Abstract
Functional molecules possessing photoluminescence (PL) and liquid-crystalline (LC) behaviors, known as photoluminescent liquid crystals, along with a small molecular structure, have attracted significant attention. Fluorinated tolane skeletons are small π-conjugated structures, which are promising candidates for such functional molecules. These structures were revealed to exhibit strong PL in solid state but no LC behavior. Based on a report on hydrogen-bonded dimer-type LC molecules of carboxylic acid, in this study, we designed and synthesized a series of fluorinated tolanecarboxylic acids (2,3,5,6-tetrafluoro-4-[2-(4-alkoxyphenyl)ethyn-1-yl]benzoic acids) as promising PLLC molecules. Evaluation of the LC behavior revealed that fluorinated tolanecarboxylic acids with a longer alkoxy chain than a butoxy chain exhibited nematic LC behavior. Additionally, fluorinated tolanecarboxylic acids showed intense PL in the solution and crystalline states. Notably, fluorinated tolanecarboxylic acid with an aggregated structure in the nematic LC phase also exhibited PL with a slight blue shift in PL maximum wavelength compared to the crystalline state. The present fluorinated tolanecarboxylic acid exhibiting PL and LC characteristics in a single molecule can be applied to thermoresponsive PL materials, such as a PL thermosensor.
1. Introduction
Photoluminescent liquid crystals, which possess photoluminescence (PL) and liquid-crystalline (LC) characteristics in a single molecule, have gained recognition as essential organic functional molecules owing to their extensive applicability in PL thermometers and thermoresponsive PL sensors [1,2,3]. To date, many PLLC molecules have been developed [4,5], which consist of large molecular structures with a π-conjugated structure, mesogenic core, and flexible unit that result in PL and LC behaviors. Therefore, developing PLLC molecules with a small molecular structure is necessary for practical applications considering the manufacturing costs and processes. An effective approach to searching for PLLC molecules with a small molecular structure is designing a common π-conjugated structure that functions as the core structure of PL and LC molecules.
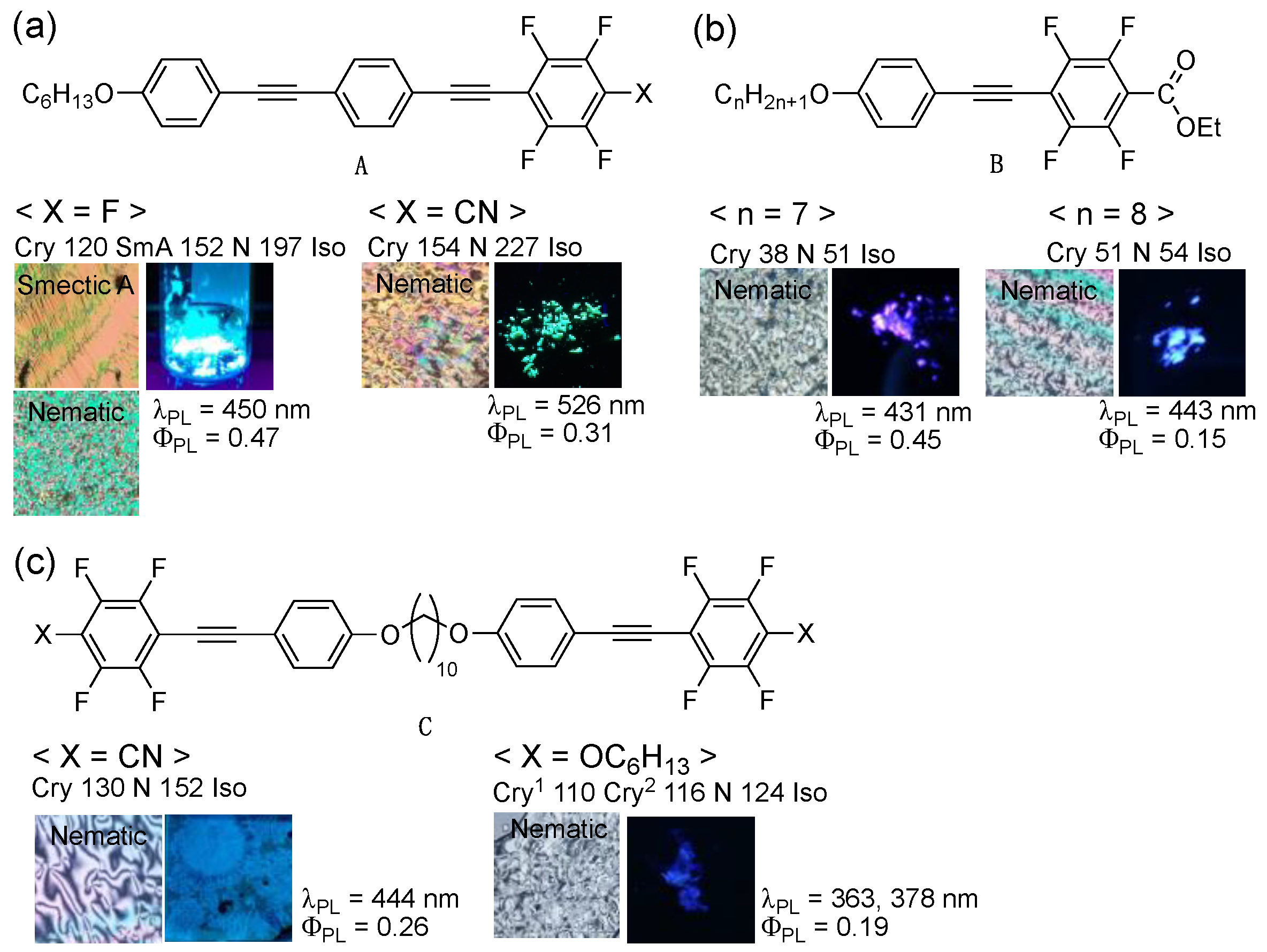
Over the past few years, our group has focused on developing fluorine-containing organic functional molecules with a PL and an LC characteristic [6,7,8,9,10,11,12,13,14]. Our recent study revealed that fluorinated bistolane-based PLLC molecules (A) exhibit PL and LC behaviors in a single molecule. The PL behavior is switched depending on the structural changes in the molecular aggregates through phase transition between the crystalline (Cry) and LC phases (Figure 1a) [6,7]. However, several issues were to be resolved, thus requiring multiple reaction steps to synthesize bistolane-based PLLC molecules. Because fluorinated tolane derivatives exhibit intense PL in the Cry phase through intermolecular H⋯F hydrogen bonds [8,9,10,11], we suggested that a fluorinated tolane skeleton, which contains a small and common π-conjugated structure, is effective as the core structure of the PL and LC molecules. Several attempts revealed that alkoxy-substituted fluorinated tolanes with a cyano (CN) [8], a trifluoromethyl (CF3) group [8], and a fluorine (F) atom [9] show intense PL in the Cry phase but no LC phase, whereas fluorinated tolanecarboxylates B with a long flexible alkoxy chain, such as C7H15O and C8H17O, reportedly exhibit intense PL in the Cry phase and the nematic (N) LC phase after the cooling process (Figure 1b) [12]. Additionally, fluorinated tolane dimer C, which is composed of two fluorinated tolane skeletons connected by a flexible chain, successfully exhibits the PL and LC phases (Figure 1c) [13,14].
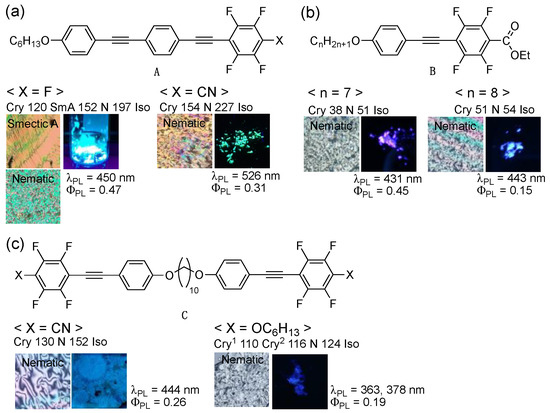
Figure 1.
Chemical structure, phase transition behavior, and crystalline-state photoluminescence (PL) behavior of (a) fluorinated bistolane-based PL liquid crystals (PLLCs) (A), (b) fluorinated tolane-based PLLCs (B), and (c) fluorinated tolane dimer-type PLLCs (C).
