Quantum Chemical Studies on the Adsorption of Hexachlorobenzene, Decachlorobiphenyl, Benzene, and Biphenyl by BN-Doped Graphene and C-Doped Hexagonal Boron Nitride Modified with β-Cyclodextrin
Abstract
1. Introduction
2. Computational Details
3. Results and Discussions
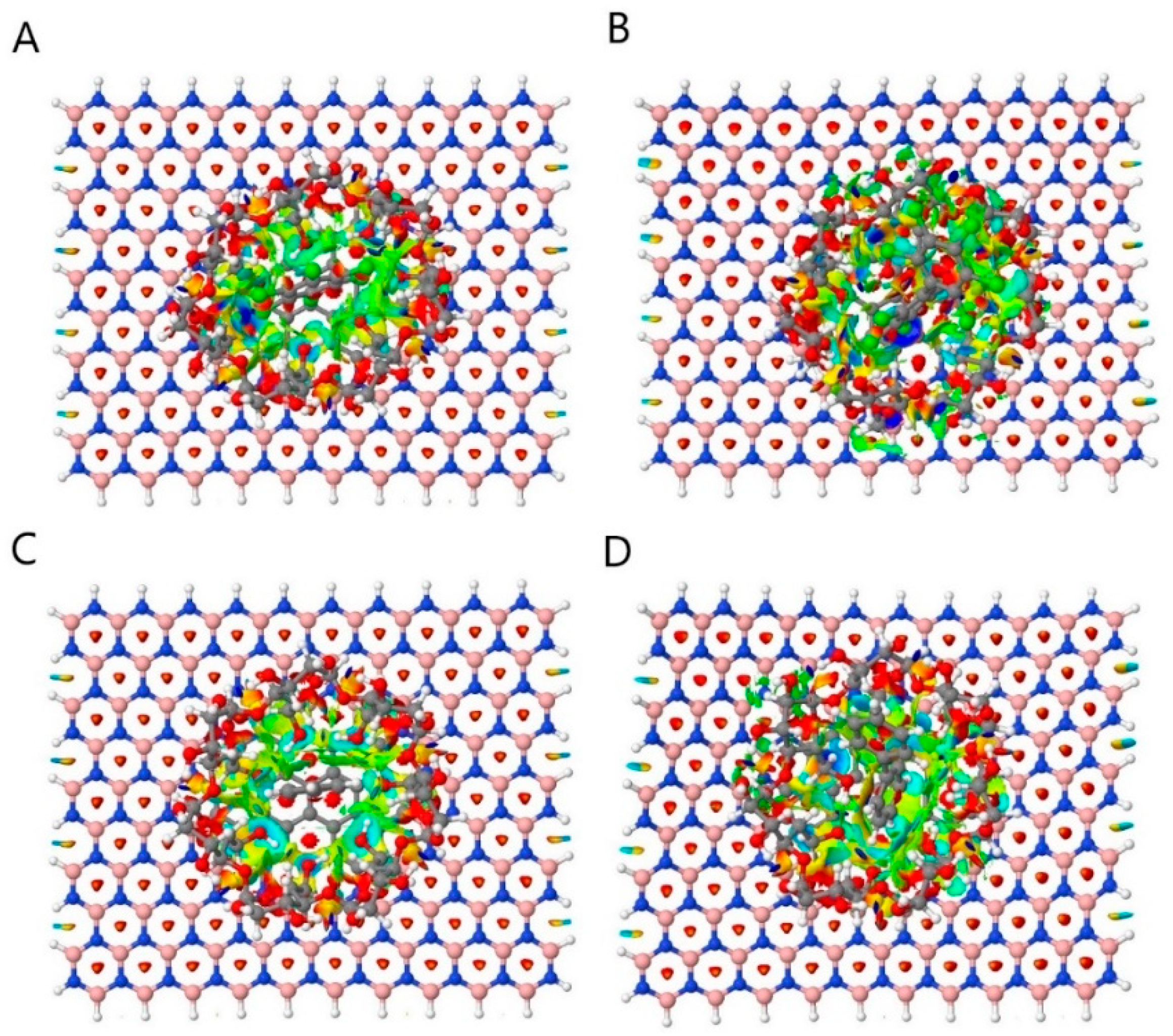
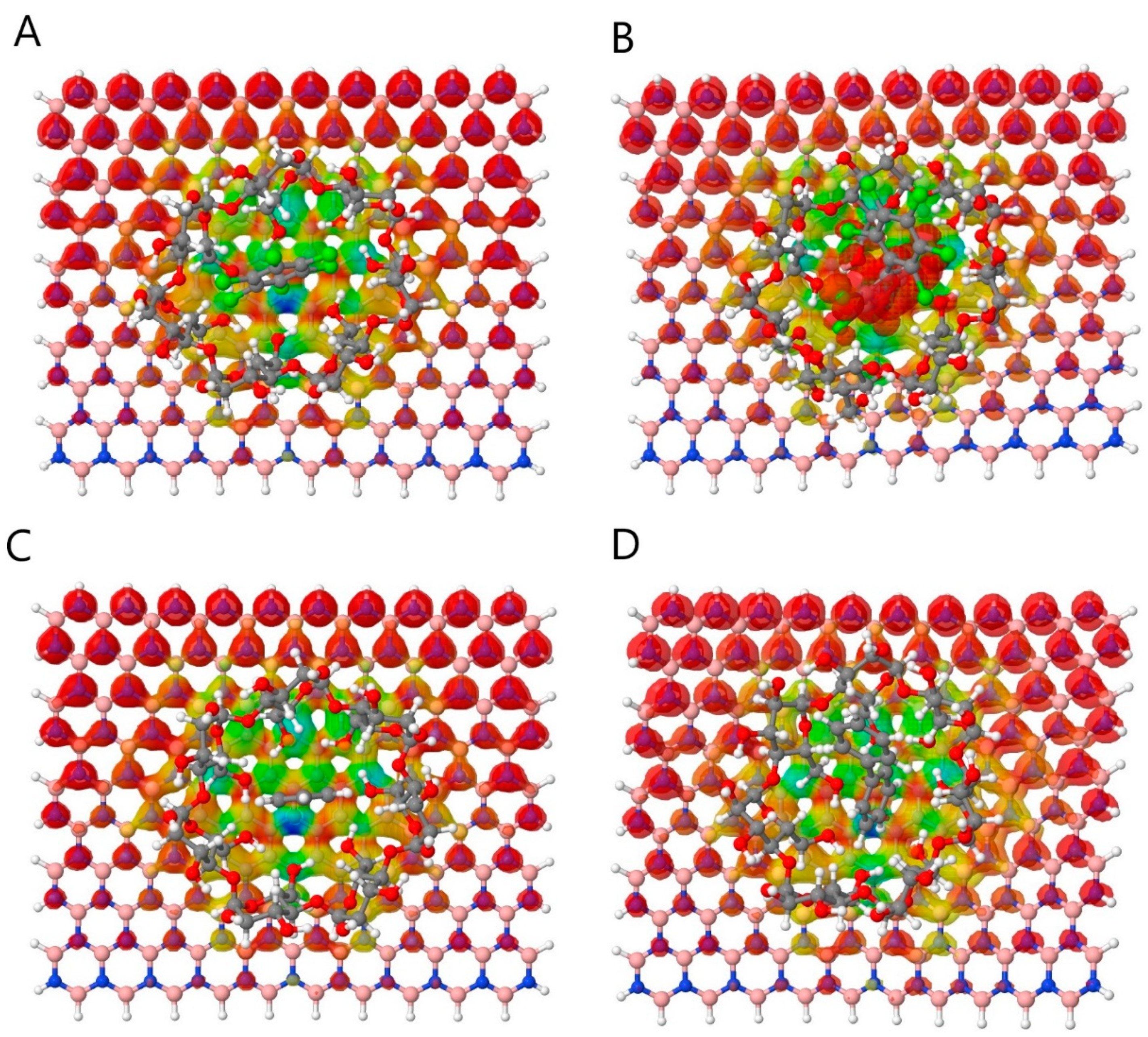
3.1. Non-Covalent Interaction (NCI)
3.2. Equilibrium Structure and Reaction Enthalpy
3.3. Molecular Electrostatic Potential
3.4. Frontier Molecular Orbital Electrophilic Susceptibility
3.5. Hard–Soft Acid-Base (HSAB) Reactivity Descriptor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Zhang, D.; Zhan, J. Investigation on the inclusions of PCB52 with cyclodextrins by performing DFT calculations and molecular dynamics simulations. J. Phys. Chem. A 2010, 114, 13122–13128. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Melville, C.C.; Della Vechia, J.F.; de Andrade, D.J.; Fraceto, L.F. Chitosan nanoparticles functionalized with β-cyclodextrin: A promising carrier for botanical pesticides. Sci. Rep. 2018, 8, 2067. [Google Scholar] [CrossRef] [PubMed]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-Y.; Shen, M.-X.; Yang, A.-W.; Liang, C.-Q.; Wang, N.; Cao, G.-P. Introduction of β-cyclodextrin into poly(aspartic acid) matrix for adsorption and time-release of ibuprofen. Chem. Commun. 2011, 47, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675. [Google Scholar] [CrossRef]
- Song, W.; Shao, D.; Lu, S.; Wang, X. Simultaneous removal of uranium and humic acid by cyclodextrin modified graphene oxide nanosheets. Sci. China Chem. 2014, 57, 1291–1299. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; El-Aassar, M.R.; El Fawal, G.F.; Soliman, H.M.A. Fabrication of polyacrylonitrile/β-cyclodextrin/graphene oxide nanofibers composite as an efficient adsorbent for cationic dye. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100207. [Google Scholar] [CrossRef]
- Lee, J.-u.; Lee, S.-S.; Lee, S.; Oh, H.B. Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase. Molecules 2020, 25, 4048. [Google Scholar] [CrossRef]
- Shen, C.; Yang, X.; Wang, Y.; Zhou, J.; Chen, C. Complexation of capsaicin with β-cyclodextrins to improve pesticide formulations: Effect on aqueous solubility, dissolution rate, stability and soil adsorption. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 263–274. [Google Scholar] [CrossRef]
