, Z.; Bilal, M.; Shah, S.H.; Waseem, A.; Shaikh, A.J. Interactive behavior of graphene quantum dots towards noble metal surfaces. Phys. E Low-Dimens. Syst. Nanostructures 2023, 147, 115596. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.K.; Gawande, M.B.; Zboril, R.; Varma, R.S. Microwave-assisted synthesis–catalytic applications in aqueous media. Coord. Chem. Rev. 2015, 291, 68–94. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Khan, J.; Ilyas, S.; Akram, B.; Ahmad, K.; Hafeez, M.; Siddiq, M.; Ashraf, M.A. Zno/NiO coated multi-walled carbon nanotubes for textile dyes degradation. Arab. J. Chem. 2018, 11, 880–896. [Google Scholar] [CrossRef]
- Khan, J.; Siddiq, M.; Akram, B.; Ashraf, M.A. In-situ synthesis of CuO nanoparticles in P(NIPAM-co-AAA) microgel, structural characterization, catalytic and biological applications. Arab. J. Chem. 2018, 11, 897–909. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, P.; Mukhopadhyay, A.; Savitha, G.; Moorthy, J.N. Remarkably selective and enantiodifferentiating sensing of histidine by a fluorescent homochiral Zn-MOF based on pyrene-tetralactic acid. Chem Sci 2016, 7, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Dong, N.; Zhang, M.; Zhu, K.; Na, R.; Zhang, S.; Sun, N.; Wang, G.; Wang, J. Covalent functionalization of graphene oxide with porphyrin and porphyrin incorporated polymers for optical limiting. Phys. Chem. Chem. Phys. 2017, 19, 2252–2260. [Google Scholar] [CrossRef]
- Akram, B.; Ahmad, K.; Khan, J.; Khan, B.A.; Akhtar, J. Low-temperature solution-phase route to sub-10 nm titanium oxide nanocrystals having super-enhanced photoreactivity. New J. Chem. 2018, 42, 10947–10952. [Google Scholar] [CrossRef]
- Lange, B.M.; Croteau, R. Genetic engineering of essential oil production in mint. Curr. Opin. Plant Biol. 1999, 2, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Andro, A.R.; Atofani, D.; Boz, I.; Zamfirache, M.; Burzo, I.; Toma, C. Studies concerning the histo-anatomy and biochemistry of Mentha longifolia (L.) Huds. during vegetative phenophase. An. Stiintifice Ale Univ. Al. I. Cuza Din Iasi 2011, 57, 25. [Google Scholar]
- Ertaş, A.; Gören, A.C.; Haşimi, N.; Tolan, V.; Kolak, U. Evaluation of Antioxidant, Cholinesterase Inhibitory and Antimicrobial Properties of Mentha longifolia subsp. noeana and Its Secondary Metabolites. Rec. Nat. Prod. 2015, 9, 105–115. [Google Scholar]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Faruque, M.R.I.; Khan, A.; Din, I.U.; Alotaibi, M.A.; Alzimami, K. Enhanced Optical and Antibacterial Activity of Hydrothermally Synthesized Cobalt-Doped Zinc Oxide Cylindrical Microcrystals. Materials 2021, 14, 3223. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Rehman, M.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A.; Alzimami, K. Structural, Optical and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide against Pathogenic Bacteria. Nanomaterials 2021, 11, 451. [Google Scholar] [CrossRef]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef]
