Abstract
The high-pressure growth technique generally plays an important role in the improvement of sample quality and the enhancement of various physical and magnetic properties of materials. The high gas pressure technique provides a large sample space (10–15 cm) to grow various kinds of materials. In this paper, we introduce the high gas pressure and high-temperature synthesis (HP-HTS) technique that is present at our institute and is applied to the growth process of different kinds of superconducting materials, particularly iron-based superconductors. More details and the working principle of this HP-HTS technique are discussed. We have also demonstrated the current results based on iron-based superconductors by using this unique HP-HTS technique. These results demonstrate the enhancement of the superconducting properties with improved sample quality compared to the conventional synthesis process at ambient pressure.
1. Introduction
High-pressure synthesis of the material is an important area for physics, chemistry, and material sciences [1,2,3]. This method reduces the chemical reaction time, controls the evaporation of lighter elements [4,5], and can also be used to grow new materials that cannot be prepared at ambient pressure, such as superhard materials like diamond and cubic boron nitride [6]. Generally, the sample space is very tiny for the pressure growth process, and due to this, a small sample size is always obtained [7]. This issue has been clearly observed with the growth process of iron-based superconductor (FBS) [8]. To overcome this problem, we need to find a good high-pressure growth method that can provide large crystals and bulk samples with high superconducting properties. However, the question is: which technique is more suitable to resolve these problems? [9,10].
There are two kinds of pressure techniques: (a) Solid-medium pressure techniques such as Hot Isostatic Pressure (HIP), Diamond Anvil Cell (DAC) Technique [11], Multi-Anvil High-Pressure Apparatus, and cold synthesis [11]. The properties of this technique are as follows: (i) It has a limited sample space of up to 0.5 cm3, and due to this, the prepared sample size is usually small. (ii) The pressure and temperature distribution are not homogeneous. It generates undefined preparation conditions. (iii) Due to the pressure medium touching the sample, there is a great possibility of introducing the impurity phases. (iv) Also, the temperature gradient is not easy to control in the multizone furnace. (b) Gas pressure technique: The properties are as follows: (i) it has several cm3 of sample space, and (ii) it is easy to create homogeneous temperature and pressure stability for a practically long growth time. (iii) The spatial temperature profile may be controlled, and it may be easy to control the partial gas pressure; (iv) the growth of the crystal is a comparatively easy process; (v) there is no possibility of introducing impurities from the pressure medium; (vi) sample chamber with an internal three-zone furnace with pressure up to 2–3 GPa and temperature ~2000 °C.
In the solid-medium pressure growth process, interactions between materials and instrument parts can introduce contamination and other issues due to the applied physical force, potentially leading to morphology problems like cracks and pores [1]. Therefore, it is crucial to explore alternative techniques to address these challenges. The above-mentioned discussion suggests that the gas pressure technique can be a unique and attractive way to grow high-quality and large amounts of samples and to improve the superconducting properties of FBS [12,13]. These reasons motivated us to use high-pressure techniques for the growth of iron-based superconductors. One can note that the gas pressure techniques can be used to create a maximum pressure of up to 3 GPa with a small pressure chamber, whereas the solid-medium pressure technique can generate much higher pressure than the gas pressure techniques. Furthermore, in the gas pressure technique, the used gas must be highly pure because a large volume of gas is compressed in a small pressure chamber to generate a high gas pressure. Consequently, even a tiny quantity of gas impurity can have a significant impact on the sample’s growth and is undesirable from a safety perspective. Gas pressure techniques, like other pressure techniques, are also complex during sample loading because every part of the chamber needs to be positioned correctly for optimal operation.
In the case of FBS, few studies have been reported based on solid-pressure medium techniques where the sample size is enhanced from 10 μm to 300 μm with improved superconducting properties [7,8]. These reports suggest that more studies are needed in this direction by using different pressure techniques, such as the high-gas growth method, so that we can also prepare a large sample with high superconducting properties. In this paper, we have introduced the principle and more details about our high gas pressure and high-temperature synthesis (HP-HTS) technique that is available at our institute, “UNIPRESS”. Also, the current results based on iron-based superconductors using this HP-HTS method will be presented and discussed.
