Nanostructure, Mechanical Properties, and Corrosion Resistance of Super Duplex Stainless Steel 2507 Aged at 500 °C
Abstract
1. Introduction
2. Materials and Methods
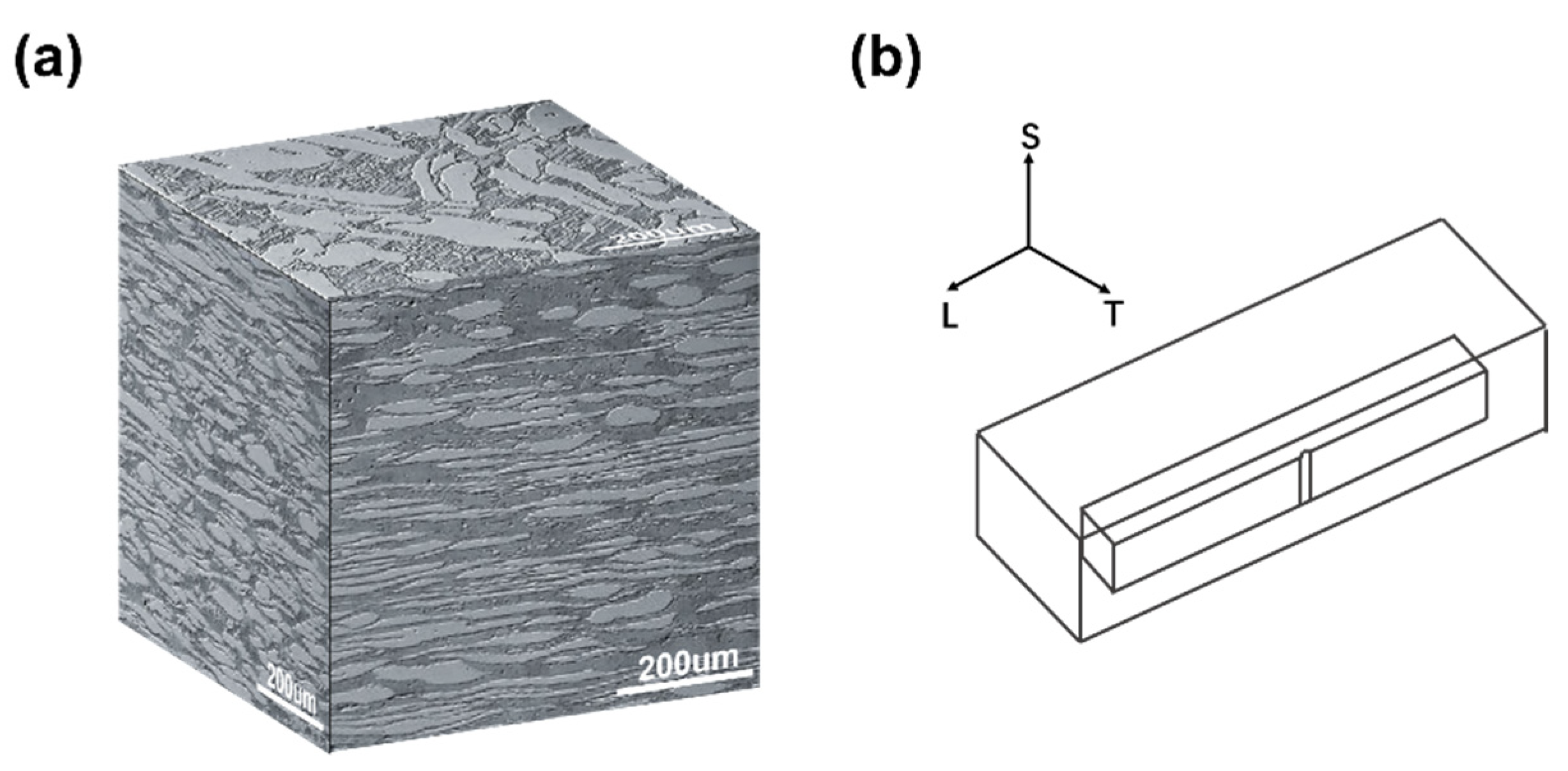
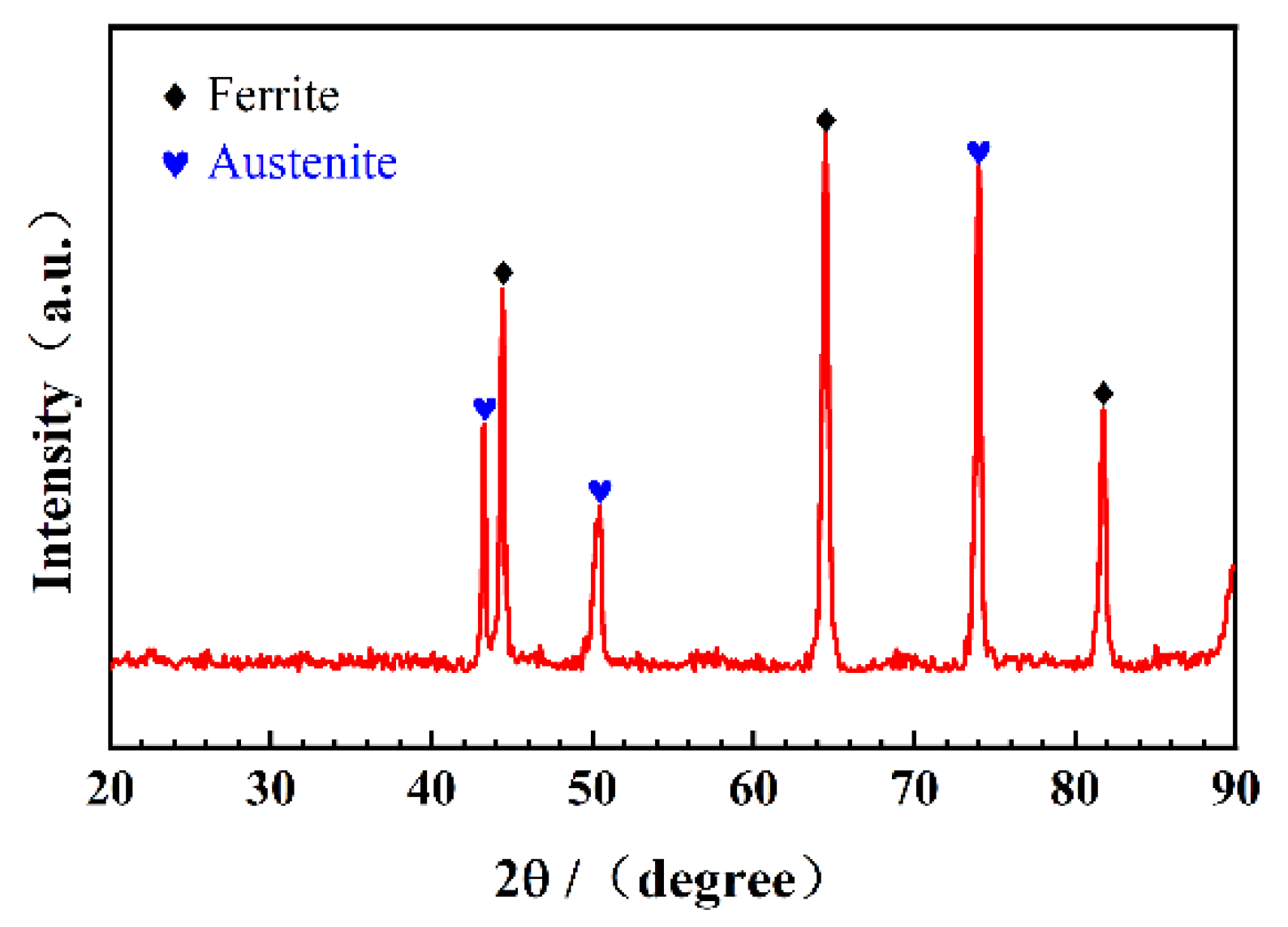
2.1. Materials
2.2. Mechanical Testing
2.3. Small-Angle Neutron Scattering (SANS) Measurements
2.3.1. Experimental Details
2.3.2. Quantitative Analysis of SANS Data
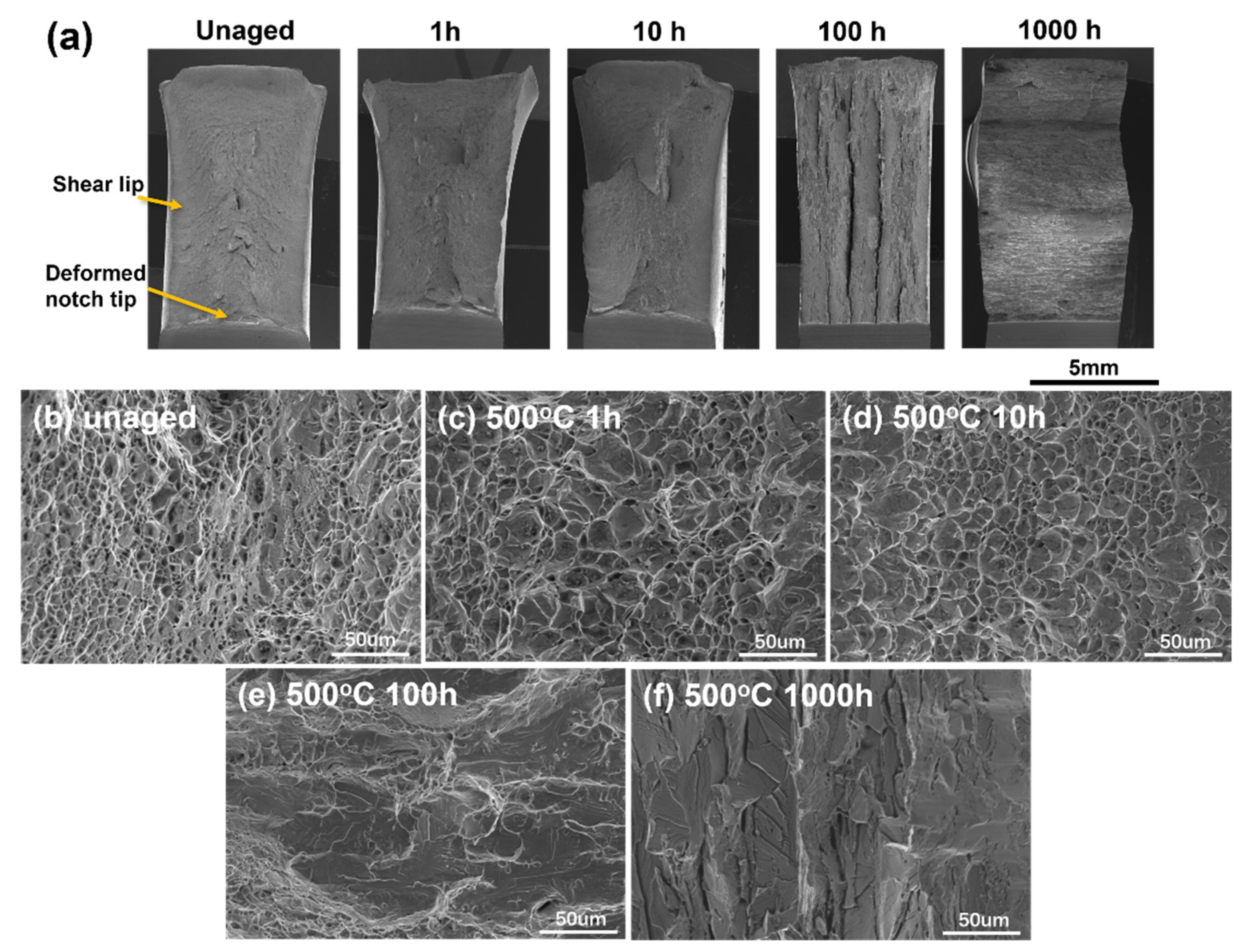
2.4. Fractograph Observation of Impact Toughness Specimens
2.5. Electrochemical Methods
3. Results
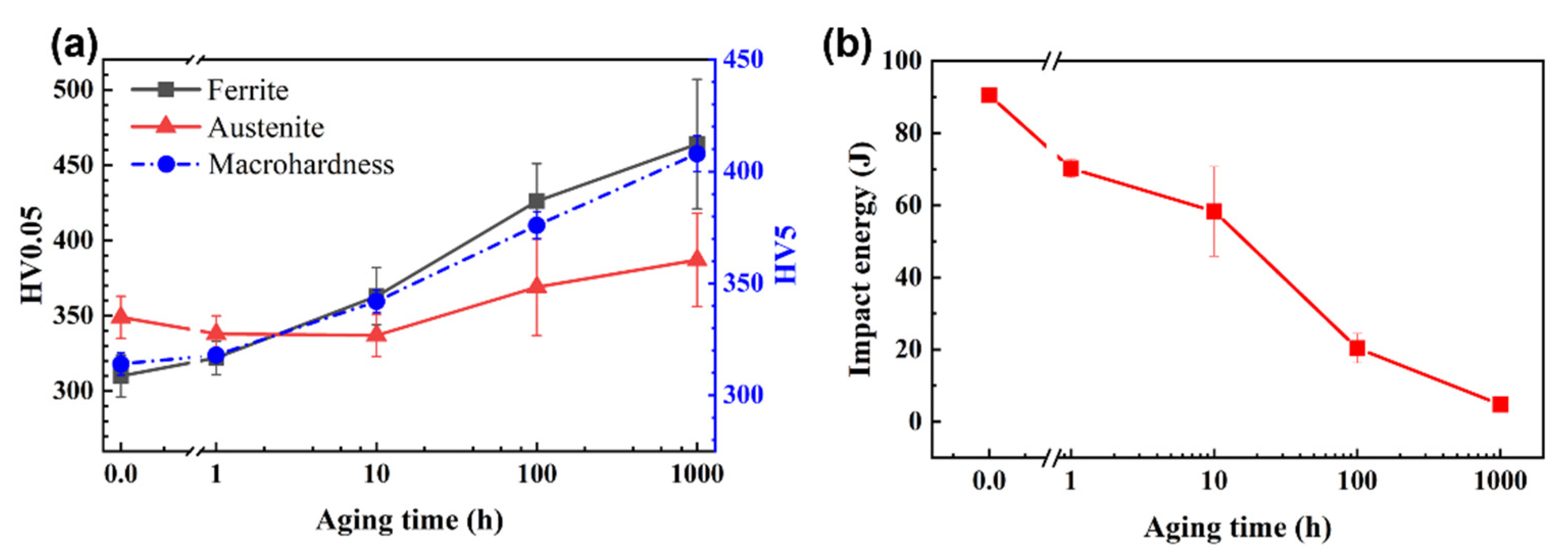
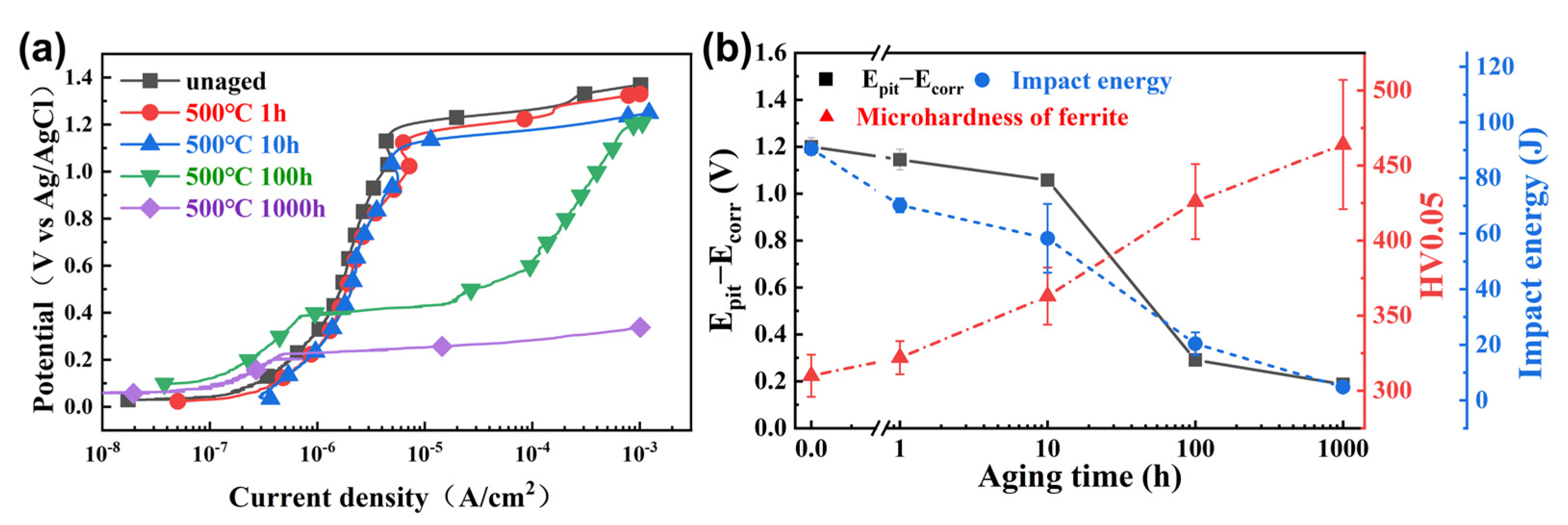
3.1. Vickers-Hardness
3.2. Impact Toughness and Fracture Behavior
3.3. Corrosion Behavior
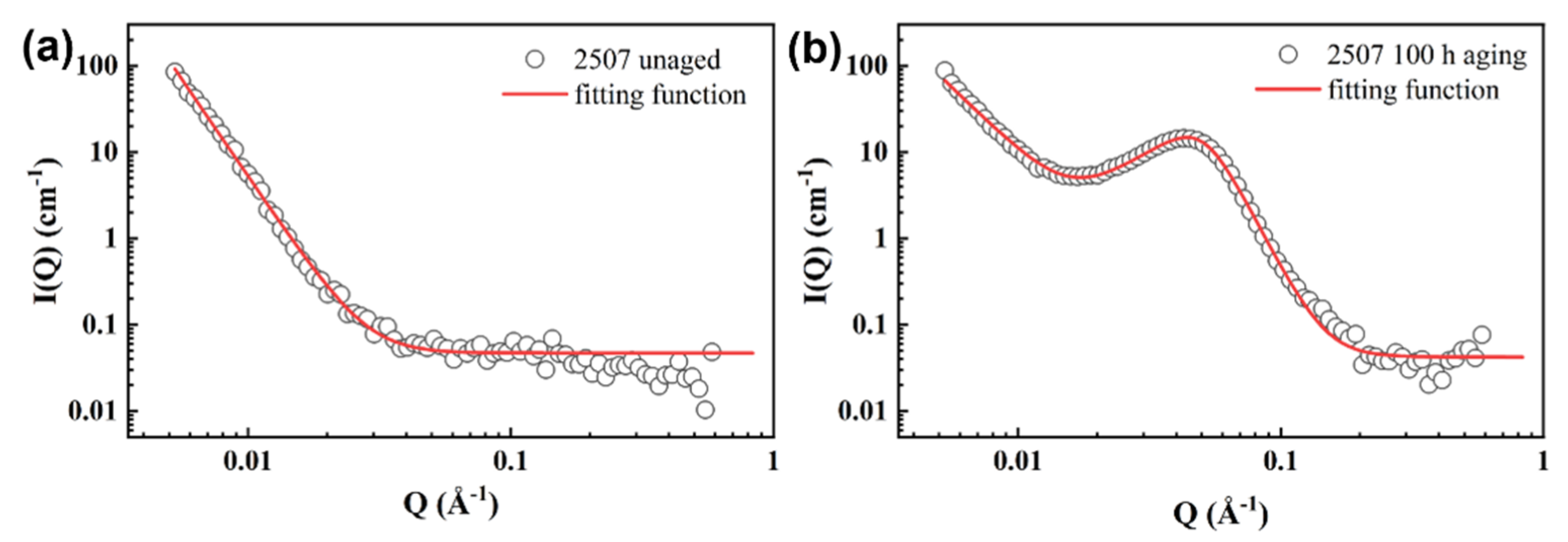
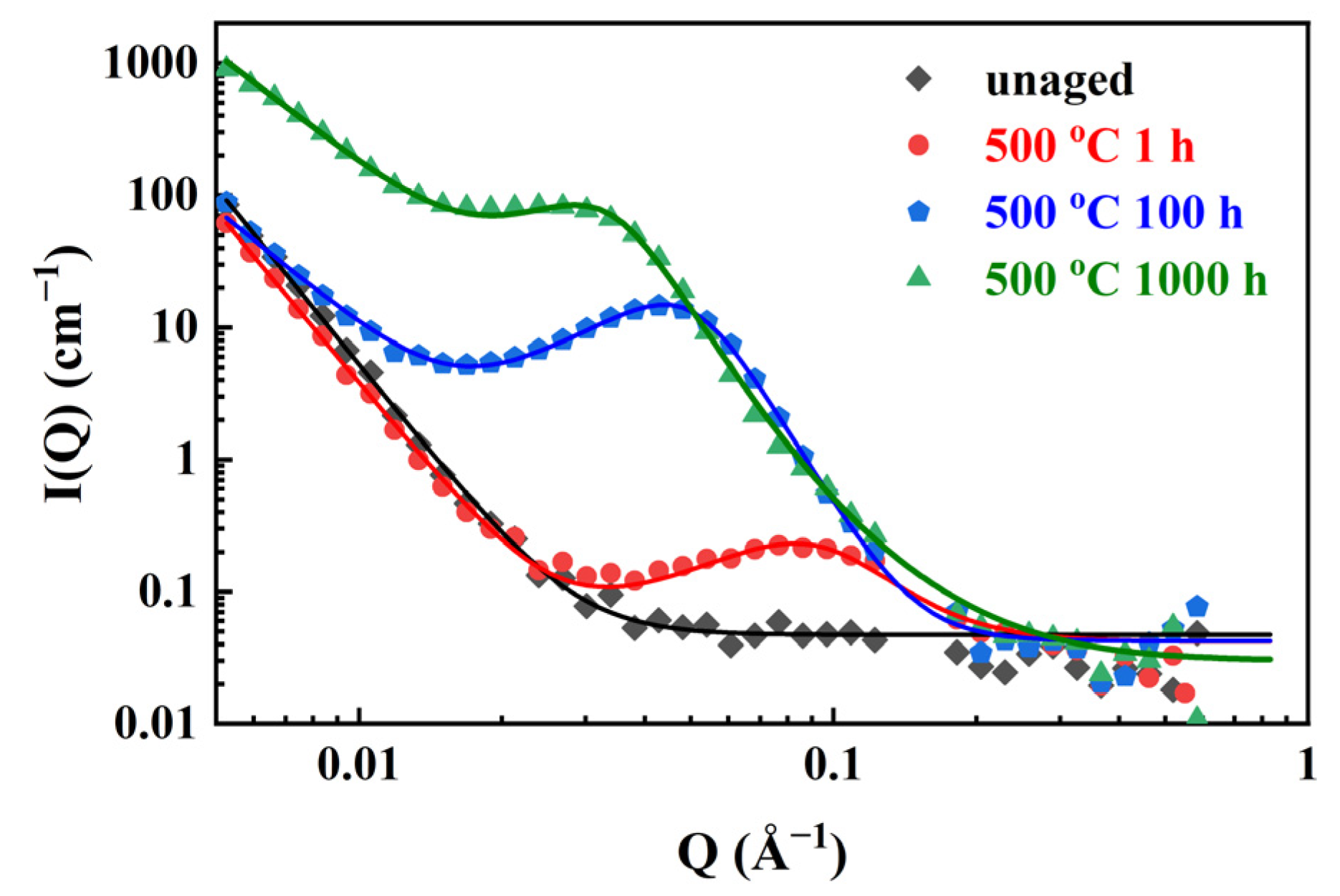
3.4. Small-Angle Neutron Scattering
4. Discussion
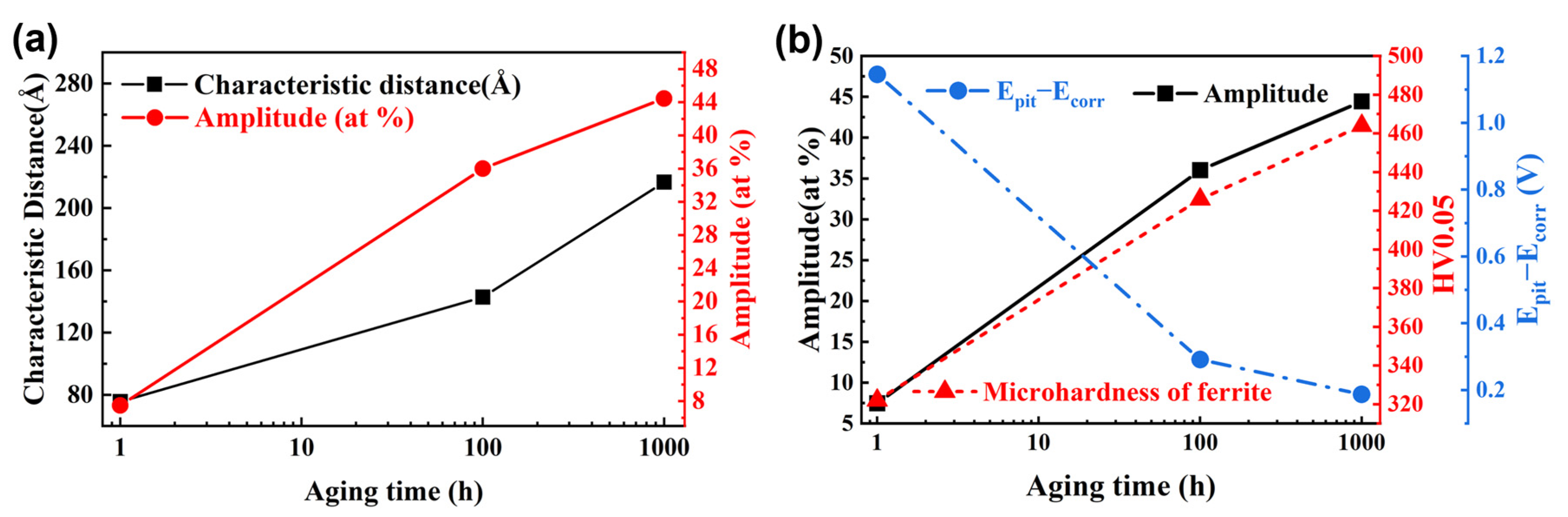
4.1. Evolution of Mechanical Properties Due to Nanostructural Development
4.2. Relationship between Corrosion Resistance and Phase Separation
