Abstract
This review paper focuses on present issues concerning the use of polyaniline–TiO2 heterostructures as potentially efficient photocatalysts. Conducting polymers such as polyaniline (PANI) are used in the preparation of heterojunction systems with metal oxides like titania to overcome their inherent limitations, e.g., their sole absorption of UV light and overly fast recombination of charge carriers. This review discusses preparation methods, the properties of resultant products and mechanistic aspects. An important part of this paper is its presentation of the major challenges and future perspectives of such photocatalytic materials.
1. Introduction
Titanium dioxide (TiO2; titania) has been recognized as the most promising photocatalytic material, with high application potential in the fields of water or air treatment (mainly in the photooxidation of organic compounds) and in solar energy’s conversion into chemical energy in the obtention of valuable products such as hydrogen and hydrocarbons [1,2,3,4,5]. Its main strengths include the optimal positions of conduction (CB) and valence bands (VB) in a semiconductor band structure, corresponding to the redox potentials of various important chemical transformations; its low cost and perfect stability toward photocorrosion and relative nontoxicity are the reasons for researchers’ widespread interest.
However, to better utilize light (solar or artificial cheap sources, e.g., light-emitting diodes (LEDs)) and thus increase photocatalytic efficiency, it is necessary to overcome the weak points of titania. The first is the relatively wide band gap of TiO2 (3.0–3.2 eV), which only corresponds to the photoabsorption of UV light. As this has been calculated in Abe’s review, if all UV light (in solar spectrum) up to 400 nm were utilized, the solar light conversion efficiency for photocatalytic water splitting would only be 2% [4]. The possibility of utilizing visible light up to 600 nm or 800 nm will lead to efficiencies of 16% and 32%, respectively (Figure 1). The second limitation is the recombination of photogenerated charge carriers (electrons and holes), which reduces overall quantum efficiency [6]. During the recombination process, the excited electron reverts to the valence band without reacting to adsorbed species.
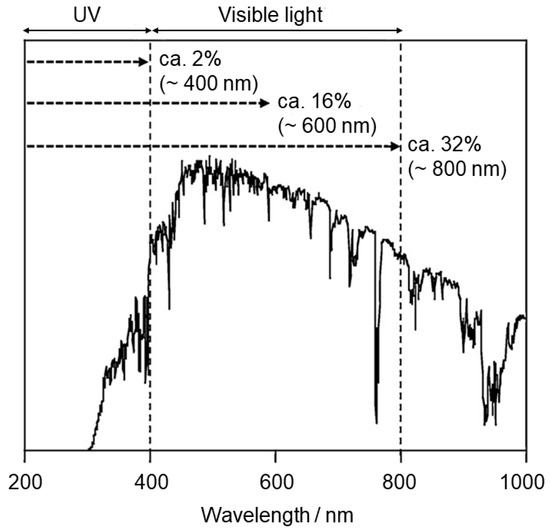
Figure 1.
Solar spectrum and maximum solar light conversion efficiencies for a photocatalytic water-splitting reaction. Reprinted with permission from [4]. Copyright 2011, Elsevier.
Many strategies have been designed to improve the photocatalytic performance of titania and overcome the above-mentioned problems. These range from intrinsic modifications of TiO2 in fields such as particle morphology, particle size, specific surface area, porosity and density of surface or bulk defects [3,7,8,9,10,11] to chemical modifications. Therefore, the introduction of visible light activity into titania chemical modifications is required, although the preparation of defective titanium dioxide could also be a promising direction [3]. Most of these chemical modifications concern the metal and non-metal modification/doping of TiO2, dye-sensitization and the design of titania–other semiconductor heterojunction systems [1,3,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. In comparison to other modification strategies, the usage of non-metal components is still very promising. The use of noble metals or metal complexes could be an unreasonable solution because of the cost of these components, which can limit their practical applications. The use of carbon-, nitrogen-, boron- or sulfur- doping/modification is much cheaper than the metals enumerated above. It is necessary to mention that a consequence of the application of some carbon (e.g., pentaerythritol) or nitrogen (e.g., urea) precursors in the modification process of TiO2 can be the formation of specific organic sensitizers on the titania surface, which is responsible for visible-light-induced photocatalytic activity [14,16]. Another possibility regarding the use of an organic sensitizer for titania is the application of conducting polymers such as polyaniline. The conductivity of polyaniline changes upon its addition to TiO2; therefore, perspectives regarding the application of such materials not only focus on photocatalysis but also on other fields, e.g., metal oxide semiconductor devices and microactuators [27]. In this review, a brief overview of titania–polyaniline systems as photocatalysts is presented in relation to current knowledge and perspectives of application. This review also indicates the main problems in interpreting the research (including mechanistic aspects) and the reproducibility of the results.
2. Electronic and Optical Properties of Polyaniline
Polyaniline (PANI) is an example of a polymer belonging to the conducting polymer (CP) group. One can classify this group as containing electrically conducting organic polymeric compounds. Other examples of CPs are polypyrrole (PPy), polythiophene (PTh), poly(3,4-ethylenedioxythiophene) (PEDOT) and polyacetylene (PAc). CPs can act as a semiconductor or a conductor. These compounds are conjugated and show π electron delocalization along their polymer backbone. This main property determines their unique electrical and optical properties [26,28,29,30,31]. The conjugated structure of CPs is a necessary condition for the formation of delocalized electronic states. The degree of delocalization influences the value of band gap energy, which determines the conductivity of CPs. Single and double bonds contain a localized σ-bond (chemically strong). In the case of double bonds, it is also possible to distinguish a localized π-bond (less strong than the σ-bond). Therefore, π-bonds are responsible for an easier delocalization of electrons (Figure 2) [29,32].
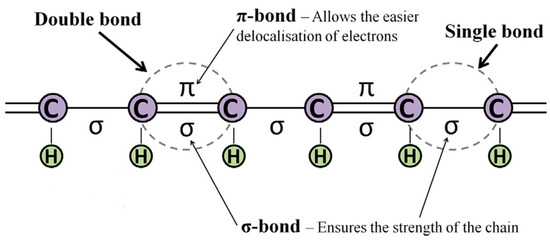
Figure 2.
A representation of a conjugated backbone [32].
PANI has many advantages, such as its low cost, its high environmental stability and its ability to enact electric switching between its conductive and resistive states via doping processes; it is important to emphasize that PANI is characterized by its diversity of structural forms [33]. Polyaniline is a mixed oxidation state-conducting polymer, which consists of reduced benzenoid amine units (–NH–) and oxidized quinoid imine units (=N–) (Figure 3). PANI’s average oxidation state is denoted as 1 − x. When 1 − x = 0 PANI exists as fully reduced leucoemeraldine (LE). If the value 1 − x is equal to 0.5, the half-oxidized emeraldine base (EB) is present. The fully oxidized form of PANI is pernigraniline (PE) when 1 − x = 1 [28,34].
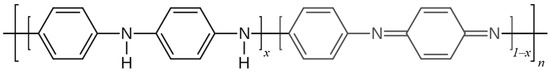
Figure 3.
General structure of polyaniline.
If the EB form of PANI is doped with inorganic acids and organic acids such as phosphoric acid, hydrochloric acid, sulfosalicylic acid and sulfuric acid, emeraldine salt (ES) is then formed. In this process, imine nitrogen is protonated by acid, and the conductivity of ES significantly increases in comparison to other forms of PANI (Table 1) [35]. Furthermore, as has been presented in Table 1, enumerated forms of PANI are characterized by different values of band gap energy. The colors of these materials are white, blue/violet, blue and green for LE, PE, EB and ES, respectively [34,35].

Table 1.
The main properties of different forms of PANI [34,35,36,37,38].
The ES form of PANI is the main candidate for a good photocatalytic material, owing to its high electron transfer properties in conjunction with its low band gap energy. The reason for this can be the polaron band formation that occurs. Furthermore, the result of partial oxidation is the occurrence of crystal lattice distortion, which can be responsible for the formation of polaron and bipolaron bands. Polaron formation is due to an upward shift in the highest occupied molecular orbital (HOMO) and a downward shift in the lowest unoccupied molecular orbital (LUMO), which results in a reduction in band gap energy. In general, the π–π* conjugate electron and polaron band transition system improves electron mobility, positively influencing photocatalytic properties through a reduction in the charge carrier recombination effect [35,39]. The low band gap is mainly due to the charge transfer exciton, such as the transition of the benzenoid rings from the HOMO (πb) to the LUMO (πq). Another transition, referred to as the UV region, is assigned to the πb → π* transition. Furthermore, ES-PANI behaves as a p-type semiconductor [37].
The optical properties of PANI are closely related to the form of PANI and preparation/modification conditions. For example, Figure 4 shows changes in the absorption spectrum of EB under different conditions of its protonation [34]. EB has an absorption peak at 2.1 eV due to electronic excitation from the benzenoid (πb) to the quinoid rings (πq). When pH changes to 3, absorption at 2.1 eV shifts to 1.5 eV. The reason for this is the lattice distortion of polyaniline into a polaronic structure as a result of the protonation of imine nitrogen atoms. Its peak at 3.9 eV can be attributed to the following transitions: (1) π–π* transition, which is similar to that of LE; (2) the transition from low-lying orbitals to the πq orbital. However, an absorption study on the different concentrations of ES in 80% acetic acid did not show any interchain interactions [34]. Generally, regardless of the form, PANI has a high absorbance coefficient over a wide spectral range, from the Vis to IR region [35].
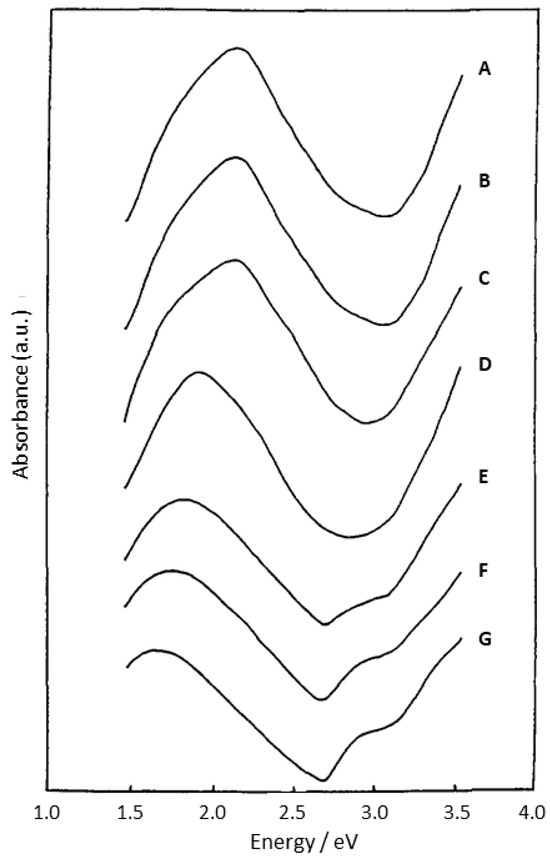
Figure 4.