- Cruickshank, D.; Rougier, N.M.; Vico, R.V.; Rossi, R.H.d.; Buján, E.I.; Bourne, S.A.; Caira, M.R. Solid-state structures and thermal properties of inclusion complexes of the organophosphate insecticide fenitrothion with permethylated cyclodextrins. Carbohydr. Res. 2010, 345, 141–147. [Google Scholar] [CrossRef]
- Cruickshank, D.; Rougier, N.; Raquel, V.; Bourne, S.; Buján, E.; Caira, M.; de Rossi, R. Inclusion of the insecticide fenitrothion in dimethylated-β-cyclodextrin: Unusual guest disorder in the solid state and efficient retardation of the hydrolysis rate of the complexed guest in alkaline solution. Beilstein J. Org. Chem. 2013, 9, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, L.; Wang, D.; Tang, C.; Deng, W.; Wang, Z. High-Capacity Amidoxime-Functionalized β-Cyclodextrin/Graphene Aerogel for Selective Uranium Capture. Environ. Sci. Technol. 2021, 55, 9181–9188. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cai, X.; Wang, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Sadegh, H.; Ali, G.A.M.; Bharti, A.K.; Hamdy Makhlouf, A.S. Facile route synthesis of novel graphene oxide-β-cyclodextrin nanocomposite and its application as adsorbent for removal of toxic bisphenol A from the aqueous phase. J. Mol. Liq. 2017, 237, 466–472. [Google Scholar] [CrossRef]
- Liu, X.; Yan, L.; Yin, W.; Zhou, L.; Tian, G.; Shi, J.; Yang, Z.; Xiao, D.; Gu, Z.; Zhao, Y. A magnetic graphene hybrid functionalized with beta-cyclodextrins for fast and efficient removal of organic dyes. J. Mater. Chem. A 2014, 2, 12296–12303. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Qiu, H.; Li, X. Synthesis of magnetic β-cyclodextrin–chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surf. B Biointerfaces 2013, 103, 601–607. [Google Scholar] [CrossRef]
- Li, L.; Fan, L.; Duan, H.; Wang, X.; Luo, C. Magnetically separable functionalized graphene oxide decorated with magnetic cyclodextrin as an excellent adsorbent for dye removal. RSC Adv. 2014, 4, 37114–37121. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Designing a novel targeted-release nano-container based on the silanized graphene oxide decorated with cerium acetylacetonate loaded beta-cyclodextrin (β-CD-CeA-MGO) for epoxy anti-corrosion coating. Chem. Eng. J. 2020, 400, 125860. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Synthesis of a non-hazardous/smart anti-corrosion nano-carrier based on beta-cyclodextrin-zinc acetylacetonate inclusion complex decorated graphene oxide (β-CD-ZnA-MGO). J. Hazard. Mater. 2020, 398, 122962. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: Properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef]