2. Principle of High-Pressure Technique
This HP-HTS has a compressor called the reciprocating compressor, which is based on the oil gas piston. The main working principle of the reciprocating compressor is based on Boyel’s law [14], which states that the absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the occupied volume. The piston compresses the gas and increases the gas pressure inside the chamber. The piston’s movement essentially operates in two modes of operation, depending on the required pressure. In the first mode, the oil is primarily located at the bottom of the piston, through which the piston can move in an upward direction to compress the gas by pushing more oil into the oil chamber, whereas by releasing oil into the oil tank through a key valve, the piston can return to its regular position. However, when high pressure (>0.4 GPa) is required, the piston must be operated in both forward and backward directions through the oil pressure. So, the lower side of the piston must have two separate oil chambers that can be operated through the oil pump to compress or release the gas pressure by adding or removing oil from one of the oil chambers. Generally, in our HP-HTS system, there are three piston cavities (piston chambers) attached to each other in a series to achieve the required high pressure. In order to safely generate high pressure and lower the possibility of mechanical problems, a significant volume of gas is compressed inside the pressure chamber through three-stage pistons. The systematic block diagram of the working principle of these pistons is shown below in various stages, especially to create high gas pressure.
(a) First-stage piston (S1): First, the gas bottle is opened, and the gas enters through the intake valve (Vi) into all piston cavities and the high-pressure chamber. Figure 1i demonstrates the gas refilling process in the first piston cavity (S1) and chamber (C). During this process, gas is filled in the upper part of the S1 by moving the piston down to the oil tank. Basically, the piston moves down with the gas bottle pressure of ~200 bar. After 4–5 min, we close the intake valve (Vi) and move the piston in an upward direction slowly to pressurize the gas inside the chamber. Figure 1ii depicts the movement of the piston in an upward direction in the S1 cavity. Through this first stage, we can reach the maximum pressure of up to 800 bar, i.e., the gas pressure can increase from 200 bar to 800 bar.
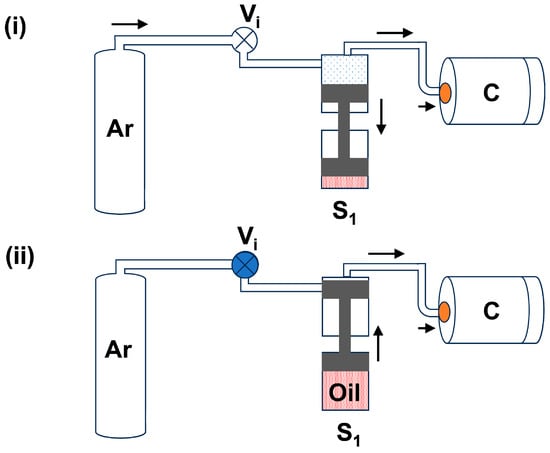
Figure 1.
The block diagram of the first-stage gas compression process: (i) Gas flow into the S1 cavity and chamber (C); (ii) S1 piston compressing the gas into chamber (C) up to 800 bar. The arrow is used to indicate the direction of gas flow.
(b) Second-stage piston (S2): This stage is connected to the first-stage piston (S1) and has a pressure of around 800 bar. There is again an oil-gas piston that moves down with the first-stage pressure of 800 bar, which is shown in Figure 2i. Now, the intake valve (Vi) between S1 and S2 is closed, and the piston of S2 moves upward slowly, which creates gas pressure inside the chamber up to 4000 bar. This process is similar to the first stage and is shown in Figure 2ii.
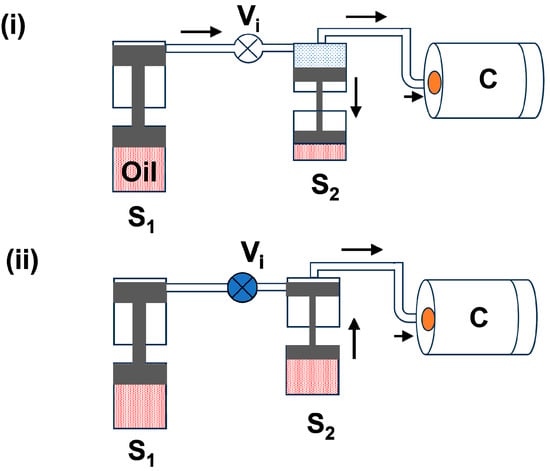
Figure 2.
The block diagram of the second-stage gas compression process: (i) Gas flow into the S2 cavity and chamber (C); (ii) S2 piston compressing the gas into chamber (C) up to 4000 bars. The arrow is used to indicate the direction of gas flow.
