Abstract
High-quality pentacene-doped p-terphenyl bulk crystals were grown by the selective self-seeding vertical Bridgman technique (SSVBT). The lattice structure and crystal properties of the samples of different doping concentrations and their relations with p-terphenyl single crystals were tested and analyzed. The doping effects of pentacene doping at different concentrations in p-terphenyl molecular crystals are discussed. The powder X-ray diffraction, FTIR, and 1H NMR studies show that no additional peaks (except for p-terphenyl) are observed in the spectra of two doped crystals. The results indicate that guest molecules appear as defects in the form of irregularly oriented molecules which do not significantly change the crystal structures. As the doping concentration increases, the average crystallite size decreases, and the crystallinity declines. The ultraviolet–visible absorption and fluorescence spectra show that with added pentacene molecules, the characteristic peak intensities decrease in the spectra owing to the p-terphenyl molecular transition. Meanwhile, characteristic peaks appear due to the pentacene molecular transition. Moreover, with the increase of doping concentration, the intensities of characteristic peaks of host molecules decrease continuously, and those of guest molecules increase accordingly.
1. Introduction
Organic optoelectronic functional material is a continuously active and cutting-edge research field [1,2]. Numerous practical advances have been achieved in the study of the properties of organic compounds and devices [3,4,5]. In particular, as a vital organic semiconductor material, pentacene is extensively used in organic thin-film transistors [6], organic field-effect transistors [7], organic solar cells [8], and other devices. To study the phenomenon of co-crystallization and doping effects, and obtain materials with superior performance, pentacene is often added to high-purity organic functional materials as adopant to prepare doped crystals [9,10].
The growth of large-sized organic crystals takes a long time by the vapor phase method [11]. With the solution method, the growth of organic crystals is limited by solvent absorption and solubility [12]. The melt method has none of these drawbacks, and the Bridgman method is widely used to grow large organic crystals [13]. Oxborrow et al. used the Bridgeman technique to grow a cone-shaped pentacene-doped p-terphenyl crystal (pentacene, 0.01%) with a length of about 7 cm and a maximum diameter of about 1.8 cm [14]. A room-temperature maser was fabricated with this crystal as the core [13,14,15]. The reports showed that the maser could be operated at room temperature without needing for high vacuum and strong magnetic field [16,17].
Based on preliminary studies, with p-terphenyl as the host and pentacene as the guest, crystal growth experiments of pentacene-doped p-terphenyl with different doping concentrations were carried out using an optimized double-wall ampoule. The results of XRD, FTIR, 1H NMR, UV–Vis absorption, and fluorescence spectra studies of pentacene-doped p-terphenyl crystals are reported. The lattice parameters and crystal properties of the samples with different doping concentrations and their relations with p-terphenyl single crystal were analyzed to explore possible doping effects upon molecular conformational changes in the crystals. The data of single p-terphenyl crystal and pentacene-doped p-terphenyl crystal (pentacene, 0.01 wt.%) involved in the comparison are from our previous research [18,19,20].
2. Materials and Methods
2.1. Design of Growth Container
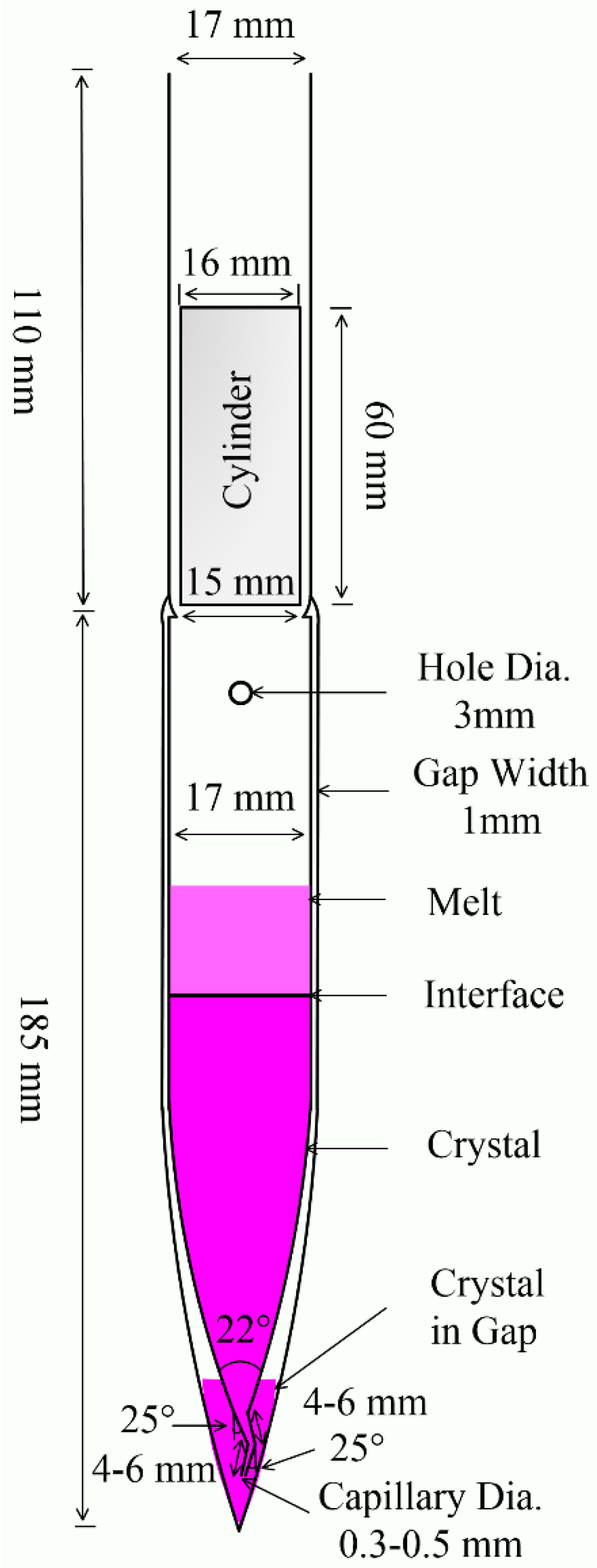
After several modifications to the ampoule, we designed a double-wall ampoule for crystal growth, as shown in Figure 1. The ampoule’s interlayer vacuum has thermal isolation, effectively resisting thermal fluctuations during the growth process. This measure can reduce defects, improve crystallinity, and ensure the growth of high-quality crystals. To ensure the isolation effect, the interlayer width was reduced to 1 mm to maximize the size of the inner tube crystal.
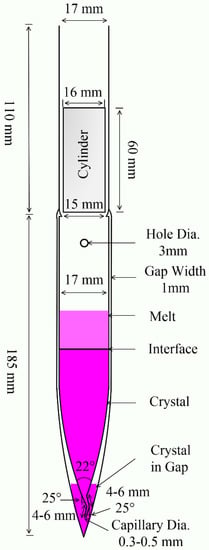
Figure 1.
Vertical section of the double-wall ampoule for production of crystals from melt.
The cone diameter of the inner tube was gradually shrunk to 2 mm before entering the capillary tube. The capillary was bent twice with a turning angle of 25°, and the length of both sections was 4–6 mm. The capillary diameter was gradually reduced to 0.3–0.5 mm at the end opening. At the initial moment of crystal growth, several initial seed crystals were produced at the bottom of the outer tube of the ampoule. The open complex capillary limits the entry of poor nucleation and ensures that only one good crystal nucleus enters the inner tube at the beginning of growth.
A small hole with a diameter of 3 mm was drilled into the inner tube near the long neck. This small hole allows air to be pumped out of the interlayer even after the ampoule is filled with material. This avoids oxidation of the material during high-temperature growth.
The ampoule was sealed with a quartz cylinder using an acetylene flame. The tube wall was integrated with the quartz cylinder through acetylene flame sealing. Compared with conventional sealing, this melting–sealing time is shortened. In addition, the strength of the tube wall is enhanced, reducing rupture and leakage. Due to the plugging effect of the quartz cylinder, the trace decomposition and oxidation impurities produced in the sealing process do not easily fall into the ampoule.
2.2. Growth of Pure and Pentacene-Doped p-Terphenyl Crystals
The double-wall ampoule was used as a growth container, and pentacene-doped p-terphenyl crystals were grown through a Bridgman furnace. Trace pentacene can dissolve in the p-terphenyl melt at high temperatures; thus, the mixed raw material melts at p-terphenyl’s melting point [18]. Therefore, the process and parameter setting of the growth of pentacene-doped p-terphenyl crystals were the same as those of p-terphenyl single-crystal growth.
