Investigation of Preparation of Slag Wool from Melting-Separated Red Mud
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Analysis of Macroscopic Properties of Modified Melting-Separated Red Mud
2.3. Preparation and Performance Characterization of Slag Wool
3. Results and Analysis
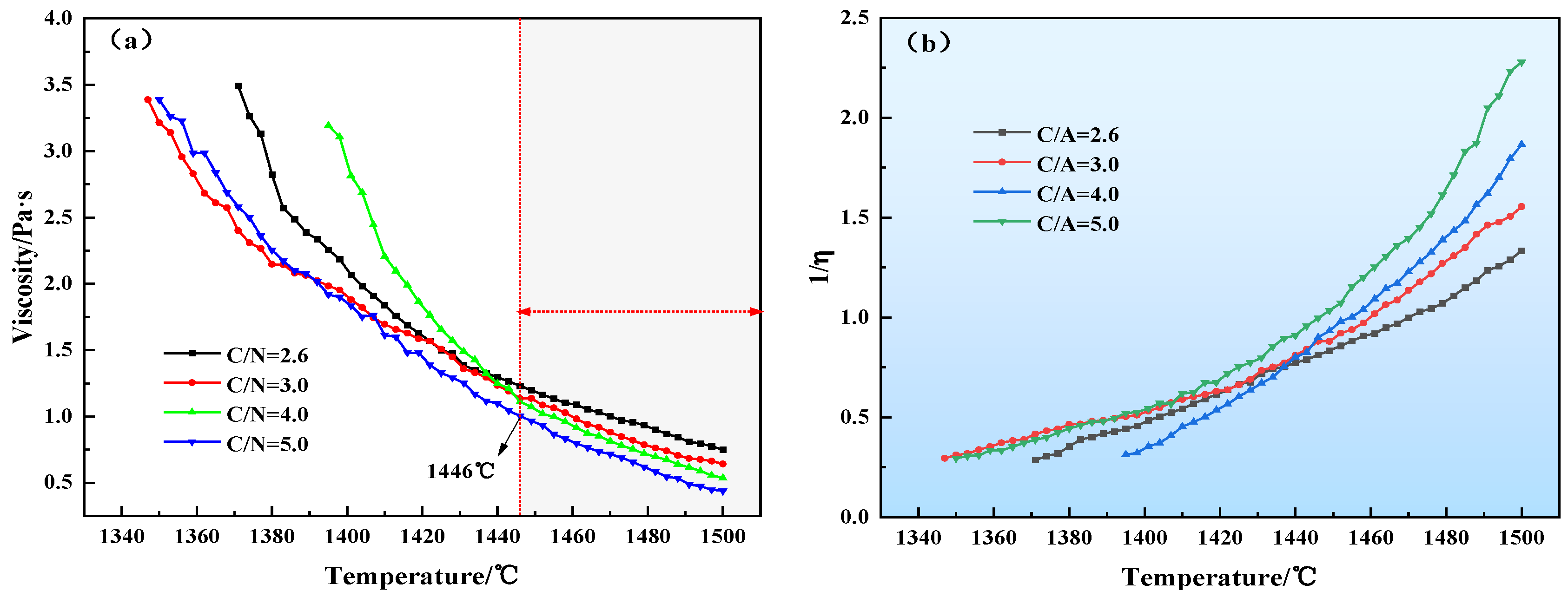
3.1. Viscosity and Fluidity
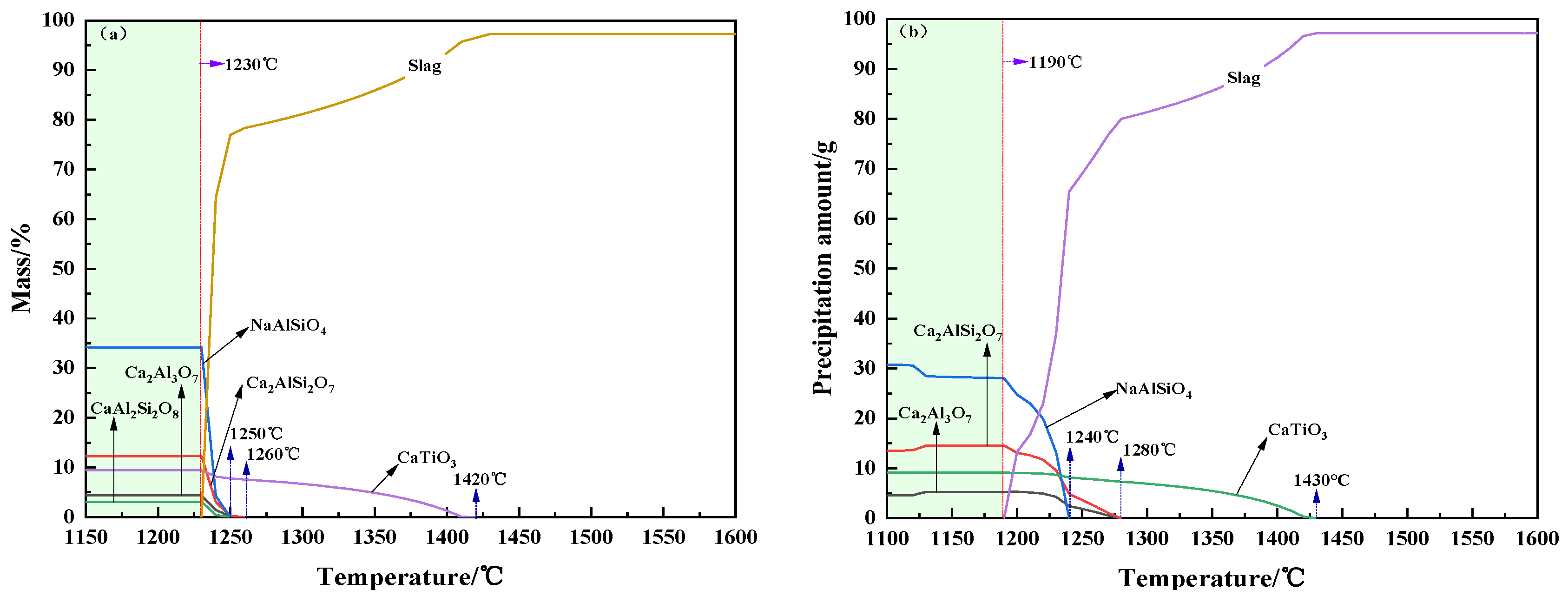
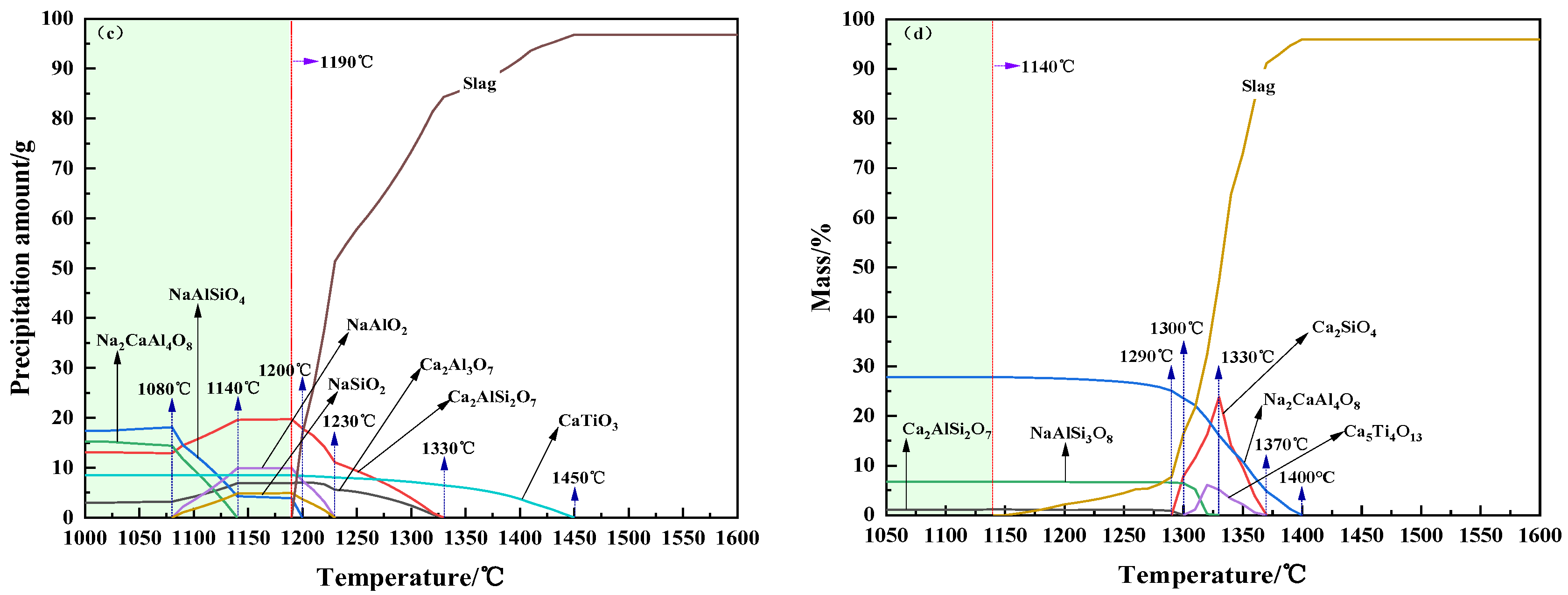
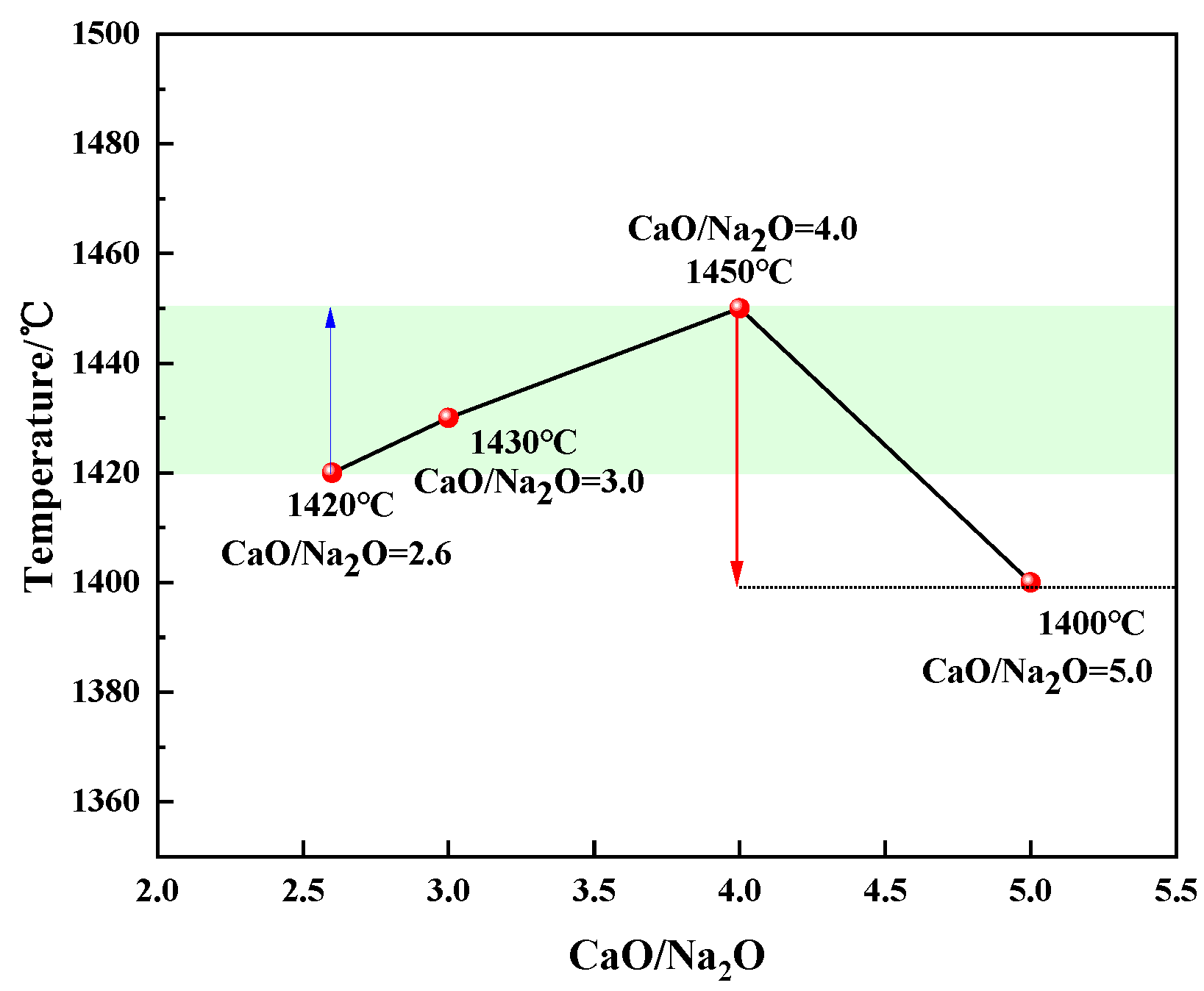
3.2. Crystallization Performance
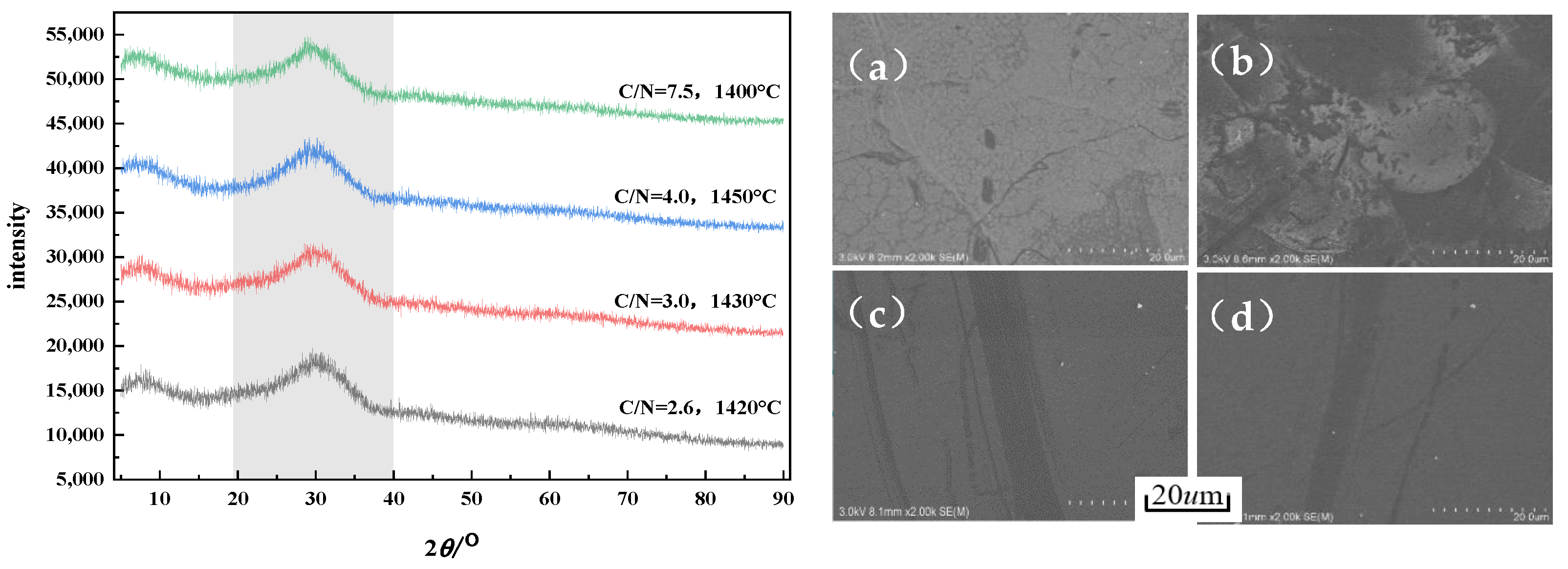
