Petrogenesis of Chatoyant Green Nephrite from Serpentinite-Related Deposits, Ospinsk, Russia: Insights from Mineralogy and Geochemistry
(This article belongs to the Section Mineralogical Crystallography and Biomineralization)
Abstract
:1. Introduction
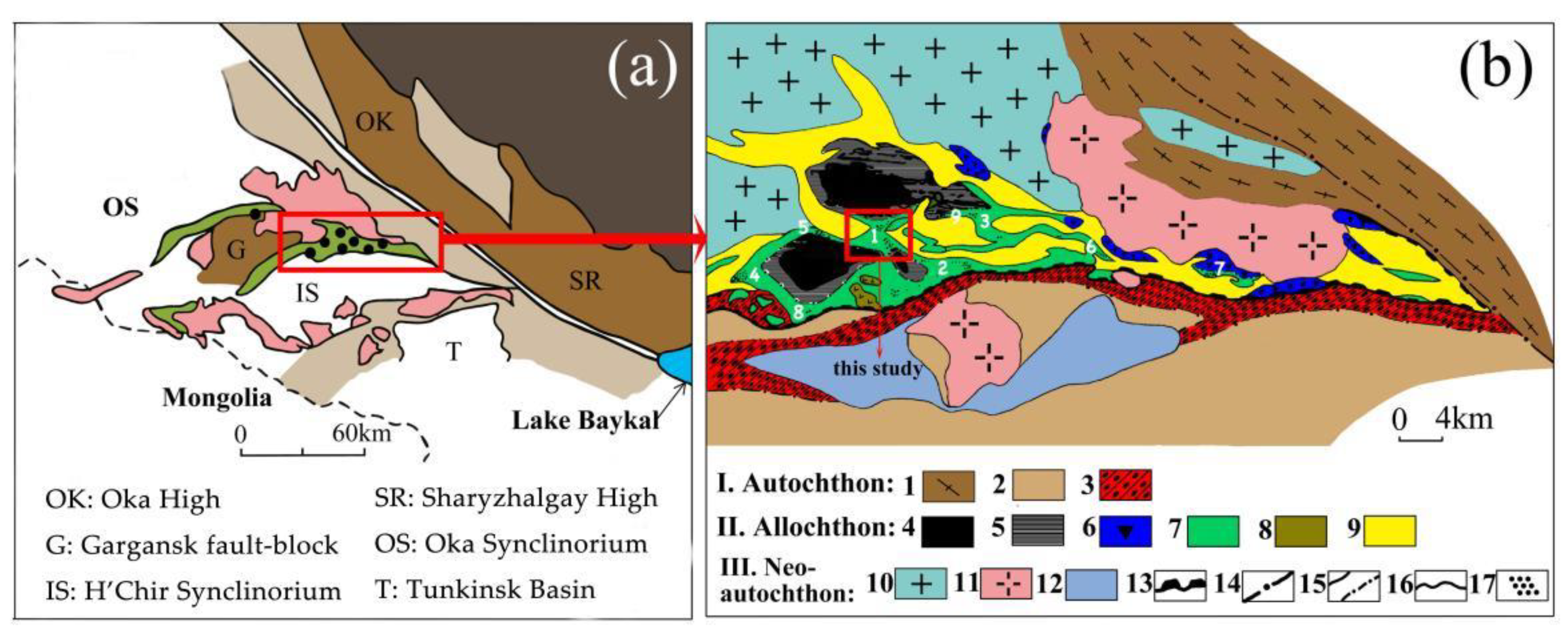
2. Geological Setting and Sample Description
3. Methods
4. Results
4.1. Mineralogy
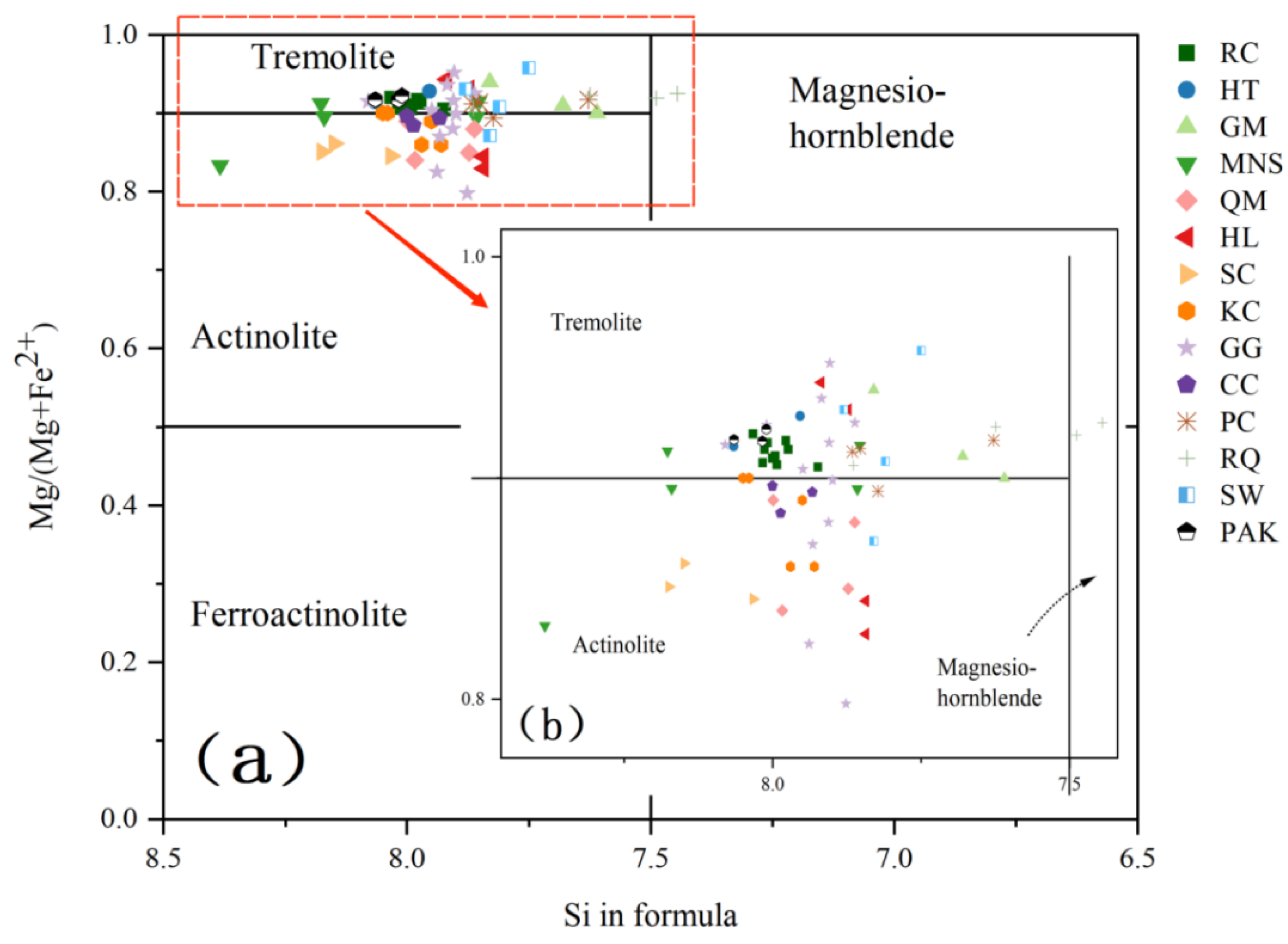
4.1.1. Tremolite
4.1.2. Subordinate Minerals
4.2. Spectroscopy
4.2.1. Tremolite
4.2.2. Magnetite
4.2.3. Graphite
4.3. Mineral Chemistry
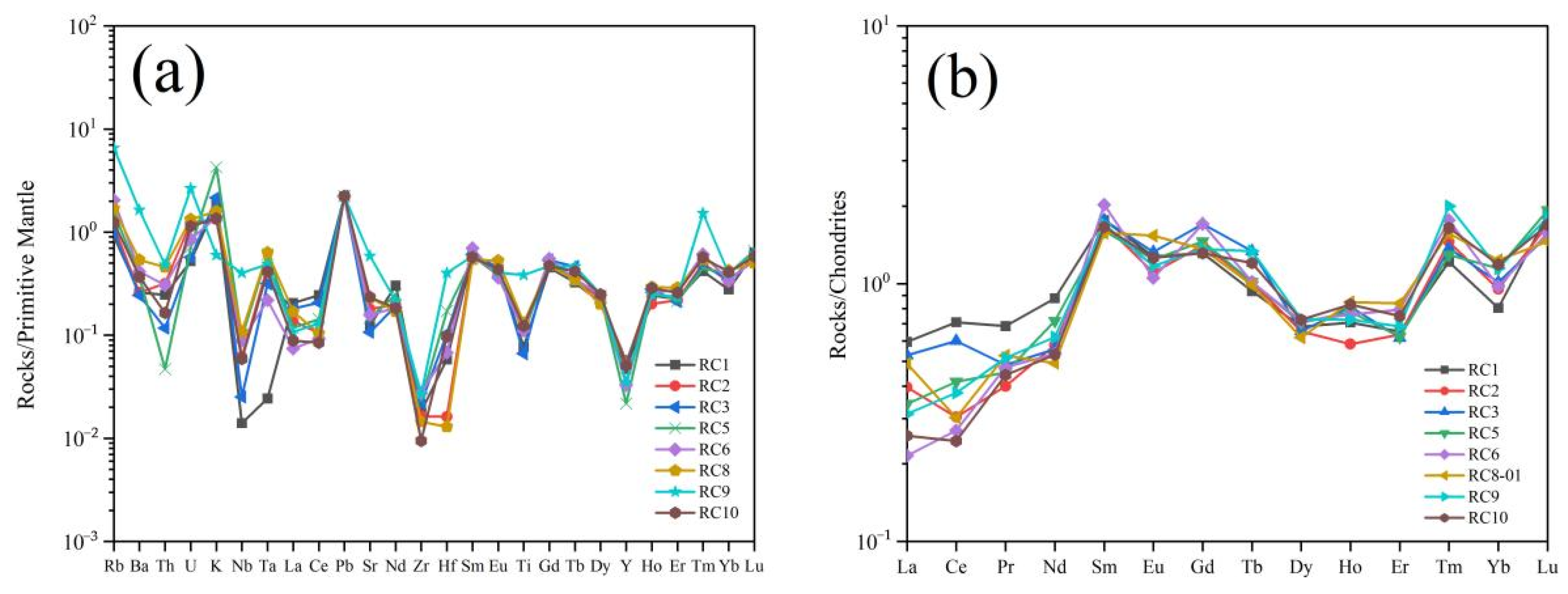
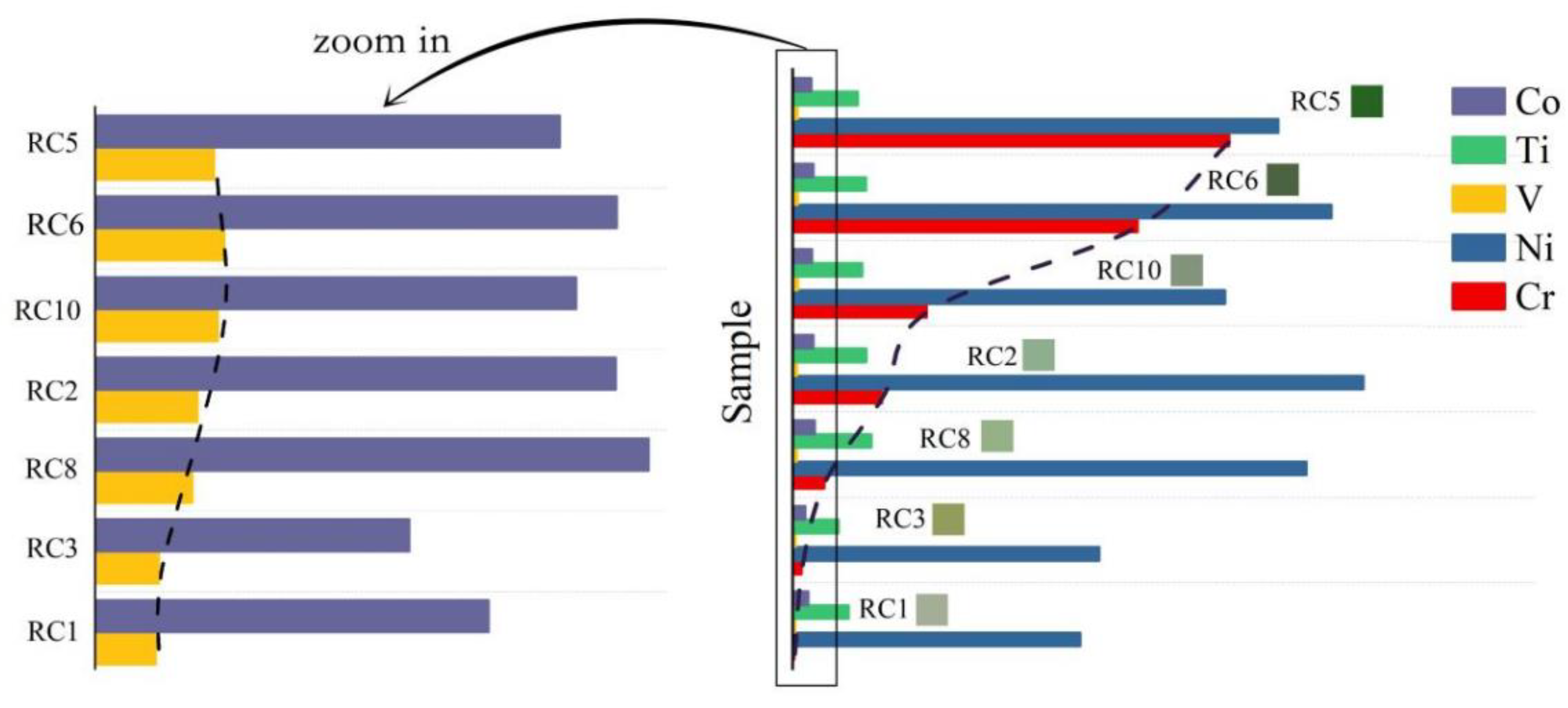
4.4. Geochemistry
5. Discussion
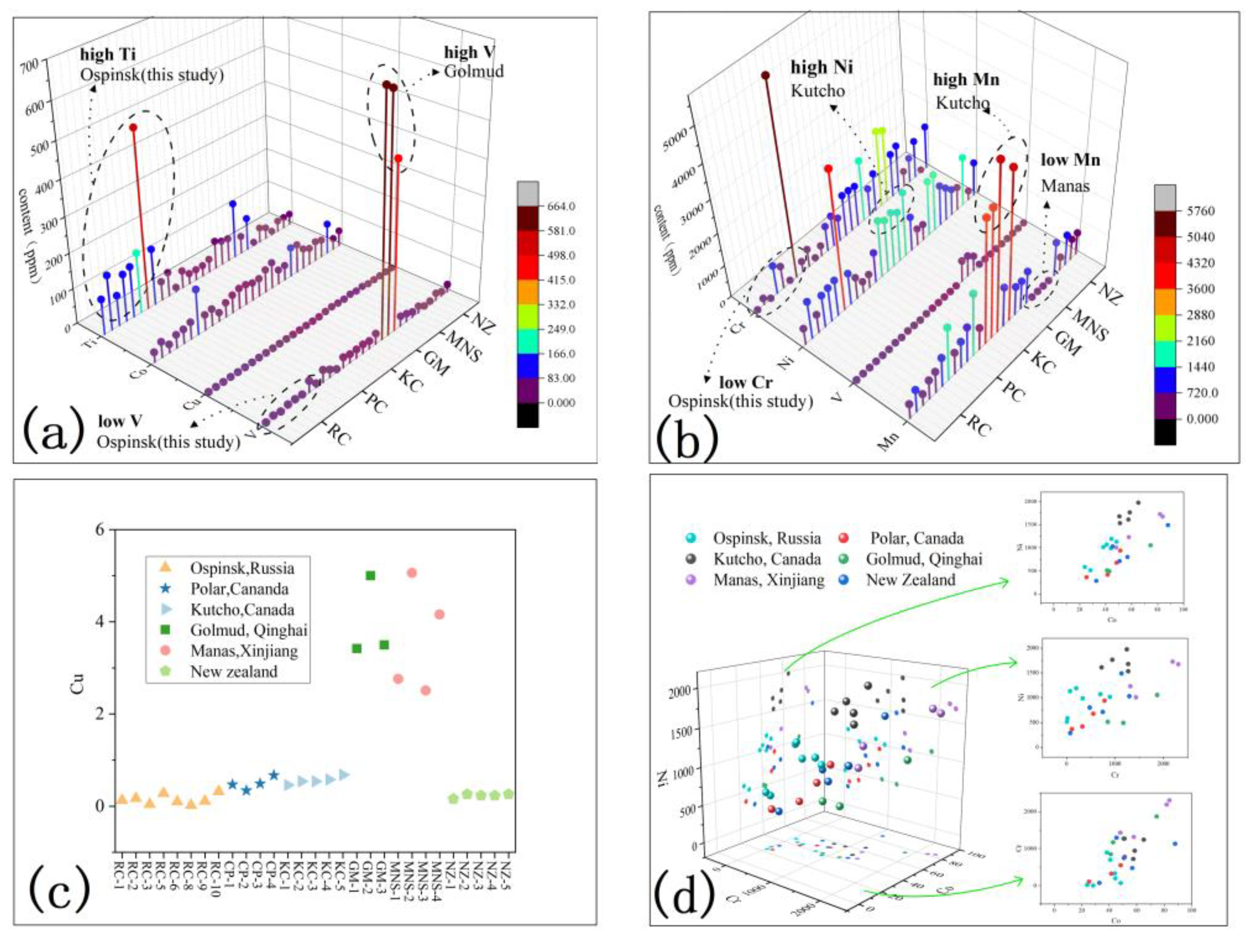
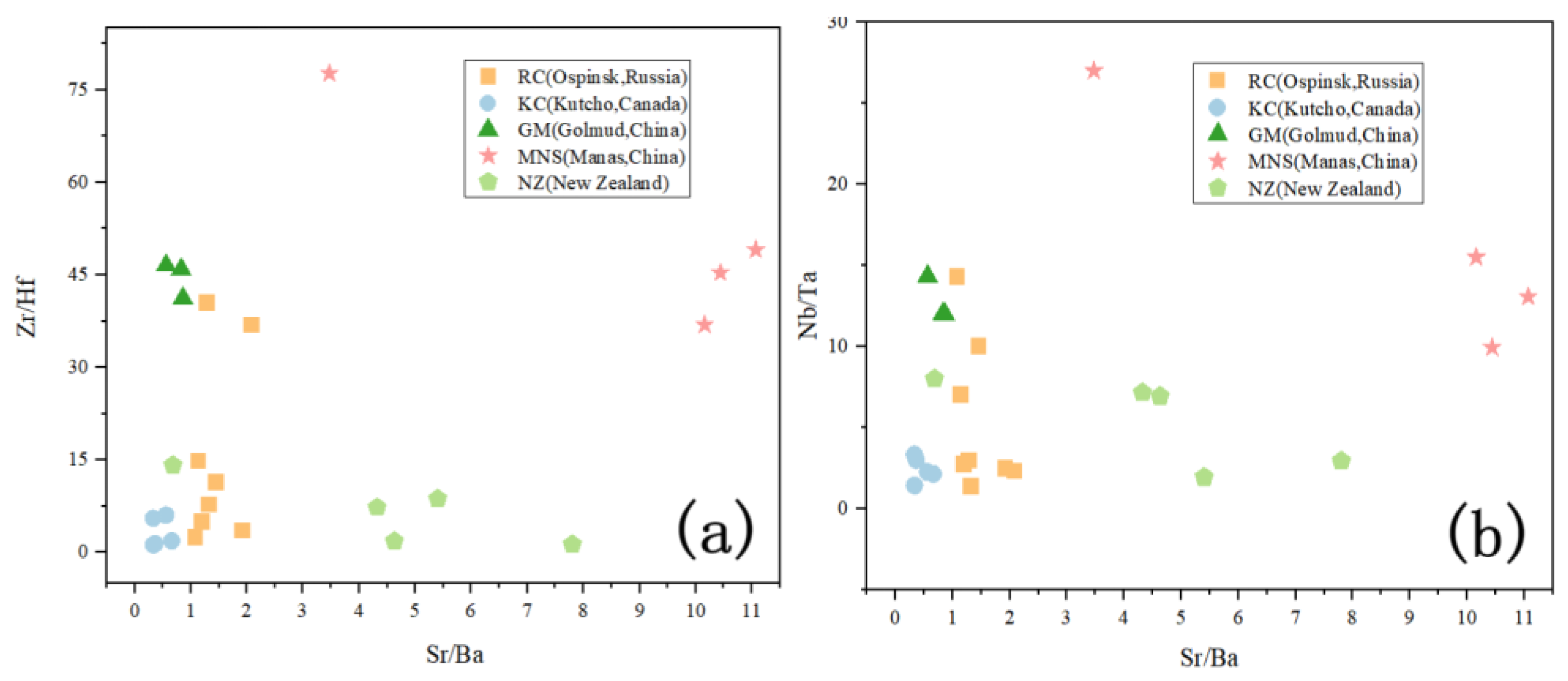
5.1. Comparison of Geochemical Characteristics
5.1.1. Comparison of Characteristics of Major Elements
5.1.2. Trace Elements
5.2. Formation Temperature of Graphite
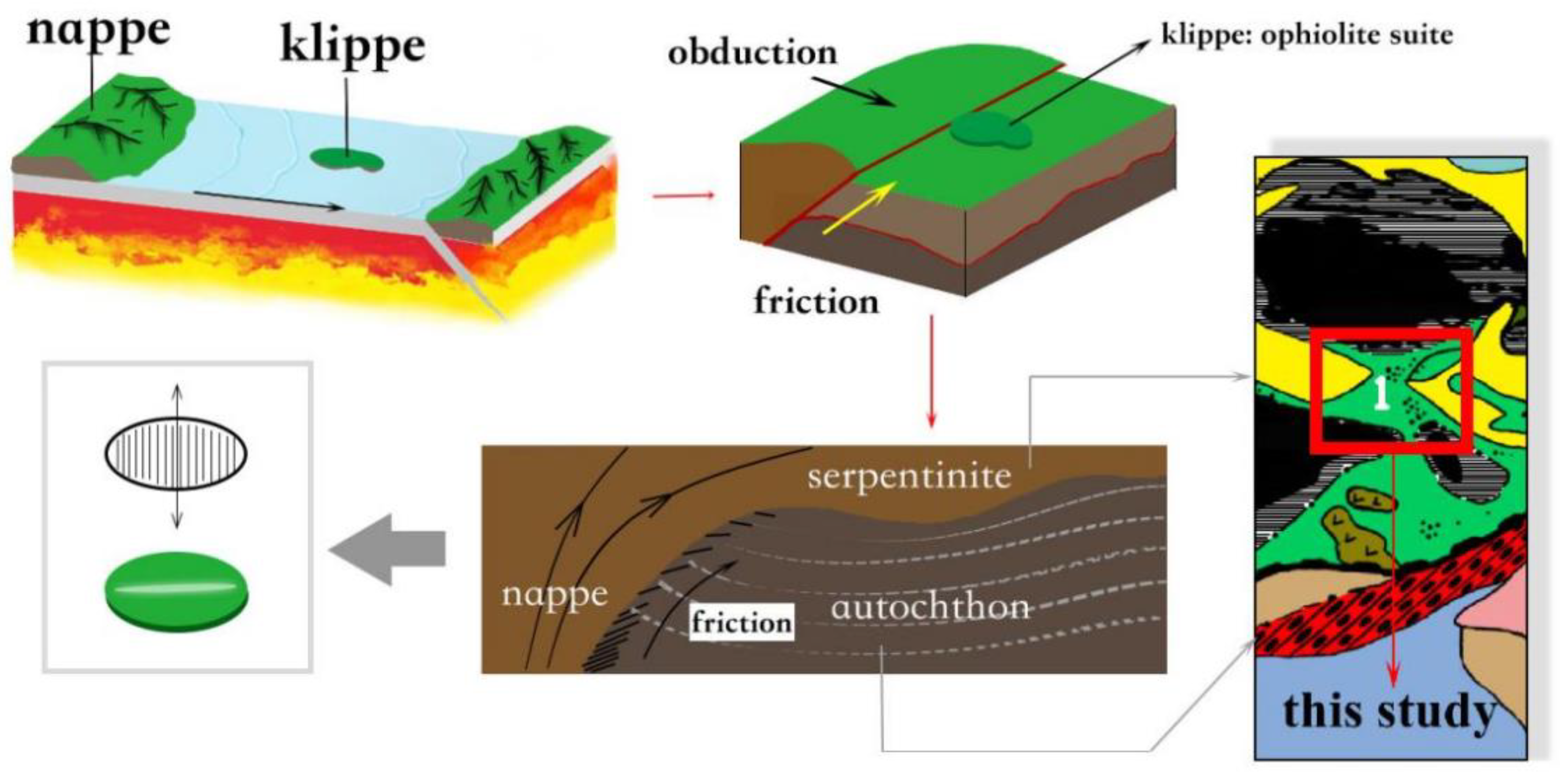
5.3. Deposit-Forming Process of Ospinsk Chatoyant Green Nephrite
5.3.1. Serpentinization of Ultramafic Rock
5.3.2. Nephritization of Serpentinite
5.3.3. Alteration
5.4. Reasons for Chatoyancy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| SiO2 | Al2O3 | MgO | CaO | FeO | Si | Al | Mg | Ca + K + Na | Fe2+ + Fe3+ | Fe2+ | Mg/(Mg + Fe2+) | Fe2+/(Mg + Fe2+) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RC1 | 56.65 | 0.31 | 21.75 | 12.17 | 3.90 | 8.35 | 0.05 | 4.78 | 1.95 | 0.48 | 0.48 | 0.91 | 0.09 |
| RC2 | 58.17 | 0.30 | 22.30 | 12.30 | 3.45 | 8.38 | 0.05 | 4.79 | 1.92 | 0.42 | 0.42 | 0.92 | 0.08 |
| RC3 | 57.78 | 0.27 | 22.27 | 12.16 | 3.79 | 8.36 | 0.05 | 4.81 | 1.91 | 0.46 | 0.46 | 0.91 | 0.09 |
| RC4 | 56.34 | 0.56 | 21.91 | 11.82 | 3.71 | 8.32 | 0.10 | 4.82 | 1.92 | 0.46 | 0.46 | 0.91 | 0.09 |
| RC5 | 55.64 | 0.65 | 21.54 | 12.13 | 4.03 | 8.27 | 0.11 | 4.77 | 1.98 | 0.50 | 0.50 | 0.91 | 0.10 |
| RC6 | 56.32 | 0.38 | 21.41 | 12.17 | 3.97 | 8.34 | 0.07 | 4.73 | 1.96 | 0.49 | 0.49 | 0.91 | 0.09 |
| RC7 | 56.19 | 0.34 | 21.36 | 12.27 | 3.78 | 8.34 | 0.06 | 4.73 | 1.98 | 0.47 | 0.47 | 0.91 | 0.09 |
| RC8 | 56.05 | 0.37 | 21.43 | 11.59 | 3.91 | 8.37 | 0.07 | 4.77 | 1.89 | 0.49 | 0.49 | 0.91 | 0.09 |
| RC9 | 56.68 | 0.43 | 21.68 | 12.47 | 3.49 | 8.33 | 0.07 | 4.75 | 1.98 | 0.43 | 0.43 | 0.92 | 0.08 |
| RC10 | 57.63 | 0.29 | 22.34 | 12.10 | 3.67 | 8.36 | 0.05 | 4.83 | 1.91 | 0.45 | 0.45 | 0.92 | 0.08 |
| HT-1 | 57.56 | 0.81 | 22.35 | 12.79 | 3.09 | 7.95 | 0.13 | 4.60 | 1.93 | 0.36 | 0.36 | 0.93 | 0.07 |
| HT-2 | 57.71 | 0.72 | 21.46 | 12.49 | 3.58 | 8.07 | 0.12 | 4.47 | 1.90 | 0.42 | 0.42 | 0.91 | 0.09 |
| GM-1 | 56.91 | 0.96 | 21.45 | 13.30 | 5.45 | 7.61 | 0.15 | 4.46 | 1.93 | 0.52 | 0.52 | 0.90 | 0.10 |
| GM-2 | 57.09 | 0.65 | 21.61 | 13.06 | 4.48 | 7.68 | 0.10 | 4.49 | 1.91 | 0.48 | 0.48 | 0.91 | 0.09 |
| GM-3 | 56.37 | 1.33 | 21.72 | 13.49 | 3.22 | 7.83 | 0.17 | 4.56 | 1.95 | 0.40 | 0.40 | 0.94 | 0.07 |
| MNS-1-2 | 55.87 | 0.85 | 21.68 | 12.50 | 4.53 | 7.86 | 0.14 | 4.55 | 1.91 | 0.53 | 0.53 | 0.90 | 0.10 |
| MNS-3-5 | 58.58 | 0.49 | 20.70 | 12.76 | 4.32 | 8.17 | 0.08 | 4.30 | 1.94 | 0.50 | 0.50 | 0.90 | 0.10 |
| MNS-3-7 | 58.14 | 0.05 | 21.11 | 12.89 | 3.62 | 8.18 | 0.01 | 4.43 | 1.96 | 0.43 | 0.43 | 0.91 | 0.09 |
| MNS-4-2 | 60.11 | 0.51 | 18.74 | 12.17 | 6.69 | 8.38 | 0.08 | 3.90 | 1.85 | 0.78 | 0.78 | 0.83 | 0.17 |
| MNS-5-1 | 56.62 | 0.10 | 22.71 | 13.36 | 3.77 | 7.85 | 0.02 | 4.70 | 1.99 | 0.44 | 0.44 | 0.91 | 0.09 |
| QM-1-2 | 56.40 | 0.30 | 20.90 | 12.50 | 4.70 | 8.00 | 0.05 | 4.42 | 1.92 | 0.56 | 0.56 | 0.89 | 0.11 |
| QM-3-2 | 56.60 | 0.74 | 21.50 | 12.60 | 5.14 | 7.86 | 0.12 | 4.45 | 1.90 | 0.60 | 0.60 | 0.88 | 0.12 |
| QM-5-3 | 57.00 | 0.86 | 20.30 | 13.30 | 6.59 | 7.87 | 0.14 | 4.18 | 1.98 | 0.76 | 0.76 | 0.85 | 0.15 |
| QM-5-4 | 56.80 | 0.77 | 19.40 | 13.00 | 6.65 | 7.98 | 0.13 | 4.07 | 1.97 | 0.78 | 0.78 | 0.84 | 0.16 |
| HL-1-1 | 55.96 | 0.49 | 20.21 | 12.59 | 7.75 | 7.84 | 0.08 | 4.22 | 1.96 | 0.91 | 0.78 | 0.84 | 0.16 |
| HL-1-2 | 55.59 | 0.53 | 19.56 | 12.85 | 8.37 | 7.84 | 0.09 | 4.11 | 2.01 | 0.99 | 0.85 | 0.83 | 0.17 |
| HL-4-1 | 56.96 | 0.32 | 22.56 | 12.88 | 4.24 | 7.92 | 0.03 | 4.75 | 1.87 | 0.40 | 0.29 | 0.94 | 0.06 |
| HL-4-2 | 57.58 | 0.19 | 23.18 | 12.72 | 3.49 | 7.87 | 0.05 | 4.65 | 1.94 | 0.15 | 0.34 | 0.93 | 0.07 |
| SC2 | 59.02 | 0.28 | 20.01 | 5.74 | 5.74 | 8.15 | 0.04 | 4.12 | 1.97 | 0.66 | 0.66 | 0.86 | 0.14 |
| SC3 | 58.89 | 0.55 | 19.09 | 5.97 | 5.97 | 8.17 | 0.09 | 3.95 | 2.03 | 0.69 | 0.69 | 0.85 | 0.15 |
| SC4 | 58.41 | 0.48 | 19.45 | 6.35 | 6.35 | 8.03 | 0.08 | 3.99 | 2.07 | 0.73 | 0.73 | 0.85 | 0.15 |
| KC1-2 | 57.96 | 0.27 | 11.99 | 11.99 | 4.24 | 8.04 | 0.04 | 4.58 | 1.79 | 0.49 | 0.49 | 0.90 | 0.10 |
| KC2-4 | 57.24 | 0.24 | 20.89 | 12.79 | 4.46 | 8.05 | 0.04 | 4.38 | 1.95 | 0.49 | 0.49 | 0.90 | 0.10 |
| KC3-2 | 55.47 | 0.80 | 19.74 | 12.21 | 6.08 | 7.93 | 0.14 | 4.21 | 1.92 | 0.67 | 0.67 | 0.86 | 0.14 |
| KC4-1 | 57.04 | 0.76 | 20.35 | 12.40 | 6.00 | 7.97 | 0.13 | 4.24 | 1.89 | 0.67 | 0.67 | 0.86 | 0.14 |
| KC5-4 | 56.41 | 0.24 | 21.05 | 12.83 | 4.85 | 7.95 | 0.04 | 4.42 | 1.97 | 0.57 | 0.57 | 0.89 | 0.11 |