The ampoule was cleaned with detergent (1%), acetone, and deionized water in sequence for three rounds of ultrasonic cleaning. After cleaning, the ampoule was dried by evaporation, and the temperature was set at 393 K. Because the ampoule structure was complex, the drying time was long (about 24–36 h). The chemicals of p-terphenyl (>99.5%, purified by sublimation) and pentacene (99.999%, purified by sublimation) were purchased from Tokyo Chemical Industry. In doped crystal growth, the raw materials of p-terphenyl and pentacene (0.1 wt.%) were weighed precisely and mixed evenly. After loading the materials, the ampoule was pumped for 10 h with a molecular pump and sealed at 10−5 Pa. Due to the oxyacetylene flame, the melting–sealing needed to be fast to avoid the decomposition of organic materials at high temperatures; the duration should not exceed 1 min [19].
The sealed ampoule was then placed in the hot zone of the furnace, and the temperature was slowly increased to the pre-melting temperature (488 K). It was kept at this temperature for 10 h to ensure the material was completely melted and then cooled naturally to the ambient temperature. The ampoule was removed from the furnace, and the melt solidified into polycrystalline. The crystal size was measured to determine the subsequent growth program.
After the pre-melting process, the furnace temperature was slowly raised to the growth temperature according to the heating plan. The highest temperature in the hot zone of the furnace was 493 K, and the temperature in the cold zone was 468 K with a temperature gradient of 1.38 K/cm. The ampoule was placed in the hot area of the furnace to melt for 20 h so that the melt was fully homogenized to avoid bubble formation. Because of the low thermal conductivity of organic materials, only a very small crystal growth rate can match it [20]. The initial growth rate of the crystals was set as 1 mm/h. With the continuous growth, the crystal diameter gradually increased, and the growth rate slowly decreased to 0.5 mm/hand maintained this rate until the end of the growth. After the growth was completed, annealing treatment was carried out at a temperature of 465 K. Finally, the furnace temperature was slowly lowered to room temperature at a rate of 2 K/h to avoid cracks caused by the different thermal expansion coefficients of glass and crystal. After removal from the ampoule, the crystals were trimmed and polished.
2.3. Characterization Techniques
The middle of the crystals was high quality and carefully ground in an agate mortar and sieved with a 300 mesh sieve. The crystallinity of the samples was analyzed at room temperature by powder XRD with an Empyrean X-ray diffractometer from Panalytical B.V. using Cu Kα (λ = 1.54059 Å) radiation in a tube voltage and current of 40 kV and 40 mA, respectively. A Jasco FP-6500 fluorescence spectrometer was used to analyze the fluorescence characteristics of the solid powder samples at room temperature with excitation wavelengths of 270 nm and 345 nm, respectively. The ultraviolet–visible absorption spectra of the solid powder samples were collected at room temperature by a PerkinElmer Lambda 35 UV–Vis spectrophotometer with a scanning range of 200~800 nm. The Fourier transform infrared spectra of the samples were measured by a Bruker Vertex 70 FTIR spectrometer in the wavenumber range 400~4000 cm−1. The 1H NMR spectra of the samples were recorded by a Bruker AscendTM 600 MHz NMR spectrometer with chloroform (CDCl3) as solvent.
3. Results and Discussion
3.1. Morphology Analyses of Pentacene-Doped p-Terphenyl Crystals
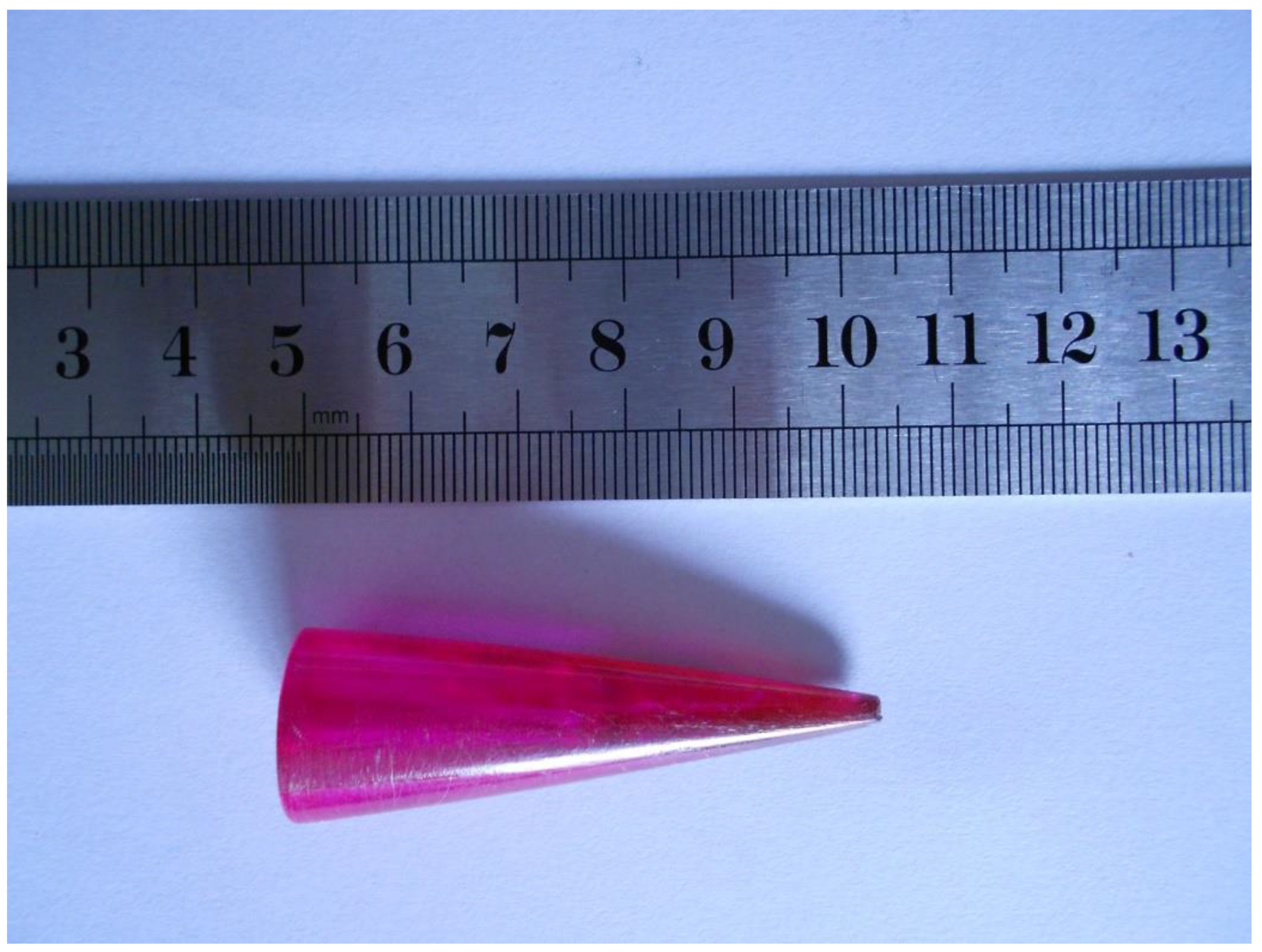
The 0.1 wt.% pentacene-doped p-terphenyl crystal was purple-red with a length of 55 mm and a maximum diameter of 18 mm, as shown in Figure 2. The uneven color of the surface indicated the non-uniform distribution of pentacene in the crystal due to the continuous action of crystallization driving forces and gravity during the growth period. The previous research work of our lab demonstrated that the 0.01 wt.% pentacene-doped p-terphenyl crystal was light purple [18]. As the doping amount increases, the heavy particle deposition portion of the bottom changes from pale yellow to deep purple-red. Compared with the colorless transparent p-terphenyl single crystal in our previous study [19], the crystals presented a color change from colorless to light purple to purplish red as the doping concentration increased. Since the trace dopant is soluble in the crystal, the damage of guest molecules to the host lattice is relatively small, as in co-crystallization [21]. The extent of crystal color change caused by guest molecules depends on the shape, size, number, and location of their functional groups [22].
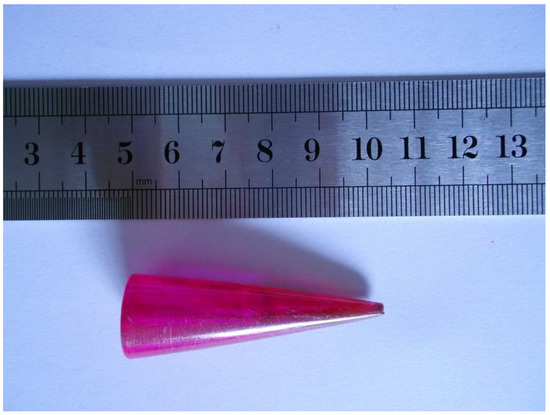
Figure 2.