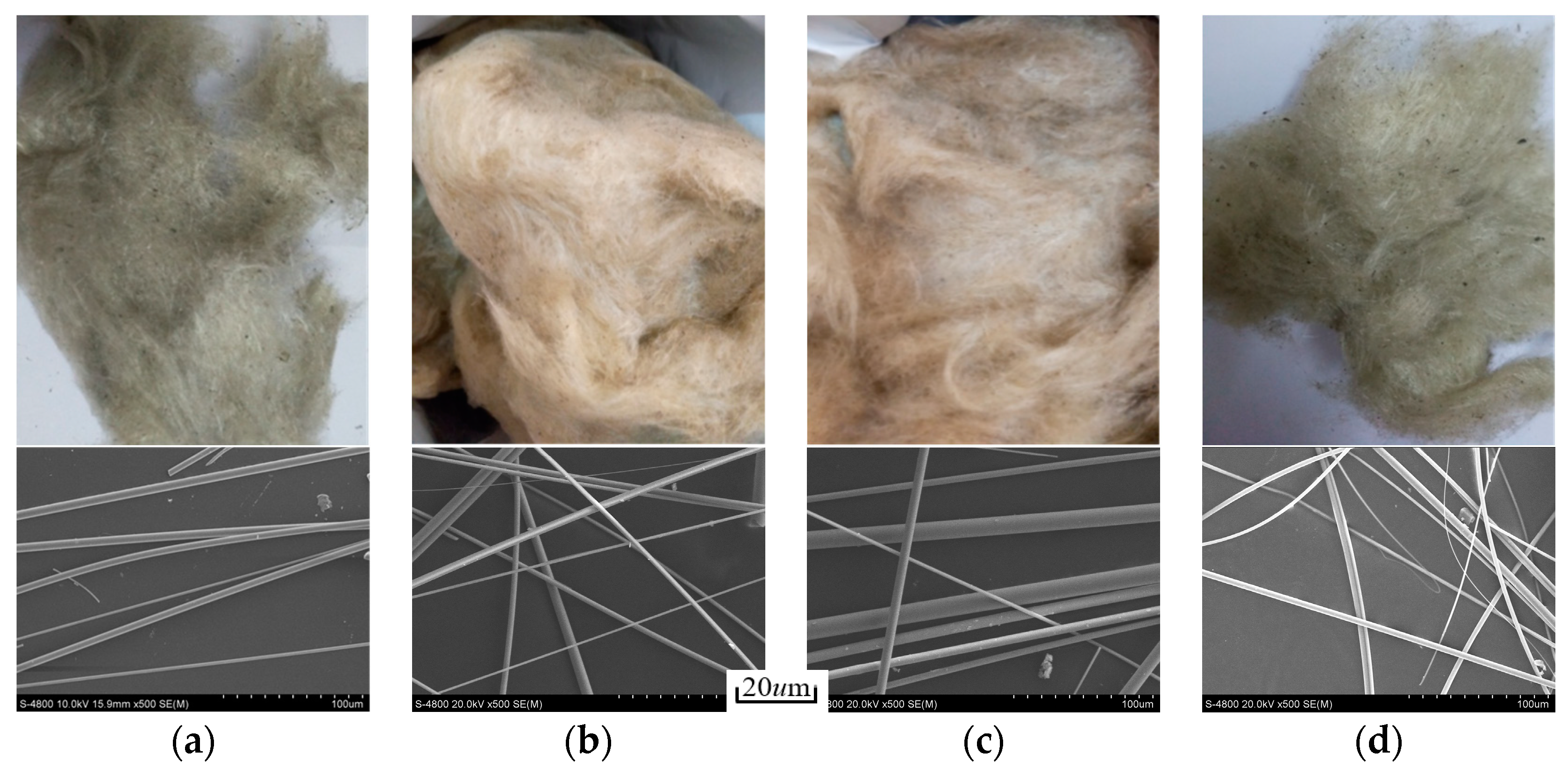
3.3. Morphology and Properties of Slag Wool
4. Conclusions
- (1)
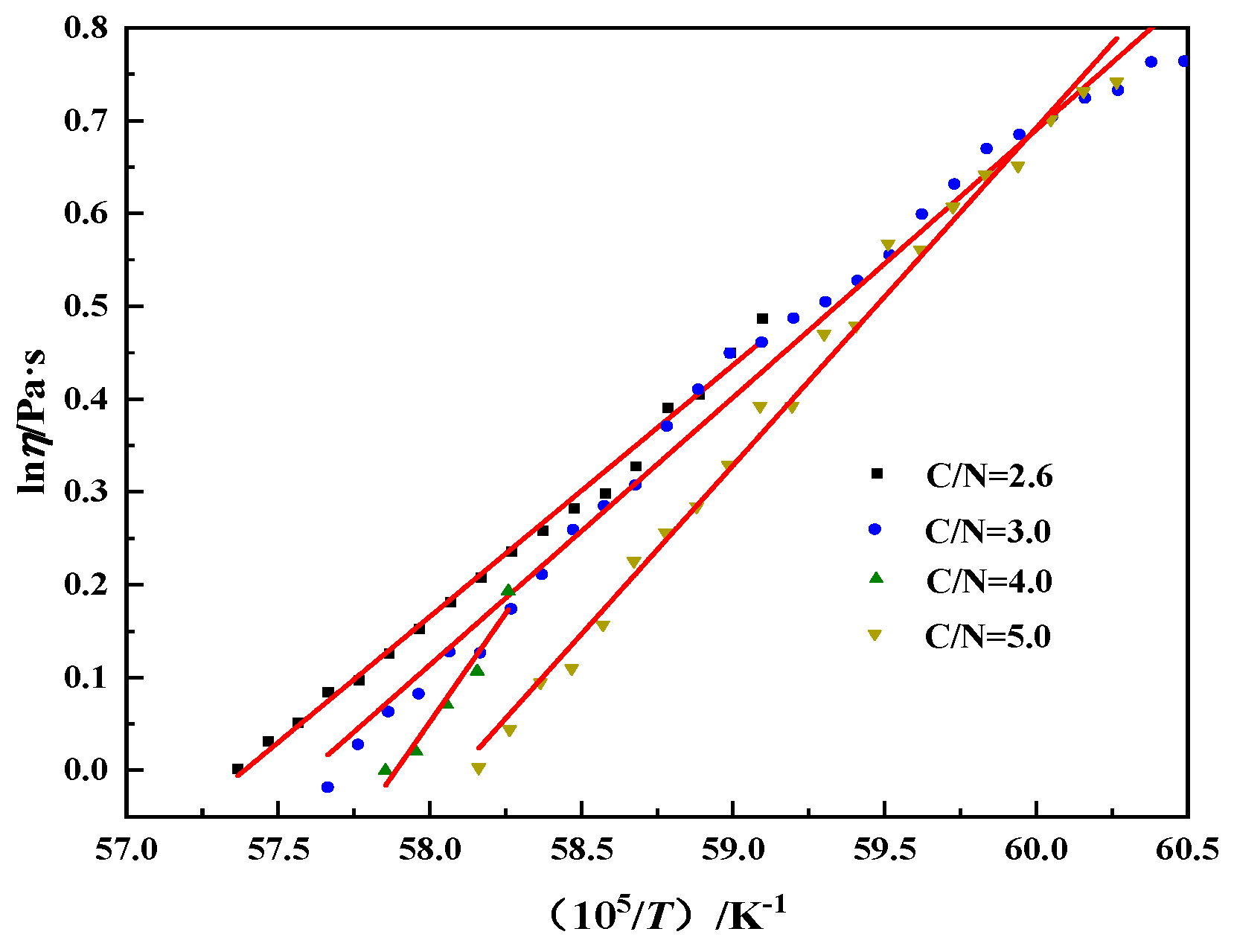
- For CaO/Na2O ratios from 2.6 to 5.0, the viscosity of the modified melting-separated red mud decreases with increasing temperature; the activation energy of the particle movement of the modified melting-separated red mud shows a trend of slowly increasing–sharply increasing–decreasing with increasing CaO/Na2O, which is consistent with the trend of the melting temperature of modified melting-separated red mud.