5. Conclusions
- New SANS data of SAF 2507 aged at 500 °C were obtained, indicating that the PS nanostructure developed rapidly during the first 100 h of aging. The characteristic distance (d) and Cr fluctuation amplitude (AP) increased from 75.7 to 142.8 Å (30.9% increase) and from 7.4% to 36.0% (64.2% increase), respectively. However, the kinetics of PS slowed down for a longer aging time. The occurrence of PS in ferrite during the aging at 500 °C had a significant influence on the mechanical properties and corrosion resistance of SAF 2507. The evolution of the PS nanostructure was highly correlated with the variation trend of mechanical properties and corrosion resistance of SAF 2507.
- The evolution of the mechanical behavior of aged SAF 2507 was mainly attributed to the progress of PS in ferrite. The early stage of PS had a more significant impact on the nanostructure and the properties of SAF 2507. The fracture behavior of the alloy was likely determined by the mechanical properties of ferrite for aged conditions.
- The pitting corrosion resistance of SAF 2507 aged at 500 °C was closely related to the Cr depletion due to PS, and the resistance became weaker with the progression of PS. The evolution of the passivation region (Epit − Ecorr) with aging time correlated well with the results of mechanical tests and characteristic parameters of PS. The passivation region may become a new index for the extent of PS, and it is possible to use the passivation region to develop a new nondestructive electrochemical method to quantify the evolution of PS in duplex stainless steels (DSSs). Therefore, the service lifetime of DSSs may be reasonably evaluated without irreversible destruction using the method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Xu, X.; Wessman, S.; Odqvist, J.; King, S.M.; Hedström, P. Nanostructure, Microstructure and Mechanical Properties of Duplex Stainless Steels 25Cr-7 Ni and 22Cr-5Ni (Wt.%) Aged at 325 °C. Mater. Sci. Eng. A 2019, 754, 512–520. [Google Scholar] [CrossRef]