Changes in the photoabsorption properties of PANI (emeraldine base) during its protonation: (A) pH 6, 16 h; (B) 10−4 M HCl, 24 h; (C) 2 × 10−4 M HCl, 3 h; (D) 4 × 10−4 M HCl, 4.5 h; (E) 6 × 10−4 M HCl, 2 h; (F) 8 × 10−4 M HCl, 16 h; (G) 10−3 M HCl, 2 h. Reprinted with permission from [34]. Copyright 1993, Elsevier.
3. The Concept of Improving Photocatalytic Activity via Binary System: Metal Oxide—Polyaniline Heterostructure Nanocomposites
Referring to the previous chapter, the chemical structure and form of PANI determine its electronic and optical properties. It is known that PANI usually behaves as a p-type semiconductor. This means that PANI has an Ef (Fermi level) close to the HOMO—it can receive electrons and demonstrate downward band bending [38]. PANI has the LUMO level in the range of −1 to −2 V vs. NHE and has the HOMO level in the range of 0.4–0.6 V [35,37,40]. Therefore, the formation of heterojunctions between PANI and metal oxides such as TiO2 is possible.
First, if two semiconductors with different redox energy levels are coupled, it can lead to an increase in photocatalytic performance by improving charge carrier separation. Second, it is important that the conditions for an efficient interparticle transfer between the semiconductor and TiO2 are met; the CB of titania is more anodic than the corresponding band of the sensitizer. Then, if one only considers the visible light activation of a semiconductor–sensitizer, this component is excited, and electrons photoexcited to its CB are injected into the conduction band of the titania [22,24,41,42,43].
The coupling of an n-type semiconductor (e.g., TiO2) with a p-type semiconductor (e.g., PANI) is advantageous from a photocatalytic point of view. This combination of semiconductors can ensure an efficient electron transfer between them [43]. Given the band positions of PANI and n-type semiconductors, such as metal oxides, it is possible to design a heterojunction system with a high photocatalytic performance (Figure 5, left). The band positions of PANI and TiO2 form a type II heterojunction (staggered band pattern) (Figure 5, right). Considering the fact that PANI can be activated by visible light, the following main advantages of TiO2 properties can be mentioned: (1) the visible light sensitization of a titania-containing system, (2) the improvement of charge carrier separation. Therefore, a synergistic effect from these two semiconductor components can be expected. As for the PANI/TiO2 ratio, the influence of this issue on photocatalytic activity is often studied; in most research reports, the amount of titania is higher than PANI.
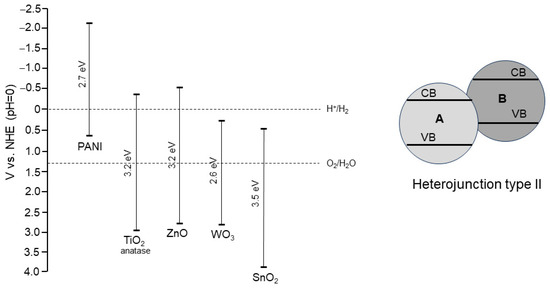
Figure 5.
Band positions and band gap energies of PANI and exemplary n-type metal oxide semiconductors. Data collected from [43,44] (left). Symbolic presentation of a type II heterojunction system of coupled semiconductors; A—TiO2, B—PANI (right).
4. PANI–TiO2 Binary Heterojunction Systems—The State of the Art
A prospective photocatalytic material with high application potential should meet three main criteria: (1) high photocatalytic performance over a broad light spectrum in major reactions, such as the degradation of organic pollutants, hydrogen production and CO2 reduction in high-value-added products; (2) reasonable cost for reproducible and large-scale production; (3) high stability (the retention of photocatalytic properties after many cycles). A major problem concerning these standards is that, as more components are introduced into the structure of the resultant material, its stability tends to decrease. Therefore, this review focuses on binary PANI and TiO2 heterojunctions to better understand the great potential of this type of material. However, many examples of ternary or multicomponent composites containing both PANI and TiO2 can be found in the literature [45,46,47,48,49,50,51,52,53,54,55,56]. Table 2 summarizes the binary PANI–TiO2 heterostructures acting as photocatalysts. Three main areas were considered: preparation conditions, photocatalytic efficiency and aspects of the photocatalytic mechanism.
4.1. Preparation Conditions
The main method for the preparation of PANI–TiO2 composites is the in situ chemical oxidative polymerization of aniline, using ammonium persulfate (APS) as an oxidant. This process occurs in the presence of TiO2 or a titania precursor. Key reaction parameters, such as the molar ratio of aniline/APS and aniline/TiO2, are different. Furthermore, polymerization conditions vary as well: polymerization time ranges from 1 h to 20 days, and process temperature ranges from ice bath conditions to room temperature. It is therefore difficult to find the optimal variant for this method. However, there are several reports that take into account the dominant amount of PANI. It is usually possible to find the best PANI/TiO2 ratio for photocatalytic performance. Figure 6 shows this type of study with 2% PANI content as the best amount for photocatalytic activity [57]. In general, these values vary in other reports according to experimental conditions.
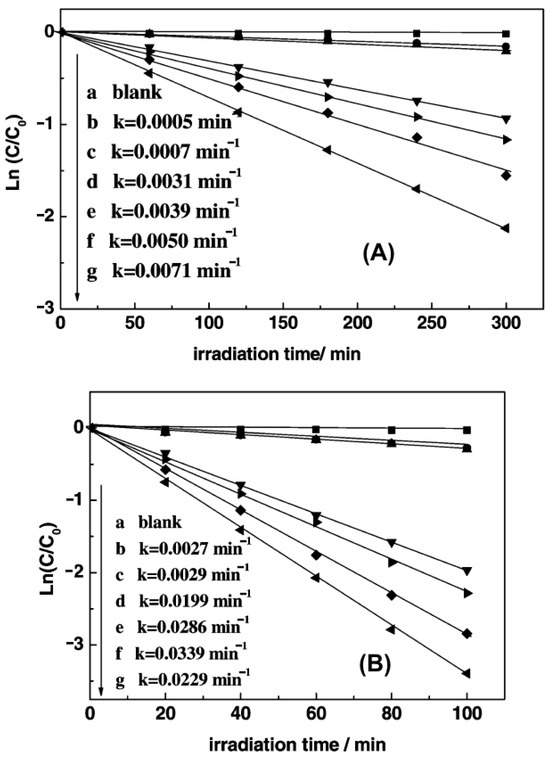
Figure 6.
Plots of the photodegradation of (A) methylene blue and (B) Rhodamine B over PANI/TiO2 photocatalysts under visible light irradiation with λ > 450 nm: (a) blank, (b) TiO2, (c) mechanical mixture of PANI and TiO2 (3:100), (d) PANI (1.0%)/TiO2, (e) PANI (5.0%)/TiO2, (f) PANI (2.0%)/TiO2, (g) PANI (3.0%)/TiO2. Reprinted with permission from [57]. Copyright 2008, American Chemical Society.
A much smaller number of papers consider other preparation methods. Some reports include PANI and TiO2 impregnation procedures [58,59,60,61]. A novel, green and promising procedure for the preparation of PANI/TiO2 was proposed by Cionti et al. [62]. Figure 7 shows the main steps of this procedure: (1) UV light-mediated growth of PANI oligomers on a TiO2 surface, (2) polymerization of the oligomers with H2O2.
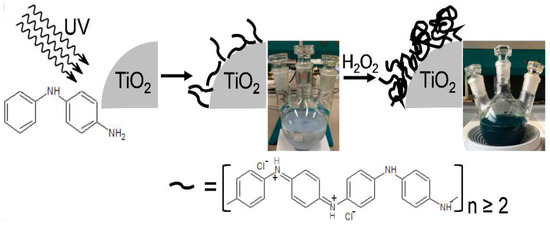
Figure 7.
UV light-mediated PANI/TiO2 preparation method. Reprinted with permission from [62]. Copyright 2018, Royal Chemical Society.
As for the form of TiO2 that forms a part of the composites discussed, it is a commercial material in many cases, such as P25, which contains a mixture of anatase (85%) and rutile (15%). Some authors directly used a titania precursor in a polymerization procedure. Attempts have also been made to obtain special morphological forms of TiO2, such as a self-assembled monolayer [63], mesoporous material [64,65,66], flowerlike NPs [67], nanotubes [68,69], nanosheets [60], nanofiber membranes [70], fiber films [59] and nanorods [71]. Some works have described the possibility of using defective forms of titania, e.g., grey or black titania [72,73]. There are also reports of modified forms of TiO2 with noble metals such as Au, Pt and Ag [58,74,75,76], core–shell structures with SiO2 [77] and combinations with reduced graphene oxide [78]. Furthermore, several applicable forms of PANI/TiO2 have been considered, such as gypsum plasters [79], acrylic pseudo-paints [80], cotton fabric [81] and polyurethane foam [82].
The identification of the PANI form (oxidation state) present in a resultant product is not a common procedure. Only some authors include information about the PANI form. As explained earlier, the form of PANI directly affects its photocatalytic properties. Therefore, it is difficult to discuss the mechanism of photocatalytic action without this knowledge. There are also a very limited number of approaches for obtaining PANI with different morphological forms [83].
4.2. Photocatalytic Efficiency
Testing the photocatalytic efficiencies of PANI/TiO2 is another crucial issue. As many as 65% of reports concern the use of organic dyes as model compounds, such as methylene blue, rhodamine B or methyl orange. This approach is highly controversial, especially for testing under visible light conditions [84]. The photosensitizing effect of the dye itself, induced by visible light, can cause the misconception that a given photocatalyst enacts real activity under visible light. Fortunately, authors included colorless organic compounds, such as phenol or 4-chlorophenol, in some of their reports. Another issue is the need to study the effect of dye adsorption and include this information in the graphs. Therefore, the selection of dyes as model compounds for photocatalytic experiments requires careful planning.