Image of Bridgman-grown 0.1 wt.% pentacene-doped p-terphenyl crystal.
3.2. Powder X-ray Diffraction Analyses
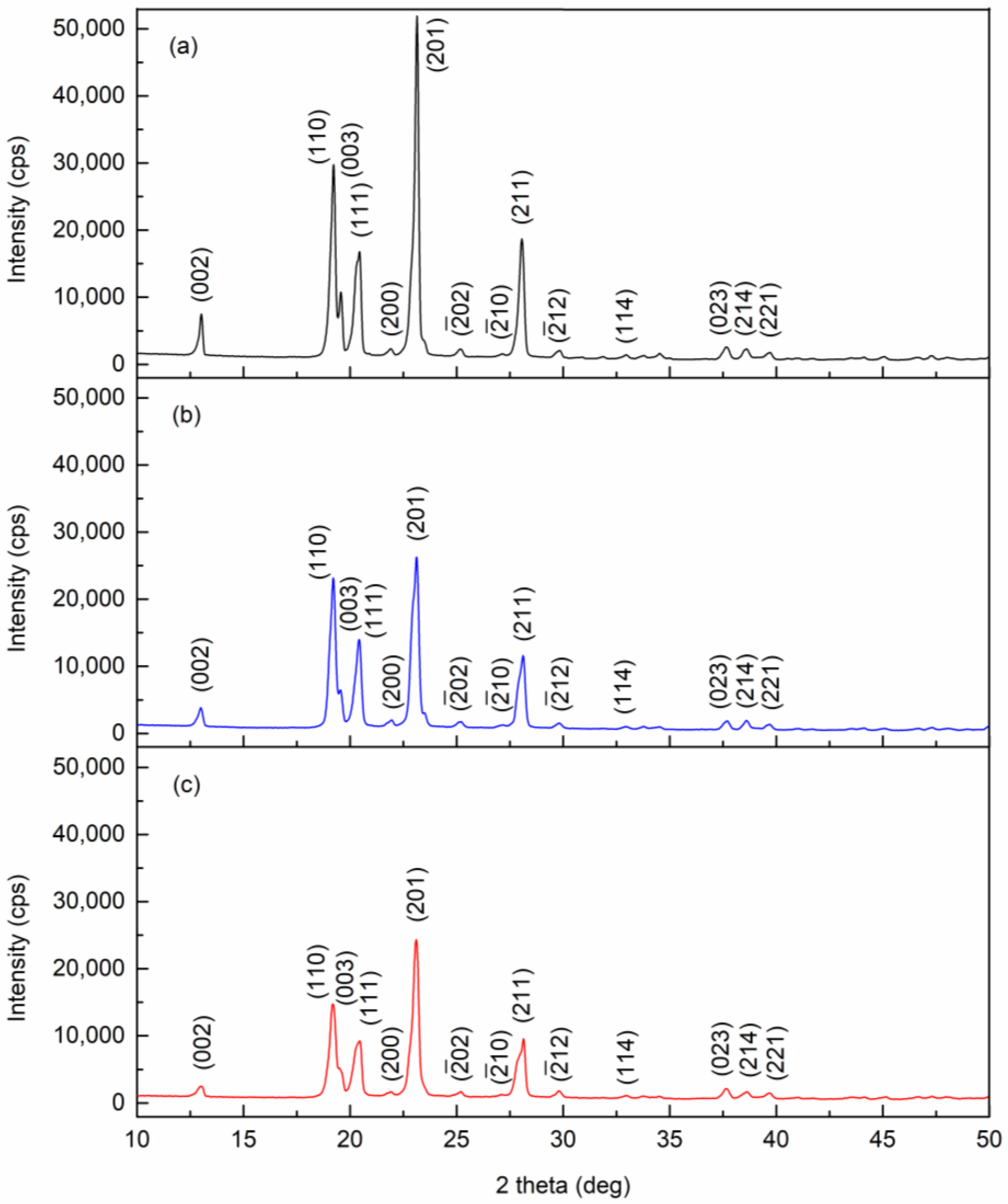
The powder X-ray diffraction patterns of pure, 0.01 wt.%, and 0.1 wt.% pentacene-doped p-terphenyl crystals are shown in Figure 3. The lattice constants were calculated by MDI Jade 6.5 and compared with CCDC data [23,24], as shown in Table 1. The two doped crystals were still of monoclinic and space group P21/a. No diffraction peaks (except p-terphenyl) were observed in the diffraction patterns of the two doped crystals. Due to the trace amount, guest molecules only randomly filled the vacancies or defects in the crystal lattice, which is insufficient to change the host lattice structure [25].
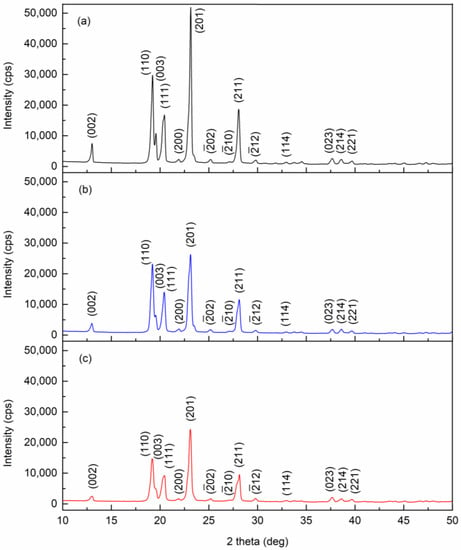
Figure 3.
Powder X-ray diffraction patterns of (a) pure, (b) 0.01 wt.%, and (c) 0.1 wt.% pentacene-doped p-terphenyl crystals.

Table 1.
Lattice parameters of pure and pentacene-doped p-terphenyl crystals.
The crystallite sizes of pure and two doped samples were calculated using the Scherrer equation. The changes in crystallite sizes corresponding to the five main crystal planes of the doped crystals, as compared to the p-terphenyl single crystal, are given in Table 2. The results suggest that the average crystallite size decreases with increased doping concentration. Trace guest molecules act as impurities, increasing lattice defects and decreasing crystallinity.

Table 2.
Change in crystallite size corresponding to different planes of pentacene-doped p-terphenyl crystals.