| GG-2-1 | 56.80 | 0.27 | 19.78 | 13.44 | 6.49 | 7.93 | 0.04 | 4.12 | 2.09 | 0.76 | 0.62 | 0.87 | 0.13 |
| GG-3-1 | 56.93 | bdl | 20.48 | 13.32 | 6.25 | 7.91 | 0.08 | 4.24 | 2.02 | 0.73 | 0.58 | 0.88 | 0.12 |
| GG-4-1 | 58.27 | 0.05 | 22.49 | 12.86 | 3.31 | 8.01 | 0.03 | 4.61 | 1.93 | 0.38 | 0.38 | 0.92 | 0.08 |
| GG-5-1 | 57.22 | 0.07 | 21.89 | 13.49 | 3.81 | 7.92 | 0.06 | 4.52 | 2.03 | 0.44 | 0.31 | 0.94 | 0.06 |
| GG-6-1 | 56.61 | 0.05 | 19.21 | 13.38 | 8.20 | 7.94 | 0.02 | 4.02 | 2.04 | 0.96 | 0.85 | 0.83 | 0.18 |
| GG-6-2 | 58.59 | 0.05 | 21.68 | 12.85 | 3.61 | 8.08 | 0.02 | 4.46 | 1.91 | 0.42 | 0.41 | 0.92 | 0.08 |
| GG-7-2 | 56.63 | 0.10 | 21.61 | 13.17 | 5.40 | 7.86 | 0.02 | 4.47 | 1.98 | 0.63 | 0.36 | 0.93 | 0.08 |
| GG-8-1 | 56.99 | 0.15 | 21.26 | 13.30 | 4.77 | 7.95 | 0.02 | 4.42 | 2.01 | 0.56 | 0.47 | 0.90 | 0.10 |
| GG-8-2 | 56.70 | 0.03 | 20.87 | 13.50 | 5.97 | 7.90 | 0.01 | 4.33 | 2.04 | 0.70 | 0.49 | 0.90 | 0.10 |
| GG-10-1 | 57.10 | 0.13 | 21.43 | 13.48 | 4.85 | 7.90 | 0.04 | 4.42 | 2.02 | 0.56 | 0.41 | 0.92 | 0.08 |
| GG-10-2 | 55.61 | 0.28 | 17.89 | 13.56 | 9.83 | 7.88 | 0.04 | 3.78 | 2.09 | 1.16 | 0.96 | 0.80 | 0.20 |
| CC1-1 | 57.54 | 0.58 | 21.07 | 12.92 | 4.34 | 8.00 | 0.10 | 4.37 | 1.98 | 0.50 | 0.50 | 0.90 | 0.10 |
| CC1-2 | 57.01 | 0.79 | 20.87 | 13.29 | 4.43 | 7.93 | 0.13 | 4.33 | 2.03 | 0.52 | 0.52 | 0.89 | 0.11 |
| CC2-1 | 57.60 | 0.63 | 20.75 | 12.97 | 4.85 | 7.99 | 0.10 | 4.29 | 1.99 | 0.56 | 0.56 | 0.88 | 0.12 |
| PC-1-1 | 56.69 | 0.15 | 22.21 | 13.04 | 4.69 | 7.82 | 0.02 | 4.57 | 2.00 | 0.54 | 0.54 | 0.89 | 0.11 |
| PC-2-1 | 57.03 | 0.21 | 22.51 | 12.99 | 3.81 | 7.85 | 0.03 | 4.62 | 2.00 | 0.44 | 0.44 | 0.91 | 0.09 |
| PC-3-1 | 58.11 | 0.04 | 22.94 | 13.34 | 3.95 | 7.87 | 0.01 | 4.63 | 1.99 | 0.45 | 0.45 | 0.91 | 0.09 |
| PC-4-1 | 57.47 | 0.16 | 22.83 | 13.20 | 3.68 | 7.63 | 0.03 | 4.52 | 1.95 | 0.41 | 0.41 | 0.92 | 0.08 |
| RQ-1 | 58.07 | 0.29 | 23.17 | 12.69 | 4.30 | 7.86 | 0.05 | 4.68 | 1.89 | 0.49 | 0.49 | 0.91 | 0.09 |
| RQ-2 | 56.32 | 0.27 | 26.45 | 12.85 | 3.82 | 7.45 | 0.04 | 5.21 | 1.88 | 0.42 | 0.42 | 0.93 | 0.07 |
| RQ-3 | 56.89 | 0.29 | 26.74 | 12.19 | 4.18 | 7.49 | 0.04 | 5.25 | 1.75 | 0.46 | 0.46 | 0.92 | 0.08 |
| RQ-4 | 57.43 | 0.39 | 25.15 | 13.03 | 3.73 | 7.62 | 0.06 | 4.98 | 1.91 | 0.41 | 0.41 | 0.92 | 0.08 |
| Rium | 56.74 | 0.39 | 21.45 | 12.88 | 5.64 | 7.83 | 0.06 | 4.41 | 1.99 | 0.65 | 0.65 | 0.87 | 0.13 |
| SW-2 | 56.84 | 0.07 | 23.03 | 12.12 | 4.15 | 7.81 | 0.01 | 4.72 | 1.93 | 0.48 | 0.48 | 0.91 | 0.09 |
| SW-3 | 57.68 | 0.04 | 22.86 | 12.22 | 4.36 | 7.88 | 0.01 | 4.66 | 1.93 | 0.50 | 0.50 | 0.93 | 0.07 |
| Vitim-3 | 57.40 | 1.10 | 24.90 | 12.40 | 0.66 | 7.83 | 0.18 | 5.07 | 1.84 | 0.08 | 0.08 | 0.99 | 0.01 |
| PAK-1 | 58.18 | 0.15 | 21.92 | 13.37 | 3.55 | 8.02 | 0.02 | 4.50 | 2.01 | 0.43 | 0.41 | 0.92 | 0.08 |
| PAK-2 | 58.22 | 0.19 | 21.49 | 13.37 | 3.45 | 8.07 | 0.03 | 4.44 | 2.02 | 0.43 | 0.40 | 0.92 | 0.08 |
| PAK-3 | 57.60 | 0.29 | 21.51 | 13.23 | 3.24 | 8.01 | 0.05 | 4.46 | 2.03 | 0.44 | 0.38 | 0.92 | 0.08 |
| Ti | V | Cr | Mn | Co | Ni | Cu | Sr/Ba | Zr/Hf | Nb/Ta | |
|---|---|---|---|---|---|---|---|---|---|---|
| RC-1 | 100.40 | 4.38 | 2.30 | 472.26 | 28.26 | 514.73 | 0.13 | 1.45 | 11.33 | 1bdl |
| RC-2 | 156.15 | 8.60 | 196.73 | 724.43 | 44.43 | 1190.88 | 0.17 | 2.09 | 36.80 | 2.32 |
| RC-3 | 86.47 | 4.90 | 15.57 | 372.91 | 23.89 | 584.88 | 0.04 | 1.32 | 7.72 | 1.38 |
| RC-5 | 134.93 | 9.75 | 893.38 | 614.44 | 38.19 | 1014.43 | 0.28 | 1.20 | 4.96 | 2.72 |
| RC-6 | 144.68 | 10.03 | 697.53 | 687.16 | 40.75 | 1070.42 | 0.10 | 1.13 | 14.76 | 7.00 |
| RC-8 | 172.04 | 8.57 | 68.67 | 933.49 | 48.33 | 1131.03 | 0.02 | 1.29 | 40.50 | 2.96 |
| RC-9 | 500.06 | 32.51 | 5749.78 | 1692.79 | 128.94 | 4204.46 | 0.11 | 1.08 | 2.43 | 14.30 |
| RC-10 | 159.93 | 11.47 | 330.61 | 692.58 | 44.06 | 987.73 | 0.32 | 1.92 | 3.53 | 2.47 |
| CP-1 | 53.82 | 34.97 | 549.86 | 847.82 | 48.32 | 675.36 | 0.47 | - | - | - |
| CP-2 | 68.69 | 22.17 | 106.03 | 1112.58 | 25.25 | 363.06 | 0.34 | - | - | - |
| CP-3 | 12.88 | 9.55 | 322.94 | 1965.42 | 41.47 | 417.02 | 0.49 | - | - | - |
| CP-4 | 49.90 | 29.55 | 780.68 | 703.64 | 51.23 | 939.17 | 0.67 | - | - | - |
| KC-1 | 30.18 | 24.57 | 942.02 | 3797.89 | 58.49 | 1763.75 | 0.46 | 0.36 | 1.39 | 3.00 |
| KC-2 | 29.90 | 24.72 | 720.36 | 3908.12 | 57.45 | 1610.58 | 0.54 | 0.33 | 1.22 | 3.33 |
| KC-3 | 30.40 | 25.72 | 1269.59 | 5006.82 | 50.55 | 1678.26 | 0.54 | 0.56 | 6.04 | 2.25 |
| KC-4 | 38.02 | 22.19 | 1268.99 | 1235.53 | 50.98 | 1531.30 | 0.58 | 0.33 | 5.53 | 1.43 |
| KC-5 | 80.82 | 30.04 | 1241.58 | 4501.67 | 65.08 | 1973.64 | 0.68 | 0.66 | 1.85 | 2.14 |
| GM-1 | 69.76 | 663.81 | 1869.31 | 889.80 | 74.49 | 1053.08 | 3.42 | 0.83 | 45.91 | 12.00 |
| GM-2 | 65.96 | 648.00 | 848.00 | 886.00 | 41.03 | 511.00 | 5.00 | 0.86 | 41.20 | 12.00 |
| GM-3 | 159.78 | 466.42 | 1169.97 | 1016.00 | 42.79 | 490.99 | 3.50 | 0.56 | 46.53 | 14.33 |
| MNS-1 | 50.54 | 28.37 | 2305.00 | 5.82 | 83.78 | 1674.00 | 2.76 | 11.07 | 49.04 | 13.06 |
| MNS-2 | 94.10 | 23.18 | 2189.00 | 9.63 | 81.79 | 1726.00 | 5.06 | 10.16 | 36.87 | 15.50 |