- Amieva, E.J.; Lópeš Barroso, J.; Martínez-Hernández, A.; Velasco-Santos, C. Graphene-based materials functionalization with natural polymeric biomolecules. Recent Adv. Graphene Res. 2016, 1, 257–298. [Google Scholar]
- Salipira, K.; Mamba, B.; Krause, R.; Malefetse, T.; Durbach, S. Carbon nanotubes and cyclodextrin polymers for removing organic pollutants from water. Environ. Chem. Lett. 2007, 5, 13–17. [Google Scholar] [CrossRef]
- Xie, F.; Yang, M.; Jiang, M.; Huang, X.-J.; Liu, W.-Q.; Xie, P.-H. Carbon-based nanomaterials—A promising electrochemical sensor toward persistent toxic substance. TrAC Trends Anal. Chem. 2019, 119, 115624. [Google Scholar] [CrossRef]
- Ding, Y.; Song, X.; Chen, J. Analysis of Pesticide Residue in Tomatoes by Carbon Nanotubes/β-Cyclodextrin Nanocomposite Reinforced Hollow Fiber Coupled with HPLC. J. Food Sci. 2019, 84, 1651–1659. [Google Scholar] [CrossRef]
- Rahemi, V.; Vandamme, J.; Garrido, J.; Borges, F.; Brett, C.; Garrido, E. Enhanced host–guest electrochemical recognition of herbicide MCPA using a β-cyclodextrin carbon nanotube sensor. Talanta 2012, 99, 288–293. [Google Scholar] [CrossRef]
- Rathour, R.K.S.; Bhattacharya, J.; Mukherjee, A. β-Cyclodextrin conjugated graphene oxide: A regenerative adsorbent for cadmium and methylene blue. J. Mol. Liq. 2019, 282, 606–616. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Chen, M.; Jing, Z.; Qiu, F. β-Cyclodextrin–graphene oxide–diatomaceous earth material: Preparation and its application for adsorption of organic dye. Mon. Chem.-Chem. Mon. 2018, 149, 1367–1377. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Liu, X.; Wu, J.; Zhu, X.; Bai, Z.; Yu, Z. Preparation of β-cyclodextrin/graphene oxide and its adsorption properties for methylene blue. Colloids Surf. B Biointerfaces 2021, 200, 111605. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Wang, L.; Li, S.; Chu, S.; Wang, J.; Li, Y.; Hou, J.; Luo, Q.; Liu, J. Design of Cyclodextrin-Based Functional Systems for Biomedical Applications. Front. Chem. 2021, 9, 635507. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, Y.; Zhu, K.; Wang, Q.; Wakeel, M.; Wahid, A.; Alharbi, N.S.; Chen, C. Investigation of the adsorption mechanisms of Pb(II) and 1-naphthol by β-cyclodextrin modified graphene oxide nanosheets from aqueous solution. J. Colloid Interface Sci. 2018, 530, 154–162. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; Mejía-Carmona, K.; Jordan-Sinisterra, M.; da Silva, L.F.; Vargas Medina, D.A.; Lanças, F.M. The Current Role of Graphene-Based Nanomaterials in the Sample Preparation Arena. Front. Chem. 2020, 8, 664. [Google Scholar] [CrossRef]