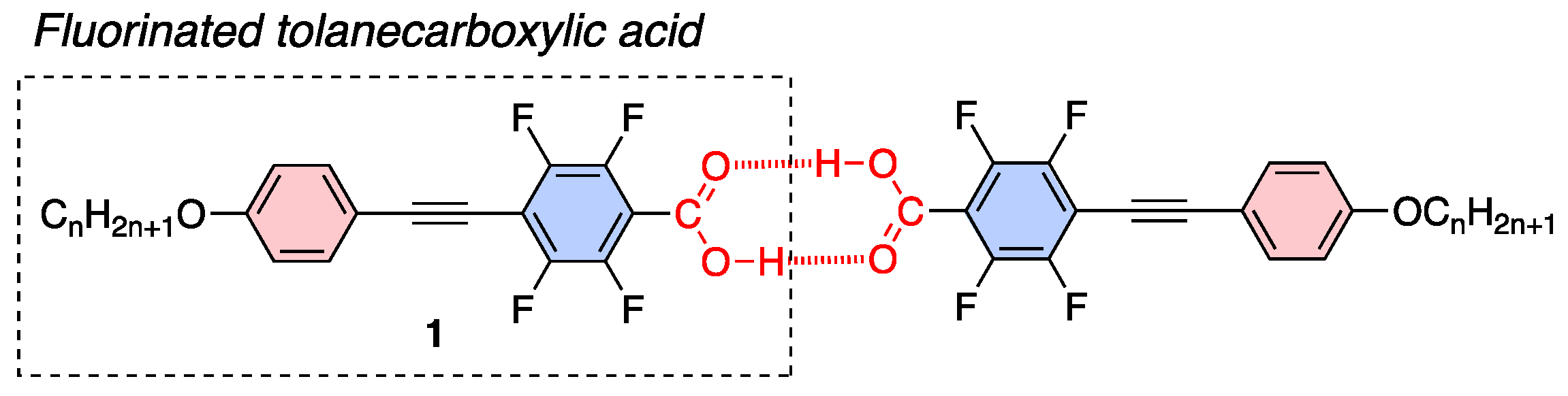
Arakawa et al. reported that aromatic carboxylic acids, including tolanecarboxylic acid, show broad LC behavior due to formation of dimer via hydrogen bonds [15,16]. Wen et al. examined fluorinated LC molecules and reported that fluorinated tolanes with an ester structure [17] or fluorinated biphenyls with a carboxy unit exhibit LC behavior [18]. Based on the molecular design of hydrogen-bonded dimer-type LC molecules, we focused on the hydrogen-bonded dimer-type LC molecules of carboxylic acid. In this study, we designed and synthesized a series of fluorinated tolanecarboxylic acids 1, such as 2,3,5,6-tetrafluoro-4-[2-(4-alkoxyphenyl)ethyn-1-yl]benzoic acids (Figure 2), and evaluated their LC and PL characteristics in detail.
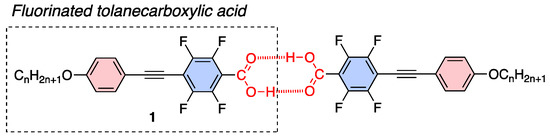
Figure 2.
Chemical structure of the fluorinated tolanecarboxylic acid 1 used in this study and plausible aggregated structure in crystalline and LC phases through hydrogen bond.
2. Materials and Methods
2.1. General
Column chromatography was performed for purification using Wakogel® 60N (38–100 μm), and thin layer chromatography (TLC) analysis was performed on silica gel TLC plates (silica gel 60F254, Merck). The melting temperature (Tm) and clearing temperature (Tc) were determined using polarized optical microscopy (POM). 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained using a Bruker AVANCE III 400 NMR spectrometer (1H: 400 MHz and 13C: 100 MHz) in chloroform-d (CDCl3) or dimethyl sulfoxide-d6 or acetone-d6, and chemical shifts were reported in parts per million (ppm) using the residual proton in the NMR solvent. 19F NMR (376 MHz) spectra were obtained using a Bruker AVANCE III 400 NMR spectrometer in CDCl3; CFCl3 (δF = 0.0 ppm) and hexafluorobenzene (δF = −163 ppm) were used as internal standards. Infrared (IR) spectra were recorded using the KBr method with a JASCO FT/IR-4100 type A spectrometer; all spectra were reported in wavenumber (cm−1) unit. High-resolution mass spectrometry (HRMS) was performed on a JEOL JMS-700MS spectrometer using the fast atom bombardment (FAB) method. Synthetic precursor ethyl 4-[2-(4-alkoxyphenyl)ethyn-1-yl]-2,3,5,6-tetrafluorobenzoate (2) was stated in a previous study and synthesized according to the reported procedure [12].
2.2. Typical Synthetic Procedure of 2,3,5,6-Tetrafluoro-2-[4-(methoxyphenyl)ethyn-1-yl]benzoic acid (1a)
Ethyl 2,3,5,6-tetrafluoro-4-[2-(4-methoxyphenyl)ethyn-1-yl]benzoate (2a, 2.0 g, 5.7 mmol), tetrahydrofuran (THF, 28 mL), and H2O (12 mL) were placed in a two-necked round-bottomed flask, followed by addition of LiOH·H2O (0.6 g, 14 mmol). The mixture was stirred at room temperature for 20 h and then acidified by adding an aqueous solution of HCl until the pH of the solution was below 1. The crude product was extracted with Et2O (10 mL, three times), while the organic layer was washed with brine (20 mL, once). The collected organic layer was dried over anhydrous Na2SO4 and separated from the drying agent by atmospheric filtration. The filtrate was evaporated using a rotary evaporator under reduced pressure and subjected to column chromatography using hexane, ethyl acetate, and acetic acid (v/v/v = 50/50/1) as an eluent, followed by recrystallization from chloroform, generating the title molecule 1a as a white solid in a 74% isolated yield (1.37 g, 4.2 mmol).
2.2.1. 2,3,5,6-Tetrafluoro-4-{2-(4-methoxyphenyl)ethyn-1-yl}benzoic acid (1a)
Yield: 74% (white solid); Tm: 223 °C (determined by POM); 1H NMR (DMSO-d6): δ 3.82 (s, 3H), 7.05 (d, J = 8.8 Hz, 2H), 7.59 (d, J = 8.8 Hz, 2H), 14.55 (brs, 1H); 13C NMR (DMSO-d6): δ 55.4, 72.8 (t, J = 4.4 Hz), 103.5 (t, J = 3.6 Hz), 105.9 (t, J = 17.6 Hz), 111.9, 113.6 (t, J = 17.6 Hz), 114.7, 133.6, 143.7 (dm, J = 253.1 Hz), 146.0 (dm, J = 250.8 Hz), 160.0, 160.8; 19F NMR (DMSO-d6, CFCl3): δ −136.3 to −136.6 (m, 2F), −138.9 to −139.1 (m, 2F); IR (KBr): ν 3730, 2844, 2221, 1698, 1601, 1475, 1247, 1174, 990, 835 cm−1; HRMS (FAB): [M+] calcd C16H8F4O3: 324.0410, found: 324.0413.
2.2.2. 2-{(4-Ethoxyphenyl)ethyn-1-yl}-2,3,5,6-tetrafluorobenzoic acid (1b)
Yield: 89% (white solid); Tm: 224 °C (determined by POM); 1H NMR (acetone-d6): δ 1.39 (t, J = 7.2 Hz, 3H), 4.12 (q, J = 7.2 Hz, 2H), 7.02 (d, J = 8.8 Hz, 2H), 7.58 (d, J = 8.8 Hz, 2H), 6.0–8.0 (brs, 1H); 13C NMR (acetone-d6): δ 14.9, 64.5, 73.4 (t, J = 3.6 Hz), 105.0 (t, J = 3.7 Hz), 108.1 (t, J = 17.6 Hz), 113.5, 113.6 (t, J = 17.6 Hz), 115.9, 134.5, 145.5 (ddt, J = 253.8, 13.2, 5.9 Hz), 147.4 (ddt, J = 250.9, 14.7, 3.6 Hz), 160.2, 161.6; 19F NMR (acetone-d6, C6F6): δ −136.92 (dd, J = 20.7, 10.9 Hz, 2F), −140.31 (dd, J = 20.7, 10.9 Hz, 2F); IR (KBr): ν 3750, 2984, 2212, 1706, 1601, 1479, 1178, 994, 844 cm−1; HRMS (FAB): [M+] calcd C17H10F4O3: 338.0566, found: 338.0563.
2.2.3. 2,3,5,6-Tetrafluoro-4-{2-(4-propyloxy)ethyn-1-yl}benzoic acid (1c)
Yield: 83% (white solid); Tm: 220 °C (determined by POM); 1H NMR (acetone-d6): δ 1.03 (t, J = 7.2 Hz, 3H), 1.81 (sext., J = 7.2 Hz, 2H), 4.031 (t, J = 6.8 Hz, 2H), 7.04 (d, J = 8.8 Hz, 2H), 7.58 (d, J = 8.8 Hz, 2H), 5.0–10 (brs, 1H); 13C NMR (acetone-d6): δ 10.7, 23.1, 70.4, 73.4 (t, J = 4.4 Hz), 105.0 (t, J = 3.6 Hz), 108.1 (t, J = 17.6 Hz), 113.5, 113.8 (t, J = 16.9 Hz), 115.9, 134.5, 145.4 (ddt, J = 253.8, 13.9, 5.1 Hz), 147.4 (ddt, J = 250.9, 15.4, 3.6 Hz), 160.3, 161.8; 19F NMR (acetone-d6, C6F6): δ −136.91 (dd, J = 20.7, 10.5 Hz, 2F), −140.3 (dd, J = 20.7, 10.5 Hz, 2F); IR (KBr): ν 3650, 2966, 2211, 1705, 1601, 1476, 1331, 1253, 993 cm−1; HRMS (FAB): [M+] calcd C18H12F4O3: 352.0723, found: 352.0733.