(c) Third stage piston (S3): This stage piston has a bigger size than the first and second-stage pistons. Due to the second-stage pressure, this piston is moved to the down position, which is depicted in Figure 3i. Once all gas pressure moves into the S3 cavity and chamber, the intake valve (Vi) between S2 and S3 is closed. In the next step, the S3 piston moves slowly in an upward direction through the oil-based pump and enhances the pressure inside the chamber (C). The maximum pressure achieved in S3 can be reached at 1.8 GPa (18,000 bar). Figure 3ii displays the block diagram of the third-stage piston movement to achieve the highest pressure. This stage plays an important role in reaching the ultimate pressure. Hence, this stage has a large piston chamber to collect more compressed gas and a powerful piston with a large oil tank to apply more force to compress the gas into the pressure chamber.
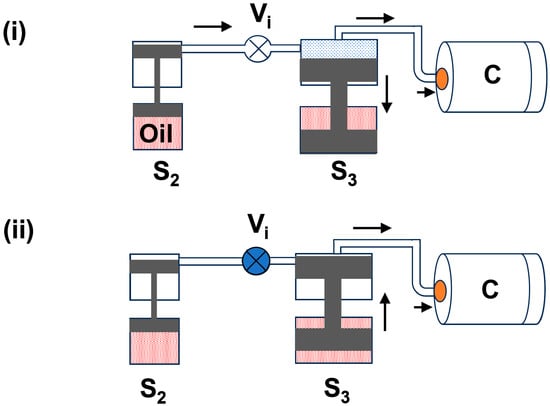
Figure 3.
The block diagram of the third-stage gas compression process: (i) Gas flow into the S3 cavity and chamber (C); (ii) S3 piston compressing the gas into chamber (C) up to 1.8 GPa (18,000 bar). The arrow is used to indicate the direction of gas flow.
3. HP-HTS Technique at UNIPRESS
The HP-HTS facility designed at UNIPRESS is capable of producing a pressure of up to 1.8 GPa and a temperature of up to 1700 °C. One can use a one- or multi-zone furnace to create different temperatures, especially for a single crystal growth process [9,10,12,15]. Our current system is based on a three-stage oil-gas compressor to create high pressure, as discussed above. More advanced designs can produce pressure up to 3 GPa, which requires three pistons and a small diameter of the pressure chamber (C). Our HP-HTS technique is vital in synthesizing high-quality materials [9,10,12,15]. With the capability to generate high gas pressure, this system has multi-zone furnaces, real-time temperature measurement, and adaptability for various sample types and positions inside the pressure chamber. It is a versatile tool for growing single and polycrystalline samples across diverse material categories [9]. The block diagram of our HP-HTS system is depicted in Figure 4. The system has four main components: a high-pressure chamber, compressor, sample holder with furnace, and controller unit and monitor, which work cohesively to ensure precise and controlled pressurization, leak-free operation, and real-time temperature and pressure monitoring. These features empower high-pressure synthesis and sintering research, providing a platform for advancements in superconductivity research and applications.
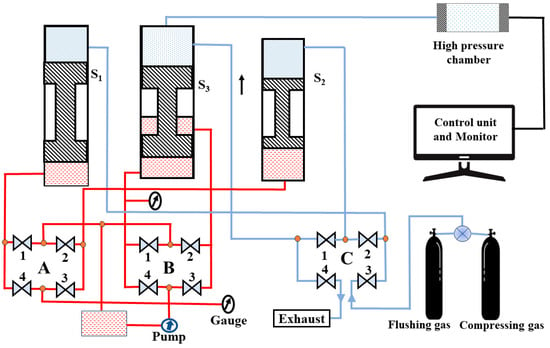
Figure 4.
The block diagram of the HP-HTS technique, presented at our institute, consists of a three-stage oil-based compressor, a high-pressure chamber (C), and a control unit monitor. “A” and “B” represent a set of key valves to operate the oil pressure through the oil pump for various stages (S1, S2, and S3), whereas “C” depicts a set of key valves to operate the gas pressure for these three stages. The numbers 1, 2, 3, and 4 are used to represent the four key valves for the systematic operation of the compressor.