3.3. Fluorescence Spectrum Analyses
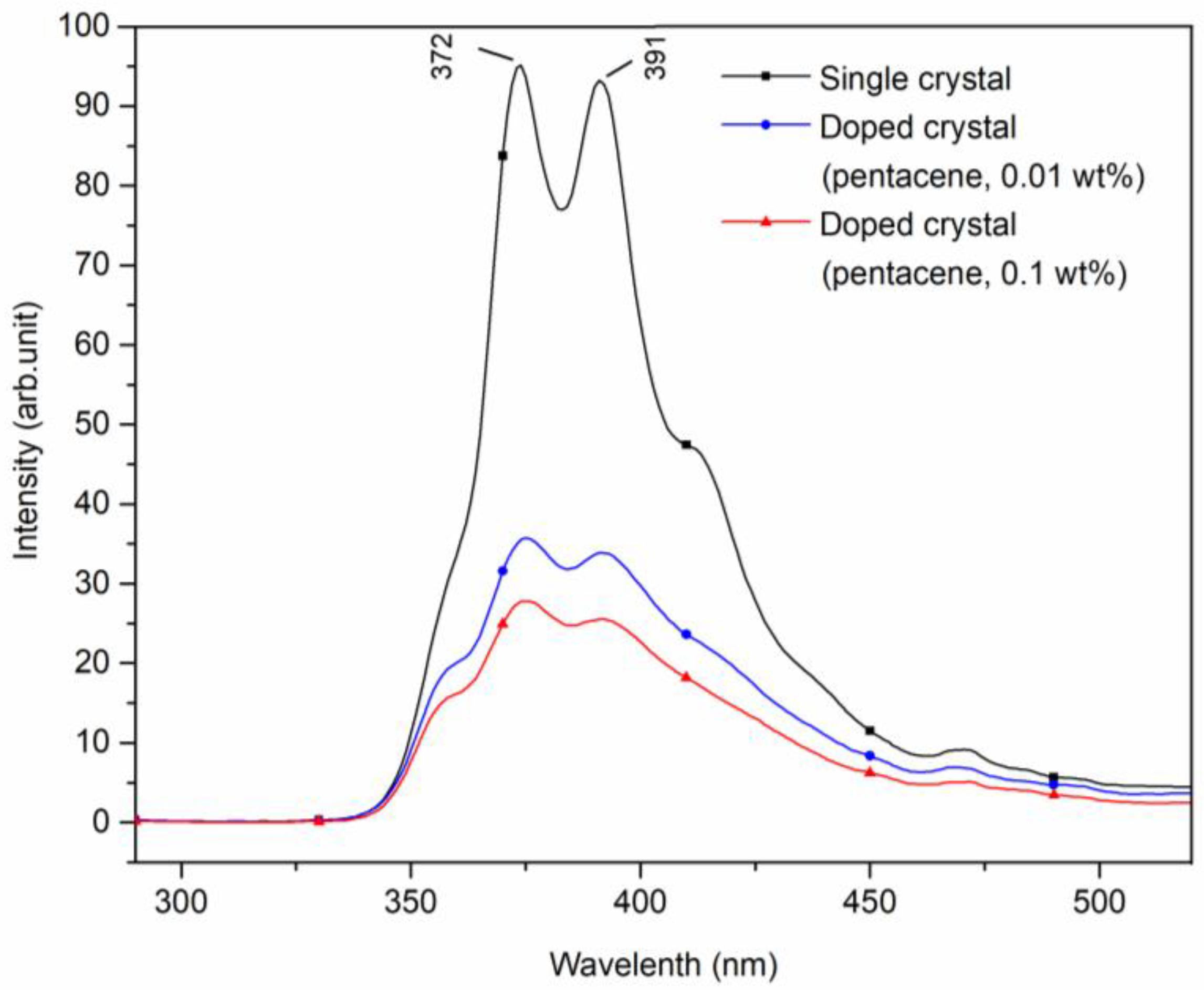
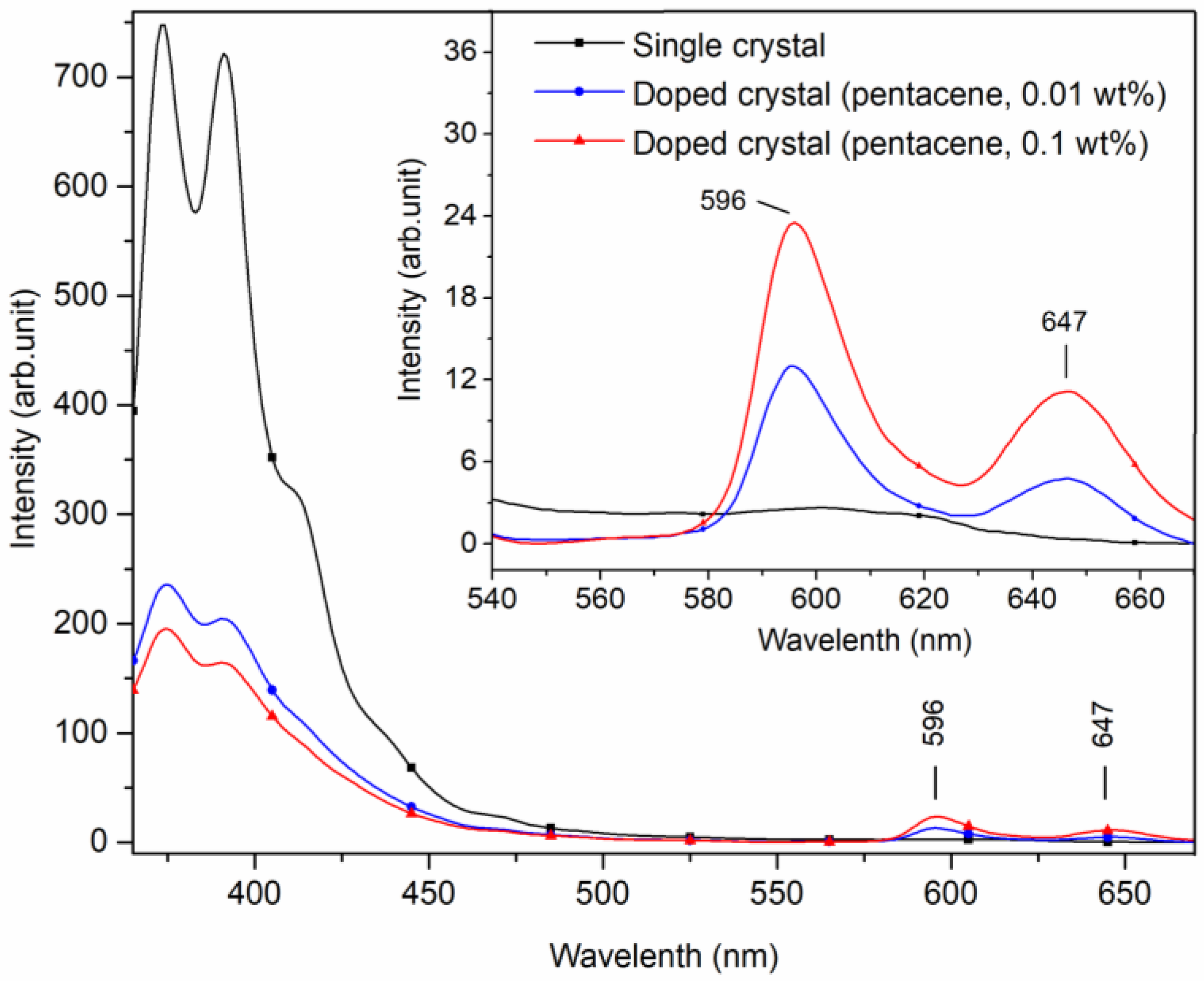
Figure 4 displays the fluorescence emission spectra of pure, 0.01 wt.%, and 0.1 wt.% pentacene-doped p-terphenyl crystals. The excitation wavelength was 270 nm, and the scanning range was 290~520 nm. Fluorescence peaks of the 0.01 wt.% doped sample appeared at 375 and 391 nm. For the 0.1 wt.% doped sample, the peaks appeared at 375 and 392 nm. In addition, two doped crystals had shoulder peaks at 361 and 412 nm. The four peaks all reflected the fluorescence characteristic of the p-terphenyl molecular transition, and their positions were very close to those of the p-terphenyl single crystal [26].

Figure 4.
Fluorescence spectra of pure and pentacene-doped p-terphenyl crystals (λex = 270 nm).
Compared with the fluorescence peak intensities of the p-terphenyl single crystal, those of the two doped samples both decrease significantly, as shown in Table 3. The comparative data show that increased doping concentration trapped guest molecules in the host lattice as chemical impurities, resulting in increased crystal defects and decreased fluorescence peak intensities of host molecules.

Table 3.
Fluorescence characteristic peaks representing p-terphenyl in pure and pentacene-doped p-terphenyl crystals.
When the excitation wavelength was adjusted to 345 nm with a scanning range of 365~670 nm, small peaks of 596 and 647 nm appeared in the fluorescence spectra of the doped crystals. In contrast, these peaks were not observed in the spectra of the p-terphenyl single crystal, as shown in Figure 5.
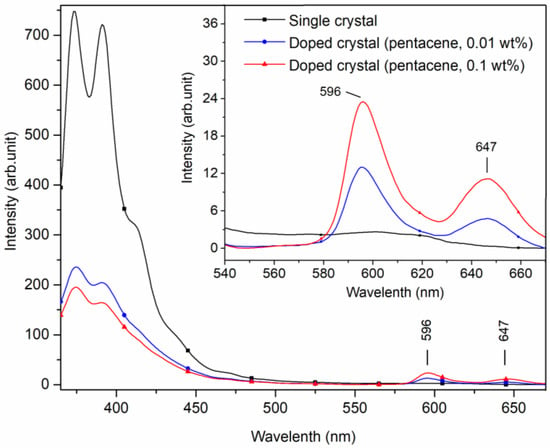
Figure 5.
Fluorescence spectra of pure and pentacene-doped p-terphenyl crystals (λex = 345 nm).
The small peaks at 596 and 647 nm of the doped crystals are characteristic of fluorescence emitting due to the pentacene molecular transition in Figure 5. Gatti et al. studied the fluorescence spectra of pentacene-doped tetracene crystals with toluene as solvent, giving peaks at 563, 616, and 670 nm. The 563 nm fluorescence peak was attributed to tetracene, while the peaks at 616 and 670 nm were likely the actions of pentacene [10]. However, the positions of the fluorescence peaks in the two studies are biased because powder samples were tested in this study, while solution samples were detected in Gatti’s study. The position and intensity of pentacene fluorescence characteristic peaks of the doped crystals are presented in Table 4. With the increase in doping concentration, the fluorescence peak intensities of host molecules decreased continuously, while those of guest molecules increased correspondingly.

Table 4.
Fluorescence characteristic peaks representing pentacene in pentacene-doped p-terphenyl crystals.