2.2.4. 2-{(4-Butoxyphenyl)ethyn-1-yl}-2,3,5,6-tetrafluorobenzoic acid (1d)
Yield: 78% (white solid); Tm: 178 °C (determined by POM); 1H NMR (acetone-d6): δ 0.97 (t, J = 7.2 Hz, 3H), 1.50 (sext., J = 7.2 Hz, 2H), 1.76 (quin, J = 7.2 Hz, 2H), 4.03 (t, J = 6.8 Hz, 2H), 6.98 (d, J = 8.8 Hz, 2H), 7.52 (d, J = 8.8 Hz, 2H), 10.0 (brs, 1H); 13C NMR (CDCl3): δ 14.1, 19.8, 31.9, 68.6, 73.4 (t, J = 5.1 Hz), 105.0 (t, J = 3.7 Hz), 108.1 (t, J = 16.1 Hz), 113.5, 113.5 (t, J = 16.2 Hz), 115.8, 134.4, 145.5 (ddt, J = 252.3, 13.2, 5.8 Hz), 147.3 (ddt, J = 253.0, 13.9, 3.6 Hz), 160.3, 161.7; 19F NMR (acetone-d6, C6F6): δ −136.9 to −137.1 (m, 2F), −140.2 to −140.4 (m, 2F); IR (KBr): ν 3743, 2950, 2209, 1707, 1600, 1477, 1252, 1177, 993 cm−1; HRMS (FAB): [M+] calcd C19H14F4O3: 366.0879, found: 366.0893.
2.2.5. 2,3,5,6-Tetrafluoro-4-{2-(4-pentyloxy)ethyn-1-yl}benzoic acid (1e)
Yield: 44% (white solid); Tm: 175 °C (determined by POM); 1H NMR (acetone-d6): δ 0.94 (t, J = 7.2 Hz, 3H), 1.35–1.52 (m, 4H), 1.80 (quin, J = 6.8 Hz, 2H), 4.07 (t, J = 6.8 Hz, 2H), 7.01 (d, J = 8.8 Hz, 2H), 7.55 (d, J = 8.8 Hz, 2H), 9.07 (brs, 1H); 13C NMR (acetone-d6): δ 14.3, 23.1, 29.0, 29.7, 69.2, 73.5 (t, J = 4.4 Hz), 105.2 (t, J = 3.6 Hz), 108.4 (t, J = 18.3 Hz), 113.8, 113.9 (t, J = 17.6 Hz), 116.1, 134.6, 145.6 (ddt, J = 255.2, 15.3, 4.4 Hz), 147.6 (ddt, J = 250.9, 14.7, 3.6 Hz), 160.3, 162.0; 19F NMR (acetone-d6, C6F6): δ −136.92 (dd, J = 20.7, 10.9 Hz, 2F), −140.28 (dd, J = 20.7, 10.9 Hz, 2F); IR (KBr): ν 3485, 2948, 2212, 1707, 1600, 1481, 1253, 1176, 996, 837 cm−1; HRMS (FAB): [M+] calcd C20H16F4O3: 380.1036, found: 380.1027.
2.2.6. 2,3,5,6-Tetrafluoro-4-{2-(4-hexyloxy)ethyn-1-yl}benzoic acid (1f)
Yield: 65% (white solid); Tm: 185 °C (determined by POM); 1H NMR (acetone-d6): δ 0.90 (t, J = 6.8 Hz, 3H), 1.30–1.38 (m, 4H), 1.48 (quin, J = 6.8 Hz, 2H), 1.79 (quin, J = 6.8 Hz, 2H), 4.06 (t, J = 6.8 Hz, 2H), 7.02 (d, J = 8.8 Hz, 2H), 7.57 (d, J = 8.8 Hz, 2H), 8.94 (brs, 1H); 13C NMR (acetone-d6): δ 14.3, 23.3, 26.4, 32.3, 68.9, 73.4 (t, J = 3.7 Hz), 105.0 (t, J = 3.6 Hz), 108.1 (t, J = 18.4 Hz), 113.5, 113.6 (t, J = 16.9 Hz), 115.9, 134.5, 145.5 (ddt, J = 252.3, 14.0, 5.1 Hz), 147.4 (ddt, J = 251.5, 14.7, 3.7 Hz), 160.2, 161.8; 19F NMR (acetone-d6, C6F6): δ −136.97 (dd, J = 20.3, 12.4 Hz, 2F), −140.29 (dd, J = 20.3, 12.4 Hz, 2F); IR (KBr): ν 3450, 2946, 2211, 1705, 1602, 1476, 1329, 1172, 993, 834 cm−1; HRMS (FAB): [M+] calcd C21H18F4O3: 394.1192, found: 394.1202.
2.2.7. 2,3,5,6-Tetrafluoro-4-{2-(4-heptyloxy)ethyn-1-yl}benzoic acid (1g)
Yield: 75% (white solid); Tm: 172 °C (determined by POM); 1H NMR (acetone-d6): δ 0.89 (t, J = 6.8 Hz, 3H), 1.26–1.42 (m, 6H), 1.48 (quin, J = 6.8 Hz, 2H), 1.80 (quin, J = 6.8 Hz, 2H), 4.07 (t, J = 6.8 Hz, 2H), 7.03 (d, J = 8.8 Hz, 2H), 7.58 (d, J = 8.8 Hz, 2H), 6.0–10 (brs, 1H); 13C NMR (acetone-d6): δ 14.3, 23.3, 26.7, 29.8, 29.9, 32.6, 68.9, 73.4 (t, J = 4.4 Hz), 105.0 (t, J = 3.7 Hz), 108.1 (t, J = 17.6 Hz), 113.5, 113.7 (t, J = 16.9 Hz), 115.9, 134.5, 145.5 (ddt, J = 253.1, 13.9, 5.8 Hz), 147.4 (ddt, J = 250.8, 14.6, 3.7 Hz), 160.3, 161.8; 19F NMR (acetone-d6, C6F6): δ −136.98 (dd, J = 20.4, 12.0 Hz, 2F), −140.31 (dd, J = 20.4, 12.0 Hz, 2F); IR (KBr): ν 3680, 2948, 2211, 1704, 1601, 1479, 1330, 1254, 1171, 995, 836 cm−1; HRMS (FAB): [M+] calcd C22H20F4O3: 408.1349, found: 408.1343.
2.2.8. 2,3,5,6-Tetrafluoro-4-{2-(4-octyloxy)ethyn-1-yl}benzoic acid (1h)
Yield: 41% (white solid); Tm: 167 °C (determined by POM); 1H NMR (DMSO-d6): δ 0.86 (t, J = 6.8 Hz, 3H), 1.23–1.34 (m, 8H), 1.40 (quin, J = 6.8 Hz, 2H), 1.72 (quin, J = 6.8 Hz, 2H), 4.02 (t, J = 6.8 Hz, 2H), 7.02 (d, J = 8.8 Hz, 2H), 7.56 (d, J = 8.8 Hz, 2H), 14.6 (brs, 1H); 13C NMR (DMSO-d6): δ 13.9, 22.0, 25.4, 24.5, 28.6, 28.7, 31.2, 67.8, 72.8 (t, J = 4.4 Hz), 103.6 (t, J = 3.6 Hz), 106.0 (t, J = 16.9 Hz), 111.8, 113.6 (t, J = 17.6 Hz), 115.2, 133.6, 142.3–145.2 (m, 1C), 144.5–147.4 (m, 1C), 160.0, 160.3; 19F NMR (DMSO-d6, CFCl3): δ −136.49 (dd, J = 23.3, 10.9 Hz, 2F), −140.50 (dd, J = 23.3, 10.9 Hz, 2F); IR (KBr): ν 3673, 2946, 2210, 1704, 1600, 1477, 1329, 1253, 1170, 995, 836 cm−1; HRMS (FAB): [M+] calcd C23H22F4O3: 422.1505, found: 422.1510.
2.3. Single-Crystal X-ray Diffraction
Single-crystal X-ray diffraction (XRD) spectra were recorded using an XtaLAB AFC11 diffractometer (Rigaku, Tokyo, Japan). The reflection data were integrated, scaled, and averaged using the CrysAlisPro program (ver. 1.171.39.43a; Rigaku Corporation, Akishima, Japan), while empirical absorption corrections were applied using the SCALE 3 ABSPACK scaling algorithm (CrysAlisPro). The structures were identified by a direct method (SHELXT-2018/2 [19]) and refined using the full matrix least-squares method (SHELXL-2018/3 [20]) visualized by Olex2 [21]. Crystallographic data were deposited in the Cambridge Crystallographic Data Centre (CCDC) database (CCDC 2193549 for 1a and 2193550 for 1e), which were obtained free of charge from the CCDC at www.ccdc.cam.ac.uk/data_request/cif (accessed on 23 November 2022).
2.4. Phase Transition Behavior
The phase transition behaviors were observed by POM using an Olympus BX53 microscope (Tokyo, Japan) equipped with a cooling and heating stage (10002L, Linkam Scientific Instruments, Surrey, UK). Thermodynamic characterization was performed by differential scanning calorimetry (DSC; DSC-60 Plus, Shimadzu, Kyoto, Japan) at heating and cooling rates of 5.0 °C min−1 under N2.