- (2)
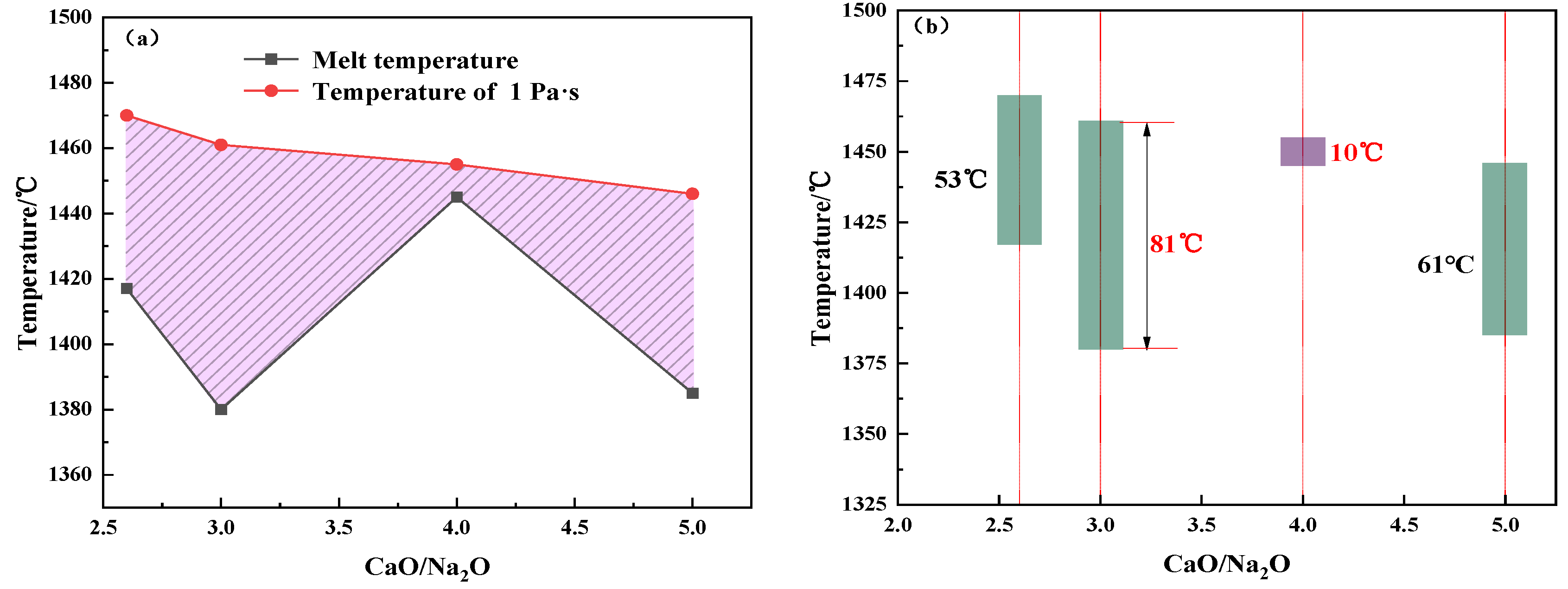
- The suitable fiber-forming temperature interval of modified melting-separated red mud shows a trend of increasing–decreasing–increasing with increasing CaO/Na2O. Considering the feasibility of preparing slag wool from modified melting-separated red mud, the CaO/Na2O ratio in the modified raw material system should not be higher than 3.0.
- (3)
- For CaO/Na2O ratios from 2.6 to 5.0, the precipitated mineral phases of modified melting-separated red mud during the cooling process mainly include nepheline (NaAlSiO4), anorthite (Ca2AlSi2O7, CaAl2Si2O8), perovskite (CaTiO3), melilite (Ca2Al3O7), and albite (Na2CaAl4O8, NaAlSi3O8), and the crystallization temperature first increases and then decreases. When the CaO/Na2O is 4.0, the maximum crystallization temperature of the modified melting-separated red mud during the cooling process is 1450 °C, and the increase in the crystallization temperature of the melt is not significant when the CaO/Na2O increases from 2.6 to 4.0. When the CaO/Na2O is greater than 4.0, the onset crystallization temperature of the melt decreases significantly.