When the types of photocatalytic reactions are considered, most of the publications are concerned with the photocatalytic oxidation of organic compounds, and the photocatalytic efficiencies reported in both UV and Vis were high in this field, but the materials from some of the papers, especially when methylene blue was used under visible light conditions, should probably be retested, using other model compounds. As shown in Figure 8, more than 80% of 4-chlorophenol was degraded after 7 h, confirming the synergistic effect of PANI and TiO2 [64]. Considering other reaction types, only 12% of the publications are concerned with testing hydrogen evolution process [72,75,76,78,85], and one report deals with photocatalytic reduction in carbon dioxide [86]. These reaction fields still require further research in order to test the suitability of these materials. It should be noted that most PANI/TiO2 photocatalysts that are active in hydrogen evolution contain modified titania (with noble metals or with defective structure—grey titania). Moreover, the work of El-Bery et al. (Figure 9) demonstrated that the PANI/TiO2 sample is more active in photocatalytic hydrogen evolution than PEDOT/TiO2 and PPy/TiO2, confirming the good prospects of PANI composites with titania compared to other conductive polymers [61].
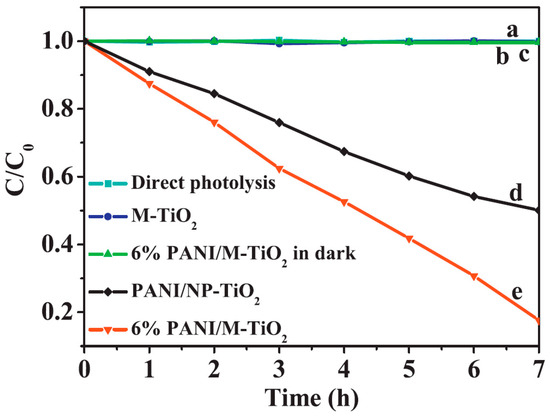
Figure 8.
Photocatalytic degradation of 4-chlorophenol under visible light irradiation (λ > 400 nm); (a) direct photolysis, (b) M(mesoporous)-TiO2, (c) 6% PANI/M-TiO2 in the dark, (d) PANI-NP-TiO2, (e) 6% PANI/M-TiO2. Reprinted with permission from [64]. Copyright 2011, Elsevier.
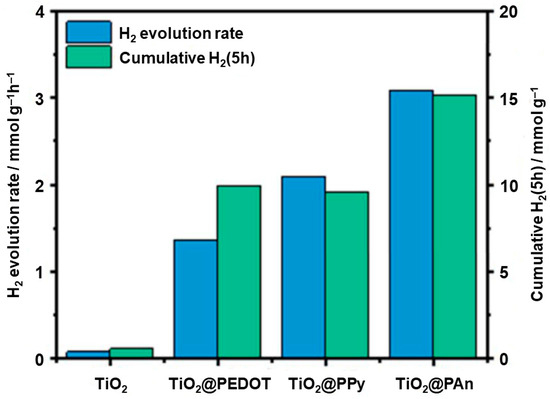
Figure 9.
Comparison of TiO2(P25), PEDOT/TiO2, PPy/TiO2, PANI/TiO2 photocatalytic activities in the process of hydrogen evolution [61].
4.3. Photocatalytic Mechanism
Upon analyzing the mechanisms of photocatalytic reactions based on the PANI/TiO2 materials presented in the publications surveyed, there is general agreement on the mechanistic routes. Figure 10 shows the mechanism of reactive oxygen species generation under UV and Vis conditions using PANI/TiO2 photocatalysts. As shown in Figure 5, the HOMO of PANI is located between the VB and CB bands of titania, while the LUMO of PANI is located higher than the CB of TiO2. This has been experimentally verified and reported by Hidalgo et al. [85]. Considering UV radiation (Figure 10a), both PANI and TiO2 absorb photons. The charge is immediately transferred from the HOMO to LUMO levels in PANI. Photogenerated electrons are transferred from the LUMO of PANI to the CB of TiO2. An electron center is formed. Charge separation is enhanced, and superoxide radicals are generated in the presence of oxygen (reduction reaction). Subsequently, these radicals react with water to form hydroxyl radicals (OH•). Simultaneously, photogenerated holes from the VB of TiO2 migrate to the surface of the HOMO of PANI. A hole center is then formed, which is involved in the oxidation of water to OH• (oxidation reaction). The resulting reactive oxygen species (mainly hydroxyl radicals) participate in the oxidation of organic compounds. The synergistic effect of PANI and TiO2 relates to an efficient charge carrier separation, which reduces the recombination effect and increases photocatalytic activity [87,88].
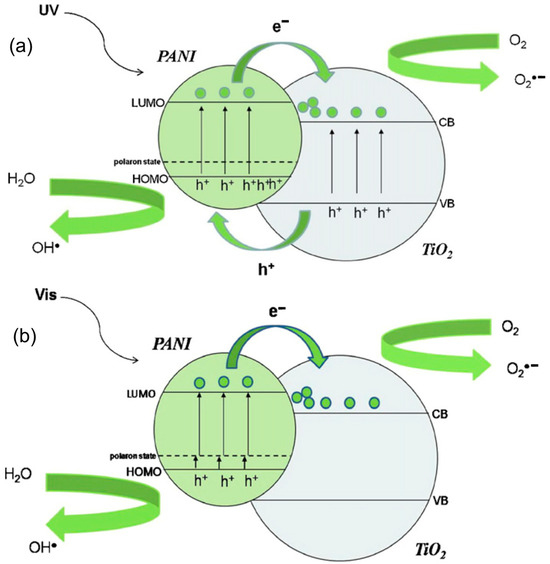
Figure 10.
Mechanism of the generation of reactive oxygen species under (a) UV and (b) visible light by a PANI–TiO2 nanocomposite photocatalyst. Reprinted with permission from [88]. Copyright 2021, Elsevier.
Under visible light irradiation, only the PANI component is excited (Figure 10b). The charge migrates from the HOMO to LUMO of PANI. The excited-state electrons from PANI can then be transferred from the LUMO of PANI to the CB of TiO2. An electron center is formed. Simultaneously, the transitions π → polaron and polaron → π* in PANI are induced. Photogenerated holes in the π orbital of PANI are transferred to the interface and react with water to form hydroxyl radicals. The synergistic effect of PANI and TiO2 is associated not only with visible light sensitization of TiO2 through PANI but also with improved charge separation [87,88].
There are also reports of modifications to the PANI–TiO2 binary system. The modification of the mechanism is the result of such a concept. For example, Zhang et al. modified the PANI–TiO2 composite with gold [58]. In this mechanism, additional hot electrons from Au NPs are injected into the CB of titania, inducing a visible-light-mediated surface plasmon resonance (LSPR) mechanism (Figure 11). The presence of Au NPs in this system enhances the charge separation and visible light sensitization effect due to the additional synergy between Au@TiO2 and PANI–TiO2.


Table 2.
Summary of binary PANI–TiO2 heterostructures with photocatalytic properties.
Table 2.
Summary of binary PANI–TiO2 heterostructures with photocatalytic properties.
| PANI–TiO2 Material | Preparation Conditions | Photocatalytic Efficiency | Type of Photocatalytic Mechanism | Ref. |
|---|---|---|---|---|
| PANI/SAM-TiO2 | A self-assembled TiO2 monolayer was used during aniline polymerization in the presence of HCl for 4 h; PANI was in ES form. | Photodegradation of methylene orange was performed under sunlight. Almost all the amounts of MO degraded during 150 min. | Proposed sensitization mechanism of TiO2 by PANI. | [63] |
| PANI/TiO2 | PANI and TiO2 (P25) were mixed in tetrahydrofuran. An optimum synergetic effect was found for 3 wt% of PANI. | Photodegradation of methylene blue and rhodamine B was performed under UV and Vis (λ > 450 nm). The activities under Vis for the PANI/TiO2 (3 wt%) sample were k = 0.0071 min−1 (MO) and k = 0.0229 min−1. The activities under UV for the PANI/TiO2 (3 wt%) sample were k = 0.0091 min−1 (MO), k = 0.099 min−1 in comparison to pure TiO2; k = 0.046 min−1 (MO) and k = 0.066 min−1. | Proposed heterojunction, providing enhanced charge separation (UV). Proposed sensitization mechanism of TiO2 by PANI (Vis). | [57] |
| PANI/TiO2 | Aniline (0.058 M) was used as a precursor; 1 h polymerization occurred at 25 °C in the presence of TiO2 (0.5 g—the best conditions) and acid—the best efficiency for H2SO4; PANI was in ES form. | Photodegradation of Allura Red (ALR) and Quinoline Yellow (QY) under UV light (λ = 254 nm). kALR = 10.7 × 10−4 s−1; kQY = 13.02 × 10−4 s−1. | Proposed heterojunction, providing enhanced charge separation. | [89] |
| PANI/TiO2 | Camphorsulfonic acid-doped PANI was prepared first; aniline was used as a precursor; 24 h polymerization occurred at 0°. The best mass ratio of PANI and TiO2 (P25) was 1:500. | Photodegradation of methylene blue was performed under visible light (λ > 400 nm). k = 0.01515 min−1 in comparison to pure TiO2, k = 0.00963 min−1. | Proposed sensitization mechanism of TiO2 by PANI. | [90] |
| PANI/TiO2 fiber film | Aniline (0.5 g) was used as a precursor; 29 h polymerization occurred at 0° in the presence of HCl and TiO2 multi-pore fiber film. Photocatalytic activity correlated to the crystal structure of titania. | Photodegradation of methylene blue was performed under sunlight. MB decolorization rate, using PANI/TiO2 film, was nearly similar to the pristine TiO2 film after 240 min irradiation. | Not studied | [59] |
| PANI/M-TiO2 (core–shell) | PANI and M-TiO2 (mesoporous titania) were mixed in tetrahydrofuran. The optimum synergetic effect was found at 6 wt% of PANI. | Photodegradation of rhodamine B and 4-chlorophenol under visible light (λ > 400 nm). The activities for the PANI/TiO2 (6 wt%) sample were 99.8% (RhB, 30 min) and 82.4% (4-CP, 7 h). | The role of the core–shell structure in visible light activity. | [64] |
| PANI nanofibers/TiO2 | Aniline (0.91 mL) was used as a precursor; 24 h polymerization occurred at 0° in the presence of HCl. Finally, the product was dispersed in absolute ethanol to form the nonaqueous suspension of PANI nanofibers. The PANI/TiO2 mass ratio of 1/100 was optimal. | Photodegradation of methylene blue and rhodamine B was performed under UV and Vis (λ > 420 nm). UV: activity (both MB and RhB) was similar to pure TiO2; Vis: activity was 8 and 2.5 times better than pure titania for MB and RhB, respectively. | UV: proposed passivation of photocatalytic activity by PANI layer; Vis: proposed sensitization mechanism of TiO2 by PANI. | [83] |
| PANI/TiO2 | Aniline (0.09 M) was dissolved in HCl; titania precursors were added to an aniline and APS solution; subsequent hydrothermal conditions were maintained at 100 °C for 4 h; next, thermal treatment occurred at different temperatures, 100–300 °C. | Photodegradation of methyl orange was performed under UV and Vis (λ > 420 nm). The best activity for the sample calcined at 200 °C; UV: k = 2.484 h−1; Vis: k = 0.5316 h−1.. | Proposed sensitization mechanism of TiO2 by PANI. | [91] |
| PANI/colloidal TiO2 NPs | Aniline was used as a precursor; 20-day polymerization occurred in the presence of TiO2; TiO2/ANI mole had ratios of 50 (TP-50), 100 (TP-100), 150 (TP-150). TP-50: PANI-ES; TP-100: both PANI-ES and PANI-EB. | Photodegradation of MB and RhB was performed under a solar simulator. The best activity reported for TP-50/TP-100 samples: RhB completely degraded after 6 h, while MB degraded 60%. | Proposed heterojunction (UV) and sensitization mechanism (Vis). | [88] |