3.4. Ultraviolet–Visible Absorption Spectrum Analyses
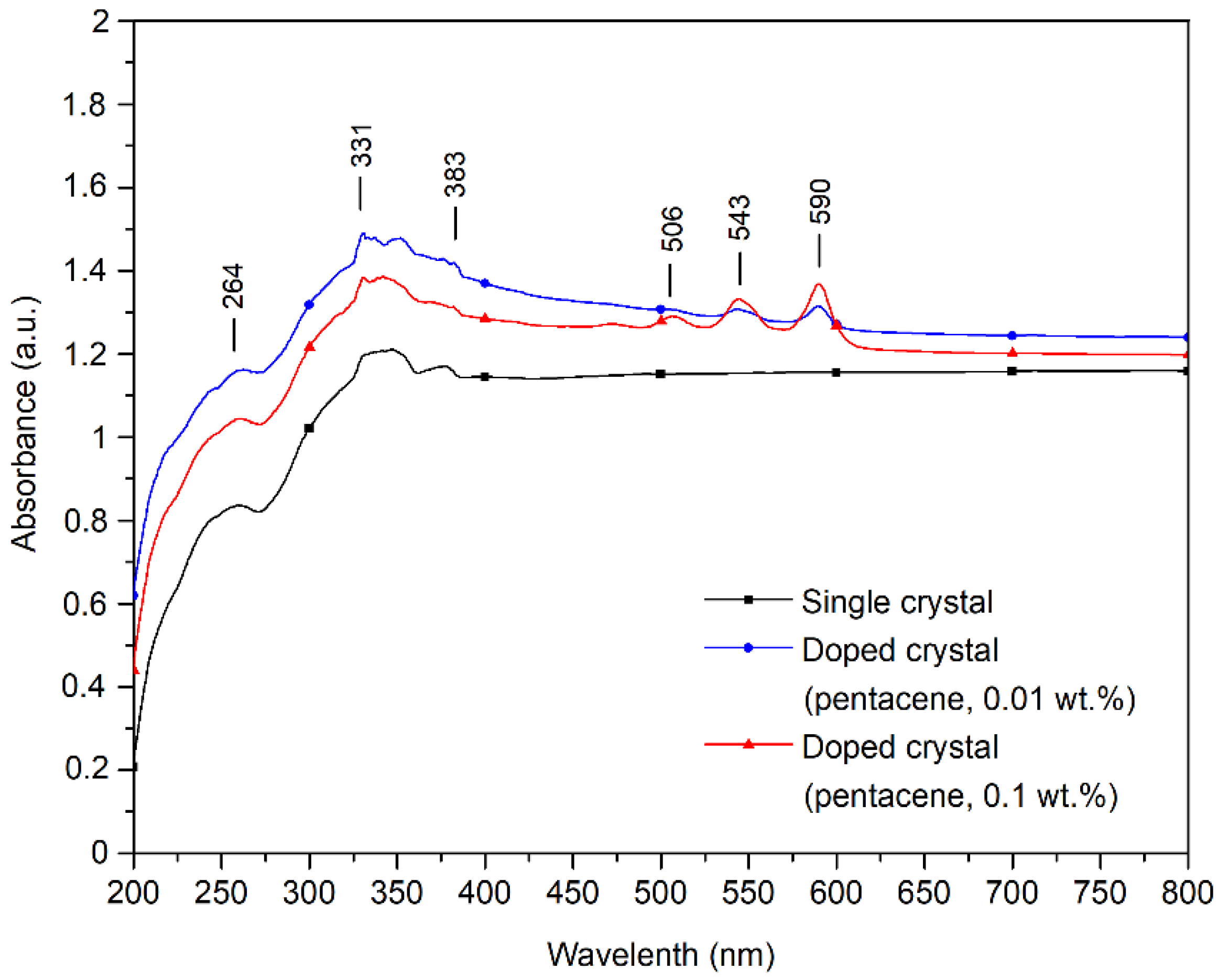
The UV–visible absorption spectra of pure and pentacene-doped p-terphenyl crystals are shown in Figure 6. Aromatic organic compounds have three absorption bands in the UV–Vis spectrum due to the ring-shaped conjugated structure: E1, E2, and B bands [27]. The p-terphenyl single crystal has the E1 band at 259 nm, the E2 band at 330 nm, and the B band at 384 nm. The position and absorption intensity of the p-terphenyl molecule UV–Vis characteristic bands of the three samples are shown in Table 5.
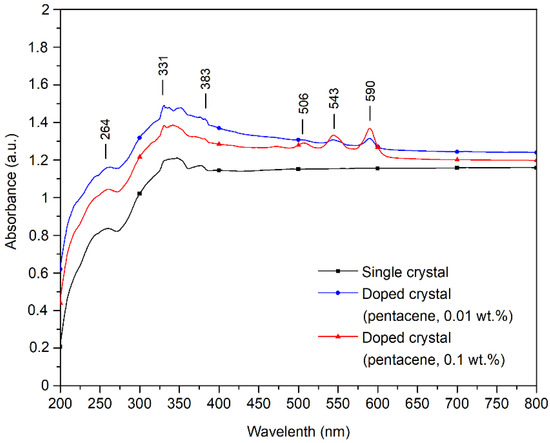
Figure 6.
UV–visible absorption spectra of pure and pentacene-doped p-terphenyl crystals.

Table 5.
UV–Vis characteristic bands representing p-terphenyl in pure and pentacene-doped p-terphenyl crystals.
Wilson showed that the UV–Vis absorption bands of pentacene films were located at 500, 542, and 584 nm [28]. Murai’s study showed that the UV–Vis absorption bands of pentacene films are situated at 545 and 592 nm [29]. The small peaks at 506, 544, and 590 nm in the doped crystals’ UV–Vis spectra represent pentacene’s characteristic UV–Vis bands. Since the doping concentration of the 0.01 wt.% sample is tiny, the UV–Vis absorption peak near 503 nm is not apparent, as shown in Table 6.

Table 6.
UV–Vis characteristic bands representing pentacene in pentacene-doped p-terphenyl crystals.
3.5. FTIR and Transmission Spectral Analyses
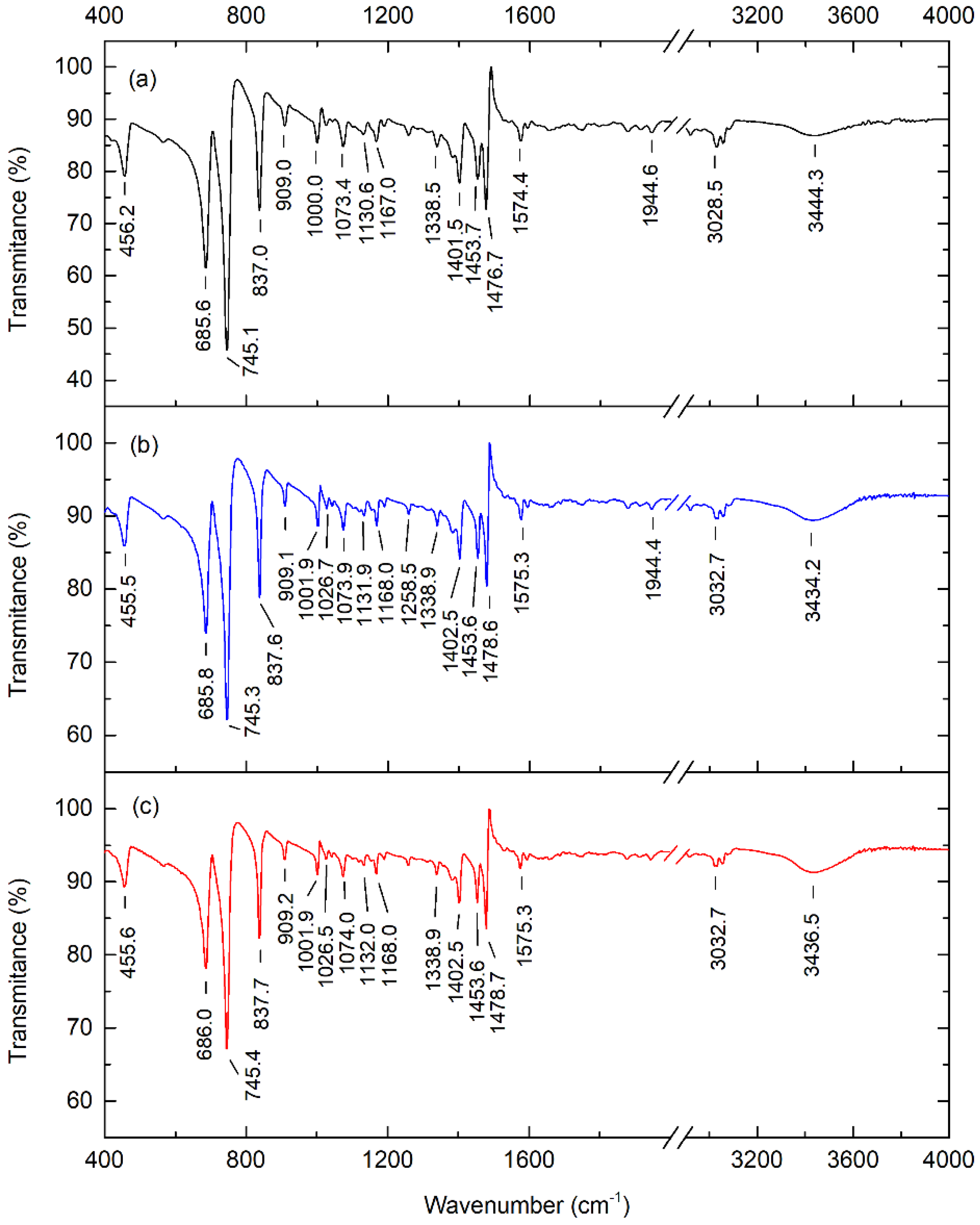
The Fourier transform infrared spectra of pure, 0.01 wt.%, and 0.1 wt.% pentacene-doped p-terphenyl crystals are shown in Figure 7. The four strongest absorption peaks of the crystals are located at 685, 745, 837, and 1478 cm−1. The absorption peaks in the spectra all reflect the vibration information of the characteristic groups of p-terphenyl [30].