- Rovere, C.A.D.; Santos, F.S.; Silva, R.; Souza, C.A.C.; Kuri, S.E. Influence of Long-Term Low-Temperature Aging on the Microhardness and Corrosion Properties of Duplex Stainless Steel. Corros. Sci. 2013, 68, 84–90. [Google Scholar] [CrossRef]
- Olsson, J.; Snis, M. Duplex—A New Generation of Stainless Steels for Desalination Plants. Desalination 2007, 205, 104–113. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, H.; Zhang, H.; Yu, Z.; Hu, J.; He, L.; Li, J. Effect of Isothermal Aging on the Pitting Corrosion Resistance of UNS S82441 Duplex Stainless Steel Based on Electrochemical Detection. Corros. Sci. 2015, 93, 120–125. [Google Scholar] [CrossRef]
- Solomon, H.D.; Levinson, L.M. Mössbauer Effect Study of ‘475°c Embrittlement’ of Duplex and Ferritic Stainless Steels. Acta Metall. 1978, 26, 429–442. [Google Scholar] [CrossRef]
- Yi, Y.S.; Shoji, T. Detection and Evaluation of Material Degradation of Thermally Aged Duplex Stainless Steels: Electrochemical Polarization Test and AFM Surface Analysis. J. Nucl. Mater. 1996, 231, 20–28. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, B.; Zhou, Y.; Wu, Y.; Zhu, H. Evaluation of Pitting Corrosion in Duplex Stainless Steel Fe20Cr9Ni for Nuclear Power Application. Acta Mater. 2020, 197, 172–183. [Google Scholar] [CrossRef]
- Iacoviello, F.; Casari, F.; Gialanella, S. Effect of “475 °C Embrittlement” on Duplex Stainless Steels Localized Corrosion Resistance. Corros. Sci. 2005, 47, 909–922. [Google Scholar] [CrossRef]
- Park, C.-J.; Kwon, H.-S. Effects of Aging at 475 °C on Corrosion Properties of Tungsten-Containing Duplex Stainless Steels. Corros. Sci. 2002, 44, 2817–2830. [Google Scholar] [CrossRef]
- Silva, R.; Vacchi, G.S.; Kugelmeier, C.L.; Santos, I.G.R.; Filho, A.A.M.; Magalhães, D.C.C.; Afonso, C.R.M.; Sordi, V.L.; Rovere, C.A.D. New Insights into the Hardening and Pitting Corrosion Mechanisms of Thermally Aged Duplex Stainless Steel at 475 °C: A Comparative Study between 2205 and 2101 Steels. J. Mater. Sci. Technol. 2022, 98, 123–135. [Google Scholar] [CrossRef]