| PANI/TiO2 | Aniline was used as a precursor; 24 h polymerization occurred at room temperature in the presence of a peroxo–titanium complex and H2SO4; optimal AN/Ti ratio of 1/1; conductivity of nanocomposite: 2.08 × 10−2 s cm−1. | Photodegradation of methylene blue was performed under UV (λ = 365 nm). Almost 17% degradation occurred during 100 min. | Not studied | [92] |
| PANI/TiO2 nanotubes | Aniline (2 mmol) was used as a precursor; 24 h polymerization occurred at room temperature in the presence of TiO2. | Photodegradation of methyl orange and orange II was performed under UV; the synergistic effect of both components was confirmed in comparison with single PANI nanotubes. | Proposed heterojunction, providing enhanced charge separation. | [68] |
| PANI/TiO2 | Aniline was used as a precursor; 24 h polymerization occurred at 4 °C in the presence of TiO2; titania/aniline ratio of 1:1 wt%; dark green composite was formed. | Photodegradation of methylene blue was performed under visible light (λ > 400 nm). More than 80% of MB degraded during 5 h; the synergistic effect of both components was confirmed in comparison with single PANI. | Proposed sensitization mechanism of TiO2 by PANI. | [93] |
| PANI/TiO2 | Aniline (0.4 M) solution in aqueous H2SO4 (one sample sonicated; another one without sonication); 24 h polymerization occurred at 0 °C in the presence of TiO2 (0.5 g). | Photodegradation of methylene blue was performed under visible light (λ > 400 nm). Photodecomposition studies of MB with PANI, TiO2, PANI–TiO2–S, and PANI–TiO2 showed efficiencies of 58, 71, 77 and 65%, respectively. | Proposed sensitization mechanism of TiO2 by PANI. | [94] |
| PANI/TiO2/SiO2 nanofiber membranes | Aniline polymerization occurred on the surface of TiO2/SiO2; aniline was dissolved in HCl solution; different polymerization times were used; PANI-ES was obtained. | Photodegradation of methyl orange blue was performed under visible light (λ > 420 nm). The best activity for the sample: PANI loading of 2.3%; 87% of MO degradation occurred during 90 min. | Proposed sensitization mechanism of TiO2 by PANI. | [70] |
| 3D flowerlike TiO2/PANI | Aniline was used as a precursor; polymerization for 4 h at 0 °C occurred in the presence of colloidal TiO2 and HCl; different Ti/ANI molar ratios were prepared: 2:1, 1:2, 1:5; dark green powder was obtained. | Photodegradation of Congo red and methyl orange was performed under UV and solar light; optimal photocatalytic performance: Ti/ANI molar ratio of 1:1: degradation under UV: 92% (MO) and 96% (CR) for 2 h, being far better than for P25 (50%); for sunlight: k = 0.031 h−1 (MO); k = 0.037 h−1 (CR) in comparison to P25: k = 0.011 h−1 (MO); k = 0.012 h−1 (CR). | UV: proposed passivation of photocatalytic activity by PANI layer; Sunlight: proposed sensitization mechanism of TiO2 by PANI. | [67] |
| PANI/TiO2 | Aniline was used as a precursor; polymerization for 3 h occurred at 0 °C in the presence of 2.5 g TiO2 and HCl; hydrothermal treatment was subsequently performed; different molar ratios of TiO2 and aniline were used: 4:1, 2:1 and 1:1. | Photodegradation of 4-nitrophenol was performed under visible light (λ > 400 nm); the molar ratio (TiO2/aniline) 2:1 was optimal for the photocatalytic performance: 90% of degradation of 4-NP. | Not studied. | [95] |
| PANI/TiO2 film | PANI was first synthesized from aniline dimer with the addition of poly (sodium 4-styrenesulfonate), which was used as an emulsioning and doping agent to stabilize a structure; next, green powder of PANI was deposited in the TiO2 films through a dip-coating method. | Photoelectrochemical behavior was evaluated under UV–Vis, using prepared photoanodes for the water photoelectrolysis reaction in a NaOH solution; an increase in photocatalytic activity was observed compared to unmodified titania; ES form of PANI was more active than the PE form. | Enhanced electron transfer and efficient charge separation. | [85] |
| PANI/TiO2 | Aniline (2.5 mmol) was used as a precursor; polymerization occurred for 12 h in the presence of various amounts of TiO2 with respect to aniline: 0, 5, 10, 15 and 20 wt%; dark green powder was obtained. | Photodegradations of rhodamine B, methylene blue and phenol were performed under UV light; photocatalyst with 20 wt% of titania displayed the highest activity (>80% for RhB after 3 h of irradiation). All modified photocatalysts were more active than pristine TiO2. | Not studied. | [96] |
| PANI/MS-TiO2 | Aniline (2.5 mmol) was used as a precursor; polymerization occurred for 12 h in the presence of 0.5 g MS (mesoporous)-TiO2 with respect to aniline molar ratios: 20:1, 40:1, 60:1 and 80:1. | Photodegradations of rhodamine B and methylene blue were performed under visible light (λ > 400 nm). The photodegradation efficiencies for RhB and MB were 99% and 97%, respectively, for the sample with the molar ratio (TiO2/ANI) 40:1. | Proposed sensitization mechanism of TiO2 by PANI. | [65] |
| Au-PANI/TiO2 | Aniline was used as a precursor; polymerization occurred for 12 h in the presence of 400 mg TiO2 and chloroauric acid; different weight ratios of aniline to titania were considered: 1/30, 1/60, 1/80, 1/100. | Photodegradation of rhodamine B was performed under visible light (λ > 420 nm). Photocatalytic efficiencies for TiO2, Au-TiO2 and Au-PANI/TiO2 (1:100) were k = 0.00379 min−1, k = 0.00379 min−1 and k = 0.01016 min−1. | Proposed sensitization mechanism of TiO2 by PANI, supported by the presence of Au NPs (surface plasmon resonance effect). | [58] |
| PANI/TiO2 | Aniline was used as a precursor; polymerization occurred for 24 h at room temperature in the presence of 0.8 g TiO2; different weight ratios of PANI to titania were considered: 10/1, 15/1, 20/1, 25/1. Highest conductivity occurred in sample 20/1. | Photodegradation of Reactive Red 45 was performed under UVA and solar irradiation (solar simulator). Photocatalytic activity under UVA (TOC removal) for sample 15/1 and pristine TiO2 were 80% and 35%; under solar irradiation, complete degradation occurred; and 83% degradation occurred for samples 15/1 and TiO2 during 90 min. | Not studied. | [97] |
| PANI/TiO2 | Aniline was used as a precursor; polymerization occurred for 24 h at 0 °C in the presence of TiO2; different amounts of titania were used at 10, 20, 40 wt%. | Photodegradation of RB5 was performed under visible light; the best activity for the sample with 10 wt% of titania content resulted in 96% of degradation. | Proposed sensitization mechanism of TiO2 by PANI. | [98] |
| Carbonized PANI/TiO2 | Aniline was used as a precursor; polymerization occurred for 3 days in the presence of TiO2; in the next step, carbonization in argon atmosphere was performed; different mole ratios of titania/aniline were considered: 20, 50 and 80. | Photodegradations of rhodamine B and methylene blue were performed under simulated solar irradiation; photocatalytic performance was optimum for molar ratio 80:1: almost 100% of MB degradation (60 min) and 97% of RhB degradation (90 min) occurred. | The role of the carbonization process—changes in TiO2 structures—for the improvement of charge separation. | [99] |
| PANI/TiO2 | Aniline was used as a precursor; polymerization occurred for 24 h at room temperature in the presence of 0.512 g of TiO2; different molar ratios of aniline to titania were considered: 1/6, 1/5, ¼, ½, 1/1, 2/1, 3/1, 4/1. | Photodegradation methylene blue was performed under UV/Vis irradiation; the optimum photocatalytic performance was for the sample with molar ratio 1/5: 60% of MB degradation occurred during 180 min, and for pristine titania, it was 30%. | Proposed sensitization mechanism of TiO2 by PANI. | [100] |
| PANI/grey-TiO2 | Aniline was used as a precursor; polymerization occurred for 3 h at 0 °C in the presence of 2 g of grey-TiO2; different weight percentages for aniline were considered: 2.5, 5 and 12 wt%. | Photodegradations of rhodamine B and hydrogen evolution were performed under visible light (λ > 420 nm). The most active sample for aniline weight content of 5 wt%; for RhB resulted in almost 100% degradation (180 min); for H2 evolution: 9 mmol/g after 5 h of irradiation; activities were better than for single grey-TiO2. | PANI serves as a co-catalyst in promoting separation of photoinduced charges, and photoelectrons and holes can efficiently transfer at the interface of PANI and grey-TiO2. | [72] |
| PANI/black anatase TiO2 (BAT) | Aniline (0.2 M) was used as a precursor; polymerization occurred for 16 h at 0–4 °C in the presence of 0.2 g of BAT. | Photodegradation of methyl orange was performed under visible light (λ > 450 nm). Photodegradation efficiency of PANI/BAT sample was better than for single BAT or single PANI: 60% of MO after 40 min. | Not studied. | [73] |
| HP-PANI/TiO2 | A novel preparation procedure of highly porous (HP) PANI/TiO2: (1) UV-mediated PANI oligomer growth on the TiO2 surface was used; (2) oligomer polymerization was conducted with H2O2. | Photodegradation of methyl orange was performed under UV irradiation; high photocatalytic efficiency was present for HP-PANI/TiO2: 97% of degradation during 20 min of irradiation. | Not studied. | [62] |