2.5. Photophysical Properties
Ultraviolet–visible (UV–vis) absorption spectra were recorded using a JASCO V-750 absorption spectrometer (JASCO, Tokyo, Japan). The PL spectra of the solutions were measured using an FP-6600 fluorescence spectrometer (JASCO, Tokyo, Japan). The PL quantum yields were measured using a Quantaurus-QY C11347-01 instrument (Hamamatsu Photonics, Hamamatsu, Japan).
2.6. Theoretical Calculations
All computations were performed using Gaussian 16 program set [22] with the density functional theory (DFT) at the M06-2X hybrid functional [23] and 6-31+G(d,p) (for all atoms) basis set with a conductor-like polarizable continuum model (CPCM) [24] for CH2Cl2. Theoretical vertical transitions were also calculated using the time-dependent DFT (TD-DFT) method at the same theory level using the same solvation model.
3. Results and Discussion
3.1. Synthesis and Crystal Structure
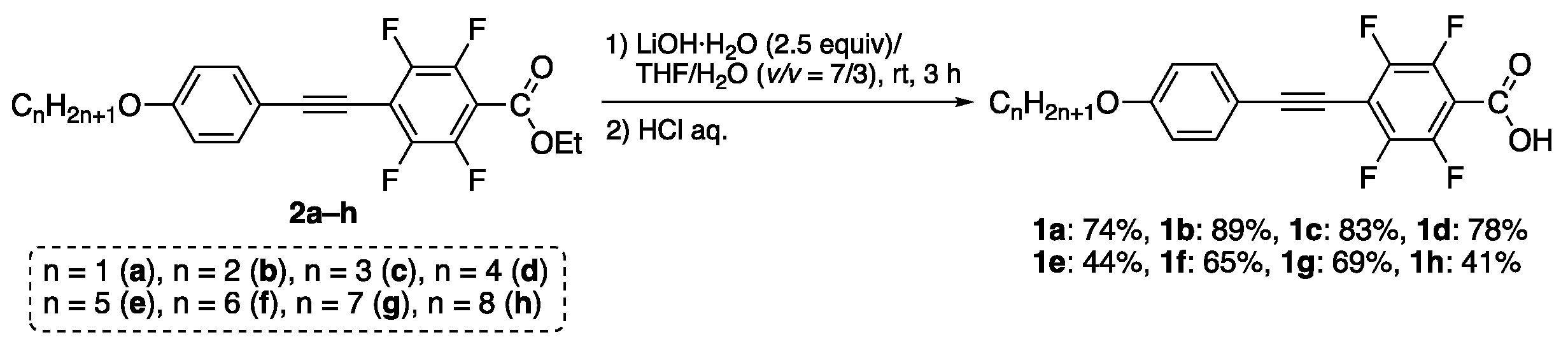
We first synthesized the fluorinated tolanecarboxylic acid 1 from the corresponding ester 2 via hydrolysis under basic conditions; synthesis of 2 was previously accomplished (Figure 3) [12].
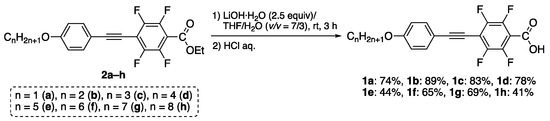
Figure 3.
Synthetic procedure of 1a–h from the corresponding ester 2.
Treatment of ester 2a with 2.5 equivalent of LiOH·H2O in a mixed solvent of THF and H2O (v/v = 7/3) at room temperature for 3 h underwent a hydrolysis reaction, which proceeded smoothly. Subsequently, treatment with an aqueous solution of concentrated HCl produced corresponding fluorinated tolanecarboxylic acid 1a. The product was purified by column chromatography and recrystallization, and the resulting 1a was generated as a white solid in a 74% isolated yield. Using a similar synthetic procedure, other analogs 1b–h bearing various alkoxy chains were also produced in 41–89% isolated yields. The molecular structures of 1a–h were assessed by 1H, 13C, and 19F-NMR, along with IR and HRMS. All structures were completely identified and sufficiently pure to evaluate their phase transition and photophysical behaviors.
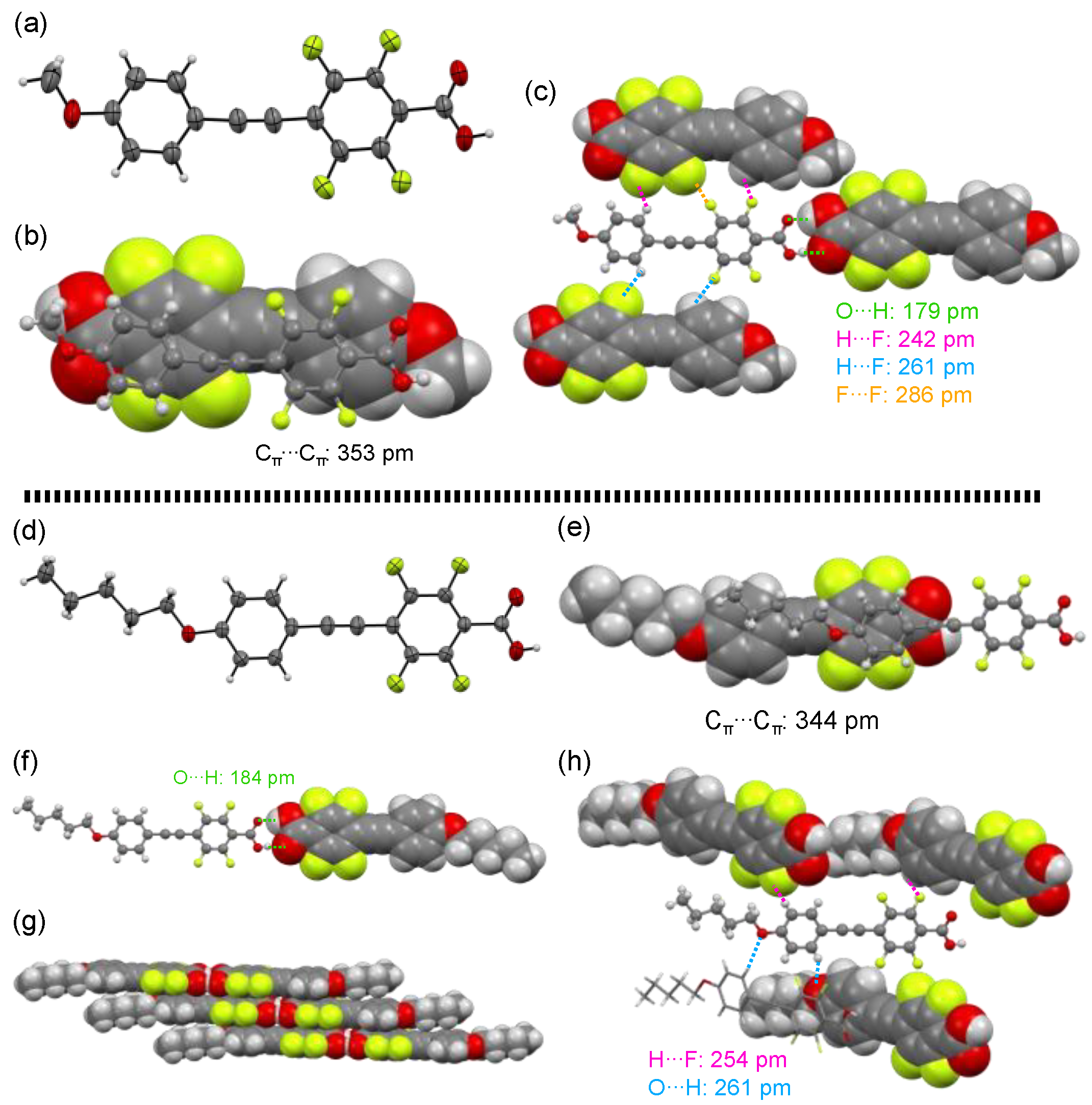
Among the fluorinated carboxylic acids 1a–h, methoxy-substituted 1a and pentyloxy-substituted 1e afforded single crystals that were appropriate for X-ray crystallographic analysis. Figure 4 shows the crystal structures of 1a and 1e and their packing structures.
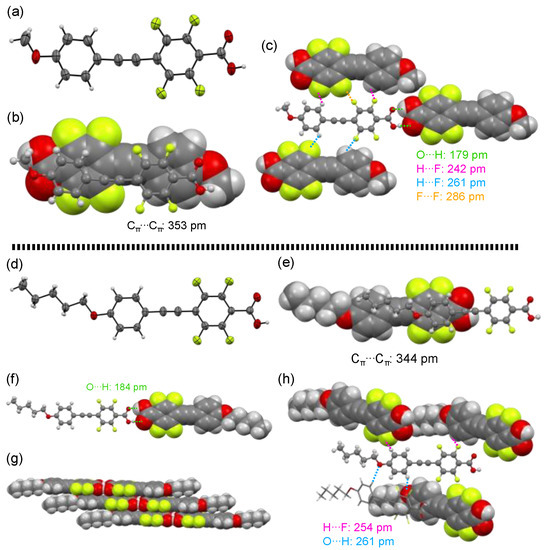
Figure 4.
(a) Crystal structure of 1a with an ORTEP drawing and (b,c) packing structures. (d) Crystal structure of 1e with an ORTEP drawing and (e–h) packing structures.