- (4)
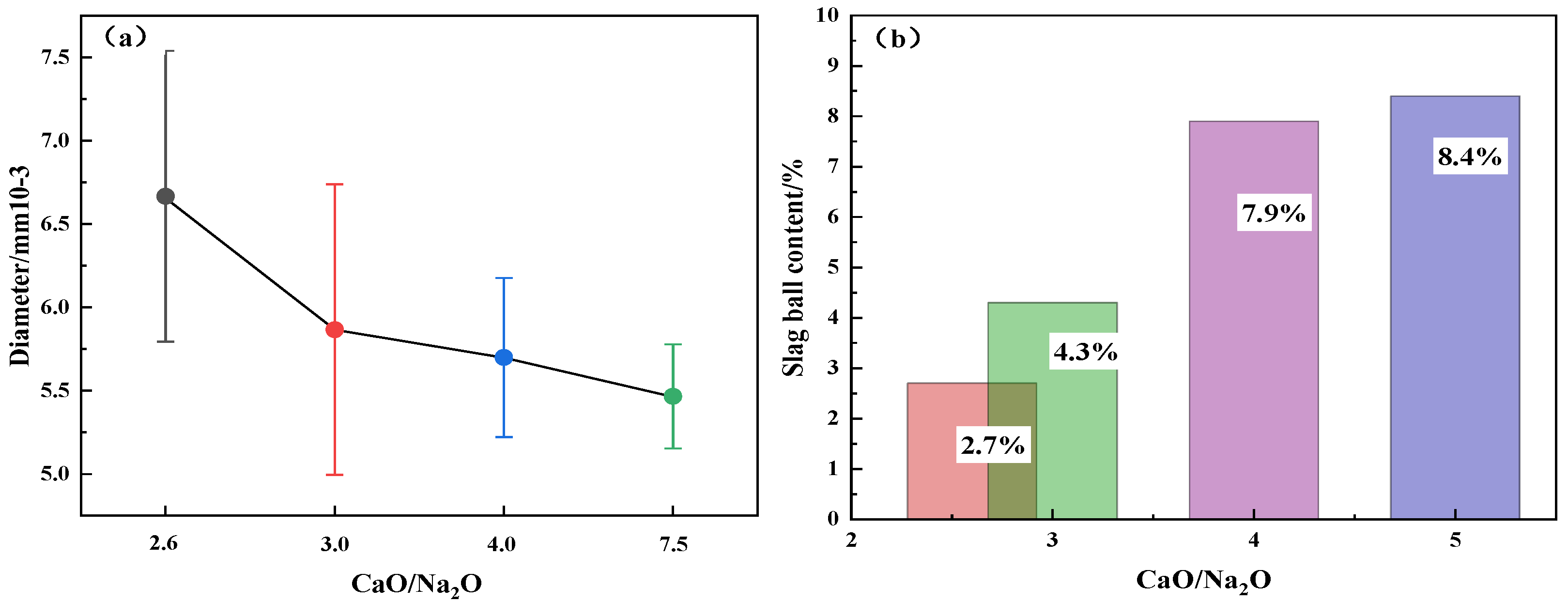
- The slag wool prepared from modified melting-separated red mud with different CaO/Na2O ratios has a smooth surface and a grayish yellow color, and the fibers are arranged crosswise and contain a small amount of slag balls. The diameters of the slag balls are all less than 7 µm, with an average diameter of 5.93 µm, and the slag ball content is 5.83%. With increasing CaO/Na2O, the diameter of the slag wool gradually decreases, but the variation is insignificant, and the slag ball content gradually increases, with a significant increase when the CaO/Na2O is greater than 3.0.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Lu, G.Z.; Zhang, T.A.; Ma, L.N.; Wang, Y.X.; Zhang, W.G.; Zhang, Z.M.; Wang, L. Utilization of Baver red mud by a calcification-carbonation method using calcium aluminate hydrate as a calcium source. Hydrometallurgy 2019, 188, 248–255. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Chen, Z.T.; Li, J.Q.; Zheng, H.N.; Wang, T.H. Recovery of aluminum and iron from red mud by low temperature roasting with sodium pyrosulfate. Chin. J. Nonferrous Met. 2023, 23, 1–16. [Google Scholar]
- Li, Y.; Ou, D.Y. Research on development countermeasures of bulk solid waste resource utilization under the background of low-carbon. Jiangxi Build. Mater. 2022, 28, 5–8+12. [Google Scholar]
- Li, S.; Zhou, B.; Liu, W.C.; Zhou, X.F.; Wang, S. Status-quo, problems and solutions of red mud comprehensive utilization. China Nonferrous Metall. 2022, 51, 32–36. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, T.; Lv, G.; Yang, J.; Wang, Y. The effect of NaOH on the direct calcification-carbonation method for processing of bayer process red mud. Green Process. Synth. 2017, 70, 546–551. [Google Scholar] [CrossRef]
- Andras, G.; Nora, K.; Beatrix, T. The red mud accident in ajka (hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Technol. EST 2011, 45, 1608–1615. [Google Scholar]
- Pan, X.; Wu, H.; Lv, Z.; Yu, H.; Tu, G. Research status and prospect of iron and aluminum recovery technology from red mud. Chin. J. Nonferrous Met. 2023, 23, 1–32. [Google Scholar]
- Liu, C.; Ma, S.; Zheng, S.; Luo, Y.; Ding, J.; Wang, X.; Zhang, Y. Combined treatment of red mud and coal fly ash by a hydro-chemical process. Hydrometallurgy 2018, 175, 224–231. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Yao, Z.; Wu, S.; Jiang, H.; Liang, M.; Qiao, Y. Environmental aspects and pavement properties of red mud waste as the replacement of mineral filler in asphalt mixture. Constr. Build. Mater. 2018, 180, 605–613. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; Wang, H.; Liu, L.; Wang, X. ANN-based structure-viscosity relationship model of multicomponent slags for production design in mineral wool. Constr. Build. Mater. 2022, 319, 126010. [Google Scholar] [CrossRef]
- Li, Z.H. Fibering Mechanism of Modified Molten Blast Furnace Slag and Experimental Research. Ph.D. Thesis, Northeastern University, Shenyang, China, 2018. [Google Scholar]