| MNS-3 | 10.26 | 15.94 | 1315.00 | 4.26 | 57.80 | 123bdl | 2.51 | 3.47 | 77.67 | 27.00 |
| MNS-4 | 43.15 | 19.99 | 143bdl | 6.31 | 48.03 | 1008.00 | 4.16 | 10.44 | 45.30 | 9.93 |
| Rium-1 | 32.89 | 8.27 | 475.19 | 1049.64 | 56.74 | 800.54 | 0.16 | 4.32 | 7.28 | 7.15 |
| Rium-2 | 10.97 | 10.08 | 744.40 | 413.60 | 50.64 | 711.86 | 0.26 | 0.68 | 14.12 | 8.00 |
| SW2 | 31.78 | 20.72 | 1131.24 | 943.57 | 87.91 | 1489.08 | 0.23 | 7.80 | 1.28 | 2.94 |
| SW3 | 32.31 | 13.55 | 72.32 | 643.65 | 32.77 | 284.27 | 0.23 | 5.40 | 8.68 | 1.94 |
References
- Harlow, G.E.; Sorensen, S.S. Jade (nephrite and jadeitite) and serpentinite: Metasomatic connections. Int. Geol. Rev. 2005, 47, 113–146. [Google Scholar] [CrossRef]
- Harlow, G.E.; Sorensen, S.S.; Sisson, V.B.; Shi, G. Chapter 10: The geology of jade deposits. In The Geology of Gem Deposits, 2nd ed.; Groat, L.A., Ed.; Mineralogical Association of Canada: Québec City, QC, Canada, 2014; pp. 305–374. [Google Scholar]
- Zhong, Q.; Liao, Z.; Qi, L.; Zhou, Z. Black Nephrite Jade from Guangxi, Southern China. Gems Gemol. 2019, 55, 198–215. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Yang, R.D.; Gao, J.B.; Chen, J.; Liu, Y.N.; Zhou, Z.R. Element geochemical characteristics of nephrite in Luodian, Guizhou Province. J. Mineral. 2015, 35, 59–67. [Google Scholar]
- Liu, Y.; Deng, J.; Shi, G.; Sun, X.; Yang, L. Geochemistry and petrogenesis of placer nephrite from Hetian, Xinjiang, Northwest China. Ore Geol. Rev. 2011, 41, 122–132. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Shi, G.; Yui, T.-F.; Zhang, G.; Abuduwayiti, M.; Yang, L.; Sun, X. Geochemistry and petrology of nephrite from Alamas, Xinjiang, NW China. J. Asian Earth Sci. 2011, 42, 440–451. [Google Scholar] [CrossRef]
- Kolesnik, Y.N. Nephrites of Siberia; Nauka: Novosibirsk, Russia, 1966. (In Russian) [Google Scholar]
- Zamaletdinov, R.S.; Yanshin, I.S. Geologic features and formationconditions of the East Sayan nephrite deposits. Razved. Okhrana Nedr. 1971, 8, 15–17. [Google Scholar]
- Suturin, A.N.; Zamaletdinov, R.S. Nephrites; Nauka: Novosibirsk, Russia, 1984. (In Russian) [Google Scholar]
- Prokhor, S.A. The Genesis of Nephrite and Emplacement of the Nephrite-Bearing Ultramafic Complexes of East Sayan. Int. Geol. Rev. 1991, 33, 290–300. [Google Scholar] [CrossRef]
- Zhang, S.Y. Gemological Characteristics of Russian Gorlik-gol Green Nephrite and Research on Composition of Melanocratic Mineral; China University of Geosciences: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Burtseva, M.; Ripp, G.; Posokhov, V.; Murzintseva, A. Nephrites of East Siberia: Geochemical features and problems of genesis. Russ. Geol. Geophys. 2015, 56, 402–410. [Google Scholar] [CrossRef]
- Tsydenova, N.; Morozov, M.V.; Rampilova, M.V.; Vasil’Ev, Y.A.; Matveeva, O.P.; Konovalov, P.B. Chemical and spectroscopic study of nephrite artifacts from Transbaikalia, Russia: Geological sources and possible transportation routes. Quat. Int. 2015, 355, 114–125. [Google Scholar] [CrossRef]
- Kislov, E.V. Russian nephrite resources and related genesis study. In Proceedings of the China International Gems & Jewelry Academic Conference, Beijing, China, 2–3 November 2019; Volume 211–213. [Google Scholar]
- Khudyakova, L.I.; Kislov, E.V.; Paleev, P.L.; Kotova, I.Y. Nephrite-Bearing Mining Waste as a Promising Mineral Additive in the Production of New Cement Types. Minerals 2020, 10, 394. [Google Scholar] [CrossRef]
- Kazak, A.P.; Dobretsov, N.L.; Moldavantsev, Y.E. Glaucophane schists, jadeites, vesuvianites and nephrites of the Rai-Iz hy-perbasite massif. Geol. I Geofiz. 1976, 2, 60–66. (In Russian) [Google Scholar]
- Arkhireev, I.E.; Makagonov, E.P.; Belyatskii, B.V.; Maslennikov, V.V. Age of nephrite-bearing dikes of the Uzunkyr Belt (South Urals): Local U-Pb isotope analysis of zircon and Sr-Nd isotope data of rock-forming minerals. Dokl. Earth Sci. 2012, 442, 70–75. [Google Scholar] [CrossRef]
- Kislov, E.V.; Erokhin, Y.V.; Popov, M.P.; Nikolayev, A.G. Nephrite of Bazhenovskoye Chrysotile–Asbestos Deposit, Middle Urals: Localization, Mineral Composition and Color. Minerals 2021, 11, 1227. [Google Scholar] [CrossRef]
- Khan, R.A.; Anwar-Ul-Haq, M.; Qasim, M.; Afgan, M.S.; Haq, S.; Hussain, S.Z. Spectroscopic and crystallographic analysis of nephrite jade gemstone using laser induced breakdown spectroscopy, Raman spectroscopy, and X-ray diffraction. Heliyon 2022, 8, e11493. [Google Scholar] [CrossRef]
- Umar, Z.A.; Liaqat, U.; Ahmed, R.; Baig, M.A. Classification of Nephrite Using Calibration-Free Laser Induced Breakdown Spectroscopy (CF–LIBS) with Comparison to Laser Ablation–Time-of-Flight–Mass Spectrometry (LA–TOF–MS). Anal. Lett. 2019, 53, 203–216. [Google Scholar] [CrossRef]
- Rehman, H.U.; Bilal, S.O.; Rahman, O.U.; Shen, A.H. Namak Mandi: A Pioneering Gemstone Market in Pakistan. Gems Gemol. 2021, 57, 138–149. [Google Scholar] [CrossRef]
- Obiadi, S.S.; Amini, M.A.; Fazli, F. Mineralogy and Geochemistry of Nephrite from Wolay Deposite, Kunar, East Afghanistan. J. Mech. Civ. Ind. Eng. 2020, 3, 56–65. [Google Scholar] [CrossRef]