Methoxy-substituted 1a crystalized with a triclinic system in the P–1 space group and two molecular units were contained in the Cry lattice. The dihedral angle between two aromatic rings in the tolane scaffold was only 4.7°, almost coplanar to each other (Figure 4a). The dihedral angle between the fluorinated aromatic ring and the carbonyl plane was reported 34° for the ester precursor 2a [12]. However, the dihedral angle of the carboxylic acid 1a was only 3.7°, resulting in an almost coplanar structure. With respect to the packing structures, the two planar tolane scaffolds were arranged in a layer structure with an antiparallel direction. This phenomenon is caused by the electrostatic weak π–π interaction (short contact of Cπ⋯Cπ: 353 pm) between the electron-rich methoxy-substituted aromatic ring and the electron-deficient fluorinated aromatic ring (Figure 4b). Additionally, the fluorinated tolanecarboxylic acid 1a formed plural intermolecular interactions (Figure 4c), such as O···H hydrogen bond (short contact of O⋯H: 179 pm), H⋯F hydrogen bond (short contact of H⋯F: 242 and 261 pm), and F⋯F interaction (short contact of F⋯F: 286 pm), wherein the short contacts mentioned above were almost identical or below the sum of van der Waals radii [25].
In contrast, pentyloxy-substituted 1e furnished single crystals with a monoclinic system in the C 2/c space group, and eight molecular units were contained in the Cry lattice. The electron-rich aromatic ring and the electron-deficient fluorinated aromatic ring were nearly coplanar, with a deviation of 3.0°. The dihedral angle between the fluorinated aromatic ring and the carbonyl plane was 11°, being almost coplanar (Figure 4d). However, unlike the π–π stacking of the antiparallel orientation in 1a, 1e formed a slipped π–π stacking (short contact of Cπ⋯Cπ: 344 pm) with a synparallel orientation induced by the electrostatic interaction between the electron-rich pentyloxy aromatic ring and the electron-deficient fluorinated aromatic ring (Figure 4e). As shown in Figure 4f,g, the carboxyl units in 1e also formed an intermolecular O⋯H hydrogen bond with a short contact of 184 pm, leading to formation of layer structures. For construction of the crystal structure of 1e, more intermolecular interactions, such as additional O⋯H and H⋯F hydrogen bonds (Figure 4h), were also observed. The interatomic distance was 254 and 261 pm for O⋯H and H⋯F, respectively, which was also almost identical or below the sum of van der Waals radii [25].
3.2. Phase Transition Behavior
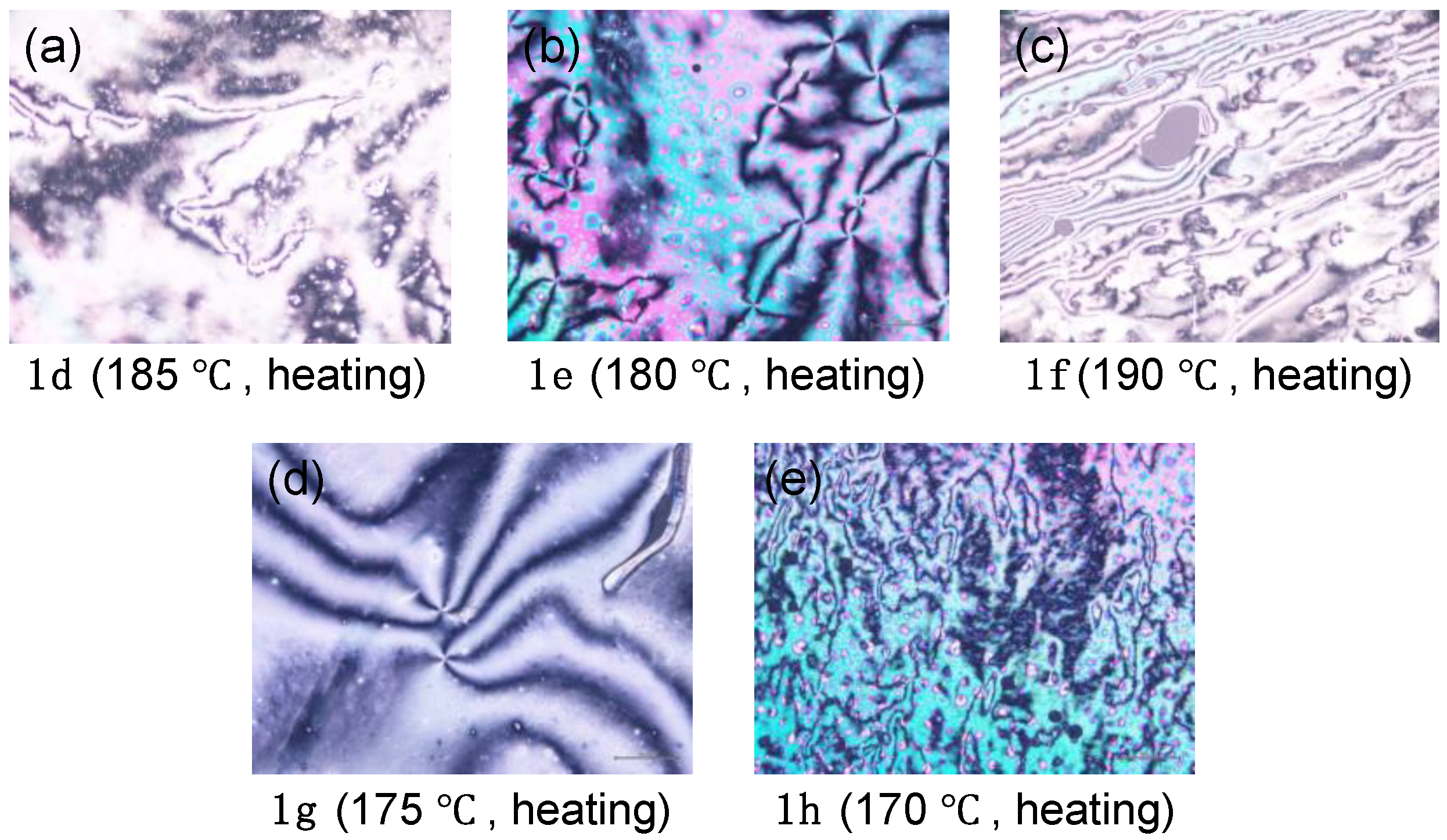
With the fluorinated tolanecarboxylic acids, 1a–h, in hand, we evaluated their phase transition behavior using DSC and POM. Table 1 summarizes the phase sequence and phase transition temperature for 1a–h during the first heating and cooling process determined by POM. Subsequent phase transition behavior is listed in Table S2 (ESI). Figure 5 shows the POM texture images of 1d–h observed in the mesophase.

Table 1.
The phase transition behavior of the fluorinated tolanecarboxylic acids, 1a–h, during the first heating and cooling process observed by POM.
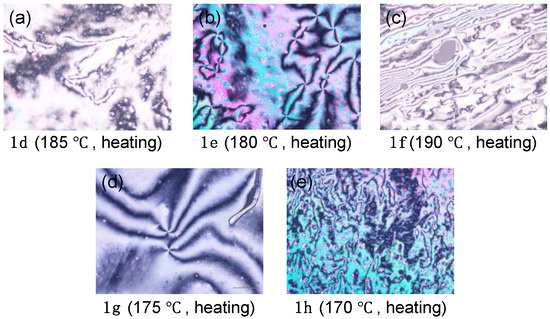
Figure 5.
Optical microphotographs of (a) 1d, (b) 1e, (c) 1f, (d) 1g, and (e) 1h in the mesophase phase.
The DSC measurement of methoxy-substituted 1a showed a large endothermic peak with an onset temperature of 223 °C during the first heating process. No sharp exothermic peak due to the Iso → Cry phase transition was observed during the subsequent cooling process. As a result of POM observation, however, a phase transition from the Iso phase to a glassy amorphous solid (G) phase was observed; 1a did not show any mesophase (Figure S25). The POM observation also proved that 1a showed no mesophase between the Cry and isotropic (Iso) phases. Additionally, no mesophase was observed for ethoxy-substituted 1b and propoxy-substituted 1c by POM and DSC measurements. In contrast, butoxy-substituted 1d showed an endothermic phase transition between the Cry and Iso phases in the first heating process of the DSC measurement and a bright-viewing field with fluidity during the heating and cooling processes of the POM observation. Thus, the phase transition behavior of butoxy-substituted 1d possessed the LC characteristic. A four-brushed Schlieren texture was observed as the optical image (Figure 5a), which is a typical texture for the N LC phase. During the subsequent cooling process, however, only the Iso → G phase transition was observed. The phase transition behavior was also supported by temperature-varying powder X-ray diffraction (VT-PXRD) measurements (Figure S26). Further POM observation was found to show similar phase transition between G and Iso phases during the second cycles. Similar to 1d, molecules 1e and 1f also exhibited an N-phase during the first heating process (Figure 5b,c), while, after the first cooling process, the LC phase disappeared, showing only a phase transition between the G and Iso phases. The other analogs, viz., 1g and 1h, with a relatively long alkoxy chain, were found to show a mesophase during both heating and cooling processes due to increasing stabilization of the mesophase. Thus, both 1g with a C7H15 chain and 1h with a C8H15 chain exhibited an N-phase with a four-brush Schlieren texture through POM measurements during both heating and cooling cycles (Figure 5d,e), in which the observed mesophase can be assigned as an N-phase by the VT-PXRD measurements (Figure S26). Focusing on the melting temperature (Tm), which is defined as the phase transition temperature from Cry to Iso or LC phases, the Tm of 1a–h was in the range of 167–224 °C for the heating process, which was much higher than that of the corresponding ester derivatives 2a–h (34–109 °C) [12]. Unlike the ester derivatives, the carboxylic acids exhibited LC phases even with relatively short alkoxy chains, particularly C4H9O, due to the increased aspect ratio of the mesogenic core induced by formation of a dimeric structure through hydrogen bonds.