- Han, X.; Yang, S.; Schröder, M. Porous metal–organic frameworks as emerging sorbents for clean air. Nat. Rev. Chem. 2019, 3, 108–118. [Google Scholar] [CrossRef]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale Metal Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef]
- Saeed, W.; Abbasi, Z.; Majeed, S.; Shahzad, S.A.; Khan, A.F.; Shaikh, A.J. An insight into the binding behavior of graphene oxide and noble metal nanoparticles. J. Appl. Phys. 2021, 129, 125302. [Google Scholar] [CrossRef]
- Razzaq, Z.; Khalid, A.; Ahmad, P.; Farooq, M.; Khandaker, M.U.; Sulieman, A.; Rehman, I.U.; Shakeel, S.; Khan, A. Photocatalytic and Antibacterial Potency of Titanium Dioxide Nanoparticles: A Cost-Effective and Environmentally Friendly Media for Treatment of Air and Wastewater. Catalysts 2021, 11, 709. [Google Scholar] [CrossRef]
- Mehta, S.S.; Nadargi, D.Y.; Tamboli, M.S.; Alshahrani, T.; Minnam Reddy, V.R.; Kim, E.S.; Mulla, I.S.; Park, C.; Suryavanshi, S.S. RGO/WO3 hierarchical architectures for improved H2S sensing and highly efficient solar-driving photo-degradation of RhB dye. Sci. Rep. 2021, 11, 5023. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Prakash, J.; Misra, M.; Sharma, A.; Gupta, R.K. Dual Functional Ta-Doped Electrospun TiO2 Nanofibers with Enhanced Photocatalysis and SERS Detection for Organic Compounds. ACS Appl. Mater. Interfaces 2017, 9, 28495–28507. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Singh, N.; Kar, P.; Tyagi, A.; Prakash, J.; Gupta, R.K. Engineering of transition metal dichalcogenide-based 2D nanomaterials through doping for environmental applications. Mol. Syst. Des. Eng. 2019, 4, 804–827. [Google Scholar] [CrossRef]
- Kar, P.; Jain, P.; Kumar, V.; Gupta, R.K. Interfacial engineering of Fe2O3@BOC heterojunction for efficient detoxification of toxic metal and dye under visible light illumination. J. Environ. Chem. Eng. 2019, 7, 102843. [Google Scholar] [CrossRef]
- Kar, P.; Shukla, K.; Jain, P.; Sathiyan, G.; Gupta, R.K. Semiconductor based photocatalysts for detoxification of emerging pharmaceutical pollutants from aquatic systems: A critical review. Nano Mater. Sci. 2021, 3, 25–46. [Google Scholar] [CrossRef]