- Liu, X.F.; Zhang, H.Q.; Liu, Y.; Zhang, Y.; Li, Z.J.; Zhang, J.H.; Zheng, F. Mineralogical Characteristics and Genesis of Green Nephrite from the World. Rock Miner. Anal. 2018, 37, 479–489, (In Chinese with English Abstract). [Google Scholar]
- Guo, L.H.; Han, J.Y. The IR analyses of M1 and M3 cation occupation of Hetian jade, Manas green jade and Xiuyan old jade. Acta Petrol. Miner. 2002, 21, 68–71, (In Chinese with English Abstract). [Google Scholar]
- Tang, Y.L.; Liu, D.Q.; Zhou, H.R. Geological characteristics of Manas green jade in Xinjiang. Acta Petrol. Miner. 2002, 21, 22–25, (In Chinese with English Abstract). [Google Scholar]
- Sun, L.H.; Yu, F.; Wang, S.Q. Gemological research on Manas green jade. Acta Petrol. Miner. 2011, 30, 35–38, (In Chinese with English Abstract). [Google Scholar]
- Jia, Y.H.; Liu, X.F.; Liu, Y.; Zhang, Q.C.; Zhang, Y.; Li, Z.J. Petrogenesis of the serpentinite-related nephrite deposit in Qiemo County, Xinjiang. Acta Petrol. Miner. 2018, 37, 824–838, (In Chinese with English Abstract). [Google Scholar]
- Liu, F.; Yu, X. Classification and mineralogical characteristics of nephrite deposits in China. Miner. Resour. Geol. 2009, 23, 375–380. [Google Scholar]
- Liu, X.L.; Yang, N.; Deng, S.L.; Meng, Q.P. Research on Gemological Characteristics of Green Nephrite in Ruoqiang Aqikekule of Xinjiang. Xinjiang Geol. 2019, 37, 359–362, (In Chinese with English Abstract). [Google Scholar]
- Yu, H.; Wang, R.; Guo, J.; Li, J.; Yang, X. Study of the minerogenetic mechanism and origin of Qinghai nephrite from Golmud, Qinghai, Northwest China. Sci. China Earth Sci. 2016, 59, 1597–1609. [Google Scholar] [CrossRef]
- Tan, L.P.; Lee, C.W.; Chen, C.C.; Tien, P.L.; Tsui, P.C.; Yui, T.F. A mineralogical study of the Fengtian nephrite deposits of Hualien, Taiwan. Nat. Sci. Coun. Spec. Publ. 1978, 1, 81. [Google Scholar]
- Yui, T.-F.; Yeh, H.-W.; Lee, C.W. Stable isotope studies of nephrite deposits from Fengtien, Taiwan. Geochim. Cosmochim. Acta 1988, 52, 593–602. [Google Scholar] [CrossRef]
- Shen, C.X.; Chen, S.Y.; Li, G.G.; Liu, D.Y. Comparative research on gemological characteristics of Hualien nephrite from Taiwan. Acta Petrol. Miner. 2014, 33, 35–40, (In Chinese with English Abstract). [Google Scholar]
- Lu, B.Q.; Qi, L.J.; Xia, Y.B.; Zhou, K.C. Mineralogy of nephrite (tremolite) cat’s eye from Sichuan Province. Acta Petrol. Miner. 2004, 23, 268–272, (In Chinese with English Abstract). [Google Scholar]
- Ji, J.H.; Lu, B.Q.; Qi, L.J. Geological and micro-texture analysis of Serpentine Cat’s Eye from Sichuan province. Shanghai Land Resour. 2015, 36, 105–108, (In Chinese with English Abstract). [Google Scholar]
- Yang, S.X.; Xiang, J.D.; Du, S.W.; Su, H.; Zhang, R. Jade-searching Potential in the Baishui River Basin, Shimian, Sichuan. Acta Geol. Sichuan 2016, 36, 381–385, (In Chinese with English Abstract). [Google Scholar]
- Gunia, P. Nephrite from south-western Poland as potential raw material of the European Neolithic artefacts. Krystallinikum 2000, 26, 166–171. [Google Scholar]
- Niichol, D. Nephrite jade from Jordanow Slaski, Poland. J. Gemmol. Lond. 2001, 27, 461–470. [Google Scholar] [CrossRef]
- Lobos, K.; Sachanbinski, M.; Pawlik, T. Nefryty z Naslawic na Dolnym slasku (Naslawice nephrite from Lower Silesia). Prze-Glad Geol. 2008, 56, 991–999. (In Polish) [Google Scholar]
- Gil, G.; Bagiński, B.; Gunia, P.; Madej, S.; Sachanbiński, M.; Jokubauskas, P.; Belka, Z. Comparative Fe and Sr isotope study of nephrite deposits hosted in dolomitic marbles and serpentinites from the Sudetes, SW Poland: Implications for Fe-As-Au-bearing skarn formation and post-obduction evolution of the oceanic lithosphere. Ore Geol. Rev. 2020, 118, 103335. [Google Scholar] [CrossRef]
- Mi, L.L. Green Nephrite Jade from Canada. J. Gems Gemmol. 2003, 5, 10–13, (In Chinese with English Abstract). [Google Scholar]
- Sun, L.H.; Wang, S.Q. Mineralogy of green nephrite jade from Canada. Acta Petrol. Miner. 2014, S1, 28–36, (In Chinese with English Abstract). [Google Scholar]
- Jiang, B.; Bai, F.; Zhao, J. Mineralogical and geochemical characteristics of green nephrite from Kutcho, northern British Columbia, Canada. Lithos 2021, 388–389, 106030. [Google Scholar] [CrossRef]
- Liu, J. A gemological study of green nephrite from the Cassiar Mine, Canada. Acta Petrol. Miner. 2014, 33, 10–14, (In Chinese with English Abstract). [Google Scholar]
- Wu, Q.M.; Wu, R.H.; Zhao, Y.Y.; Shi, W. Gemological and Mineralogical Characterisitics of Green Nephrite from Cassiar, Canada. Acta Petrol. Miner. 2014, 33, 43–47, (In Chinese with English Abstract). [Google Scholar]
- Wu, Q.M. Study of Gemological Mineralogy of Green Nephrite from Polar, Canada; China University of Geosciences: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Cooper, A.F. Nephrite and metagabbro in the Haast Schist at Muddy Creek, northwest Otago, New Zealand. N. Z. J. Geol. Geophys. 1995, 38, 325–332. [Google Scholar] [CrossRef]
- Cuthbert, J.; Wilkins, W.; Craighead, T. Spectroscopic and related evidence on the coloring and constitution of New Zealand jade. Am. Mineral. 2003, 88, 1336–1344. [Google Scholar]