- Koroli, M.A.; Ivanisova, A.R. Thermal insulation of engineering structures with mineral wool based on basalt fiber. In Proceedings of the 7th International Seminar on Advances in Materials Science and Engineering, Changsha, China, 21–23 May 2021. [Google Scholar]
- Pavlin, M.; König, K.; König, J.; Javornik, U.; Ducman, V. Sustainable alkali-activated slag binders based on alternative activators sourced from mineral wool and glass waste. Front. Mater. 2022, 9, 902139. [Google Scholar] [CrossRef]
- Kostadinović, D.; Jovanović, M.; Bakić, V.; Stepanić, N.; Todorović, M. Experimental investigation of summer thermal performance of the green roof system with mineral wool substrate. Build. Environ. 2022, 217, 109061. [Google Scholar] [CrossRef]
- Pavlin, M.; Horvat, B.; Ducman, V. Preparation of façade panels based on alkali-activated waste mineral wool, their characterization, and durability aspects. Int. J. Appl. Ceram. Technol. 2022, 19, 1227–1234. [Google Scholar] [CrossRef]
- Li, Z.J. Blast Furnace Slag Wool Used as Soilless Culture Substrates on Flowers. Ph.D. Thesis, North China University of Science and Technology, Tangshan, China, 2019. [Google Scholar]
- Balomenos, E.; Giannopoulou, I.; Gerogiorgis, D.; Panias, D.; Paspaliaris, I. Resource-efficient and economically viable pyrometallurgical processing of industrial ferrous by-products. Waste Biomass Valorization 2013, 5, 333–342. [Google Scholar] [CrossRef]
- Balomenos, E.; Panias, D. Iron recovery and production of high added value products from the metallurgical by-products of primary aluminium and ferro-nickel industries. In Proceedings of the 3rd International Slag Valorisation Symposium, Leuven, Belgium, 19–20 March 2013. [Google Scholar]
- Kim, Y.; Kim, M.; Sohn, J.; Park, H. Applicability of gold tailings, waste limestone, red mud, and ferronickel slag for producing glass fibers. J. Clean. Prod. 2018, 203, 957–965. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Wang, M.; Wu, W.; Liu, L.; Wang, X. Simulation and experimental investigation on one-step process for recovery of valuable metals and preparation of clean mineral wool from red mud. J. Clean. Prod. 2018, 380, 134982. [Google Scholar] [CrossRef]
- Bernd, F.; Frank, K.; Efthymios, B.; Dimitrios, P. Slag Design for Glass Fiber Production from Red Mud after Iron Removal; RWTH Aachen University: Berlin, Germany, 2016. [Google Scholar]
- Du, P.; Zhang, Y.; Long, Y.; Xing, L. Preparation of CaO-SiO2-Al2O3 inorganic fibers from melting-separated red mud. High Temp. Mater. Process. 2023, 42, 20220272. [Google Scholar] [CrossRef]
- Zhao, C.G. Fluidity and Structure of CaO-SiO-MgO-Al2O3-FeO Bosh Slag in Blast Furnace. Master’s Thesis, University of Science and Technology Liaoning, Anshan, China, 2016. [Google Scholar]
- Turkdogen, E.T. Physicochemical Properties of Molten Slags and Glasses, 1st ed.; The Metals Society: London, UK, 1983; pp. 81–85. [Google Scholar]