The HP-HTS system which is currently used for the growth of FBS, is based on three pistons, as depicted in Figure 4. These pistons are connected to each other and also to the pressure chamber. The first stage (S1) generates the pressure for the second stage (S2), the third stage (S3), and the pressure chamber (C). In the next step, the first stage is disconnected, and the second-stage piston creates the pressure for the third stage (S3) and pressure chamber. Finally, the third stage (S3) starts to work and generate the maximum pressure inside the chamber, up to 1.8 GPa. More details about each part are given below:
(i) High-Pressure chamber: The high-pressure chamber body is a central component, depicted in Figure 5, and is constructed from robust steel to withstand extremely high pressures. Its intricate design ensures that this camber remains impervious to leaks even under high-pressure conditions, ensuring a safe environment for high-pressure growth. To regulate temperature efficiently, the outer jacket of the chamber is integrated with a water cooling system with a water inlet and outlet. The water cooling system plays a vital role in maintaining the temperature of the pressure chamber body and ensuring its safe operation under high-temperature and high-pressure conditions. One side of the chamber is precisely connected to a capillary using a big-diameter screw, supported with O-rings and additional seals, ensuring a completely leak-free chamber. This capillary tube serves a dual purpose, functioning as both the inlet and outlet for gas, and is connected to the compressor and gas bottle. On the opposite side, a sample holder with a high-quality O-ring and metal seal facilitates the insertion of samples into the pressure chamber, and a big-diameter screw provides crucial support to secure plugs, which contain numerous components within the chamber as shown in Figure 5. Typically, we flush the chamber at least three times before initiating any growth processes. However, in some cases, the sample is highly sensitive to moisture and oxygen, so we employ a vacuum space to create a vacuum environment within the chamber and heat the entire chamber to a temperature of about 100 °C to eliminate any remaining oxygen and moisture. Within the sample holder, a variety of thermocouples are employed to monitor the temperature at different sections of the chamber precisely. Moreover, the plugs are the central hubs for all electrical connections, including heater wires, thermocouples, and pressure gauges, ensuring seamless operation and integration while securely eliminating any possibility of leakage risk. Reliability and security are ensured for the entire system by connecting these wires to the control unit and monitor. The chamber’s adaptability for single, double, or triple zone furnaces is a noteworthy feature, enhancing its utility for growing high-quality single crystals and facilitating a wide range of experiments in materials science.
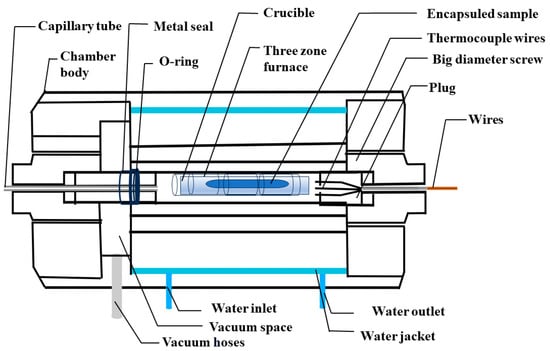
Figure 5.
Schematic diagram of a high-pressure chamber.
(ii) Compressor: The compressor is an oil-based system with a three-stage piston configuration and a pump, as shown in Figure 6. The piston moves upward to create gas pressure. Generally, this pump is connected to all the stages and moves the piston upward to create high pressure. The systematic block diagram is shown in Figure 6, and the list of maximum pressures for different stages is mentioned in Table 1. In the first stage (S1), it can generate pressure up to 800 bar. The second piston (S2) can achieve pressures of up to 4000 bar, and in the final stage (S3), it can reach an impressive pressure of up to 1.8 GPa (18,000 bar). This three-stage piston setup allows for the precise control and adjustment of pressure from low to very high, which is one of the notable strengths of our compressor. The presence of 12 key valves is the most intricate and critical aspect of the compressor, as shown in Figure 6, which plays a pivotal role during the gas compression process and ensures precise and controlled pressurization. The basic principle of the pump is already explained above.
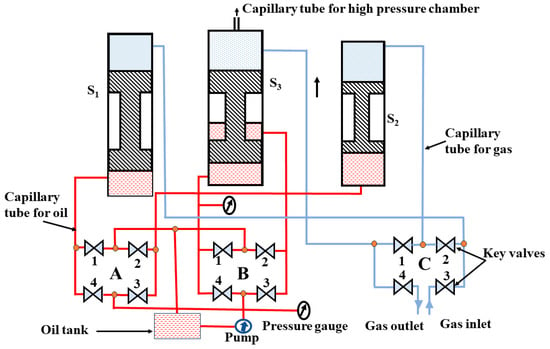
Figure 6.
Schematic diagram of a compressor based on the oil pump with three-stage pistons. “A” and “B” represent a set of key valves to operate the oil pressure through the oil pump for various stages (S1, S2, and S3), whereas “C” depicts a set of key valves to operate the gas pressure for these three stages. The numbers 1, 2, 3, and 4 are used to represent the four key valves for the systematic operation of the compressor.