- Arnold, O.; Bilheux, J.C.; Borreguero, J.M.; Buts, A.; Campbell, S.I.; Chapon, L.; Doucet, M.; Draper, N.; Ferraz Leal, R.; Gigg, M.A.; et al. Mantid—Data Analysis and Visualization Package for Neutron Scattering and μ SR Experiments. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 764, 156–166. [Google Scholar] [CrossRef]
- Wignall, G.D.; Bates, F.S. Absolute calibration of small-angle neutron scattering data. J. Appl. Crystallogr. 1987, 20, 28–40. [Google Scholar] [CrossRef]
- Penfold, J.; King, S.M. SANS at Pulsed Neutron Sources: Present and Future Prospects. J. Appl. Crystallogr. 1997, 30, 1140–1147. [Google Scholar] [CrossRef]
- Hörnqvist, M.; Thuvander, M.; Steuwer, A.; King, S.; Odqvist, J.; Hedström, P. Early Stages of Spinodal Decomposition in Fe–Cr Resolved by In-Situ Small-Angle Neutron Scattering. Appl. Phys. Lett. 2015, 106, 061911. [Google Scholar] [CrossRef]
- Xu, X.; Odqvist, J.; Colliander, M.H.; King, S.; Thuvander, M.; Steuwer, A.; Hedström, P. Effect of Cooling Rate after Solution Treatment on Subsequent Phase Separation during Aging of Fe-Cr Alloys: A Small-Angle Neutron Scattering Study. Acta Mater. 2017, 134, 221–229. [Google Scholar] [CrossRef]
- SasView. SasView. Available online: https://sasview.github.io/ (accessed on 17 October 2022).
- Xu, X.; Westraadt, J.E.; Odqvist, J.; Youngs, T.G.A.; King, S.M.; Hedström, P. Effect of Heat Treatment above the Miscibility Gap on Nanostructure Formation Due to Spinodal Decomposition in Fe-52.85 at.%Cr. Acta Mater. 2018, 145, 347–358. [Google Scholar] [CrossRef]
- Xu, X.; Odqvist, J.; Colliander, M.H.; Thuvander, M.; Steuwer, A.; Westraadt, J.E.; King, S.; Hedström, P. Structural Characterization of Phase Separation in Fe-Cr: A Current Comparison of Experimental Methods. Metall. Mater. Trans. A 2016, 47, 5942–5952. [Google Scholar] [CrossRef]
- Hammouda, B. Probing Nanoscale Structures, the SANS Toolbox; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008.