- Adams, C.; Beck, R.; Campbell, H. Characterisation and origin of New Zealand nephrite jade using its strontium isotopic signature. Lithos 2007, 97, 307–322. [Google Scholar] [CrossRef]
- Grapes, R.H.; Yun, S.-T. Geochemistry of a New Zealand nephrite weathering rind. N. Z. J. Geol. Geophys. 2010, 53, 413–426. [Google Scholar] [CrossRef]
- Cai, Q.; Zhu, Q.W. A study of gemological characteristics of green nephrite in New Zealand. Acta Petrol. Miner. 2011, 30, 95–100, (In Chinese with English Abstract). [Google Scholar]
- Hockley, J.J. Nephrite (jade) Occurrence in the Great Serpentine Belt of New South Wales, Australia. Nature 1974, 247, 364. [Google Scholar] [CrossRef]
- Hockley, J.J.; Birch, W.D.; Worner, H.K. A nephrite deposit in the great serpentine belt of New South Wales. J. Geol. Soc. Aust. 1978, 25, 249–254. [Google Scholar] [CrossRef]
- Fu, F.F.; Zhang, G.B.; Meng, L.J.; Tang, B.; Yan, J.G. A mineralogical study of Taiwan nephite. Acta Petrol. Miner. 2014, 33, 1–6, (In Chinese with English Abstract). [Google Scholar]
- Wang, J.; Shi, G. Comparative Study on the Origin and Characteristics of Chinese (Manas) and Russian (East Sayan) Green Nephrites. Minerals 2021, 11, 1434. [Google Scholar] [CrossRef]
- Zamaletdinov, R.S.; Suturin, A.N. The Ulankhoda nephrite deposit (East Sayan). Sov. Geol. 1974, 9, 90–98. (In Russian) [Google Scholar]
- Letnikov, F.A.; Sekerin, A.N. Composition and Genesis of Nephrites of the Sayan–Baikal mou ntainous lan d, in: Mineralogy and Genesis of East Siberian Gems; Nauka: Novosibirsk, Russia, 1983; pp. 96–103. (In Russian) [Google Scholar]
- Sekerin, A.P. Nephrite of the Gorlykgol deposit in East Sayan, in: Mineralogy and Genesis of East Siberian Gems; Nauka: Novosibirsk, Russia, 1983; pp. 103–110. (In Russian) [Google Scholar]
- Sekerin, A.P.; Sekerina, N.V. Nephrites and their occurrence in southern Siberia, in: The Baikal Area of Siberia at the Ancient Time. Izd. IGPU Irkutsk 2000, 2, 146–160. (In Russian) [Google Scholar]
- Dobretsov, N.L.; Tatarinov, A.V. Jadeite and Nephrite in Ophiolites; Nauka: Novosibirsk, Russia, 1983. (In Russian) [Google Scholar]
- Chen, B.W.; Chen, T.Y. Basic characteristics and mineralization of the traverse Asian giant tectonic belt. Acta Petrol. 2007, 23, 865–876, (In Chinese with English Abstract). [Google Scholar]
- Khain, E.V.; Bibikova, E.V.; Kroner, A.; Zhuravlev, D.Z.; Sklyarov, E.V.; Fedotov, A.A.; Kravchenko-Berezhnoy, I.R. The most ancient ophiolite of the Central Asian fold belt: U-Pb and Pb-Pb zircon ages for the Dunzhugur Complex, Eastern Sayan, Si-beria, and geodynamic implications. Earth Planet. Sci. Lett. 2002, 199, 311–325. [Google Scholar] [CrossRef]
- Burns, R.G.; Strens, R.G.J. Infrared Study of the Hydroxyl Bands in Clinoamphiboles. Science 1966, 153, 890–892. [Google Scholar] [CrossRef]
- Apopei, A.I.; Buzgar, N. The Raman study of amphiboles. Stiintifice De Univ. AI Cuza Din Lasi. Sect. 2 Geol. 2010, 56, 57–83. [Google Scholar]
- Potgieter-Vermaak, S.; Maledi, N.; Wagner, N.; Heerden, J.; Potgieter, J.H. Raman spectroscopy for the analysis of coal: A review. J. Raman Spectrosc. 2015, 42, 123–129. [Google Scholar] [CrossRef]
- Gao, F.; Deng, H.Z.; Wang, X.F.; Dai, F.W.; Wu, S.Y. Raman spectroscopic analysis of bituminous coal. J. Anal. Sci. 2016, 3, 377–380, (In Chinese with English Abstract). [Google Scholar]
- Asadullah, M.; Zhang, S.; Li, C.-Z. Evaluation of structural features of chars from pyrolysis of biomass of different particle sizes. Fuel Process. Technol. 2010, 91, 877–881. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2271–2288. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processe. Geol. Soc. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Wang, S.Q.; Yuan, X.M. Characteristics of Material Compositions and Geological Origin of Green Hetian Nephrite. J. Gems Gemmol. 2008, 3, 4–7, (In Chinese with English Abstract). [Google Scholar]
- Su, X.F.; Zhou, K.C.; Qi, L.J.; Qian, X.L. Gemological Characteristics of Serpentine Cat’s Eye in Sichuan Province. China Min. 2006, 5, 73–75, (In Chinese with English Abstract). [Google Scholar]
- Zhao, Y.Y. Study of Gemological Mineralogy of Green Nephrite from New Zealand; China University of Geosciences: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Leake, B.E.; Woolley, A.R.; Birch, W.D.; Burke, E.; Ferraris, G.; Grice, J.D.; Grice, F.C.; Hawthorne, H.J.; Kisch, V.G.; Krivovichev, J.C. Nomenclature of amphiboles: Additions and revisions to the international mineralogical association’s amphibole nomenclature. Eur. J. Mineral. 2004, 16, 190–195. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Oberti, R. On the classification of amphiboles. Can. Mineral. 2006, 44, 1–21. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Oberti, R. Amphiboles: Crystal Chemistry. Rev. Mineral. Geochem. 2007, 67, 1–54. [Google Scholar] [CrossRef]