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef] [PubMed]
- Putri, L.K.; Ong, W.-J.; Chang, W.S.; Chai, S.-P. Heteroatom doped graphene in photocatalysis: A review. Appl. Surf. Sci. 2015, 358, 2–14. [Google Scholar] [CrossRef]
- Mir, S.; Yadav, V.; Singh, J. Boron–Carbon–Nitride Sheet as a Novel Surface for Biological Applications: Insights from Density Functional Theory. ACS Omega 2019, 4, 3732–3738. [Google Scholar] [CrossRef] [PubMed]
- Timsorn, K.; Wongchoosuk, C. Adsorption of NO2, HCN, HCHO and CO on pristine and amine functionalized boron nitride nanotubes by self-consistent charge density functional tight-binding method. Mater. Res. Express 2020, 7, 055005. [Google Scholar] [CrossRef]
- Mirhaji, E.; Afshar, M.; Rezvani, S.; Yoosefian, M. Boron nitride nanotubes as a nanotransporter for anti-cancer docetaxel drug in water/ethanol solution. J. Mol. Liq. 2018, 271, 151–156. [Google Scholar] [CrossRef]
- García-Toral, D.; Báez, R.M.; Sánchez S, J.I.; Flores-Riveros, A.; Cocoletzi, G.H.; Rivas-Silva, J.F. Encapsulation of Pollutant Gaseous Molecules by Adsorption on Boron Nitride Nanotubes: A Quantum Chemistry Study. ACS Omega 2021, 6, 14824–14837. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Chen, D.; Cui, H.; Tang, J. Adsorption behaviour of SO2 and SOF2 gas on Rh-doped BNNT: A DFT study. Mol. Phys. 2020, 118, e1580394. [Google Scholar] [CrossRef]
- Yoosefian, M.; Rahmanifar, E.; Etminan, N. Nanocarrier for levodopa Parkinson therapeutic drug; comprehensive benserazide analysis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 434–446. [Google Scholar] [CrossRef]
- Bououden, W.; Benguerba, Y.; Darwish, A.S.; Attoui, A.; Lemaoui, T.; Balsamo, M.; Erto, A.; Alnashef, I.M. Surface adsorption of Crizotinib on carbon and boron nitride nanotubes as Anti-Cancer drug Carriers: COSMO-RS and DFT molecular insights. J. Mol. Liq. 2021, 338, 116666. [Google Scholar] [CrossRef]
- Yoosefian, M.; Mola, A.; Fooladi, E.; Ahmadzadeh, S. The effect of solvents on formaldehyde adsorption performance on pristine and Pd doped on single-walled carbon nanotube using density functional theory. J. Mol. Liq. 2017, 225, 34–41. [Google Scholar] [CrossRef]