- Hafeez, M.; Afyaz, S.; Khalid, A.; Ahmad, P.; Khandaker, M.U.; Sahibzada, M.U.K.; Ahmad, I.; Khan, J.; Alhumaydhi, F.A.; Emran, T.B. Synthesis of cobalt and sulphur doped titanium dioxide photocatalysts for environmental applications. J. King Saud Univ.-Sci. 2022, 34, 102028. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Khan, A.; Khandaker, M.U.; Kebaili, I.; Alam, M.M.; Din, I.U.; Muhammad, S.; Razzaq, Z.; Rehman, I.U.; et al. Cytotoxic and Photocatalytic Studies of Hexagonal Boron Nitride Nanotubes: A potential Candidate for Wastewater and Air Treatment. RSC Adv. 2022, 12, 6592–6600. [Google Scholar] [CrossRef]
- Vanathi, P.; Rajiv, P.; Narendhran, S.; Rajeshwari, S.; Rahman, P.K.; Venckatesh, R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater. Lett. 2014, 134, 13–15. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, M.; Mafina, M.-K.; Belousova, O.; Vakin, N.; Shchipakin, S.Y.; Morozov, I.G. Catalytically active magnetic nanoparticles in the Cu-O system. Inorg. Mater. 2015, 51, 307–318. [Google Scholar] [CrossRef]
- Padalia, H.; Baluja, S.; Chanda, S. Effect of pH on size and antibacterial activity of Salvadora oleoides leaf extract-mediated synthesis of zinc oxide nanoparticles. Bionanoscience 2017, 7, 40–49. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef]
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 2015, 9, 7–12. [Google Scholar] [CrossRef]
- Patle, S.; Kumar, P. Decision making approach to prefer route repair technique in AODV routing protocol of MANET. Int. J. Res. Eng. Technol 2015, 4, 9–16. [Google Scholar]
- Chen, C.; Yu, B.; Liu, P.; Liu, J.; Wang, L. Investigation of nano-sized ZnO particles fabricated by various synthesis routes. J. Ceram. Process. Res. 2011, 12, 420–425. [Google Scholar]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical Analysis by X-Ray Diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Widiarti, N.; Sae, J.; Wahyuni, S. Synthesis CuO-ZnO nanocomposite and its application as an antibacterial agent. In Proceedings of the IOP Conference Series: Materials Science and Engineering, 2017; IOP Publishing: Bristol, UK, 2017; p. 012036. [Google Scholar]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B Biointerfaces 2013, 101, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Gawade, V.; Gavade, N.; Shinde, H.; Babar, S.; Kadam, A.; Garadkar, K. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017, 28, 14033–14039. [Google Scholar] [CrossRef]
- Barzegar, M.; Habibi-Yangjeh, A.; Behboudnia, M. Ultrasonic-assisted preparation and characterization of CdS nanoparticles in the presence of a halide-free and low-cost ionic liquid and photocatalytic activity. J. Phys. Chem. Solids 2010, 71, 1393–1397. [Google Scholar] [CrossRef]