Table 1.
List of the maximum pressure created by the different stages of a compressor.
(iii) Sample holder with furnace: This component contains a wide setup, consisting of essential components like the sample container, furnace, thermocouples, and pressure gauge. A systematic block diagram is shown in Figure 7. The sample container, designed either in a boat or cylindrical form with a secure cap, acts as the vessel for keeping the samples inside the high-pressure chamber. The furnace is equipped with a Kanthal (FeCrAl alloy) heater wire capable of reaching temperatures up to 1100 °C under high pressure for a long time. When higher temperatures are needed, molybdenum (Mo) wires can be utilized, through which the maximum temperature can reach up to approximately 1700 °C within this high-pressure chamber [3], as listed in Table 2. Furthermore, either a single or multi-zone furnace can be used, as shown in Figure 7, to create the high temperature. However, a three-zone, or multi-zone, furnace is typically employed to establish temperature variations during the sample growth process. The length of the heater is around ~15 cm, and a thermal gradient in one zone furnace can be observed in the range of 10 °C to 30 °C at the top and bottom of the chamber compared to the center part of the chamber.
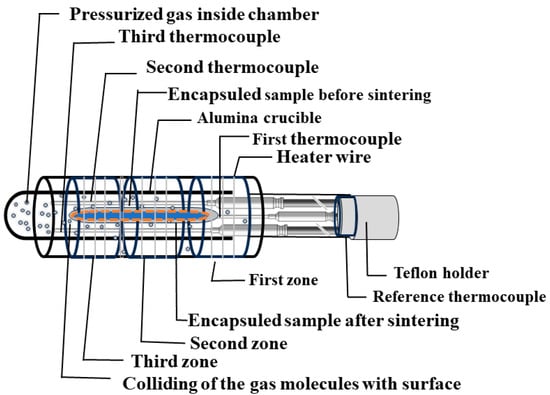
Figure 7.
Block diagram of the sample holder with the furnace.

Table 2.
Heater wires for the furnace that are used for the HP-HTS technique.
For accurate temperature monitoring, three thermocouples are employed, in addition to a reference thermocouple that provides precise temperature readings across all zones. We can use various kinds of thermocouples according to the temperature range, as mentioned in Table 3. The plug tip’s temperature is typically monitored with T-type thermocouples made of copper and constantan, commonly used as a reference thermocouple, where its temperature variation up to 50–70 °C is a common practice to ensure the safety of plug connections and suggests a good performance of the experiment during high-temperature and high-pressure conditions within the sample holder. Typically, K-type thermocouples made of chromel and nickel-aluminum, are used for three thermocouples that are placed at the bottom, center, and top positions of the sample holder. We generally use these sensors to monitor the temperature up to 1100 °C. For high temperatures ranging from 1100 °C to 1700 °C, B-type thermocouples made of platinum and rhodium are used in a similar way. However, various thermocouples can be selected based on specific requirements [3,16]. The actual pressure inside the chamber is regularly monitored using pressure gauges. These vital components, including the thermocouples, heaters, and gauges, are seamlessly connected to the controller unit and monitor, ensuring precise control and monitoring of the high-pressure chamber’s conditions. Modifying this chamber according to specific material growth conditions adds to its versatility and suitability for diverse research and experimentation needs in materials science.

Table 3.
Types of thermocouples utilized in the HP-HTS technique.
(iv) Controller unit and monitor: The final component of HP-HTS comprises a temperature controller and a pressure gauge, both of which can be connected to a computer for enhanced functionality. This capability allows for the real-time monitoring of temperature and pressure within the system. The temperature controller facilitates the monitoring of all four thermocouples, each positioned at different locations within the system. One of these thermocouples also serves as a reference temperature point, enabling precise temperature measurements near the sample. This feature provides valuable insights into the actual temperature conditions during experiments. Through the interface with the computer, the pressure gauge provides real-time data on the pressure inside the chamber. This allows for continuous monitoring and control, ensuring that pressure conditions are maintained as required for specific experiments.