| rGH-PANI/TiO2 | PANI was obtained as a chemical; TiO2 in the P25 was used; reduced graphene oxide–PANI/TiO2 composite (rGH-PANI/TiO2) with a three-dimensional (3D) network structure was synthesized by a two-step method, involving a hybridization process and a water bath approach. | The photo-electro-catalytic degradation efficiencies of rGH-PANI/TiO2 for the degradation of phenol, 2,4-dichlorophenol, and BPA were tested under UV (λ > 320 nm); this reached 100% degradation after 7 h, 3.5 h and 4.5 h, respectively. | Proposed heterojunction, providing enhanced charge separation. | [101] |
| PANI/TiO2 | Aniline (1 mL) was used as a precursor; polymerization occurred for 4 h at 0 °C in the presence of 0.1 g of TiO2 (P25). | Photodegradation of Reactive Blue-19 was performed under UV, visible and solar light; photocatalytic efficiency was similar for all sources of light: almost 100% degradation during 120 min. | Proposed heterojunction, providing enhanced charge separation (UV). Proposed sensitization mechanism of TiO2 by PANI (Vis). | [87] |
| PANI/colloidal TiO2 NPs | Aniline was used as a precursor; polymerization occurred 20 days in the presence of TiO2; TiO2/ANI mole ratios of 50 (TP-50), 100 (TP-100), 150 (TP-150). TP-50: PANI-ES; TP-100: both PANI-ES and PANI-EB. | Photodegradation of biologically active compounds was carried out in a cell under UV degradation; for amitriptyline, sample TP-100—45% degradation (60 min); for sulcotrione, sample TP-150 (same as pristine TiO2)—97% degradation (60 min); for clomazone, sample TP-150—33% degradation (60 min). | Not studied. | [102] |
| PANI/TiO2 nanotubes | Titania nanotubes (TNT) were used as a substrate for the deposition of PANI via vacuumed rotating-bed plasma-enhanced vapor deposition; different plasma powers were used: 25, 50 and 75 W. | Photodegradation of RB5 was performed under UV and visible light; under UV conditions, photoactivities for TNT, PANI(25 W)/TNT and PANI (50 W)/TNT were similar (near 100% degradation); for Vis conditions, the highest degradation was for PANI (50 W)/TNT sample (56.4% degradation). | Not studied. | [69] |
| PANI/TiO2 (acrylic pseudo-paints) | Aniline was used as a precursor; polymerization occurred for 6 h at 5 °C in the presence of 1 g of TiO2; dark green materials were obtained; for the preparation of pseudo-paint films, the aqueous dispersion of dodecyl benzene sulfonic acid and PANI/TiO2 were prepared and added to the acrylic latex. | Photodegradation of benzene was performed under UV and Vis light; adding nanocomposites into the acrylic film improved the photocatalytic performance under visible light but decreased activity under UV light. The photocatalytic pseudo paints containing PANI/TiO2 nanocomposites degraded approximately 16% benzene under visible light, suggesting its use as a practical solution for indoor air purification. | Not studied. | [80] |
| PANI/TiO2/cotton | Aniline (0.5 mol/L) was added into a TiO2 homogeneous suspension; the cotton fabrics after plasma pretreatment underwent the “dip-pad” method with the solution mentioned. Aniline-adsorbed cotton fabrics were immersed in a solution containing APS, and a polymerization process was conducted for 4 h at 0 °C. | Photodegradation of Rhodamine B was performed under simulated sunlight; PANI/ TiO2/cotton composite fabric had good adsorption and photocatalytic performance, and the total efficiency of adsorption and degradation for RhB was up to 87.7%. | Not studied. | [81] |
| PANI/Ti0.91O2 | PANI NPs were introduced into the interlayer space of titanate lattice by the addition of Ti0.91O2 colloidal suspension to the turbid PANI NPs suspension, stirring for 2 h; green products were obtained. | Photodegradation of Rhodamine B was performed under visible light (λ > 420 nm). Photocatalytic efficiency resulted in an almost complete degradation during 6 h. | Proposed sensitization mechanism of Ti0.91O2 by PANI. | [60] |
| PANI/Pt-TiO2 | Pt-loaded TiO2 NPs were prepared via photoreduction method; next, aniline was used as a precursor; polymerization occurred for 6 h at 0 °C in the presence of 0.5 g of Pt-TiO2; aniline/titania ratio was 4/100. | Photocatalytic hydrogen generation was performed under visible light (λ > 420 nm). The rates of hydrogen evolution (μmol h−1 g−1) for TiO2, Pt-TiO2, PANI–TiO2 and PANI/Pt-TiO2 were 0, 0, 3 and 60, respectively. | Proposed sensitization mechanism of Pt-TiO2 by PANI. | [76] |
| PANI/M-TiO2 | Aniline was used as a precursor; polymerization occurred for 12 h at 0 °C in the presence of 1.04 g of mesoporous TiO2; by varying aniline amounts, samples with different content of PANI were obtained: 1, 3, 5, 7, 10 wt%. | Photocatalytic reduction of Cr(VI) to Cr(III) was performed under UV; the maximum reaction rate was 0.62 min−1 for samples containing 3 wt% of PANI. | Proposed heterojunction, providing enhanced charge separation. | [66] |
| PANI/TiO2-rGO | Aniline (in different amounts: 0.225 mmol, 0.45 mmol, 0.9 mmol) was used as a precursor; polymerization occurred for 24 h at 5 °C in the presence of TiO2-rGO (rGO-reduced graphene oxide); dark green material was obtained. | Photodegradations of Rhodamine B and hydrogen production were performed under visible light (λ > 420 nm); photodegradation efficiencies after 90 min of irradiation for TiO2, TiO2-rGO, PANI/TiO2-rGO(0.225), PANI/TiO2-rGO(0.45), PANI/TiO2-rGO (0.9) were 22%, 62%, 80%, 90% and 85%, respectively; hydrogen production efficiencies for TiO2, TiO2-rGO, PANI/TiO2-rGO(0.225), PANI/TiO2-rGO(0.45), PANI/TiO2-rGO (0.9) were (in mmol h−1 g−1) 0.08, 0.45, 0.57, 0.8 and 0.72, respectively. | The enhanced photocatalytic performance is attributed to the rapid photoinduced charge separation and efficiently suppressed charge recombination caused by the synergy between PANI, TiO2, and rGO. Moreover, the interfacial charge transfer in PANI and rGO with the p-conjugated group used for photocatalytic performance is of great significance. | [78] |
| PANI/TiO2 | Aniline was used as a precursor; polymerization occurred overnight in the presence of TiO2; different contents of titania were used (0.2 M, 0.3 M, 0.4 M, 0.5 M, 0.6 M). | Photodegradation of methylene blue was performed under UV; For samples containing 0.6 M of titania, the efficiency was highest (86.35%), because large amounts of TiO2 in the PANI matrix can produce many reactive sites. | Proposed heterojunction, providing enhanced charge separation and visible light activity. | [103] |
| PANI/TiO2 nanorods | The first step: synthesis of titania nanorods (hydrothermal conditions); the second step: using aniline as a precursor; polymerization occurred for 12 h in the presence of TiO2 nanorods; green product was obtained. | Photodegradation of bisphenol A was performed under UV; photocatalytic efficiency was higher for PANI/TiO2 than for TiO2: 79.8% and 60.7% of bisphenol A degradation after 80 min. | Proposed heterojunction, providing enhanced charge separation. | [71] |
| PANI/TiO2-gypsum plaster | Aniline was used as a precursor; polymerization occurred for 24 h at 0 °C in the presence of TiO2 (molar ratio of aniline to titania was 1:5); the photocatalytic gypsum plaster was prepared by blending commercial gypsum plaster and distilled water to obtain a paste form; PANI–TiO2 in 10 wt% to the dry mass of the plaster. | Photodegradation of phenol was performed under UV/Vis and visible light ((λ > 400 nm and λ > 420 nm), using non-gypsum materials; UV/Vis—TiO2: 4.63 μmol h−1, PANI–TiO2: 2.01 μmol h−1,Vis(400)—TiO2: 0.4 μmol h−1, PANI–TiO2: 0.07 μmol h−1, Vis(420): TiO2: 0.12 μmol h−1, PANI–TiO2: 0.26 μmol h−1; photodegradation of toluene in the presence of gypsum plasters containing composites; using 460 nm LED activity of the gypsum containing PANI–TiO2 was higher than the one containing TiO2; this was the opposite if 380 nm LED was used. | Proposed heterojunction, providing enhanced charge separation and visible light activity. | [79] |
| PANI/TiO2 | Aniline was used as a precursor; polymerization occurred for 12 h in the presence of TiO2; PANI contents were: 3%, 10%, 30%; green powder was formed; prepared composite (3 wt%) was added to polystyrene dissolved in toluene. | Polystyrene composite sheets were exposed to UV radiation (λ = 253 nm); the optimum photodegradation performance was reported for TiO2-10% PANI, and it was almost two times higher than for single TiO2. | Proposed heterojunction, providing enhanced charge separation. | [104] |
| Polyurethane-PANI/TiO2 | Different methods of combining polyurethane foam with PANI/TiO2 to form floating composites were used: (1) in situ polymerization, (2) immobilization, (3) in situ UV-assisted polymerization, (4) impregnation by organic dopant-modified PANIs solutions. | Photodegradation of Rhodamine B was performed under simulated sunlight; the composite prepared by method (4) had the best activity: almost 100% of RhB degradation occurred during 30 min. | Not studied. | [82] |
| PANI/TiO2 | PANI synthesis: aniline was used as a precursor; polymerization occurred for 6 h at 5 °C; next step— the impregnation of PANI and TiO2, using porcelain crucible and a DMF solution. | Photocatalytic hydrogen production was performed under UV light (LED). The highest efficiency was for samples with 5 wt% content of PANI; 15 mmol g−1 of hydrogen evolved during 5 h; this result was better than for other titania composites containing different conducting polymers such as PEDOT (10 mmol g−1) and PPy (9.5 mmol g−1). | Proposed sensitization mechanism of TiO2 by PANI. | [61] |