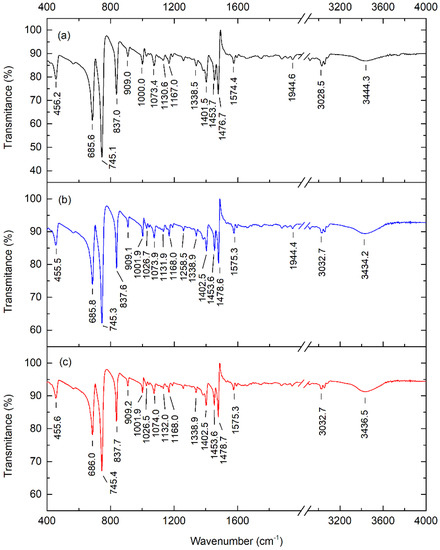
Figure 7.
FTIR spectra of (a) pure, (b) 0.01 wt.%, and (c) 0.1 wt.% pentacene−doped p-terphenyl crystals.
Ross et al. measured the Fourier transform infrared spectra of pentacene. The results showed that pentacene’s four strongest absorption peaks were located at 733, 906, 1296, and 3041 cm−1 [31]. The study of Hosoi et al. showed that the three strongest absorption peaks of pentacene were situated at 907, 1296, and 3043 cm−1 [32]. Compared with the infrared spectra of the p-terphenyl single crystal and the spectra of pentacene given by Ross and Hosoi, there were no pentacene absorption peaks (except p-terphenyl) in the two doped samples. Furthermore, the absorption peak positions did not shift significantly relative to the p-terphenyl single crystal. This is because the doping content in the two samples was insufficient to change the host lattice and cause significant conformational changes. Hence, the vibrational and rotational frequencies of the molecular groups had no apparent change.
3.6. 1H NMR Spectrum Analyses
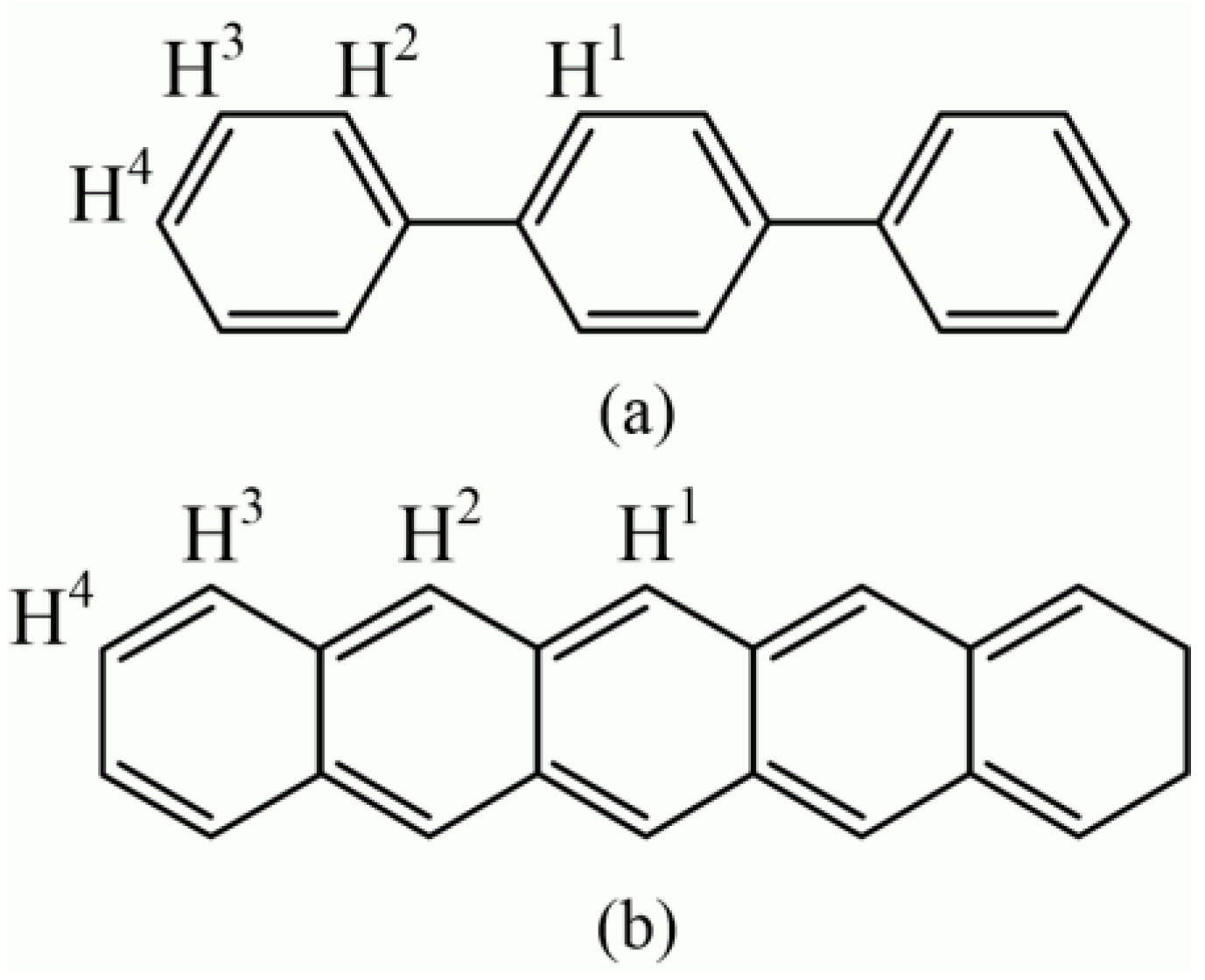
The p-terphenyl and pentacene molecules contain four structural types of equivalent hydrogen atoms, as shown in Figure 8. Nagano et al. tested the 1H NMR spectrum of pentacene using a dimethyl sulfoxide (DMSO-d6) reagent. The results show that the chemical shift values of four types of hydrogen nuclei (H1, H2, H3, and H4) were 9.2, 8.8, 8.0, and 7.4 ppm [33], respectively. According to the study of Kupka et al., the chemical shift values of four types of hydrogen nuclei (H1, H2, H3, and H4) of pentacene were 9.123, 8.782, 8.004, and 7.356 ppm [34].
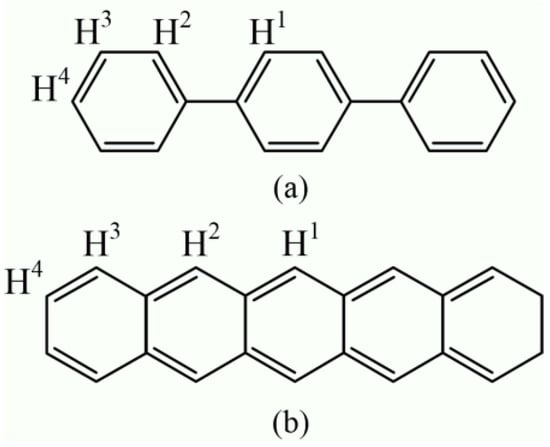
Figure 8.
Molecular structure and hydrogen atoms types of (a) p-terphenyl and (b) pentacene.