3.3. Photophysical Behavior in Solution Phase
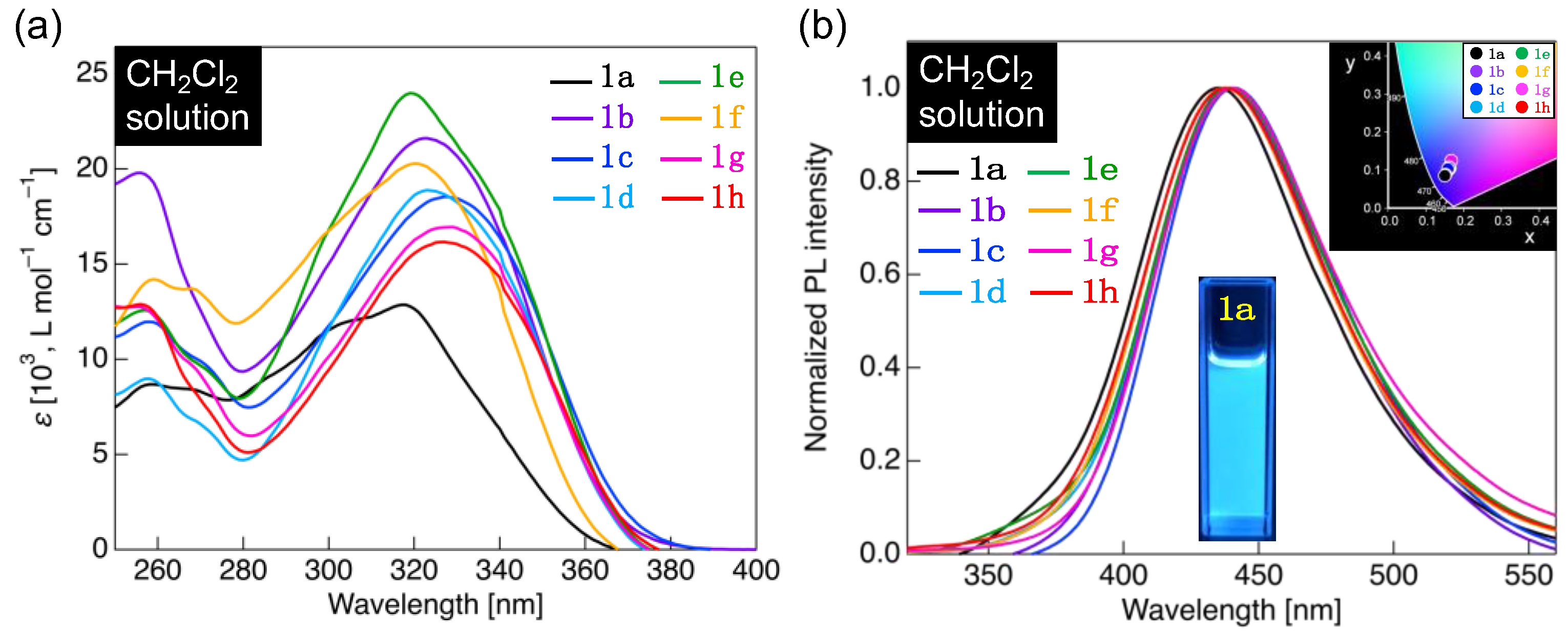
A solution sample was prepared to investigate the photophysical behavior of the fluorinated carboxylic acids, 1a–h, in the solution phase by individually dissolving 1a–h in CH2Cl2; the concentration was adjusted to 1.0 × 10−5 mol L−1. Figure 6 illustrates the photophysical behavior in the solution, and the photophysical data are summarized in Table 2.
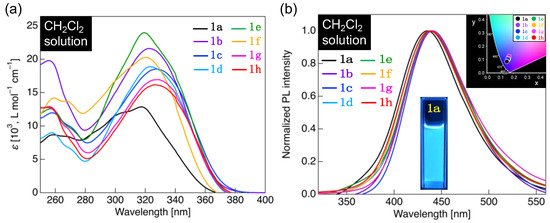
Figure 6.
(a) Ultraviolet (UV)–visible absorption spectrum of 1a–h in the CH2Cl2 solution (concentration: 1.0 × 10−5 mol L−1). (b) PL spectrum of 1a–h in the CH2Cl2 solution (concentration: 1.0 × 10−5 mol L−1) and a photograph of the PL behavior of 1a solution under UV light (λex = 365 nm). Inset: Commission Internationale de l’Eclairage (CIE) diagram for PL color of 1a–h solutions.

Table 2.
Photophysical data of 1a–h in solution state.
Methoxy-substituted 1a in CH2Cl2 absorbed UV light with a maximum absorption wavelength (λabs) near 259 nm and 317 nm (Figure 6a). Other analogs, particularly 1b–h, also showed two absorption bands: a high-energy absorption band near 255–259 nm of λabs and a low-energy absorption band near 319–329 nm of λabs (Figure 6a). Quantum chemical calculations were performed by the TD-DFT method using 1a as a representative, and two allowed transitions with theoretical absorption wavelengths of 319 and 262 nm were calculated as theoretical vertical transitions (Figure S31). The calculated absorption wavelengths were close to the experimentally obtained λabs. Thus, the result confirms that the long-wavelength absorption band of 1a in CH2Cl2 is the ππ* transition with an intramolecular charge transfer (ICT) character involving the highest occupied molecular orbital to the lowest unoccupied molecular orbital (HOMO → LUMO) transition, while the short-wavelength band is the ππ* transition with a local excitation character involving a HOMO–1 → LUMO transition.
With the λabs as the excitation wavelength, the methoxy-substituted 1a in the solution state was observed to emit blue PL, with a maximum PL wavelength (λPL) of approximately 435 nm (Figure 6b). In addition, 1b–h with varying lengths of alkoxy group were found to have a PL band with λPL in the range of 437–441 nm, leading to the blue PL. Considering the observed PL colors using the Commission Internationale de l’Eclailage (CIE) diagrams (Figure 6b, inset), the CIE coordinates for the PL colors of 1a–h were similar to each other. The PL color of the fluorinated tolanecarboxylic acids in CH2Cl2 showed a uniform blue PL in the solution state without affecting the alkoxy-chain length. PL quantum yields (ΦPL) of 1a–h in CH2Cl2 solutions were in the range of 0.27–0.38, which is higher than that of the unsubstituted tolane [26,27]. This phenomenon is observed because the donor–π–acceptor structure of the fluorinated tolanecarboxylic acid suppresses the internal conversion from the ππ* excited state to the dark πσ* excited state. In addition, we investigated the effect of solvent polarity on photophysical properties using 1a as a representative [28]. We found that, although the solvent polarity did not affect the absorption properties significantly, the PL properties shifted to longer wavelengths as the polarity increased, which is attributed to stabilization of solute–solvent interactions (Figure S28d). Considering the Lippert–Mataga plot [29,30], which is created from the orientational polarizability (Δf) and Stokes shift (Δν) on the horizontal and vertical axes, respectively, a linear relationship was obtained (Figure S28e). The dipole moment difference (μe–μg) between the excited and ground states was 14.1 D, which was calculated from the slope of the straight line. The large difference in the dipole moment proves that the radiative deactivation from the ICT excited state resulted in the PL of 1a–h.