4. Current Results Using this HP-HTS Facility
Currently, we are applying this HP-HTS technique for FBS [17,18], which has the highest Tc of 58 K, a high upper critical field (Hc2) of 100 T, and a high critical current density (Jc) of 107–108 A/cm2 at 5 K [19]. These properties make them a strong contender for practical applications [20]. Many compounds belonging to this high Tc superconductor can be categorized into six families, of which 1111 (REFeAs(O,F), RE = La, Ca, Pr, Gd) as a doped family and 1144 (AeAFe4As4; Ae = Ca; A = K) as a stoichiometric family provide the highest Tc of 58 K [21] and 36 K [22], respectively, for FBS. Hence, our current focus is on the growth process of 1111 and 1144 families using the HP-HTS technique [15]. Before using the high-pressure technique for these complicated families, the HP-HTS technique was applied to the simplest FBS, i.e., the 11 (FeSe) family. Tellurium (Te) doping at Se-sites, i.e., Fe(Se,Te), provides the highest transition temperature Tc of 15 K for FeSe0.5Te0.5 [23]. First, we synthesized the high-quality Fe(Se,Te) sample by the solid-state reaction method at ambient pressure (0 GPa). The selected composition FeSe0.5Te0.5 is prepared at 600 °C for 21 h in the first step, and in the second stage, the sample is grounded and heated again at 600 °C for 4 h, as more detail was discussed in our previous studies [12,23]. This FeSe0.5Te0.5 sample shows the transition temperature up to 15 K by the convenient synthesis method at ambient pressure, as reported by the previous report [23] and depicted in Figure 8a.
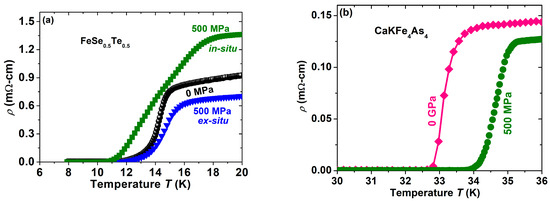
Figure 8.
The temperature dependence of the resistivity (ρ) for (a) FeSe0.5Te0.5 prepared by in-situ and ex-situ processes using HP-HTS, where the samples were sealed in a Ta-tube before being placed inside the high-pressure chamber. The sample at 0 MPa is prepared by the conventional synthesis method [12]. (b) The variation of resistivity with temperature for CaKFe4As4 (1144) bulk using the HP-HTS facility, which is presented at our institute [15].
To understand the high-pressure growth effect, Fe(Se,Te) bulks are prepared in a very broad pressure range from 0 GPa to 1 GPa at 600 °C for 1 h and 11 h, as reported in our previous study [12]. Also, these samples were prepared by in-situ and ex-situ processes and were sealed in a Ta-tube or placed in an open Ta-tube. These various conditions were used to optimize the best growth conditions so that a high-quality sample could be produced with high superconducting properties. Interestingly, the optimized conditions were obtained at 600 °C, 1 h, and 500 MPa, where samples show the highest superconducting properties [12]. The comparative graph is shown in Figure 8a. Our studies also confirm that grain connectivity can be improved by having the pure superconducting phase when the samples are sealed into a Ta-tube under an argon gas atmosphere through an ARC melter, whereas the samples placed in an open Ta-tube have a pure superconducting phase, but the grain connectivity is poor due to the high gas pressure passing through the micro- or nanopores. Fe(Se,Te) bulks prepared by the optimized conditions are depicted in Figure 8a, which shows the high superconducting transition (Tc = 17 K) compared to the samples prepared by the ambient pressure method (Tc = 15 K). These optimized study processes confirm that the high gas pressure technique can be an effective method to enhance the superconducting properties, and the synthesis process can be completed in a very short reaction time under the optimized growth pressure [12].
After having a good experience with this 11 family of FBS, we have started to work with CaKFe4As4 (1144) which is a stoichiometric compound of FBS [15,24]. On the basis of the optimization of the 11 (FeSe0.5Te0.5) family, we have prepared 1144 samples under the optimized conditions (500 MPa, 1 h) at 500 °C. Interestingly, CaKFe4As4 prepared by HP-HTS has enhanced the superconducting transition by ~2 K with improved sample quality compared to the 1144 bulks prepared under the conventional synthesis method [15] at ambient pressure. The resistivity measurements of these samples are depicted in Figure 8b, which clearly enhances the Tc value with a sharp transition. It suggests that the 1144 bulk prepared by HP-HTS has homogeneous and well-connected grain boundaries. In a similar way, we are also applying this HP-HTS to other members of the 1144 and 1111 families.