- Hawthorne, F.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Jin, B.F.; Yue, W.; Wang, K.S. The crystallochemistry characteristics and genetic analysis of amphibole in the sediments of the Huanghe River. Acta Oceanol. Sin. 2013, 35, 131–143. (In Chinese) [Google Scholar]
- Bong, W.S.K.; Mastumura, K.; Yokoyama, K. Provenance study of early and middle Bronze Age pottery from Kaman-Kalehoyuk, Turkey, by heavy mineral analysis and geochemical analysis of individual hornblende grasins. J. Archaeol. Sci. 2010, 37, 2165–2178. [Google Scholar] [CrossRef]
- Siqin, B.; Qian, R.; Zhuo, S.; Gan, F.; Dong, M.; Hua, Y. Glow discharge mass spectrometry studies on nephrite minerals formed by different metallogenic mechanisms and geological environments. Int. J. Mass Spectrom. 2012, 309, 206–211. [Google Scholar] [CrossRef]
- Kostov, R.I.; Protochristov, C.; Stoyanov, C.; Csedreki, L.; Simon, A.; Szikszai, Z.; Uzonyi, I.; Chapman, J. Micro-PIXE Geo-chemical Fingerprinting of Nephrite Neolithic Artifacts from Southwest Bulgaria. Geoarchaeology 2012, 27, 457–469. [Google Scholar] [CrossRef]
- Godfrey, L.V.; White, W.M.; Salter, V.J.M. Dissolved zirconium and hafnium distributions across a shelf break in the north-eastern Atlantic Ocean. Geochim. Et Cosmochim. Acta 1996, 60, 3995–4006. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Norisuye, K.; Nakagawa, Y.; Nakatsuka, S.; Sohrin, Y. Dissolved and labile particulate Zr, Hf, Nb, Ta, Mo and W in the western North Pacific Ocean. J. Oceanogr. 2008, 64, 247–257. [Google Scholar] [CrossRef]
- Bau, M.; Alexander, B.W. Distribution of high field strength elements (Y, Zr, REE, Hf, Ta, Th, U) in adjacent magnetite and chert bands and in reference standards FeR-3 and FeR-4 from the Temagami iron-formation, Canada, and the redox level of the Neoarchean ocean. Precambrian Res. 2009, 174, 337–346. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, D.S. Application of microelements analysis in identifying sedimentary environment—Taking Qianjiang formation in the Jianghan Basin as an example. Pet. Geol. 2007, 29, 307–310, (In Chinese with English Abstract). [Google Scholar]
- Deng, P. The application of trace amount of elements in the exploration of oil and gas. Pet. Explor. 1993, 20, 27–32, (In Chinese with English Abstract). [Google Scholar]
- Peng, L.C.; Han, D.X.; Zhou, J.J. Element geochemistry characteristics of different sedimentary environments of Lengke No.1, Caidamu Basin. Coal Geol. Explor. 2011, 29, 1–3, (In Chinese with English Abstract). [Google Scholar]
- Beyssac, O.; Goffé, B.; Chopin, C.; Rouzaud, J.N. Raman spectra of carbonaceous material in metasediments: A new geother-mometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Beyssac, O.; Brunet, F.; Petitet, J.P.; Goffé, B.; Rouzaud, J.N. Experimental study of the microtextural and structural trans-formations of carbonaceous materials under pressure and temperature. Eur. J. Mineral. 2004, 15, 937–951. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Kwiecinska, B.; Suárez-Ruiz, I.; Paluszkiewicz, C.; Rodriques, S. Raman spectroscopy of selected carbonaceous samples. Int. J. Coal Geol. 2010, 84, 206–212. [Google Scholar] [CrossRef]
- Beny-Bassez, C.; Rouzaud, J.N. Characterization of carbonaceous materials by correlated electron and optical microscopy and Raman microspectroscopy. Scanning Electron Microsc. 1985, 1, 119–132. [Google Scholar]
- Zhang, C.; Yu, X.Y. Spectral Characteristic and Origin Identification of Tremolite Jade from Sangpiyu, Liaoning Province. J. Gems Gemmol. 2018, 1, 41–53, (In Chinese with English Abstract). [Google Scholar]
- Wang, A.; Dhamenincourt, P.; Dubessy, J.; Guerard, D.; Landais, P.; Lelaurain, M. Characterization of graphite alteration in an uranium deposit by micro-Raman spectroscopy, X-ray diffraction, transmission electron microscopy and scanning electron microscopy. Carbon 1989, 27, 209–218. [Google Scholar] [CrossRef]
- Coleman, R.G. New Zealand serpentinite and associated metasomaticrocks. Bull. N. Z. Geol. Surv. 1966, 76, 102. [Google Scholar]
- Wang, X.M.; Zeng, Z.G.; Chen, J.B. Serpentine of peridotite in the southern forearc of Mariana. Prog. Nat. Sci. 2009, 19, 1287–1295. [Google Scholar] [CrossRef]
- Toft, P.B.; Arkani-Hamed, J.; Haggerty, S.E. The effects of serpentinization on density and magnetic susceptibility: A petrophysical model. Phys. Earth Planet. Inter. 1990, 65, 137–157. [Google Scholar] [CrossRef]
- Coleman, R.G. Low-temperature reaction zones and alpine ultramafic rocks of California, Oregon, and Washington. US Geol. Surv. Bull. 1967, 1247, 1–49. [Google Scholar] [CrossRef]
- Coleman, R.G. Ophiolites, Ancient Oceanic Lithosphere? Springer Verlag: Berlin, Germany, 1977; 229p. [Google Scholar]
- O’hanley, D.S. Serpentinites, recorders of tectonic and petrological history. Oxf. Mono. Geol. Geophys. 1996, 34, 256. [Google Scholar]
- Karpov, I.K.; Chudnenko, K.V.; Suturin, A.N. Physicochemical modeling of processes of contact-infiltration metasomatism. Doklady. Earth Sci. Sect. 1987, 297, 189–192. [Google Scholar]
- Liu, X.F.; Liu, Y.; Li, Z.J.; Maituohuti, A.; Tian, G.Y.; Guo, D.X. The genesis and SHRIMP U-Pb zircon dating of the Pishan brown nephrite-bearing Mg-skarn deposit in Xinjiang. Acta Petrol. Miner. 2017, 2, 259–273, (In Chinese with English Abstract). [Google Scholar]