3.4. Photophysical Behavior in Aggregated Phases
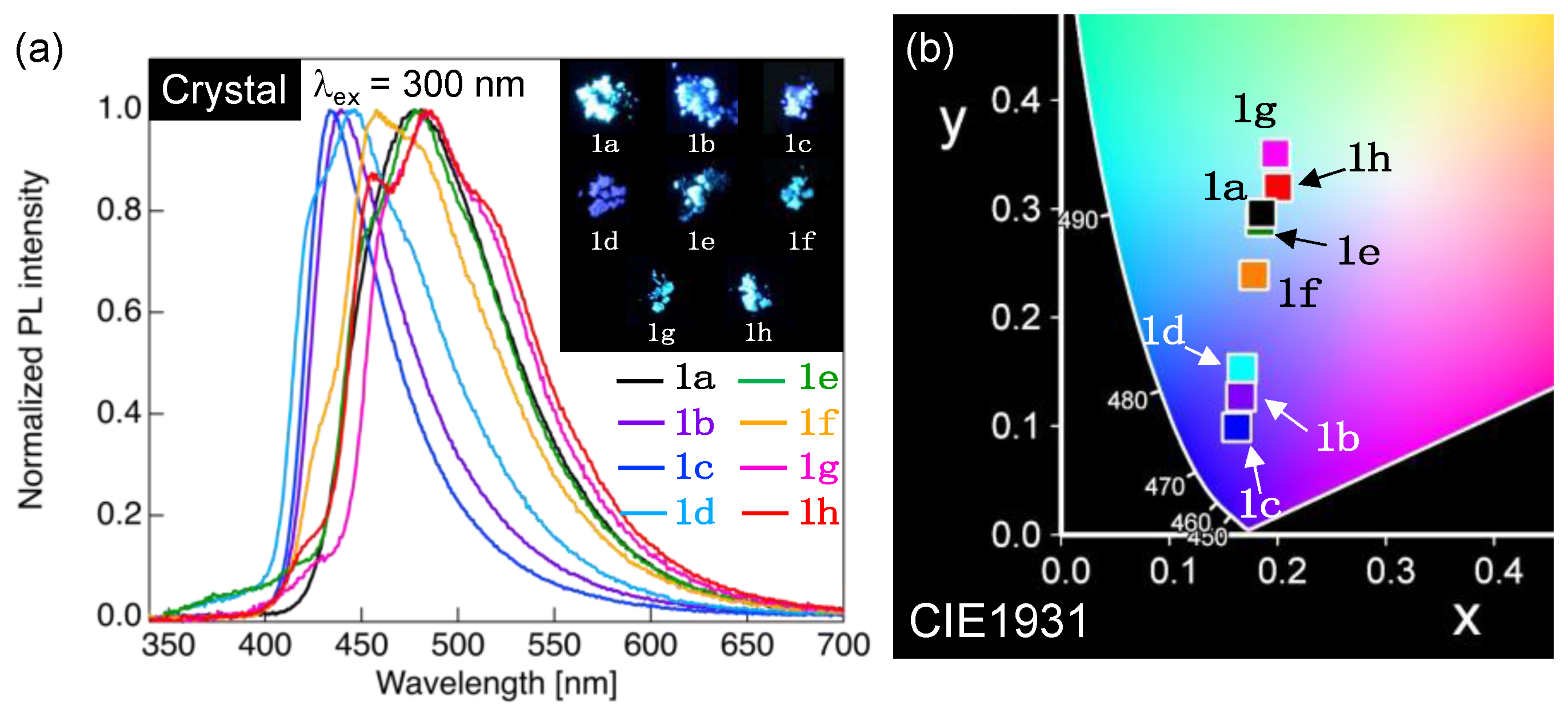
We next examined the PL behavior of fluorinated tolanecarboxylic acids, 1a–h, in the aggregated phases. Figure 7 shows the PL spectrum, photographs of the PL behavior under UV irradiation, and a CIE diagram for the PL colors. The photophysical data of 1a–h in the aggregated phases are summarized in Table 3.
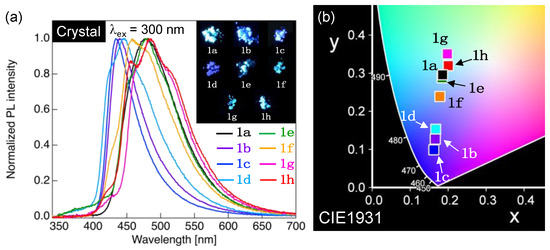
Figure 7.
(a) PL spectra of 1a–h in crystalline state. Excitation wavelength (λex): 300 nm. Inset: Photographs of the PL behavior of the 1a–h crystals under UV light (λex = 365 nm). (b) CIE color diagram of PL colors for 1a–h crystals.

Table 3.
Photophysical data of 1a–h in aggregated phases.
When methoxy-substituted 1a in the Cry phase was excited by irradiation with incident light of 300 nm, which is the maximum excitation wavelength (λex), a single PL band was observed with a λPL of approximately 481 nm (Figure 7a). As shown in Figure 8b,c, the CIE coordinate (x, y) of the PL color was (0.185, 0.296), indicating that the PL color was light blue. Notably, a CH2Cl2 solution of 1a had a ΦPL of 0.33, whereas the 1a in the Cry phase dramatically increased the ΦPL to up to 0.99. Although 1b–h with varying lengths of the alkoxy chain had almost identical λPL in dilute solutions, they exhibited various λPL in the Cry phase, ranging from 428 to 511 nm (Figure 7a). The alteration in λPL indicated a change in the PL color. Thus, various PL colors ranging from blue to light green were obtained by changing the length of the alkoxy chain, which is evident from the photographs and the CIE diagram demonstrating the PL colors (Figure 7b). The ΦPL values of 1b–h in the Cry phase were in the range of 0.49–0.71, which were higher than those in dilute solutions (up to 0.38). Considering the crystal packing structures of 1a and 1e shown in Figure 4, the change in the length of the alkoxy chain considerably altered the molecular arrangements in molecular aggregated phases; 1a–h exhibited various PL behaviors in the Cry phase. Furthermore, O⋯H and H⋯F hydrogen bonds and intermolecular interactions, such as F⋯F interactions and weak π⋯π interactions, function in the Cry phase, which possibly restricts the molecular motion to suppress non-radiative deactivation, resulting in a significant increase in the ΦPL in the Cry state.
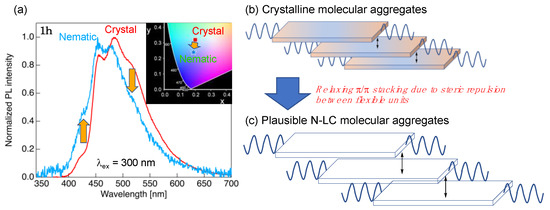
Figure 8.
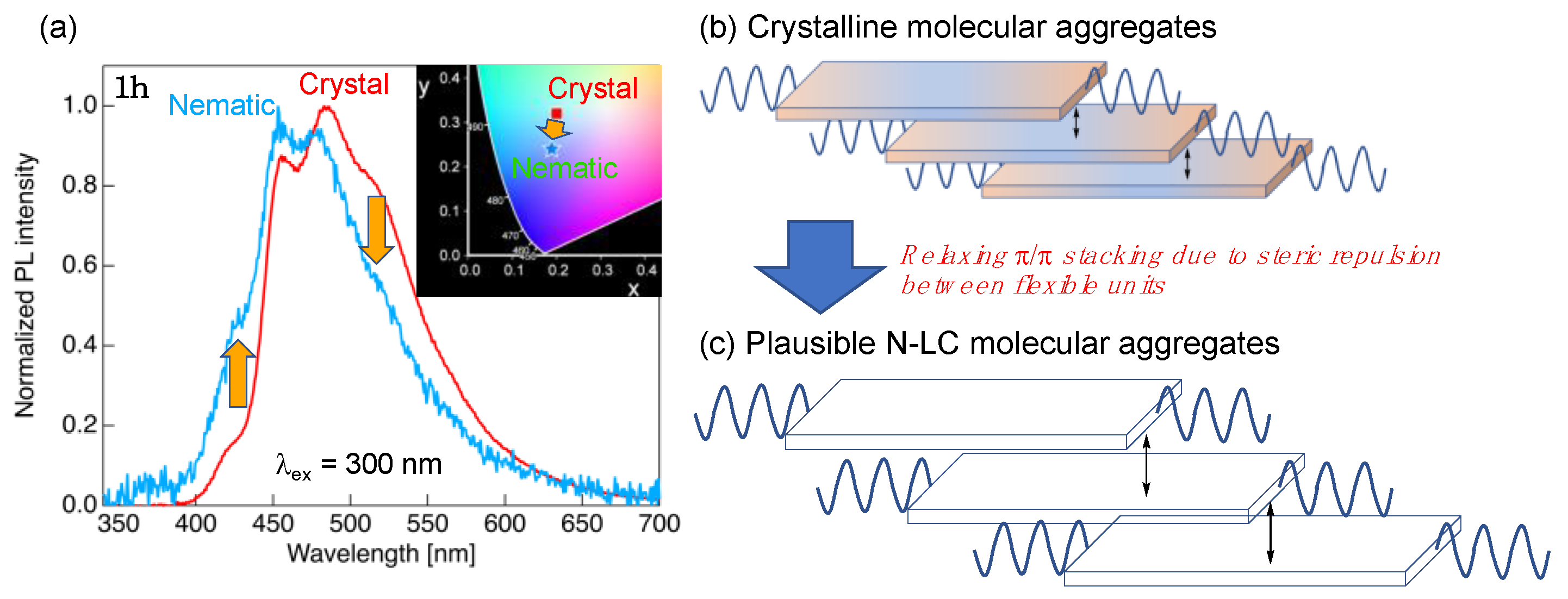
(a) PL spectra of 1h with the Cry- and N-phase molecular aggregated structures. Inset: CIE diagram of the PL colors for 1h with the Cry- and N-phase aggregated structures. (b,c) Schematic illustration of plausible structural alteration from the Cry- to N-phase molecular aggregated structures.
The PL behavior in the aggregated structures of the N-phase was evaluated using octyloxy-substituted 1h with an N LC phase. The measurement sample was prepared by quenching the sample with the N LC phase at 170 °C, which was developed during the 1st heating process, with liquid nitrogen. Figure 8 shows the PL spectra and CIE diagrams for 1h with the Cry- and N-phase molecular aggregated structures.