5. Conclusions
The high gas pressure technique can be a unique way to improve the sample quality, sample size, and material properties during the growth process. We have discussed the working principle and details about the high gas pressure and high-temperature synthesis (HP-HTS) technique that is presented at our institute. This unique HP-HTS technique provides a large sample space (~15 cm), a high growth pressure of up to 1.8 GPa, and a high heating temperature of up to 1700 °C to grow various kinds of materials. It can use different kinds of gas to generate pressure, such as inert gas, chlorine, and hydrogen, or mix different gases. Currently, we are using this technique for high Tc iron-based superconductors to grow single-crystal and polycrystalline samples. Several types of processes have been reported to improve the sample quality and the properties of FBS, but there is no report based on the HP-HTS effect on its superconducting properties. Our current research results based on FBS are also presented here, along with a detailed discussion of the HP-HTS technique. Interestingly, the observed results depict the enhancement of superconducting properties and also the improved sample quality of HP-HTS. Our studies suggest that the high-pressure synthesis works well for the high Tc material and can be useful for other kinds of materials to improve their properties.
Author Contributions
Conceptualization, Supervision and Formal analysis, S.J.S.; methodology, S.J.S., M.A. and M.M.; data collection, M.M. and M.A.; High-pressure experiments, M.A., M.M., A.M. and T.C.; investigation and writing—original draft preparation, S.J.S., M.A. and M.M.; writing—review and editing, S.J.S., M.A. and M.M.; Comments and Suggestions, M.A., M.M., A.M. and S.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre (NCN), Poland, grant number “2021/42/E/ST5/00262” (SONATA-BIS 11). S.J.S. acknowledges financial support from the National Science Centre (NCN), Poland, through research project number: 2021/42/E/ST5/00262.
Data Availability Statement
Data are available upon request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bocannegra-Bernal, M.H. Review: Hot Isostatic Pressing (HIP) technology and its applications to metals and ceramics. J. Mater. Sci. 2004, 39, 6399. [Google Scholar] [CrossRef]
- Brazhkin, V.V. High-pressure synthesized materials: Treasures and hints. High Press. Res. 2007, 27, 333–351. [Google Scholar] [CrossRef]
- Koizumi, M. Hot isostatic pressing- Theory and applications. In Proceedings of the Third International Conference, Osaka, Japan, 10–14 June 1991. [Google Scholar]
- Fujioka, M.; Denholme, S.J.; Tanaka, M.; Takeya, H.; Yamaguchi, T.; Takano, Y. The effect of exceptionally high fluorine doping on the anisotropy of single crystal single crystalline SmFeAsO1-xFx. Appl. Phys. Lett. 2014, 105, 102602. [Google Scholar] [CrossRef]
- Niska, J.V.; Loberg, B. The Role of Oxygen in the Hot Isostatic Pressing of High Tc Superconductors. MRS Online Proc. Libr. 1991, 251, 335–340. [Google Scholar] [CrossRef]
- Atkinson, H.V.; Davies, S. Fundamental aspects of hot isostatic pressing: An overview. Metall. Mater. Trans. A 2000, 31, 2981–3000. [Google Scholar] [CrossRef]
- Karpinski, J.; Zhigadlo, N.D.; Katrych, S.; Bukowski, Z.; Moll, P.; Weyeneth, S.; Keller, H.; Puzniak, R.; Tortello, M.; Daghero, D.; et al. Single crystals of LnFeAsO1-xFx (Ln = La, Pr, Nd, Sm, Gd) and Ba1-xRbxFe2As2. Phys. C 2009, 469, 370. [Google Scholar] [CrossRef]
- Sang, L.N.; Li, Z.; Yang, G.S.; Yue, Z.J.; Liu, J.X.; Cai, C.B.; Wu, T.; Dou, S.X.; Ma, Y.W.; Wang, X.L. Pressure effects on iron-based superconductor families: Superconductivity, flux pinning and vortex dynamics. Mater. Today Phys. 2021, 19, 100414. [Google Scholar] [CrossRef]