| PANI/TiO2-Ag | The first step: the preparation of Ag-TiO2 NPs; The second step: the use of aniline as a precursor; polymerization occurred for 4 h in ice bath conditions in the presence of Ag-TiO2; different amounts of Ag-TiO2 were used: 5 wt%, 9 wt%, 11 wt% and 13 wt%. | Photocatalytic disinfection activity was tested using E. coli under visible light; the best sample for disinfection efficiency was a composite with 13 wt% content of Ag-TiO2; photodegradation of lipopolysaccharides were performed under visible light; ca. 40% of endotoxin was degraded in 180 min. | Not studied. | [74] |
| PANI/SiO2@TiO2 | The first step was the preparation of core–shell SiO2@TiO2 particles; subsequently, PANI was deposited on SiO2@TiO2; aniline was used as a precursor; polymerization occurred in the presence of SiO2@TiO2; molar ratios between aniline and TiO2 were as follows: 1:80, 1:60, 1:40; mesoporous and non-porous SiO2 were used. | Photodegradation of phenol was tested under visible light (LED). The highest photocatalytic performance was reported in samples with 1:80 molar ratio and a mesoporous core; 35% of phenol degradation occurred during 190 min. | Not studied. | [77] |
| PANI/TiO2-Au | Polymeric nanocomposite films were produced via layer-by-layer technique using poly(allyamine hydrochloride), poly(acrylic acid), TiO2 NPs, tetrachloroauric(III) acid and mercaptoethane sodium sulfonate solutions; the films were assembled on FTO glass; PANI was in the form of ES. | Photocatalytic reaction for hydrogen production was performed in a quartz reactor, using a solar simulator; hydrogen evolution rate for the best sample was 1300 μmol g−1 during 180 min. | Proposed sensitization mechanism of TiO2 by PANI, supported by the presence of Au NPs (surface plasmon resonance effect). | [75] |
| PANI/TiO2 | Aniline (1 mL) was used as a precursor; polymerization occurred for 4 h in the presence of TiO2 (P25); different weight contents of titania were considered: 9.4%, 12%, 18.2%, 29.1% and 35%. | Photocatalytic reduction in carbon dioxide was performed under visible light; the yield of formaldehyde and formic acid formed per gram of catalyst increased as the TiO2 content in the PANI–TiO2 nanocomposites varied from 9.4% to 18.2%; however, a further increase in TiO2 content to 29.1 or 35% resulted in a decrease in the yields of products (formaldehyde and formic acid). | Proposed heterojunction, providing enhanced charge separation and visible light activity. | [86] |
| PANI/TiO2, PANI/TiO2/GO | Combination of aniline, titanium isopropoxide and graphene oxide; polymerization occurred for 2 h; finally, HCl treatment was used to obtain conductive PANI. | Photodegradations of Thymol Blue and Rose Bengal dyes were performed under visible light; the photodegradation efficiencies of TiO2/PANI/GO, TiO2/PANI, and TiO2 for Thymol Blue were 96%, 87% and 21%, respectively, after irradiation for 180 min; for Rose Bengal, they were 97%, 93% and 14%, respectively. | Not studied. | [105] |
| PANI/TiO2 | PANI-ES/TiO2 composites were synthesized via in situ chemical oxidation polymerization method. | Photodegradation of RfOM (refractory organic matter) was performed under simulated sunlight; the synergistic effect between PANI and TiO2 was more pronounced in lower PANI ratios, whereas higher PANI ratios reflected a retardation effect up to a specific mass ratio of polyaniline to TiO2. | Not studied. | [106] |
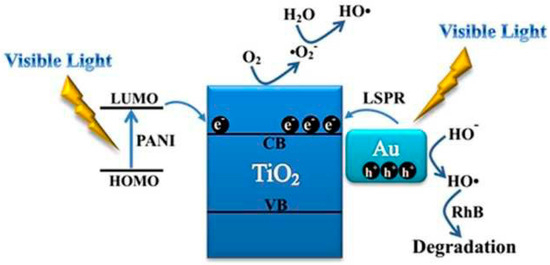
Figure 11.
Mechanism of the photocatalytic degradation of RhB by the Au-PANI@TiO2 NPs under visible light irradiation. Reprinted with permission from [58]. Copyright 2016, Royal Chemical Society.
5. Conclusions
Binary PANI–TiO2 heterostructures are very promising photocatalytic materials. They can be used in many types of reactions, such as the photooxidation of organic compounds, photocatalytic hydrogen generation and carbon dioxide reduction. These materials can be efficient in both UV and Vis due to the synergistic effect of both components. This effect manifests itself in two aspects: (1) the presence of PANI as an effective visible light sensitizer of titania and (2) the formation of a heterojunction (type II) between PANI and TiO2, which is a decisive factor in reducing the charge carrier recombination effect and increasing the efficiency of UV-induced reactions. Based on the literature survey, the following conclusions and remarks can be drawn: (I) there are reports on the use of different morphological forms of titania, but there are still too few reports on the influence of different forms of TiO2 used in heterostructures with PANI on the efficiency of these binary systems; (II) there are almost no reports on the evaluation of the influence of PANI with different properties, e.g., particle size on photocatalytic activity, including the use of different preparation methods of PANI or PANI–TiO2 heterostructures; (III) the model organic compounds in the photooxidation reactions were mainly dyes, making it advisable to use other, preferably colorless, organics, e.g., phenols, especially for visible light-induced reactions; (IV) mechanistic studies of photocatalytic reactions based on PANI–TiO2 materials should be further investigated via experiments to confirm existing concepts or to propose other mechanism variants; (V) research into alternative, simple and low-cost preparation methods of PANI–TiO2 structures with high application potential is highly desirable; (VI) studies on the stability of these heterostructures should be more frequently included in research reports.
Author Contributions
Y.F. and M.J. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Polish Ministry of Education and Science subsidy from the Poznan University of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C 2011, 11, 179–209. [Google Scholar] [CrossRef]
- Hossen, M.A.; Solayman, H.M.; Leong, K.H.; Sim, L.C.; Yaacof, N.; Aziz, A.A.; Wu, L.; Monir, M.U. Recent progress in TiO2-Based photocatalysts for conversion of CO2 to hydrocarbon fuels: A systematic review. Results Eng. 2022, 16, 100795. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Sheppard, L.R.; Bak, T.; Nowotny, J. Defect chemistry of titanium dioxide. Application of defect engineering in processing of TiO2-based photocatalysts. J. Phys. Chem. C 2008, 112, 5275–5300. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Sucher, N.J.; Wachsman, E. Effect of crystal imperfections on reactivity and photoreactivity of TiO2 (rutile) with oxygen, water, and bacteria. J. Phys. Chem. C 2011, 115, 15711–15738. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Zolnhofer, E.M.; Meyer, K.; Schmuki, P. Noble-metal-free photocatalytic H2 generation: Active and inactive ‘black’ TiO2 nanotubes and synergistic effects. Chem. Eur. J. 2016, 22, 13810–13814. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Ząbek, P.; Eberl, J.; Kisch, H. On the origin of visible light activity in carbon-modified titania. Photochem. Photobiol. Sci. 2009, 8, 264–269. [Google Scholar] [CrossRef]
- Kisch, H.; Sakthivel, S.; Janczarek, M.; Mitoraj, D. A low-band gap, nitrogen-modified titania visible-light photocatalyst. J. Phys. Chem. C 2007, 111, 11445–11449. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. On the mechanism of urea-induced titania modification. Chem. Eur. J. 2010, 16, 261–269. [Google Scholar] [CrossRef]
- Kowalska, E.; Prieto Mahaney, O.O.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Wysocka, I.; Janczarek, M.; Stampor, W.; Hupka, J. Preparation and characterization of Pt–N/TiO2 photocatalysts and their efficiency in degradation of recalcitrant chemicals. Sep. Purif. Technol. 2015, 156, 369–378. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Artiglia, L.; Granozzi, G.; Ohtani, B.; Selli, E. Photocatalytic activity vs structural features of titanium dioxide materials singly doped or codoped with fluorine and boron. J. Phys. Chem. C 2014, 118, 25579–25589. [Google Scholar] [CrossRef]
- Diaz-Angulo, J.; Gomez-Bonilla, I.; Jimenez-Tohapanta, C.; Mueses, M.; Pinzon, M.; Machuca-Martinez, F. Visible-light activation of TiO2 by dye-sensitization for degradation of pharmaceutical compounds. Photochem. Photobiol. Sci. 2019, 18, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Bessekhouad, Y.; Robert, D.; Weber, J.-V. Photocatalytic activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 heterojunctions. Catal. Today 2005, 101, 315–321. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial performance of cuprous oxide/titania: The effect of titania matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Sun, W.; Zhang, S.; Zheng, X.; Fu, X.; Chen, S. Insight into the transfer mechanism of photogenerated carriers for WO3/TiO2 heterojunction photocatalysts: Is it the transfer of band–band or Z-scheme? Why? J. Phys. Chem. C 2018, 122, 26326–26336. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Ghosh, S.; Remita, H.; Basu, R.N. Conducting polymers nanostructures for solar-light harvesting. In Visible Light-Active Photocatalysis: Nanostructured Catalyst Design, Mechanisms, and Applications; Wiley: Hoboken, NJ, USA, 2018; pp. 227–252. [Google Scholar] [CrossRef]