- Zhao, Y.Y.; Wu, R.H.; Wu, Q.M.; Shi, W. A study of gemological characteristics of green nephrite in Ospa 11# mining area of Russia. Acta Petrol. Miner. 2014, 33, 37–42, (In Chinese with English Abstract). [Google Scholar]








| Sample | RC-1 | RC-2 | RC-3 | RC-4 | RC-5 | RC-6 | RC-7 | RC-8 | RC-9 | RC-10 |
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 56.65 | 58.17 | 57.78 | 56.34 | 55.64 | 56.32 | 56.19 | 56.05 | 56.68 | 57.63 |
| Na2O | 0.06 | 0.07 | 0.06 | 0.03 | 0.08 | 0.08 | 0.07 | 0.09 | 0.06 | 0.06 |
| Cr2O3 | 0.01 | 0.03 | 0.04 | 0.03 | 0.19 | 0.13 | 0.14 | 0.03 | 0.31 | 0.11 |
| K2O | 0.06 | 0.04 | 0.07 | 0.20 | 0.13 | 0.04 | 0.02 | 0.05 | 0.02 | 0.04 |
| MgO | 21.75 | 22.30 | 22.27 | 21.91 | 21.54 | 21.41 | 21.36 | 21.43 | 21.68 | 22.34 |
| MnO | 0.11 | 0.12 | 0.11 | 0.12 | 0.10 | 0.11 | 0.12 | 0.13 | 0.10 | 0.12 |
| FeO | 3.90 | 3.45 | 3.79 | 3.71 | 4.03 | 3.97 | 3.78 | 3.91 | 3.49 | 3.67 |
| Al2O3 | 0.31 | 0.30 | 0.27 | 0.56 | 0.65 | 0.38 | 0.34 | 0.37 | 0.43 | 0.29 |
| NiO | 0.08 | 0.16 | 0.12 | 0.10 | 0.13 | 0.14 | 0.13 | 0.11 | 0.16 | 0.11 |
| CaO | 12.17 | 12.30 | 12.16 | 11.82 | 12.13 | 12.17 | 12.27 | 11.59 | 12.47 | 12.10 |
| TiO2 | 0.01 | 0.01 | 0.03 | 0.02 | 0.02 | 0.01 | 0.04 | 0.04 | 0.02 | bdl |
| Cl | bdl | 0.01 | 0.02 | 0.01 | bdl | bdl | 0.01 | 0.02 | 0.01 | bdl |
| Total | 95.10 | 96.95 | 96.71 | 94.85 | 94.64 | 94.75 | 94.46 | 93.81 | 95.41 | 96.46 |
| Si | 8.00 | 8.03 | 8.01 | 7.97 | 7.92 | 7.99 | 8.00 | 8.02 | 7.98 | 8.01 |
| Na | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Cr | bdl | bdl | 0.01 | bdl | 0.02 | 0.01 | 0.02 | bdl | 0.03 | 0.01 |
| K | 0.01 | 0.01 | 0.01 | 0.04 | 0.02 | 0.01 | bdl | 0.01 | bdl | 0.01 |
| Mg | 4.58 | 4.59 | 4.61 | 4.62 | 4.57 | 4.53 | 4.53 | 4.57 | 4.55 | 4.63 |
| Mn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 |
| Fe | 0.46 | 0.40 | 0.44 | 0.44 | 0.48 | 0.48 | 0.45 | 0.47 | 0.41 | 0.43 |
| Al | 0.05 | 0.05 | 0.04 | 0.09 | 0.11 | 0.06 | 0.06 | 0.06 | 0.07 | 0.05 |
| Ni | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 |
| Ca | 1.84 | 1.82 | 1.81 | 1.79 | 1.85 | 1.85 | 1.87 | 1.78 | 1.88 | 1.80 |
| Ti | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.01 | bdl | bdl |
| Cl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Sum | 14.98 | 14.95 | 14.98 | 15.00 | 15.03 | 14.98 | 14.98 | 14.97 | 14.98 | 14.97 |
| Mg + Fe | 5.04 | 4.99 | 5.04 | 5.06 | 5.05 | 5.00 | 4.98 | 5.04 | 4.96 | 5.05 |
| Mg/(Mg + Fe) | 0.91 | 0.92 | 0.91 | 0.91 | 0.90 | 0.91 | 0.91 | 0.91 | 0.92 | 0.92 |
| Fe/(Mg + Fe) | 0.09 | 0.08 | 0.09 | 0.09 | 0.10 | 0.09 | 0.09 | 0.09 | 0.08 | 0.08 |
| Sample | RC1 | RC2 | RC3 | RC5 | ||||||||
| 01 | 02 | 03 | 01 | 02 | 03 | 01 | 02 | 03 | 01 | 02 | 03 | |
| Li | 0.26 | 0.32 | 0.28 | 0.60 | 0.47 | 0.44 | 0.13 | 0.16 | 0.14 | 0.49 | 0.55 | 0.51 |
| Be | 0.30 | 0.33 | 0.20 | 0.49 | 0.65 | 0.35 | 0.23 | 0.35 | 0.25 | 0.51 | 0.49 | 0.37 |
| Sc | 0.43 | 0.65 | 0.46 | 1.75 | 1.62 | 1.77 | 0.47 | 0.56 | 0.55 | 2.44 | 2.62 | 2.16 |
| Ti | 100.40 | 130.51 | 107.13 | 156.15 | 140.65 | 148.25 | 86.47 | 93.79 | 95.65 | 134.93 | 141.64 | 115.80 |
| V | 4.38 | 5.69 | 4.55 | 8.60 | 7.95 | 8.05 | 4.90 | 5.24 | 5.21 | 9.75 | 10.41 | 8.45 |
| Cr | 2.30 | 1.84 | 2.76 | 196.73 | 174.51 | 167.93 | 15.57 | 17.81 | 19.30 | 893.38 | 967.12 | 778.95 |
| Mn | 472.26 | 619.23 | 504.52 | 724.43 | 664.68 | 692.81 | 372.91 | 407.63 | 413.32 | 614.44 | 642.69 | 521.54 |
| Ni | 514.73 | 672.90 | 550.83 | 1190.88 | 1105.36 | 1155.08 | 584.88 | 633.07 | 636.04 | 1014.43 | 1056.54 | 865.86 |
| Co | 28.26 | 36.58 | 29.95 | 44.43 | 39.83 | 41.06 | 23.89 | 26.12 | 25.63 | 38.19 | 40.80 | 32.92 |
| Cu | 0.13 | bdl | 0.19 | 0.17 | 0.12 | 0.53 | 0.04 | 0.12 | 0.10 | 0.28 | 0.01 | 0.27 |
| Zn | 68.30 | 90.72 | 72.90 | 119.80 | 107.91 | 111.88 | 57.46 | 62.26 | 62.34 | 93.96 | 96.83 | 78.96 |
| Ga | 0.66 | 0.86 | 0.61 | 0.86 | 0.91 | 1.02 | 0.60 | 0.63 | 0.69 | 0.89 | 0.98 | 0.87 |
| Rb | 0.57 | 0.94 | 0.55 | 0.73 | 0.76 | 0.95 | 0.63 | 0.69 | 0.67 | 0.99 | 1.09 | 0.96 |
| Sr | 2.65 | 3.44 | 2.91 | 3.79 | 3.74 | 3.83 | 2.26 | 2.40 | 2.46 | 3.11 | 3.52 | 2.81 |
| Zr | 0.06 | 0.20 | 0.15 | 0.18 | 0.21 | 0.21 | 0.25 | 0.22 | 0.28 | 0.26 | 0.18 | 0.15 |
| Nb | 0.01 | 0.02 | 0.01 | 0.04 | 0.05 | 0.04 | 0.02 | 0.02 | 0.03 | 0.03 | 0.07 | 0.02 |
| Ba | 1.82 | 2.37 | 1.94 | 1.82 | 2.18 | 2.97 | 1.71 | 2.01 | 2.19 | 2.60 | 3.24 | 2.89 |
| Hf | 0.02 | 0.03 | 0.03 | 0.01 | 0.01 | 0.05 | 0.03 | 0.03 | 0.03 | 0.05 | 0.03 | bdl |
| Ta | bdl | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | bdl | bdl | 0.01 | 0.03 | bdl |
| Pb | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 |
| Th | 0.02 | 0.01 | 0.02 | 0.03 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | bdl | bdl |
| U | 0.01 | 0.01 | bdl | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | bdl | 0.02 | 0.02 |
| Sample | RC6 | RC8 | RC9 | RC10 | ||||||||
| 01 | 02 | 03 | 01 | 02 | 03 | 01 | 02 | 03 | 01 | 02 | 03 | |