- He, K.Y. Physical Chemistry of Silicate, 1st ed.; Wuhan University of Technology Press: Wuhan, China, 2010; pp. 227–228. [Google Scholar]
- Liang, Z.Y.; Ning, X.J. Analysis of viscosity and thermodynamic properties of high alumina slag. Met. World 2019, 26, 16–19+35. [Google Scholar]
- Du, P.P. Mechanism and Experiment Research of Centrifugal Spinning Method of Blast Slag. Master’s Thesis, North China University of Science and Technology, Tangshan, China, 2016. [Google Scholar]
- Fan, X.Y.; Zhang, J.L.; Xu, R.Z.; Jiao, K.; Wang, K. Effect of B2O3 on fluidity of low MgO slag containing titanium. J. Cent. South Univ. (Sci. Technol.) 2018, 49, 1863–1868. [Google Scholar]
- Lu, P.W. Fundamentals of Inorganic Materials Science, 1st ed.; Wuhan University of Technology Press: Wuhan, China, 2010; pp. 264–271. [Google Scholar]
- Zhang, Z.Q. Study on Technology of Fiber Formation of Molten Blast Furnace Slag and Fiber Products. Ph.D. Thesis, Yanshan University, Qinhuangdao, China, 2015. [Google Scholar]
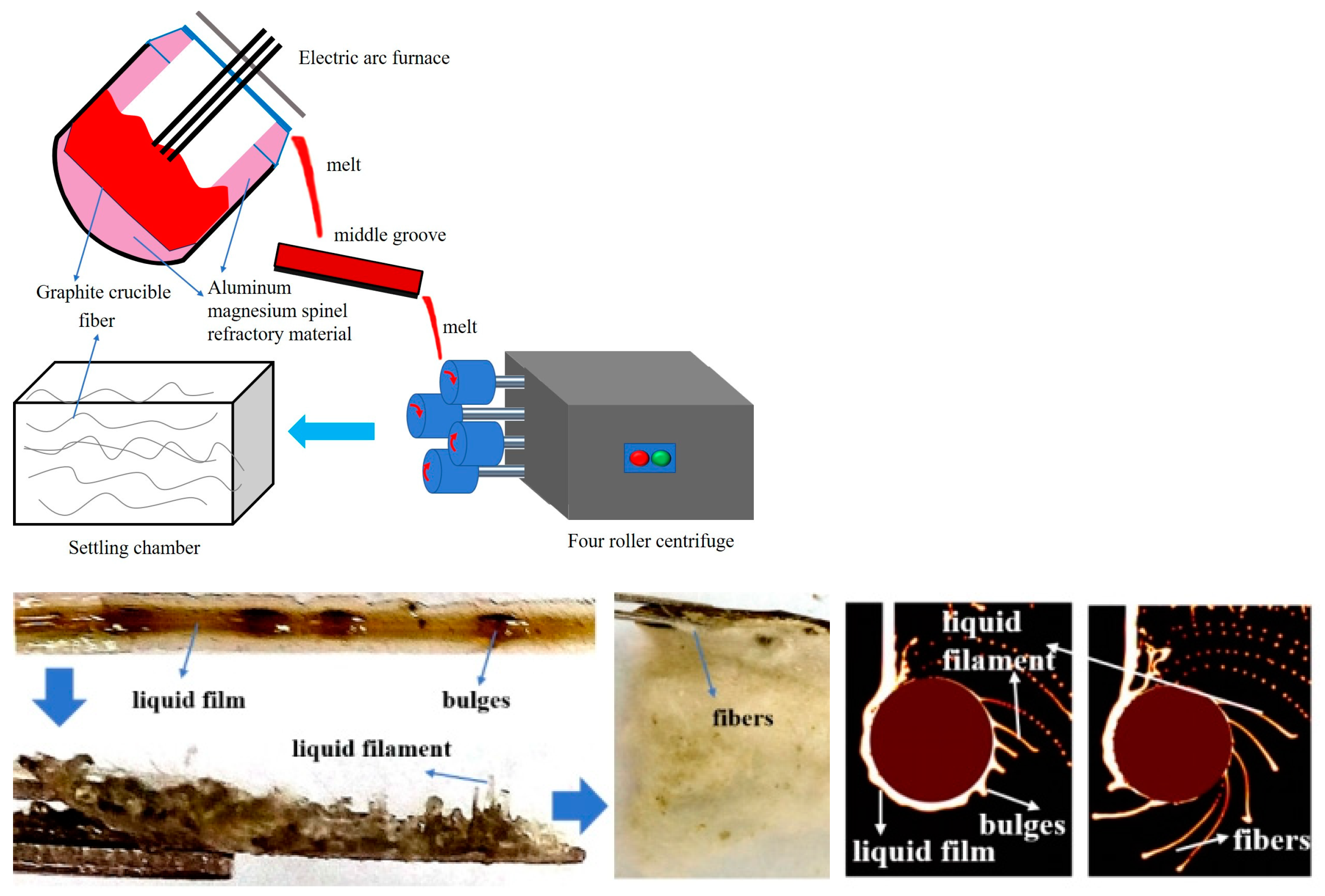
| Raw Material | Al2O3 | SiO2 | CaO | Na2O | TiO2 | Fe2O3 | MgO | K2O | MnO | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Melting-separated red mud | 32.31 | 27.26 | 10.62 | 11.16 | 7.23 | 7.56 | 2.14 | 0.30 | 0.13 | 1.29 |
| Water-quenched blast furnace slag | 14.47 | 28.32 | 38.72 | 0.32 | 2.16 | -- | 11.28 | 0.46 | 0.38 | 3.89 |
| Quicklime | -- | 1.28 | 90.0 | -- | -- | -- | 1.56 | -- | -- | 7.16 |
| CaO/Na2O | Quicklime | Chemical Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Na2O | TiO2 | Fe2O3 | MgO | K2O | MnO | Other | ||
| 2.6 | 0 | 27.61 | 26.36 | 19.98 | 7.55 | 5.54 | 5.04 | 5.19 | 0.35 | 0.21 | 2.17 |
| 3.0 | 2.88% | 26.86 | 25.60 | 22.00 | 7.33 | 5.38 | 4.89 | 5.08 | 0.34 | 0.21 | 2.31 |
| 4.0 | 10.20% | 24.92 | 23.67 | 27.12 | 6.78 | 4.98 | 4.52 | 4.82 | 0.32 | 0.19 | 2.68 |
| 5.0 | 16.49% | 23.27 | 22.01 | 31.53 | 6.31 | 4.63 | 4.21 | 4.59 | 0.70 | 0.29 | 2.46 |
| CaO/Na2O | 2.6 | 3.0 | 4.0 | 5.0 |
| Activation energy of particle movement | 225.38 | 239.78 | 389.20 | 302.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, P.; Zhang, Y.; Long, Y.; Xing, L. Investigation of Preparation of Slag Wool from Melting-Separated Red Mud. Crystals 2023, 13, 1645. https://doi.org/10.3390/cryst13121645
Du P, Zhang Y, Long Y, Xing L. Investigation of Preparation of Slag Wool from Melting-Separated Red Mud. Crystals. 2023; 13(12):1645. https://doi.org/10.3390/cryst13121645
Chicago/Turabian StyleDu, Peipei, Yuzhu Zhang, Yue Long, and Lei Xing. 2023. "Investigation of Preparation of Slag Wool from Melting-Separated Red Mud" Crystals 13, no. 12: 1645. https://doi.org/10.3390/cryst13121645
APA StyleDu, P., Zhang, Y., Long, Y., & Xing, L. (2023). Investigation of Preparation of Slag Wool from Melting-Separated Red Mud. Crystals, 13(12), 1645. https://doi.org/10.3390/cryst13121645