- Dey, A.; De, S.; De, A.; De, S.K. Characterization and dielectric properties of polyaniline–TiO2 nanocomposites. Nanotechnology 2004, 15, 1277–1283. [Google Scholar] [CrossRef]
- Molapo, K.M.; Ndangili, P.M.; Ajayi, R.F.; Mbambisa, G.; Mailu, S.M.; Njomo, N.; Maskini, M.; Baker, P.; Iwuoha, E.I. Electronics of conjugated polymers (I): Polyaniline. Int. J. Electrochem. Sci. 2012, 7, 11859–11875. [Google Scholar] [CrossRef]
- Abdelhamid, M.E.; O’Mullane, A.P.; Snook, G.A. Storing energy in plastics: A review on conducting polymers & their role in electrochemical energy storage. RSC Adv. 2015, 5, 11611–11626. [Google Scholar]
- Ghosh, S.; Kouame, N.A.; Remita, S.; Ramos, L.; Goubard, F.; Aubert, P.-H.; Dazzi, A.; Deniset-Besseau, A.; Remita, H. Visible-light active conducting polymer nanostructures with superior photocatalytic activity. Sci. Rep. 2015, 5, 18002. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Huang, W.S.; MacDiarmid, A.G. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Ekande, O.S.; Kumar, M. Review on polyaniline as reductive photocatalyst for the construction of the visible light active heterojunction for the generation of reactive oxygen species. J. Environ. Chem. Eng. 2021, 9, 105725. [Google Scholar] [CrossRef]
- Kwon, O.; McKee, M.L. Calculations of band gaps in polyaniline from theoretical studies of oligomers. J. Phys. Chem. B 2000, 104, 1686–1694. [Google Scholar] [CrossRef]
- Belabed, C.; Abdi, A.; Benabdelghani, Z.; Rekhila, G.; Etxeberria, A.; Trari, M. Photoelectrochemical properties of doped polyaniline: Application to hydrogen photoproductio. Int. J. Hydrogen Energy 2013, 38, 6593–6599. [Google Scholar] [CrossRef]
- Huang, H.G.; Zheng, Z.X.; Luo, J.; Zhang, H.P.; Wu, L.L.; Lin, Z.H. Internal photoemission in polyaniline revealed by photoelectrochemistry. Synth. Met. 2001, 123, 321–325. [Google Scholar] [CrossRef]
- Deb, K.; Bera, A.; Saha, B. Tuning of electrical and optical properties of polyaniline incorporated functional paper for flexible circuits through oxidative chemical polymerization. RSC Adv. 2016, 6, 94795–94802. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Zeng, G.; Gong, X.; Xue, W.; Li, J.; Li, T. Modifying delafossite silver ferrite with polyaniline: Visible-light-response Z-scheme heterojunction with charge transfer driven by internal electric field. Chem. Eng. J. 2019, 370, 1087–1100. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.V. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. J. Photochem. Photobiol. A 2004, 163, 569–580. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Sharma, S.; Khare, N. Sensitization of narrow band gap Bi2S3 hierarchical nanostructures with polyaniline for its enhanced visible-light photocatalytic performance. Colloid Polym. Sci. 2018, 296, 1479–1489. [Google Scholar] [CrossRef]
- Mou, J.; Xu, Y.; Zhong, D.; Chang, H.; Li, J.; Xu, C.; Wang, H.; Shen, H. A highly efficient visible-light-driven photocatalytic fuel cell with ZnIn2S4/PANI/TiO2/Ti photoanode for simultaneous degradation of rhodamine B and electricity generation. New J. Chem. 2023, 47, 4277–4287. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A.; Yubuta, K.; Vadivel, S. Fabrication of TiO2/CoMoO4/PANI nanocomposites with enhanced photocatalytic performances for removal of organic and inorganic pollutants under visible light. Mater. Chem. Phys. 2019, 224, 10–21. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.; Zhao, C.; Zhang, Q.; Geng, W. Synthesis of magnetically separable Fe3O4 at PANI/TiO2 photocatalyst with fast charge migration for photodegradation of EDTA under visible-light irradiation. Chem. Eng. J. 2016, 303, 282–291. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Chauhan, N.; Tahir, M.; Kumari, K.; Mittal, A.; Kumar, N. TiO2/Bi2O3/PANI nanocomposite materials for enhanced photocatalytic decontamination of organic pollutants. Inorg. Chem. Commun. 2022, 146, 110093. [Google Scholar]
- Zeynali, S.; Taghizadeh, M.T. Highly efficient TiO2/AgBr/PANI heterojunction with enhanced visible light photocatalytic activity towards degradation of organic dyes. J. Mater. Sci. Mater. Electron. 2019, 30, 17020–17031. [Google Scholar] [CrossRef]
- Zhao, J.; Biswas, M.R.U.D.; Oh, W.-C. A novel BiVO4-GO-TiO2-PANI composite for upgraded photocatalytic performance under visible light and its non-toxicity. Environ. Sci. Pollut. Res. 2019, 26, 11888–11904. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, D.; Sun, Y.; Sheng, X.; Zhao, J.; Guo, J.; Zhou, B. g-C3N4-and polyaniline-co-modified TiO2 nanotube arrays for significantly enhanced photocatalytic degradation of tetrabromobisphenol A under visible light. Chemosphere 2020, 252, 126468. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Photocatalytic activity of TiO2/Nb2O5/PANI and TiO2/Nb2O5/RGO as new nanocomposites for degradation of organic pollutants. J. Hazard. Mater. 2018, 351, 147–159. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Zhang, T.C.; Xiang, G.; Wang, X.; Pehkonen, S.; Yuan, S. A magnetic γ-Fe2O3@PANI@TiO2 core–shell nanocomposite for arsenic removal via a coupled visible-light-induced photocatalytic oxidation–adsorption process. Nanoscale Adv. 2020, 2, 2018–2024. [Google Scholar] [CrossRef]
- Nekooie, R.; Shamspur, T.; Mostafavi, A. Novel CuO/TiO2/PANI nanocomposite: Preparation and photocatalytic investigation for chlorpyrifos degradation in water under visible light irradiation. J. Photochem. Photobiol. A 2021, 407, 113038. [Google Scholar] [CrossRef]
- Mousli, F.; Chaouchi, A.; Jouini, M.; Maurel, F.; Kadri, A.; Chehimi, M.M. Polyaniline-grafted RuO2-TiO2 heterostructure for the catalysed degradation of methyl orange in darkness. Catalysts 2019, 9, 578. [Google Scholar] [CrossRef]
- Alenizi, M.A.; Rajeev, K.; Aslam, M.; Alseroury, F.A.; Barakat, M.A. Construction of a ternary g-C3N4/TiO2@polyaniline nanocomposite for the enhanced photocatalytic activity under solar light. Sci. Rep. 2019, 9, 12091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zong, R.; Zhao, J.; Zhu, Y. Dramatic visible photocatalytic degradation performances due to synergetic effect of TiO2 with PANI. Environ. Sci. Technol. 2008, 42, 3803–3807. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, Z.; Tang, Y.; Yang, M.; Wang, G. One-step modified method for a highly efficient Au-PANI@TiO2 visible-light photocatalyst. New J. Chem. 2016, 40, 8587–8592. [Google Scholar] [CrossRef]
- Yu, Q.-Z.; Wang, M.; Chen, H.-Z.; Dai, Z.-W. Polyaniline nanowires on TiO2 nano/microfiber hierarchical nano/microstructures: Preparation and their photocatalytic properties. Mater. Chem. Phys. 2011, 129, 666–672. [Google Scholar] [CrossRef]
- Zhou, T.; Ma, L.; Gan, M.; Wang, H.; Hao, C. Sandwich-structured hybrids: A facile electrostatic self-assembly of exfoliated titania nanosheets and polyaniline nanoparticles and its high visible-light photocatalytic performance. J. Phys. Chem. Solids 2019, 125, 123–130. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Salah, M.R.; Ahmed, S.M.; Soliman, S.A. Efficient non-metal based conducting polymers for photocatalytic hydrogen production: Comparative study between polyaniline, polypyrrole and PEDOT. RSC Adv. 2021, 11, 13229. [Google Scholar] [CrossRef]
- Cionti, C.; Pina, C.D.; Meroni, D.; Falletta, E.; Ardizzone, S. Triply green polyaniline: UV irradiation-induced synthesis of a highly porous PANI/TiO2 composite and its application in dye removal. Chem. Commun. 2018, 54, 10702–10705. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, L.; Wu, Y.; Harima, Y.; Zhang, A.; Tang, H. Hybrid composites of conductive polyaniline and nanocrystalline titanium oxide prepared via self-assembling and graft polymerization. Polymer 2006, 47, 7361–7367. [Google Scholar] [CrossRef]
- Liao, G.; Chen, S.; Quan, X.; Zhang, Y.; Zhao, H. Remarkable improvement of visible light photocatalysis with PANI modified core-shell mesoporous TiO2 microspheres. Appl. Catal. B Environ. 2011, 102, 126–131. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Dong, H.; Yan, M.; Wang, J.; Hu, W.; Wang, J.; Zhou, Y.; Tang, J. Enhanced visible light photocatalytic performance of polyaniline modified mesoporous single crystal TiO2 microsphere. Appl. Surf. Sci. 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Y.; Wen, J.; Xu, Y.; Zhu, J.; Bian, Z. Polyaniline-TiO2 composite photocatalysts for light-driven hexavalent chromium ions reduction. Sci. Bull. 2020, 65, 105–112. [Google Scholar] [CrossRef]
- Guo, N.; Liang, Y.; Lan, S.; Liu, L.; Zhang, J.; Ji, G.; Gan, S. Microscale hierarchical three-dimensional flowerlike TiO2/PANI composite: Synthesis, characterization, and its remarkable photocatalytic activity on organic dyes under UV-light and sunlight irradiation. J. Phys. Chem. C 2014, 118, 18343–18355. [Google Scholar] [CrossRef]
- Cheng, Y.; An, L.; Gao, F.; Wang, G.; Li, X.; Chen, X. Simplified synthesis of polyaniline–TiO2 composite nanotubes for removal of azo dyes in aqueous solution. Res. Chem. Intermed. 2013, 39, 3969–3979. [Google Scholar] [CrossRef]