- Cao, M.; Wu, D.; Yoosefian, M.; Sabaei, S.; Jahani, M. Comprehensive study of the encapsulation of Lomustine anticancer drug into single walled carbon nanotubes (SWCNTs): Solvent effects, molecular conformations, electronic properties and intramolecular hydrogen bond strength. J. Mol. Liq. 2020, 320, 114285. [Google Scholar] [CrossRef]
- Yoosefian, M.; Etminan, N. Leucine/Pd-loaded (5,5) single-walled carbon nanotube matrix as a novel nanobiosensors for in silico detection of protein. Amino Acids 2018, 50, 653–661. [Google Scholar] [CrossRef]
- Srihakulung, O.; Maezono, R.; Toochinda, P.; Kongprawechnon, W.; Intarapanich, A.; Lawtrakul, L. Host-Guest Interactions of Plumbagin with β-Cyclodextrin, Dimethyl-β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin: Semi-Empirical Quantum Mechanical PM6 and PM7 Methods. Sci. Pharm. 2018, 86, 20. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Archimandritis, A.S.; Papadimitriou, T.; Kormas, K.A.; Laspidou, C.S.; Yannakopoulou, K.; Lazarou, Y.G. Theoretical investigation of microcystin-LR, microcystin-RR and nodularin-R complexation with α-, β-, and γ-cyclodextrin as a starting point for the targeted design of efficient cyanotoxin traps. Sustain. Chem. Pharm. 2016, 3, 25–32. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Braga, L.S.; Leal, D.H.; Kuca, K.; Ramalho, T.C. Perspectives on the Role of the Frontier Effective-for-Reaction Molecular Orbital (FERMO) in the Study of Chemical Reactivity: An Updated Review. Curr. Org. Chem. 2020, 24, 314–331. [Google Scholar] [CrossRef]
- Park, J.H.; Nah, T.H. Binding forces contributing to the complexation of organic molecules with β-cyclodextrin in aqueous solution. J. Chem. Soc. Perkin Trans. 2 1994, 6, 1359–1362. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. Novel Prediction for the Driving Force and Guest Orientation in the Complexation of α- and β-Cyclodextrin with Benzene Derivatives. J. Phys. Chem. B 1999, 103, 3461–3467. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, H.; Hou, P.; Qian, B.; Wang, X.; Guo, C.; Wang, L. Efficient Graphene/Cyclodextrin-Based Nanocontainer: Synthesis and Host–Guest Inclusion for Self-Healing Anticorrosion Application. ACS Appl. Mater. Interfaces 2018, 10, 36229–36239. [Google Scholar] [CrossRef] [PubMed]
- Fu, L. Cyclodextrin Functionalized Graphene and Its Applications. In Graphene Functionalization Strategies: From Synthesis to Applications; Khan, A., Jawaid, M., Neppolian, B., Asiri, A.M., Eds.; Springer: Singapore, 2019; pp. 193–213. [Google Scholar]