- Dai, D.; Wang, L.; Xiao, N.; Li, S.; Xu, H.; Liu, S.; Xu, B.; Lv, D.; Gao, Y.; Song, W.; et al. In-situ synthesis of Ni2P co-catalyst decorated Zn0.5Cd0.5S nanorods for high-quantum-yield photocatalytic hydrogen production under visible light irradiation. Appl. Catal. B Environ. 2018, 233, 194–201. [Google Scholar] [CrossRef]
- Wali, L.A.; Alwan, A.M.; Dheyab, A.B.; Hashim, D.A. Excellent fabrication of Pd-Ag NPs/PSi photocatalyst based on bimetallic nanoparticles for improving methylene blue photocatalytic degradation. Optik 2019, 179, 708–717. [Google Scholar] [CrossRef]
- Ravichandran, K.; Mohan, R.; Sakthivel, B.; Varadharajaperumal, S.; Devendran, P.; Alagesan, T.; Pandian, K. Enhancing the photocatalytic efficiency of sprayed ZnO thin films through double doping (Sn+F) and annealing under different ambiences. Appl. Surf. Sci. 2014, 321, 310–317. [Google Scholar] [CrossRef]
- Wu, X.; Wen, L.; Lv, K.; Deng, K.; Tang, D.; Ye, H.; Du, D.; Liu, S.; Li, M. Fabrication of ZnO/graphene flake-like photocatalyst with enhanced photoreactivity. Appl. Surf. Sci. 2015, 358, 130–136. [Google Scholar] [CrossRef]
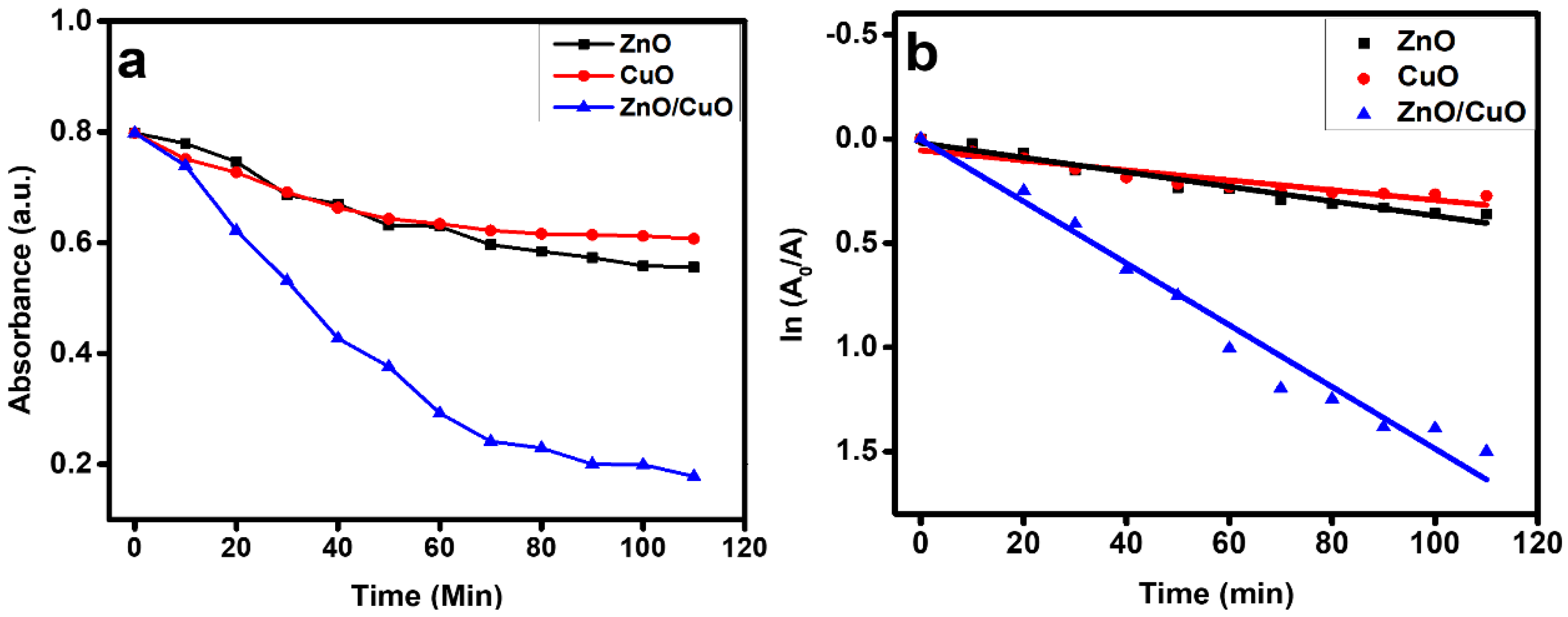
| Sr. No | Type of Catalyst | Rate Constant (k) min−1 | R2 |
|---|---|---|---|
| 1 | ZnO/CuO | 0.0148 | 0.9749 |
| 2 | CuO | 0.0025 | 0.9568 |
| 3 | ZnO | 0.0036 | 0.9696 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basit, R.A.; Abbasi, Z.; Hafeez, M.; Ahmad, P.; Khan, J.; Khandaker, M.U.; Al-Mugren, K.S.; Khalid, A. Successive Photocatalytic Degradation of Methylene Blue by ZnO, CuO and ZnO/CuO Synthesized from Coriandrum sativum Plant Extract via Green Synthesis Technique. Crystals 2023, 13, 281. https://doi.org/10.3390/cryst13020281
Basit RA, Abbasi Z, Hafeez M, Ahmad P, Khan J, Khandaker MU, Al-Mugren KS, Khalid A. Successive Photocatalytic Degradation of Methylene Blue by ZnO, CuO and ZnO/CuO Synthesized from Coriandrum sativum Plant Extract via Green Synthesis Technique. Crystals. 2023; 13(2):281. https://doi.org/10.3390/cryst13020281
Chicago/Turabian StyleBasit, Raja Abdul, Zeeshan Abbasi, Muhammad Hafeez, Pervaiz Ahmad, Jahanzeb Khan, Mayeen Uddin Khandaker, Kholoud Saad Al-Mugren, and Awais Khalid. 2023. "Successive Photocatalytic Degradation of Methylene Blue by ZnO, CuO and ZnO/CuO Synthesized from Coriandrum sativum Plant Extract via Green Synthesis Technique" Crystals 13, no. 2: 281. https://doi.org/10.3390/cryst13020281
APA StyleBasit, R. A., Abbasi, Z., Hafeez, M., Ahmad, P., Khan, J., Khandaker, M. U., Al-Mugren, K. S., & Khalid, A. (2023). Successive Photocatalytic Degradation of Methylene Blue by ZnO, CuO and ZnO/CuO Synthesized from Coriandrum sativum Plant Extract via Green Synthesis Technique. Crystals, 13(2), 281. https://doi.org/10.3390/cryst13020281