The PL spectrum of 1h with N-phase aggregated structures was also obtained by irradiation with incident light of 300 nm, in which the λPL was approximately 454 and 479 nm, along with a shoulder peak of approximately 428 nm. Compared to the Cry phase, the PL spectra of the N-phase aggregated structure yielded a slight short-wavelength shift with weakened long-wavelength shoulder peaks and increased short-wavelength peaks. In the Cry phase, the dimer mesogens formed a dense packing structure due to the weak π⋯π interactions (Figure 8b), as shown in Figure 4g. Conversely, the phase transition to the N-phase increased the molecular fluidity, allowing the increase in the two molecular distances (Figure 8c). The increased spacing between the dimer mesogens in the N-phase aggregated structure and the promotion of the molecular motion drastically reduced the ΦPL compared to that in the Cry phase.
4. Conclusions
In conclusion, we designed and synthesized a series of fluorinated tolanecarboxylic acids bearing various lengths of alkoxy chains and investigated their phase transition and photophysical behaviors. The fluorinated tolanecarboxylic acids exhibited the N LC phase due to formation of the dimer-type mesogen of the carboxylic acid moiety via O···H hydrogen bonds. Furthermore, regarding photophysical measurements, the fluorinated tolanecarboxylic acids emitted blue PL in the solution phase. The PL quantum yield (ΦPL) was approximately 0.33, which was higher than that of the unsubstituted tolane. The fluorinated tolanecarboxylic acid exhibited remarkably strong PL even in the Cry phase, and its ΦPL was much higher than that in the dilute-solution state, which could be attributed to the O⋯H and H⋯F hydrogen bonds and the weak π⋯π and F⋯F intermolecular interactions. Investigation of the PL behavior in the N-phase molecular aggregated structure revealed a slight short-wavelength shift and a significant decrease in ΦPL, which is attributable to the wider spacing between the dimer-type mesogens caused by increasing the molecular fluidity in the N-phase. These findings will offer a new molecular design for PLLC molecules effectively using intermolecular interactions and pave the way for developing new thermo-responsive luminescent materials in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13010025/s1, Figures S1–S24: NMR spectra of 1a–h; Figure S25: DSC thermograms of 1a–h; Figure S26: TG thermograms of 1a–h; Figure S27: PXRD patterns of 1d–h on the mesophase; Figure S28: UV–vis absorption and PL spectra of 1a–h in CH2Cl2 solution; Figure S29: UV–vis absorption and PL spectra of 1a in different solvents; Figure S30: Excitation and PL spectra of 1a–h in the Cry phase; Figure S31: Excitation and PL spectra of 1h in the aggregated structure of the nematic phase; Figures S32 and S33: Optimized structure of 1a and 1e and their orbital distributions; Table S1: Crystallographic data of 1a and 1e; Table S2: Phase transition behaviors of 1a–h observed by DSC measurements; Table S3: Solvent effect on the photophysical behavior; Tables S4 and S5: Cartesian coordinate for 1a and 1e.
Author Contributions
Conceptualization, S.Y.; methodology, S.Y.; validation, S.Y., M.K., K.Y. and T.K.; investigation, S.Y., M.K. and K.Y.; resources, S.Y. and T.K.; data curation, S.Y.; writing—original draft preparation, S.Y. and M.K.; writing—review and editing, S.Y., M.K., K.Y., M.N., T.A., H.F. and T.K.; visualization, S.Y.; supervision, S.Y.; project administration, S.Y.; funding acquisition, S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Murata Science Foundation and Shorai Foundation for Science and Technology.
Data Availability Statement
Not applicable.
Acknowledgments
We are deeply grateful to Sakurai and Shimizu (Kyoto Inst. Tech.) for their valuable cooperation in the PXRD measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Irfan, M.; Sumra, I.; Zhang, M.; Song, Z.; Liu, T.; Zeng, Z. Thermochromic and highly tunable color emitting bis-tolane based liquid crystal materials for temperature sensing devices. Dye. Pigment. 2021, 190, 109272. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional liquid crystals towards the next generation of materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Chen, J.; Zhu, W.; Baranoff, E. Recent progress in luminescent liquid crystal materials: Design, properties and application for linearly polarized emission. J. Mater. Chem. C 2015, 3, 7993–8005. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Stimuli-responsive luminescent liquid crystals: Change of photoluminescent colors triggered by a shear-induced phase transition. Angew. Chem. Int. Ed. 2008, 120, 5253–5256. [Google Scholar] [CrossRef]
- Zhao, D.; Fan, F.; Cheng, J.; Zhang, Y.; Wong, K.S.; Chigrinov, V.G.; Kwok, H.S.; Guo, L.; Tang, B.Z. Light-emitting liquid crystal displays based on an aggregation-induced emission luminogen. Adv. Opt. Mater. 2015, 3, 199–202. [Google Scholar] [CrossRef]
- Yamada, S.; Miyano, K.; Konno, T.; Agou, T.; Kubota, T.; Hosokai, T. Fluorine-containing bistolanes as light-emitting liquid crystalline molecules. Org. Biomol. Chem. 2017, 15, 5949–5958. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Agou, T.; Kubota, T.; Konno, T. Luminescence tuning of fluorinated bistolanes via electronic or aggregated-structure control. Appl. Sci. 2019, 9, 1905. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Fluorine-induced emission enhancement of tolanes via formation of tight molecular aggregates. New J. Chem. 2020, 44, 6704–6708. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Systematic studies on the effect of fluorine atoms in fluorinated tolanes on their photophysical properties. Molecules 2021, 26, 2274. [Google Scholar] [CrossRef]
- Yamada, S.; Kobayashi, K.; Morita, M.; Konno, T. D–π–A-type fluorinated tolanes with a diphenylamio group: Crystal polymorphism formation and photophysical behavior. CrystEngComm 2022, 24, 936–941. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Halogen atom effect of fluorinated tolanes on their luminescence characteristics. New J. Chem. 2022, 46, 4562–4569. [Google Scholar] [CrossRef]
- Yamada, S.; Kataoka, M.; Yoshida, K.; Nagata, M.; Agou, T.; Fukumoto, H.; Konno, T. Photophysical and thermophysical behavior of D-p-A-type fluorinated diphenylacetylenes bearing an alkoxy and an ethoxycarbonyl group at both longitudinal molecular terminals. J. Fluor. Chem. 2022, 261–262, 110032. [Google Scholar] [CrossRef]
- Yamada, S.; Uto, E.; Sakurai, T.; Konno, T. Development of thermoresponsive near-ultraviolet photoluminescent liquid crystals using hexyloxy-terminated fluorinated tolane dimers connected with an alkylene spacer. J. Mol. Liq. 2022, 362, 119755. [Google Scholar] [CrossRef]
- Yamada, S.; Uto, E.; Yoshida, K.; Sakurai, T.; Konno, T. Development of photoluminescent liquid-crystalline dimers bearing two fluorinated tolane-based luminous mesogens. J. Mol. Liq. 2022, 363, 119884. [Google Scholar] [CrossRef]
- Arakawa, Y.; Sasaki, Y.; Igawa, K.; Tsuji, H. Hydrogen bonding liquid crystalline benzoic acids with alkylthio groups: Phase transition behavior and insights into the cybotactic nematic phase. New J. Chem. 2017, 41, 6514–6522. [Google Scholar] [CrossRef]
- Arakawa, Y.; Kang, S.; Watanabe, J.; Konishi, G. Assembly of thioether-containing rod-like liquid crystalline materials assisted by hydrogen-bonding terminal carboxyl groups. RSC Adv. 2015, 5, 8056–8062. [Google Scholar] [CrossRef]
- Wen, J.; Tian, M.; Yu, H.; Guo, Z.; Chen, Q. Novel fluorinated liquid crystals. Part 9.—Synthesis and mesomorphic properties of 4-(n-alkoxycarbonyl)phenyl 4-[(4-n-alkoxy-2,3,5,6-tetrafluorophenyl)ethynyl]benzoates. J. Mater. Chem. 1994, 4, 327–330. [Google Scholar] [CrossRef]
- Wen, J.X.; Tian, M.Q.; Chen, Q. Synthesis and mesomorphic properties of 4′-n-alkoxy-2,3,5,6-tetrafluorobiphenyl-4-carboxylic acids. J. Fluor. Chem. 1994, 67, 207–210. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement, and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jensen, J.H. Improving the efficiency and convergence of geometry optimization with the polarizable continuum model: New energy gradients and molecular surface tesselation. J. Comput. Chem. 2004, 25, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Zgierski, M.Z.; Lim, E.C. Nature of the ‘dark’ state in diphenylacetylene and related molecules: State switch from the linear ππ* state to the bent πσ* state. Chem. Phys. Lett. 2004, 387, 352–355. [Google Scholar] [CrossRef]
- Saltiel, J.; Kumar, V.K.R. Photophysics of diphenylacetylene: Light from the “dark state”. J. Phys. Chem. A 2012, 116, 10548–10558. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. The solvent effect on fluorescence spectrum, change of solute-solvent interaction during the lifetime of excited solute molecule. Bull. Chem. Soc. Jpn. 1955, 28, 690–691. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent effects upon fluorescence spectra and dipole moments of excited molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).