- Shao, D.; Sheng, G.; Chen, C.; Wang, X.; Nagatsu, M. Removal of polychlorinated biphenyls from aqueous solutions using β-cyclodextrin grafted multiwalled carbon nanotubes. Chemosphere 2010, 79, 679–685. [Google Scholar] [CrossRef]
- Tan, P.; Hu, Y. Improved synthesis of graphene/β-cyclodextrin composite for highly efficient dye adsorption and removal. J. Mol. Liq. 2017, 242, 181–189. [Google Scholar] [CrossRef]
- Fraga, T.M.; Carvalho, M.; Ghislandi, M.; Sobrinho, M.A.M. Functionalized Graphene-Based Materials as Innovative Adsorbents of Organic Pollutants: A Concise Overview. Braz. J. Chem. Eng. 2019, 36, 1–31. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Pang, H.; Zhang, R.; Song, W.; Fu, D.; Hayat, T.; Wang, X. Boron nitride-based materials for the removal of pollutants from aqueous solutions: A review. Chem. Eng. J. 2018, 333, 343–360. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, W.; Peng, Y.; Chen, Z.; Chen, J.; Xiao, Z.; Zhao, X.; Qu, Y.; Li, J. Predicting the adsorption of organic pollutants on boron nitride nanosheets via in silico techniques: DFT computations and QSAR modeling. Environ. Sci. Nano 2021, 8, 795–805. [Google Scholar] [CrossRef]
- Huang, Q.; Chai, K.; Zhou, L.; Ji, H. A phenyl-rich β-cyclodextrin porous crosslinked polymer for efficient removal of aromatic pollutants: Insight into adsorption performance and mechanism. Chem. Eng. J. 2020, 387, 124020. [Google Scholar] [CrossRef]
- Chen, X.; Jia, S.; Ding, N.; Shi, J.; Wang, Z. Capture of aromatic organic pollutants by hexagonal boron nitride nanosheets: Density functional theoretical and molecular dynamic investigation. Environ. Sci. Nano 2016, 3, 1493–1503. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin Functionalized Graphene Nanosheets with High Supramolecular Recognition Capability: Synthesis and Host−Guest Inclusion for Enhanced Electrochemical Performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef]
- Gao, Y.-S.; Wu, L.-P.; Zhang, K.-X.; Xu, J.-K.; Lu, L.-M.; Zhu, X.-F.; Wu, Y. Electroanalytical method for determination of shikonin based on the enhancement effect of cyclodextrin functionalized carbon nanotubes. Chin. Chem. Lett. 2015, 26, 613–618. [Google Scholar] [CrossRef]
- Jaiyong, P.; Bryce, R.A. Approximate quantum chemical methods for modelling carbohydrate conformation and aromatic interactions: β-cyclodextrin and its adsorption on a single-layer graphene sheet. Phys. Chem. Chem. Phys. 2017, 19, 15346–15355. [Google Scholar] [CrossRef] [PubMed]