- Furukawa, H. Dynamics-Scaling Theory for Phase-Separating Unmixing Mixtures: Growth Rates of Droplets and Scaling Properties of Autocorrelation Functions. Phys. A Stat. Mech. Its Appl. 1984, 123, 497–515. [Google Scholar] [CrossRef]
- Das, Y.; Liu, J.; Wessman, S.; Xu, X.; Odqvist, J.; King, S.; Hedström, P. Small-Angle Neutron Scattering Quantification of Phase Separation and the Corresponding Embrittlement of a Super Duplex Stainless Steel after Long-Term Aging at 300 °C. Materialia 2020, 12, 100771. [Google Scholar] [CrossRef]
- Hashimoto, T.; Takenaka, M.; Jinnai, H. Scattering Studies of Self-Assembling Processes of Polymer Blends in Spinodal Decomposition. J. Appl. Crystallogr. 1991, 24, 457–466. [Google Scholar] [CrossRef]
- Meier, H.; Strobl, G.R. Small-Angle X-ray Scattering Study of Spinodal Decomposition in Polystyrene/Poly(Styrene-Co-Bromostyrene) Blends. Macromolecules 1987, 20, 649–654. [Google Scholar] [CrossRef]
- Yamada, T.; Okano, S.; Kuwano, H. Mechanical Property and Microstructural Change by Thermal Aging of SCS14A Cast Duplex Stainless Steel. J. Nucl. Mater. 2006, 350, 47–55. [Google Scholar] [CrossRef]
- Chen, T.H.; Weng, K.L.; Yang, J.R. The Effect of High-Temperature Exposure on the Microstructural Stability and Toughness Property in a 2205 Duplex Stainless Steel. Mater. Sci. Eng. A 2002, 338, 259–270. [Google Scholar] [CrossRef]
- Park, J.S.; Yoon, Y.K. Evaluation of Thermal Aging Embrittlement of Duplex Stainless Steels by Electrochemical Method. Scr. Metall. Mater. 1995, 32, 1163–1168. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandström, R.; Westin, E.M. Fracture Toughness of the Lean Duplex Stainless Steel LDX 2101. Metall. Mater. Trans. A 2006, 37, 2975–2981. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandström, R. Fracture Toughness of a Welded Duplex Stainless Steel. Eng. Fract. Mech. 2006, 73, 377–390. [Google Scholar] [CrossRef]
- Chandra, K.; Singhal, R.; Kain, V.; Raja, V.S. Low Temperature Embrittlement of Duplex Stainless Steel: Correlation between Mechanical and Electrochemical Behavior. Mater. Sci. Eng. A 2010, 527, 3904–3912. [Google Scholar] [CrossRef]
- Cahn, J.W. Hardening by spinodal decomposition. Acta Metall. 1963, 11, 1275–1282. [Google Scholar] [CrossRef]
- Park, K.-H.; LaSalle, J.C.; Schwartz, L.H.; Kato, M. Mechanical Properties of Spinodally Decomposed Fe-30 Wt% Cr Alloys: Yield Strength and Aging Embrittlement. Acta Metall. 1986, 34, 1853–1865. [Google Scholar] [CrossRef]
- Weng, K.L.; Chen, H.R.; Yang, J.R. The Low-Temperature Aging Embrittlement in a 2205 Duplex Stainless Steel. Mater. Sci. Eng. A 2004, 379, 119–132. [Google Scholar] [CrossRef]
- Bonnet, S.; Bourgoin, J.; Champredonde, J.; Guttmann, D.; Guttmann, M. Relationship between Evolution of Mechanical Properties of Various Cast Duplex Stainless Steels and Metallurgical and Aging Parameters: Outline of Current EDF Programmes. Mater. Sci. Technol. 1990, 6, 221–229. [Google Scholar] [CrossRef]


| Element | Fe | C | N | Si | P | Cr | Mn | Ni | Cu | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | Bal. | 0.015 | 0.30 | 0.47 | 0.024 | 25.35 | 0.71 | 6.40 | 0.20 | 3.68 |
| Aging Time (h) | IPS (cm−1) | QP (Å−1) | d (Å) | SLD (× 10−6/Å2) | AP (at.%) |
|---|---|---|---|---|---|
| 1 | 0.2 | 0.083 | 75.7 | 0.372 | 7.5 |
| 100 | 14.7 | 0.044 | 142.8 | 1.80 | 36.0 |
| 1000 | 75. 4 | 0.029 | 216.7 | 2.22 | 44.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Yuan, S.; Wang, Y.; Li, Q.; Deng, Z.; Xie, Y.; Ke, Y.; Xu, J.; Yu, H.; Sun, D.; et al. Nanostructure, Mechanical Properties, and Corrosion Resistance of Super Duplex Stainless Steel 2507 Aged at 500 °C. Crystals 2023, 13, 243. https://doi.org/10.3390/cryst13020243
Yuan Y, Yuan S, Wang Y, Li Q, Deng Z, Xie Y, Ke Y, Xu J, Yu H, Sun D, et al. Nanostructure, Mechanical Properties, and Corrosion Resistance of Super Duplex Stainless Steel 2507 Aged at 500 °C. Crystals. 2023; 13(2):243. https://doi.org/10.3390/cryst13020243
Chicago/Turabian StyleYuan, Ye, Sui Yuan, Yifei Wang, Qikang Li, Zize Deng, Yinsong Xie, Yubin Ke, Jian Xu, Hongying Yu, Dongbai Sun, and et al. 2023. "Nanostructure, Mechanical Properties, and Corrosion Resistance of Super Duplex Stainless Steel 2507 Aged at 500 °C" Crystals 13, no. 2: 243. https://doi.org/10.3390/cryst13020243
APA StyleYuan, Y., Yuan, S., Wang, Y., Li, Q., Deng, Z., Xie, Y., Ke, Y., Xu, J., Yu, H., Sun, D., & Xu, X. (2023). Nanostructure, Mechanical Properties, and Corrosion Resistance of Super Duplex Stainless Steel 2507 Aged at 500 °C. Crystals, 13(2), 243. https://doi.org/10.3390/cryst13020243