- Subramanian, M.N.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Gursoy, M.; Karaman, M. Synthesis of titania nanotubes/polyaniline via rotating bed-plasma enhanced chemical vapor deposition for enhanced visible light photodegradation. Appl. Surf. Sci. 2019, 484, 740–750. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, Y.; Liu, M.; Ding, Q.; Tjiu, W.W.; Cui, X.; Liu, T. Flexible polyaniline-coated TiO2/SiO2 nanofiber membranes with enhanced visible-light photocatalytic degradation performance. J. Colloid Interface Sci. 2014, 424, 49–55. [Google Scholar] [CrossRef]
- Sambaza, S.S.; Maity, A.; Pillay, K. Polyaniline-coated TiO2 nanorods for photocatalytic degradation of bisphenol A in water. ACS Omega 2020, 5, 29642–29656. [Google Scholar] [CrossRef]
- Xu, S.; Han, Y.; Meng, H.; Xu, J.; Wu, J.; Xu, Y.; Zhang, X. Fabrication of polyaniline sensitized grey-TiO2 nanocomposites and enhanced photocatalytic activity. Sep. Purif. Technol. 2017, 184, 248–256. [Google Scholar] [CrossRef]
- Kavil, J.; Ullattil, S.G.; Alshahrie, A.; Periyat, P. Polyaniline as photocatalytic promoter in black anatase TiO2. Solar Energy 2017, 158, 792–796. [Google Scholar] [CrossRef]
- Gadgil, D.J.; Kodialbail, V.S. Suspended and polycaprolactone immobilized Ag@TiO2/polyaniline nanocomposites for water disinfection and endotoxin degradation by visible and solar light-mediated photocatalysis. Environ. Sci. Pollut. Res. 2021, 28, 12780–12791. [Google Scholar] [CrossRef] [PubMed]
- Galvao, R.A.; Silva, G.M.M.; Coelho, N.C.F.; Santa-Cruz, L.A.; Machado, G. Hydrogen production by the layer-by-layer assembled films of PAni TiO2-AuNP. Mater. Today Chem. 2022, 26, 101072. [Google Scholar] [CrossRef]
- Liu, X.; Lai, H.; Li, J.; Peng, G.; Yi, Z.; Zeng, R.; Wang, M.; Liu, Z. Polyaniline sensitized Pt@TiO2 for visible-light-driven H2 generation. Int. J. Hydrogen Energy 2019, 44, 4698–4706. [Google Scholar] [CrossRef]
- Acosta-Alejandro, J.A.; Lopez-Gonzalez, R.; Frías-Marquez, D.M.; De La Rosa-Vazquez, J.M.; Uribe-Lopez, M.C.; Gomez, R. Polyaniline-sensitized SiO2/TiO2 photocatalysts for the degradation of phenol using visible light. J. Photochem. Photobiol. A 2022, 425, 113696. [Google Scholar] [CrossRef]
- Ma, J.; Dai, J.; Duan, Y.; Zhang, J.; Qiang, L.; Xue, J. Fabrication of PANI-TiO2/rGO hybrid composites for enhanced photocatalysis of pollutant removal and hydrogen production. Renew. Energ. 2020, 156, 1008–1018. [Google Scholar] [CrossRef]
- Sulowska, A.; Wysocka, I.; Pelczarski, D.; Karczewski, J.; Zielińska-Jurek, A. Hybrid TiO2–polyaniline photocatalysts and their application in building gypsum plasters. Materials 2020, 13, 1516. [Google Scholar] [CrossRef]
- Monafared, A.H.; Jamshidi, M. Synthesis of polyaniline/titanium dioxide nanocomposite (PAni/TiO2) and its application as photocatalyst in acrylic pseudo paint for benzene removal under UV/VIS lights. Prog. Org. Coat. 2019, 136, 105257. [Google Scholar] [CrossRef]
- Yu, J.; Pang, Z.; Zheng, C.; Zhou, T.; Zhang, J.; Zhou, H.; Wei, Q. Cotton fabric finished by PANI/TiO2 with multifunctions of conductivity, anti-ultraviolet and photocatalysis activity. Appl. Surf. Sci. 2019, 470, 84–90. [Google Scholar] [CrossRef]
- Falletta, E.; Bruni, A.; Sartirana, M.; Boffito, D.C.; Cerrato, G.; Giordana, A.; Djellabi, R.; Khatibi, E.S.; Bianch, C.L. Solar light photoactive floating polyaniline/TiO2 composites for water remediation. Nanomaterials 2021, 11, 3071. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wang, J.; Qi, R.; Wang, X.; Xu, P.; Han, X. A novel incorporating style of polyaniline/TiO2 composites as effective visible photocatalysts. J. Mol. Catal. A 2012, 357, 19–25. [Google Scholar] [CrossRef]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef]
- Hidalgo, D.; Bocchini, S.; Fontana, M.; Saracco, G.; Hernandez, S. Green and low-cost synthesis of PANI–TiO2 nanocomposite mesoporous films for photoelectrochemical water splitting. RSC Adv. 2015, 5, 49429–49438. [Google Scholar] [CrossRef]
- Teja, K.P.; Kodialbail, V.S. Visible light mediated photocatalytic reduction of CO2 to non-fossil fuel and valuable products by polyaniline-TiO2 nanocomposites. Arab. J. Sci. Eng. 2022, 47, 6591–6603. [Google Scholar] [CrossRef]
- Kalikeri, S.; Kamath, N.; Gadgil, D.J.; Kodialbail, V.S. Visible light-induced photocatalytic degradation of Reactive Blue-19 over highly efficient polyaniline-TiO2 nanocomposite: A comparative study with solar and UV photocatalysis. Environ. Sci. Pollut. Res. 2018, 25, 3731. [Google Scholar] [CrossRef]
- Radoicic, M.; Saponjic, Z.; Jankovic, I.A.; Ciric-Marjanovic, G.; Ahrenkiel, S.P.; Comor, M.I. Improvements to the photocatalytic efficiency of polyaniline modified TiO2 nanoparticles. Appl. Catal. B Environ. 2013, 136–137, 133–139. [Google Scholar] [CrossRef]
- Salem, M.A.; Al-Ghonemiy, A.F.; Zaki, A.B. Photocatalytic degradation of Allura red and Quinoline yellow with Polyaniline/TiO2 nanocomposite. Appl. Catal. B Environ. 2009, 91, 59–66. [Google Scholar] [CrossRef]
- Wang, F.; Min, S.; Han, Y.; Feng, L. Visible-light-induced photocatalytic degradation of methylene blue with polyaniline-sensitized TiO2 composite photocatalysts. Superlattices Microstruct. 2010, 48, 170–180. [Google Scholar] [CrossRef]
- Lin, Y.; Li, D.; Hu, J.; Xiao, G.; Wang, J.; Li, W.; Fu, X. Highly efficient photocatalytic degradation of organic pollutants by PANI-modified TiO2 composite. J. Phys. Chem. C 2012, 116, 5764–5772. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Wu, L.; Zhi, J. Processable polyaniline/titania nanocomposites with good photocatalytic and conductivity properties prepared via peroxo-titanium complex catalyzed emulsion polymerization approach. Appl. Surf. Sci. 2013, 273, 135–143. [Google Scholar] [CrossRef]
- Jeong, W.-H.; Amna, T.; Ha, Y.-M.; Shamshi Hassan, M.; Kim, H.-C.; Khil, M.-S. Novel PANI nanotube@TiO2 composite as efficient chemical and biological disinfectant. Chem. Eng. J. 2014, 246, 204–210. [Google Scholar] [CrossRef]
- Subramanian, E.; Subbulakshmi, S.; Murugan, C. Inter-relationship between nanostructures of conducting polyaniline and the photocatalytic methylene blue dye degradation efficiencies of its hybrid composites with anatase TiO2. Mater. Res. Bull. 2014, 51, 128–135. [Google Scholar] [CrossRef]
- Sandhya, K.P.; Sugunan, S. Heterogeneous photocatalytic degradation of 4-nitrophenol by visible light responsive TiO2-polyaniline nanocomposites. J. Water Supply Res. Technol. AQUA 2015, 64, 74–84. [Google Scholar] [CrossRef]
- Reddy, K.R.; Karthik, K.V.; Benaka Prasad, S.B.; Soni, S.K.; Jeong, H.M.; Raghu, A.V. Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 2016, 120, 169–174. [Google Scholar] [CrossRef]
- Gilja, V.; Novakovic, K.; Travas-Sejdic, J.; Hrnjak-Murgic, Z.; Rokovic, M.K.; Zic, M. Stability and synergistic effect of polyaniline/TiO2 photocatalysts in degradation of azo dye in wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef]
- Jumat, N.A.; Wai, P.S.; Ching, J.J.; Basirun, W.J. Synthesis of polyaniline-TiO2 nanocomposites and their application in photo-catalytic degradation. Polym. Polym. Compos. 2017, 25, 507–514. [Google Scholar] [CrossRef]
- Radoicic, M.; Ciric-Marjanovic, G.; Spasojevic, V.; Ahrenkiel, S.P.; Mitric, M.N.; Novakovic, K.; Saponjic, Z. Superior photocatalytic properties of carbonized PANI/TiO2 nanocomposites. Appl. Catal. B Environ. 2017, 213, 155–166. [Google Scholar] [CrossRef]
- Yang, C.; Dong, W.; Cui, G.; Zhao, Y.; Shi, X.; Xia, X.; Tang, B.; Wang, W. Enhanced photocatalytic activity of PANI/TiO2 due to their photosensitization-synergetic effect. Electrochim. Acta 2017, 247, 486–495. [Google Scholar] [CrossRef]
- Cui, W.; He, J.; Wang, H.; Hu, J.; Liu, L.; Liang, Y. Polyaniline hybridization promotes photo-electro-catalytic removal of organic contaminants over 3D network structure of rGH-PANI/TiO2 hydrogel. Appl. Catal. B Environ. 2018, 232, 232–245. [Google Scholar] [CrossRef]
- Sojic Merkulov, D.V.; Despotovic, V.N.; Banic, N.D.; Armakovic, S.J.; Fincur, N.L.; Lazarevic, M.J.; Cetojevic-Simin, D.D.; Orcic, D.Z.; Radoicic, M.; Saponjic, Z.; et al. Photocatalytic decomposition of selected biologically active compounds in environmental waters using TiO2/polyaniline nanocomposites: Kinetics, toxicity and intermediates assessment. Environ. Pollut. 2018, 239, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.H.; Kar, A.K. Effect of band gap variation and sensitization process of polyaniline (PANI)-TiO2 p-n heterojunction photocatalysts on the enhancement of photocatalytic degradation of toxic methylene blue with UV irradiation. J. Environ. Chem. Eng. 2020, 8, 104181. [Google Scholar] [CrossRef]
- Lal, S.D.; Jose, T.S.; Rajesh, C.; Puthukkara, P.A.R.; Unnikrishnan, K.S.; Arun, K.J. Accelerated photodegradation of polystyrene by TiO2-polyaniline photocatalyst under UV radiation. Eur. Polym. J. 2021, 153, 110493. [Google Scholar]
- Kumar, A.; Raorane, C.J.; Syed, A.; Bahkali, A.H.; Elgorban, A.M.; Raj, V.; Kim, S.C. Synthesis of TiO2, TiO2/PAni, TiO2/PAni/GO nanocomposites and photodegradation of anionic dyes Rose Bengal and thymol blue in visible light. Envron. Res. 2023, 216, 114741. [Google Scholar] [CrossRef] [PubMed]
- Uyguner-Demirel, C.S.; Turkten, N.; Karatas, Y.; Bekbolet, M. Photocatalytic performance of PANI modified TiO2: Degradation of refractory organic matter. Environ. Sci. Pollut. Res. 2023, 30, 85626–85638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).