| Reaction Enthalpy (∆Hf) (kcal/mol) | |||
|---|---|---|---|
| CC_BN1 | +BCD | →CC_BN1_BCD | −72.1674 |
| CC_BN2 | +BCD | →CC_BN2_BCD | −92.0323 |
| h-BN_CC1 | +BCD | →h-BN_CC1_BCD | −76.3327 |
| h-BN_CC2 | +BCD | →h-BN_CC2_BCD | −77.2604 |
| CC_BN1_BCD | +cb | →CC_BN1_BCD_cb | −60.9593 |
| CC_BN1_BCD | +pcb | →CC_BN1_BCD_pcb | −94.6094 |
| CC_BN1_BCD | +b | →CC_BN1_BCD_b | −24.2197 |
| CC_BN1_BCD | +bp | →CC_BN1_BCD_bp | −22.9383 |
| CC_BN2_BCD | +cb | →CC_BN2_BCD_cb | −52.5011 |
| CC_BN2_BCD | +pcb | →CC_BN2_BCD_pcb | −88.8037 |
| CC_BN2_BCD | +b | →CC_BN2_BCD_b | −28.7710 |
| CC_BN2_BCD | +bp | →CC_BN2_BCD_bp | −41.4766 |
| h-BN_CC1_BCD | +cb | →h-BN_CC1_BCD_cb | −50.0348 |
| h-BN_CC1_BCD | +pcb | →h-BN_CC1_BCD_pcb | −97.4077 |
| h-BN_CC1_BCD | +b | →h-BN_CC1_BCD_b | −31.9365 |
| h-BN_CC1_BCD | +bp | →h-BN_CC1_BCD_bp | −46.1465 |
| h-BN_CC2_BCD | +cb | →h-BN_CC2_BCD_cb | −45.7307 |
| h-BN_CC2_BCD | +pcb | →h-BN_CC2_BCD_pcb | −110.203 |
| h-BN_CC2_BCD | +b | →h-BN_CC2_BCD_b | −14.9105 |
| h-BN_CC2_BCD | +bp | →h-BN_CC2_BCD_bp | −55.5254 |
| GAP (eV) | I (eV) | A (eV) | χ (eV) | η (eV) | μ (eV) | S (eV−1) | ω (eV) | |
|---|---|---|---|---|---|---|---|---|
| CC_BN1 | 2.796 | 6.418 | 3.622 | 5.020 | 1.398 | −5.020 | 0.358 | 9.013 |
| CC_BN2 | 2.736 | 6.343 | 3.607 | 4.975 | 1.368 | −4.975 | 0.365 | 9.046 |
| h-BN_CC1 | 5.477 | 7.396 | 1.919 | 4.658 | 2.739 | −4.658 | 0.183 | 3.961 |
| h-BN_CC2 | 4.957 | 6.964 | 2.007 | 4.486 | 2.479 | −4.486 | 0.202 | 4.059 |
| BCD | 11.113 | 10.177 | −0.936 | 4.621 | 5.557 | −4.621 | 0.090 | 1.921 |
| CC_BN1_BCD | 2.788 | 6.463 | 3.675 | 5.069 | 1.394 | −5.069 | 0.359 | 9.216 |
| CC_BN2_BCD | 2.727 | 6.388 | 3.661 | 5.025 | 1.364 | −5.025 | 0.367 | 9.258 |
| h-BN_CC1_BCD | 5.351 | 7.320 | 1.969 | 4.645 | 2.676 | −4.645 | 0.187 | 4.031 |
| h-BN_CC2_BCD | 4.842 | 6.890 | 2.048 | 4.469 | 2.421 | −4.469 | 0.207 | 4.125 |
| cb | 8.542 | 10.180 | 1.638 | 5.909 | 4.271 | −5.909 | 0.117 | 4.088 |
| pcb | 8.358 | 10.117 | 1.759 | 5.938 | 4.179 | −5.938 | 0.120 | 4.219 |
| b | 10.060 | 9.824 | −0.236 | 4.794 | 5.030 | −4.794 | 0.099 | 2.285 |
| bp | 9.134 | 9.292 | 0.158 | 4.725 | 4.567 | −4.725 | 0.109 | 2.444 |
| CC_BN1_BCD_cb | 2.792 | 6.478 | 3.686 | 5.082 | 1.396 | −5.082 | 0.358 | 9.250 |
| CC_BN1_BCD_pcb | 2.741 | 6.441 | 3.700 | 5.071 | 1.371 | −5.071 | 0.365 | 9.380 |