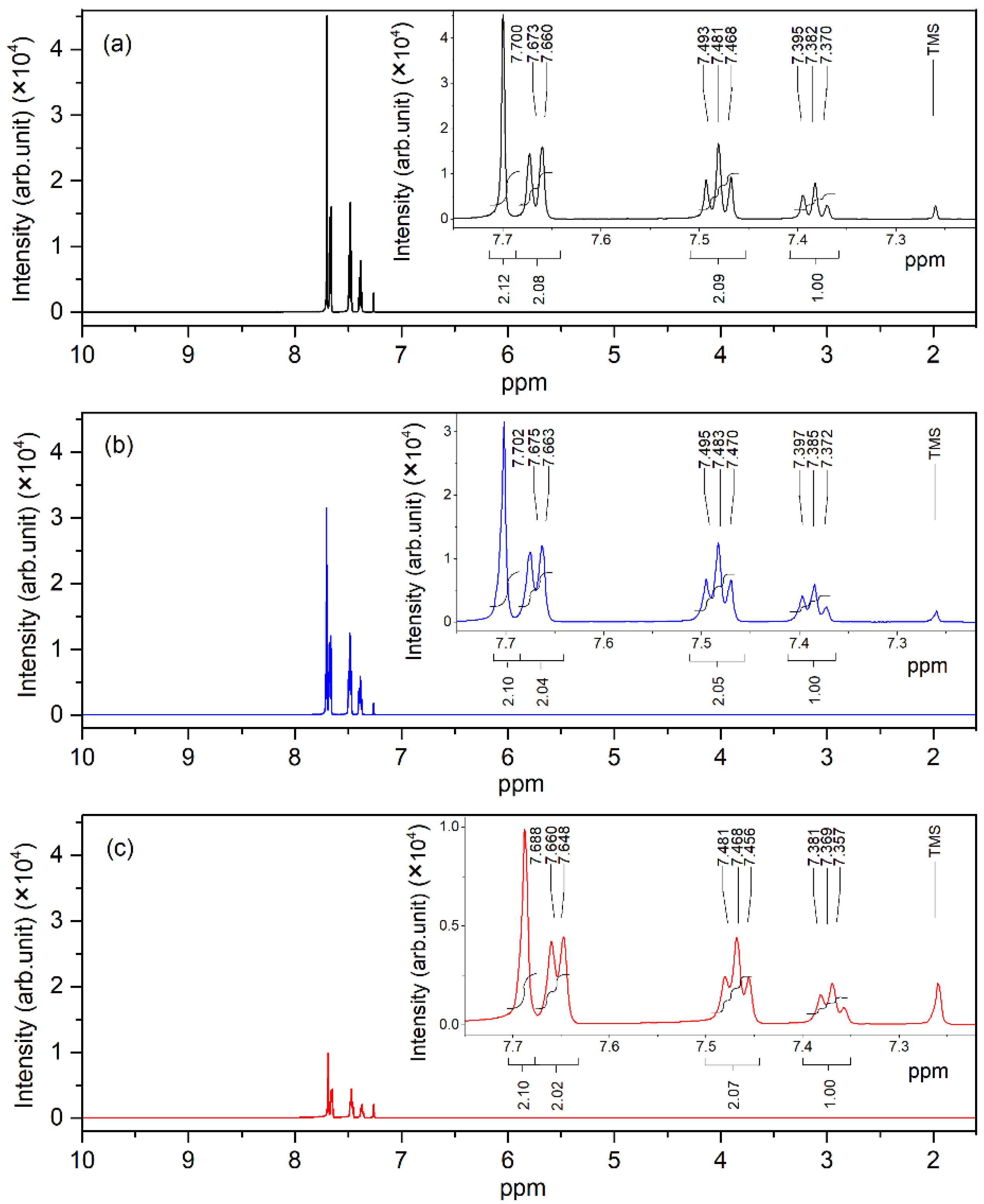
The 1H NMR spectra of pure, 0.01 wt.%, and 0.1 wt.% pentacene-doped p-terphenyl crystals are shown in Figure 9. The chemical shift values of four types of hydrogen nuclei were 7.702, 7.675, 7.483, and 7.385 ppm. These resonance signal peaks in the spectra reflect the information of hydrogen atoms in the p-terphenyl molecule [35].
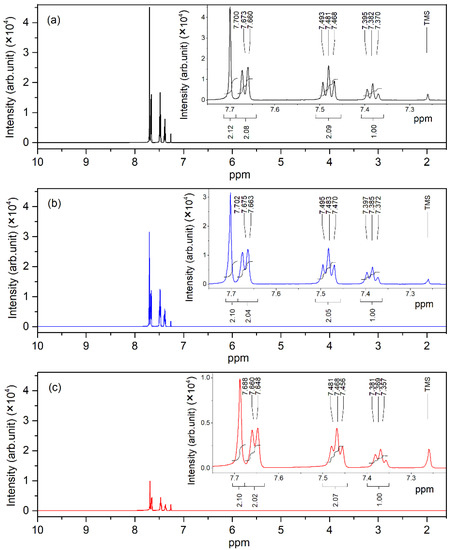
Figure 9.
1H NMR spectra of (a) pure, (b) 0.01 wt.%, and (c) 0.1 wt.% pentacene-doped p-terphenyl crystals.
Compared with the NMR spectra of p-terphenyl single crystals and the spectra of pentacene given by Nagano and Kupka, it can be concluded that there are no resonance signal peaks of the pentacene molecule (except p-terphenyl) in the spectra of the two doped samples. In addition, the chemical shifts of the signal peak did not change significantly compared with the NMR spectra of the p-terphenyl single crystal. Since the doping content of the two samples was too small under the action of the external magnetic field, there was no nuclear energy level transition, and the nuclear magnetic resonance signal could not be generated.
4. Conclusions
The crystal growth experiments of pentacene-doped p-terphenyl crystals were conducted by the Bridgman method. The sample properties of different doping concentrations and their relations with p-terphenyl single crystals were tested and analyzed. The effects of pentacene doping at different concentrations in p-terphenyl molecular crystals are discussed. As the dopant concentration increased, the crystals changed from colorless to light purple to purplish red. The XRD, FTIR, and NMR studies showed that no additional peaks (except for p-terphenyl) in the doped crystal spectra. The results indicate that guest molecules appear as defects in irregularly oriented molecules, which do not significantly change the crystal structure. As the doping concentration increased, the average crystallite size and crystallinity decreased. The fluorescence and UV–visible absorption spectra show that the p-terphenyl molecular transition decreased with added pentacene guest molecules, and characteristic peaks appeared due to the pentacene molecular transition. Moreover, with increased doping concentration, the host molecule’s characteristic peak intensities decreased continuously while the guest molecule’s increased correspondingly.