| Li | 0.67 | 0.58 | 0.70 | 0.48 | 0.47 | 0.38 | 3.92 | 1.41 | 1.54 | 0.57 | 0.64 | 0.31 |
| Be | 0.50 | 0.21 | 0.76 | 0.75 | 0.22 | 0.38 | 1.52 | 0.58 | 0.19 | 0.50 | 0.04 | 0.35 |
| Sc | 2.46 | 2.33 | 2.90 | 1.42 | 1.31 | 1.37 | 14.38 | 5.32 | 6.23 | 1.83 | 1.94 | 1.03 |
| Ti | 144.68 | 141.06 | 156.00 | 172.04 | 156.95 | 145.79 | 500.06 | 202.33 | 230.21 | 159.93 | 168.53 | 92.22 |
| V | 10.03 | 9.52 | 11.51 | 8.57 | 7.59 | 7.19 | 32.51 | 12.16 | 13.38 | 11.47 | 11.52 | 6.52 |
| Cr | 697.53 | 640.51 | 747.27 | 68.67 | 61.51 | 59.92 | 5749.78 | 2119.58 | 2398.10 | 330.61 | 320.36 | 158.56 |
| Mn | 687.16 | 679.60 | 792.57 | 933.49 | 838.70 | 789.74 | 1692.79 | 744.78 | 798.29 | 692.58 | 718.40 | 402.83 |
| Ni | 1070.42 | 1040.15 | 1148.30 | 1131.03 | 1019.83 | 956.90 | 4204.46 | 1758.10 | 1973.65 | 987.73 | 1063.48 | 563.60 |
| Co | 40.75 | 40.16 | 44.69 | 48.33 | 43.32 | 41.70 | 128.94 | 53.63 | 61.25 | 44.06 | 46.75 | 24.98 |
| Cu | 0.10 | 0.14 | 0.29 | 0.02 | 0.16 | 0.14 | 0.11 | 0.33 | 0.22 | 0.32 | 0.13 | 1.80 |
| Zn | 105.87 | 106.29 | 118.73 | 139.38 | 125.25 | 116.72 | 288.88 | 120.35 | 130.71 | 112.57 | 112.17 | 75.17 |
| Ga | 1.00 | 0.84 | 1.13 | 1.24 | 1.15 | 1.15 | 3.29 | 1.48 | 1.78 | 11.06 | 0.97 | 0.67 |
| Rb | 1.30 | 1.78 | 1.26 | 1.07 | 0.82 | 0.90 | 4.15 | 1.94 | 2.39 | 0.79 | 1.17 | 0.37 |
| Sr | 3.31 | 3.16 | 3.71 | 4.88 | 4.30 | 3.96 | 12.39 | 5.28 | 6.10 | 4.95 | 3.52 | 1.78 |
| Zr | 0.31 | 0.25 | 0.31 | 0.16 | 0.15 | 0.20 | 0.30 | 0.23 | 0.41 | 0.11 | 0.06 | 0.06 |
| Nb | 0.06 | 0.05 | 0.06 | 0.08 | 0.04 | 0.09 | 0.29 | 0.10 | 0.07 | 0.04 | 0.04 | 0.04 |
| Ba | 2.91 | 3.07 | 3.69 | 3.78 | 3.48 | 3.09 | 11.47 | 5.14 | 5.22 | 51.93 | 2.57 | 2.39 |
| Hf | 0.02 | 0.08 | 0.02 | bdl | 0.08 | 0.06 | 0.12 | 0.04 | 0.01 | 0.03 | 0.02 | 0.01 |
| Ta | 0.01 | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 | 0.02 | 0.03 | 0.02 | 0.02 | 0.01 | bdl |
| Pb | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 |
| Th | 0.03 | 0.03 | 0.05 | 0.04 | 0.01 | 0.01 | 0.04 | 0.03 | 0.03 | 0.01 | 0.03 | 0.01 |
| U | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.01 | 0.06 | 0.02 | 0.04 | 0.02 | 0.03 | 0.01 |
| Sample | RC1-1 | RC2-1 | RC3-1 | RC5-1 | RC6-1 | RC8-1 | RC9-1 | RC10-1 |
|---|---|---|---|---|---|---|---|---|
| La | 0.14 | 0.09 | 0.13 | 0.08 | 0.05 | 0.12 | 0.07 | 0.06 |
| Ce | 0.43 | 0.19 | 0.37 | 0.26 | 0.17 | 0.19 | 0.23 | 0.15 |
| Pr | 0.07 | 0.04 | 0.05 | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 |
| Nd | 0.41 | 0.27 | 0.26 | 0.34 | 0.25 | 0.23 | 0.29 | 0.25 |
| Sm | 0.27 | 0.26 | 0.27 | 0.25 | 0.31 | 0.24 | 0.26 | 0.25 |
| Eu | 0.07 | 0.06 | 0.08 | 0.07 | 0.06 | 0.09 | 0.07 | 0.07 |
| Gd | 0.27 | 0.29 | 0.35 | 0.30 | 0.35 | 0.28 | 0.28 | 0.27 |
| Tb | 0.04 | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | 0.05 | 0.05 |
| Dy | 0.17 | 0.17 | 0.17 | 0.18 | 0.18 | 0.16 | 0.19 | 0.18 |
| Ho | 0.04 | 0.03 | 0.05 | 0.04 | 0.04 | 0.05 | 0.04 | 0.05 |
| Er | 0.11 | 0.11 | 0.10 | 0.10 | 0.13 | 0.14 | 0.11 | 0.12 |
| Tm | 0.03 | 0.04 | 0.04 | 0.03 | 0.05 | 0.04 | 0.05 | 0.04 |
| Yb | 0.14 | 0.16 | 0.17 | 0.20 | 0.17 | 0.21 | 0.20 | 0.20 |
| Lu | 0.05 | 0.04 | 0.04 | 0.05 | 0.04 | 0.04 | 0.05 | 0.04 |
| Y | 0.21 | 0.26 | 0.25 | 0.10 | 0.15 | 0.24 | 0.16 | 0.23 |
| ΣREE | 2.24 | 1.79 | 2.11 | 1.97 | 1.88 | 1.86 | 1.93 | 1.79 |
| LREE | 1.39 | 0.91 | 1.15 | 1.03 | 0.88 | 0.91 | 0.97 | 0.83 |
| HREE | 0.84 | 0.87 | 0.96 | 0.94 | 1.00 | 0.95 | 0.96 | 0.96 |
| LREE/HREE | 1.66 | 1.04 | 1.19 | 1.10 | 0.89 | 0.96 | 1.01 | 0.87 |
| LaN/YbN | 0.74 | 0.42 | 0.52 | 0.30 | 0.22 | 0.40 | 0.27 | 0.22 |
| δEu | 0.83 | 0.71 | 0.77 | 0.81 | 0.56 | 1.05 | 0.77 | 0.85 |
| δCe | 1.11 | 0.76 | 1.19 | 1.05 | 0.78 | 0.60 | 0.91 | 0.70 |
| Peak Area | BeginPointX (cm−1) | EndPointX (cm−1) | FWHM (cm−1) | Raman Shift (cm−1) | Intensity (Counts) | |
|---|---|---|---|---|---|---|
| D1 | 6585.20 | 1288.44 | 1402.74 | 44.35 | 1352.14 | 134.34 |
| G | 10,289.37 | 1523.25 | 1619.01 | 20.75 | 1582.07 | 362.34 |
| D2 | 698.97 | 1619.01 | 1646.60 | 11.80 | 1619.01 | 57.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Bai, F.; Zhao, C.; Qu, H.; Li, X. Petrogenesis of Chatoyant Green Nephrite from Serpentinite-Related Deposits, Ospinsk, Russia: Insights from Mineralogy and Geochemistry. Crystals 2023, 13, 252. https://doi.org/10.3390/cryst13020252
He W, Bai F, Zhao C, Qu H, Li X. Petrogenesis of Chatoyant Green Nephrite from Serpentinite-Related Deposits, Ospinsk, Russia: Insights from Mineralogy and Geochemistry. Crystals. 2023; 13(2):252. https://doi.org/10.3390/cryst13020252
Chicago/Turabian StyleHe, Weishi, Feng Bai, Chen Zhao, Hongting Qu, and Xuemei Li. 2023. "Petrogenesis of Chatoyant Green Nephrite from Serpentinite-Related Deposits, Ospinsk, Russia: Insights from Mineralogy and Geochemistry" Crystals 13, no. 2: 252. https://doi.org/10.3390/cryst13020252
APA StyleHe, W., Bai, F., Zhao, C., Qu, H., & Li, X. (2023). Petrogenesis of Chatoyant Green Nephrite from Serpentinite-Related Deposits, Ospinsk, Russia: Insights from Mineralogy and Geochemistry. Crystals, 13(2), 252. https://doi.org/10.3390/cryst13020252