| CC_BN1_BCD_b | 2.789 | 6.467 | 3.678 | 5.073 | 1.395 | −5.073 | 0.359 | 9.226 |
| CC_BN1_BCD_bp | 2.794 | 6.462 | 3.668 | 5.065 | 1.397 | −5.065 | 0.358 | 9.182 |
| CC_BN2_BCD_cb | 2.720 | 6.390 | 3.670 | 5.030 | 1.360 | −5.030 | 0.368 | 9.302 |
| CC_BN2_BCD_pcb | 2.805 | 6.407 | 3.602 | 5.005 | 1.403 | −5.005 | 0.357 | 8.929 |
| CC_BN2_BCD_b | 2.743 | 6.387 | 3.644 | 5.016 | 1.372 | −5.016 | 0.365 | 9.171 |
| CC_BN2_BCD_bp | 2.738 | 6.384 | 3.646 | 5.015 | 1.369 | −5.015 | 0.365 | 9.186 |
| h-BN_CC1_BCD_cb | 5.365 | 7.345 | 1.980 | 4.663 | 2.683 | −4.663 | 0.186 | 4.052 |
| h-BN_CC1_BCD_pcb | 5.428 | 7.409 | 1.981 | 4.695 | 2.714 | −4.695 | 0.184 | 4.061 |
| h-BN_CC1_BCD_b | 5.375 | 7.343 | 1.968 | 4.656 | 2.688 | −4.656 | 0.186 | 4.032 |
| h-BN_CC1_BCD_bp | 5.378 | 7.350 | 1.972 | 4.661 | 2.689 | −4.661 | 0.186 | 4.040 |
| h-BN_CC2_BCD_cb | 4.833 | 6.911 | 2.078 | 4.495 | 2.417 | −4.495 | 0.207 | 4.180 |
| h-BN_CC2_BCD_pcb | 4.949 | 7.001 | 2.052 | 4.527 | 2.475 | −4.527 | 0.202 | 4.140 |
| h-BN_CC2_BCD_b | 4.810 | 6.871 | 2.061 | 4.466 | 2.405 | −4.466 | 0.208 | 4.147 |
| h-BN_CC2_BCD_bp | 4.887 | 6.946 | 2.059 | 4.503 | 2.444 | −4.503 | 0.205 | 4.148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Chang, T.-C.; Chang, C.M. Quantum Chemical Studies on the Adsorption of Hexachlorobenzene, Decachlorobiphenyl, Benzene, and Biphenyl by BN-Doped Graphene and C-Doped Hexagonal Boron Nitride Modified with β-Cyclodextrin. Crystals 2023, 13, 266. https://doi.org/10.3390/cryst13020266
Lee C-L, Chang T-C, Chang CM. Quantum Chemical Studies on the Adsorption of Hexachlorobenzene, Decachlorobiphenyl, Benzene, and Biphenyl by BN-Doped Graphene and C-Doped Hexagonal Boron Nitride Modified with β-Cyclodextrin. Crystals. 2023; 13(2):266. https://doi.org/10.3390/cryst13020266
Chicago/Turabian StyleLee, Chien-Lin, Tai-Chao Chang, and Chia Ming Chang. 2023. "Quantum Chemical Studies on the Adsorption of Hexachlorobenzene, Decachlorobiphenyl, Benzene, and Biphenyl by BN-Doped Graphene and C-Doped Hexagonal Boron Nitride Modified with β-Cyclodextrin" Crystals 13, no. 2: 266. https://doi.org/10.3390/cryst13020266
APA StyleLee, C.-L., Chang, T.-C., & Chang, C. M. (2023). Quantum Chemical Studies on the Adsorption of Hexachlorobenzene, Decachlorobiphenyl, Benzene, and Biphenyl by BN-Doped Graphene and C-Doped Hexagonal Boron Nitride Modified with β-Cyclodextrin. Crystals, 13(2), 266. https://doi.org/10.3390/cryst13020266