Author Contributions
Conceptualization, P.C.; methodology, Y.X.; investigation, Q.A.; resources, P.C.; data curation, Q.A.; writing—original draft preparation, Q.A.; writing—review and editing, Y.X.; visualization, Q.A.; supervision, Y.X.; project administration, Q.A.; funding acquisition, L.Z. and Q.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China under grant number 61871178 and the Scientific Research Project of the Education Department of Hubei Province under grant number B2019229.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to acknowledge the Analytical and Testing Center at Huazhong University of Science and Technology for XRD, FTIR, NMR, UV–Vis, and fluorescence spectra analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, Z.; Chen, J.; Li, D. Crystal alignment for high performance organic electronics devices. J. Vac. Sci. Technol. A 2019, 37, 040801. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Yang, F.; Zhang, X.; Hu, W. Cocrystal engineering: A collaborative strategy toward functional materials. Adv. Mater. 2019, 31, 1902328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, S.; Xu, S.; Li, A.; Li, B.; Ye, L.; Geng, Y.; Tian, Y.; Xu, W. Morphology-dependent luminescence and optical waveguide property in large-size organic charge transfer cocrystals with anisotropic spatial distribution of transition dipole moment. Adv. Opt. Mater. 2019, 8, 1901280. [Google Scholar] [CrossRef]
- Mercier, G.M.; Robeyns, K.; Tumanov, N.; Champagne, B.; Wouters, J.; Leyssens, T. New insights into photochromic properties of n-salicylideneaniline derivatives using a cocrystal engineering approach. Cryst. Growth Des. 2019, 19, 5544–5556. [Google Scholar] [CrossRef]
- Jalili, B.; Ghafoori, H.; Jalili, P. Investigation of carbon nano-tube (CNT) particles effect on the performance of a refrigeration cycle. Int. J. Mater. Sci. Innov. 2014, 2, 8–17. [Google Scholar]
- Fan, C.; Lin, W.; Chang, H.; Lin, Y.; Huang, B. Effects of the F4TCNQ-doped pentacene interlayers on performance improvement of top-contact pentacene-based organic thin-film transistors. Materials 2016, 9, 46. [Google Scholar] [CrossRef]
- Maeda, Y.; Ohmi, S. Steep subthreshold swing of pentacene-based organic field-effect transistor with nitrogen-doped LaB6 interfacial layer. Jpn. J. Appl. Phys. 2017, 56, 04CL06. [Google Scholar] [CrossRef]
- Lee, H.; Huang, H. Investigation performance and mechanisms of inverted polymer solar cells by pentacene doped P3HT: PCBM. Int. J. Photoenergy 2014, 2014, 812643. [Google Scholar] [CrossRef]
- Raghuwanshi, V.; Bharti, D.; Tiwari, S.P. Flexible organic field-effect transistors with TIPS-Pentacene crystals exhibiting high electrical stability upon bending. Org. Electron. 2016, 31, 177–182. [Google Scholar] [CrossRef]
- Gatti, T.; Brambilla, L.; Tommasini, M.; Villafiorita-Monteleone, F.; Botta, C.; Sarritzu, V.; Mura, A.; Bongiovanni, G.; Zoppo, M.D. Near IR to red up-conversion in tetracene/pentacene host/guest cocrystals enhanced byenergy transfer from host to guest. J. Phys. Chem. C 2015, 119, 17495–17501. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Jin, J.; Wang, W.; Liu, X.; Xu, H.; Zhang, J.; Huang, W. Pentacene derivative/DTTCNQ cocrystals: Alkyl-confined mixed heterojunctions with molecular alignment and transport property tuning. Chem. Sci. 2019, 10, 11125–11129. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yang, X.; Yan, D. Vapor-phase π-π molecular recognition: A fast and solvent-free strategy towards the formation of co-crystalline hollow microtube with 1D optical waveguide and up-conversion emission. J. Mater. Chem. C 2017, 5, 1632–1637. [Google Scholar] [CrossRef]
- Cui, S.; Liu, Y.; Li, G.; Han, Q.; Ge, C.; Zhang, L.; Guo, Q.; Ye, X.; Tao, X. Growth regulation of pentacene-doped p-terphenyl crystals on their physical properties for promising maser gain medium. Cryst. Growth Des. 2020, 20, 783–792. [Google Scholar] [CrossRef]
- Oxborrow, M.; Breeze, J.D.; Alford, N.M. Room-temperature solid-state maser. Nature 2012, 488, 353–356. [Google Scholar] [CrossRef]
- Breeze, J.; Tan, K.J.; Richards, B.; Sathian, J.; Oxborrow, M.; Alford, N.M. Enhanced magnetic Purcell effect in room-temperature masers. Nat. Commun. 2015, 6, 6215. [Google Scholar] [CrossRef]
- Bogatko, S.; Haynes, P.D.; Sathian, J.; Wade, J.; Kim, J.S.; Tan, K.J.; Breeze, J.; Salvadori, E.; Horsfield, A.; Oxborrow, M. Molecular design of a room-temperature maser. J. Phys. Chem. C 2016, 120, 8251–8260. [Google Scholar] [CrossRef]
- Salvadori, E.; Breeze, J.D.; Tan, K.J.; Sathian, J.; Richards, B.; Fung, M.W.; Wolfowicz, G.; Oxborrow, M.; Alford, N.M.; Kay, C.W.M. Nanosecond time-resolved characterization of a pentacene based room-temperature MASER. Sci. Rep. 2017, 7, 41836. [Google Scholar] [CrossRef]
- Ai, Q.; Chen, P.; Feng, Y.; Xu, Y. Growth of pentacene-doped p terphenyl crystals by vertical Bridgman technique and doping effect on their characterization. Cryst. Growth Des. 2017, 17, 2473–2477. [Google Scholar] [CrossRef]
- Ai, Q.; Chen, P.; Feng, Y.; Xu, Y. Effect of growth-vessel design on the properties of p-terphenyl single crystals grown by vertical Bridgman technique. In Proceedings of the 3rd International Conference on Chemical Materials and Process, Beijing, China, 25–27 May 2017. [Google Scholar]
- Ai, Q.; Chen, P.; Feng, Y.; Xu, Y. Enhanced crystalline perfection and fluorescence properties of p-terphenyl single crystals grown by the vertical Bridgman technique with a novel modified growth vessel. J. Appl. Crystallogr. 2017, 50, 278–282. [Google Scholar] [CrossRef]
- Kuwabara, T.; Nakajima, H.; Nanasawa, M.; Ueno, A. Color change indicators for molecules using methyl red-modified cyclodextrins. Anal. Chem. 1999, 71, 2844–2849. [Google Scholar] [CrossRef]
- Mitsui, M.; Kawano, Y. Electronic energy transfer in tetracene-doped p-terphenyl nanoparticles: Extraordinarily high fluorescence enhancement and quenching efficiency. Chem. Phys. 2013, 419, 30–36. [Google Scholar] [CrossRef]
- Rice, A.P.; Tham, F.S.; Chronister, E.L. A Temperature Dependent X-ray Study of the Order–Disorder Enantiotropic Phase Transition of p-Terphenyl. J. Chem. Crystallogr. 2013, 43, 14–25. [Google Scholar] [CrossRef]
- Siegrist, T.; Besnard, C.; Haas, S.; Schiltz, M.; Pattison, P.; Chernyshov, D.; Batlogg, B.; Kloc, C. A Polymorph Lost and Found: The High-Temperature Crystal Structure of Pentacene. Adv. Mater. 2007, 19, 2079–2082. [Google Scholar] [CrossRef]
- Costa, A.M.A.D.; Amado, A.M. Doping effects in p-terphenyl molecular crystals: A study by Raman spectroscopy. Solid State Ionics 1999, 125, 263–269. [Google Scholar] [CrossRef]
- Selvakumar, S.; Sivaji, K.; Arulchakkaravarthi, A.; Sankar, S. Enhanced fluorescence and time resolved fluorescence properties of p-terphenyl crystal grown by selective self seeded vertical Bridgman technique. Mater. Lett. 2007, 61, 4718–4721. [Google Scholar] [CrossRef]
- Antoine, R.; Dugourd, P. UV–Visible Activation of Biomolecular Ions. In Laser Photodissociation and Spectroscopy of Mass-Separated Biomolecular Ions; Polfer, N.C., Dugourd, P., Eds.; Springer International Publishing: Cham, Switzerland, 2013; pp. 93–116. [Google Scholar]
- Wilson, M.W.B.; Rao, A.; Ehrler, B.; Friend, R.H. Singlet exciton fission in polycrystalline pentacene: From photophysics toward devices. Acc. Chem. Res. 2013, 46, 1330–1338. [Google Scholar] [CrossRef]
- Murai, Y.; Misaki, M.; Ishida, K.; Ueda, Y. Pillarlike crystals of pentacene prepared from soluble precursor. Appl. Phys. Express 2011, 4, 121603. [Google Scholar] [CrossRef]
- Iwata, K.; Hamaguchi, H. Picosecond time-resolved Raman spectroscopy of S1 p-terphenyl and p-terphenyl-d14 in solution: Time-dependent changes of Raman band shapes. J. Raman Spectrosc. 1994, 25, 615–621. [Google Scholar] [CrossRef]
- Ross, D.; Aroca, R. Effective medium theories in surface enhanced infrared spectroscopy: The pentacene example. J. Chem. Phys. 2002, 117, 8095–8103. [Google Scholar] [CrossRef]
- Hosoi, Y.; Okamura, K.; Kimura, Y.; Ishii, H.; Niwano, M. Infrared spectroscopy of pentacene thin film on SiO2 surface. Appl. Surf. Sci. 2005, 244, 607–610. [Google Scholar] [CrossRef]
- Nagano, M.; Hasegawa, T.; Myoujin, N.; Yamaguchi, J.; Itaka, K.; Fukumoto, H.; Yamamoto, T.; Koinuma, H. The first observation of 1H-NMR spectrum of pentacene. Jpn. J. Appl. Phys. 2004, 43, L315–L316. [Google Scholar] [CrossRef]
- Kupka, T.; Stachow, M.; Nieradka, M.; Stobinski, L. DFT calculation of structures and NMR chemical shifts of simple models of small diameter zigzag single wall carbon nanotubes (SWCNTs). Magn. Reson. Chem. 2011, 49, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, S.; Sivaji, K.; Balamurugan, N.; Arulchakkaravarthi, A.; Sankar, S.; Venkateswaran, C.; Ramasamy, P. Growth and studies on SSVBT grown p-terphenyl single crystals. J. Cryst. Growth 2005, 275, e265–e271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).