- Karpinski, J.; Schwer, H.; Mangelschots, I.; Conder, K.; Morawski, A.; Lada, T.; Paszewin, A. Single crystals of Hg1−xPbxBa2Can−1CunO2n+2+δ and infinite-layer CaCuO2. synthesis at gas pressure 10 kbar, properties and structure. Phys. C 1994, 234, 10–18. [Google Scholar] [CrossRef]
- Morawski, A.; Lada, T.; Paszewin, A.; Przybylski, K. High gas pressure for HTS single crystals and thin layer technology. Supercond. Sci. Technol. 1998, 11, 193–199. [Google Scholar] [CrossRef]
- Matsumoto, R.; Terashima, K.; Nakano, S.; Nakamura, K.; Yamamoto, S.; Yamamoto, T.D.; Ishikawa, T.; Adachi, S.; Irifune, T.; Imai, M.; et al. High-Pressure Synthesis of Superconducting Sn3S4 Using a Diamond. Inorg. Chem. 2022, 61, 4476–4483. [Google Scholar] [CrossRef]
- Azam, M.; Manasa, M.; Zajarniuk, T.; Diduszko, R.; Cetner, T.; Morawski, A.; Więckowski, J.; Wiśniewski, A.; Singh, S.J. High-Pressure Synthesis and the Enhancement of the Superconducting Properties of FeSe0.5Te0.5. Materials 2023, 16, 5358. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, O.; Morawski, A.; Zaleski, A.J.; Przyslupski, P.; Dietl, T.; Diduszko, R.; Presz, A.; Werner-Malento, K. Synthesis, Crystal Growth and Epitaxial Layers Growth of FeSe0,88 Superconductor and Other Poison Materials by Use of High Gas Pressure Trap System. J. Supercond. Nov. Magn. 2009, 22, 599–602. [Google Scholar] [CrossRef]
- West, J.B. Robert Boyle’s landmark book of 1660 with the first experiments. J. Appl. Physiol. 2005, 98, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Manasa, M.; Azam, M.; Zajarniuk, T.; Diduszko, R.; Cetner, T.; Morawski, A.; Wiśniewski, A.; Singh, S.J. Enhancement of Superconducting Properties of Polycrystalline CaKFe4As4 by High-Pressure Growth. Submitt. J. (Under Rev.) 2023. [Google Scholar]
- Loh, N.L.; Sia, K.Y. An overview of hot isostatic pressing. J. Mater. Process. Technol. 1992, 30, 45–65. [Google Scholar] [CrossRef]
- Singh, S.J.; Sturza, M.I. Bulk and Single Crystal Growth Progress of Iron-Based Superconductors (FBS): 1111 and 1144. Crystals 2021, 12, 20. [Google Scholar] [CrossRef]
- Iida, K.; Hänisch, J.; Yamamoto, A. Grain boundary characteristics of Fe-based superconductors. Supercond. Sci. Technol. 2020, 33, 043001. [Google Scholar] [CrossRef]
- Singh, S.J.; Bristow, M.; Meier, W.R.; Taylor, P.; Blundell, S.J.; Canfield, P.C.; Coldea, A.I. Ultrahigh critical current densities, the vortex phase diagram, and the effect of granularity of the stoichiometric high-Tc superconductor CaKFe4As4. Phys. Rev. Mater. 2018, 2, 074802. [Google Scholar] [CrossRef]
- Shimoyama, J.-I. Potentials of iron-based superconductors for practical future materials. Supercond. Sci. Technol. 2014, 27, 44002. [Google Scholar] [CrossRef]
- Singh, S.J.; Shimoyama, J.; Yamamoto, A.; Ogino, H.; Kishio, K. Transition Temperature and Upper Critical Field in SmFeAsO1−xFx Synthesized at Low Heating Temperatures. IEEE Trans. Appl. Supercond. 2013, 23, 7300605. [Google Scholar] [CrossRef]
- Iyo, A.; Kawashima, K.; Kinjo, T.; Nishio, T.; Ishida, S.; Fujihisa, H.; Gotoh, Y.; Kihou, K.; Eisaki, H.; Yoshida, Y. New-Structure-Type Fe-Based Superconductors: CaAFe4As4 (A = K, Rb, Cs) and SrAFe4As4 (A = Rb, Cs). J. Am. Chem. Soc. 2016, 138, 3410–3415. [Google Scholar] [CrossRef] [PubMed]
- Manasa, M.; Azam, M.; Zajarniuk, T.; Diduszko, R.; Cetner, T.; Morawski, A.; Wiśniewski, A.; Singh, S.J. Cometal Addition Effect on Superconducting Properties and Granular Behaviours of Polycrystalline FeSe0.5Te0.5. Materials 2023, 16, 2892. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Cassidy, S.J.; Bristow, M.; Blundell, S.J.; Clarke, S.J.; Coldea, A.I. Optimization of superconducting properties of the stoichiometric CaKFe4As4. Supercond. Sci. Technol. 2020, 33, 025003. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).