Organic Nonlinear Optical Crystals for Highly Efficient Terahertz-Wave Generation
Abstract
1. Introduction
2. Discussion
2.1. Pyridinium-Based THz Crystals
| Crystal Name | Space Group | λmax(nm) in Methanol | βmax (Cation) | SHG Intensity | Ref. |
|---|---|---|---|---|---|
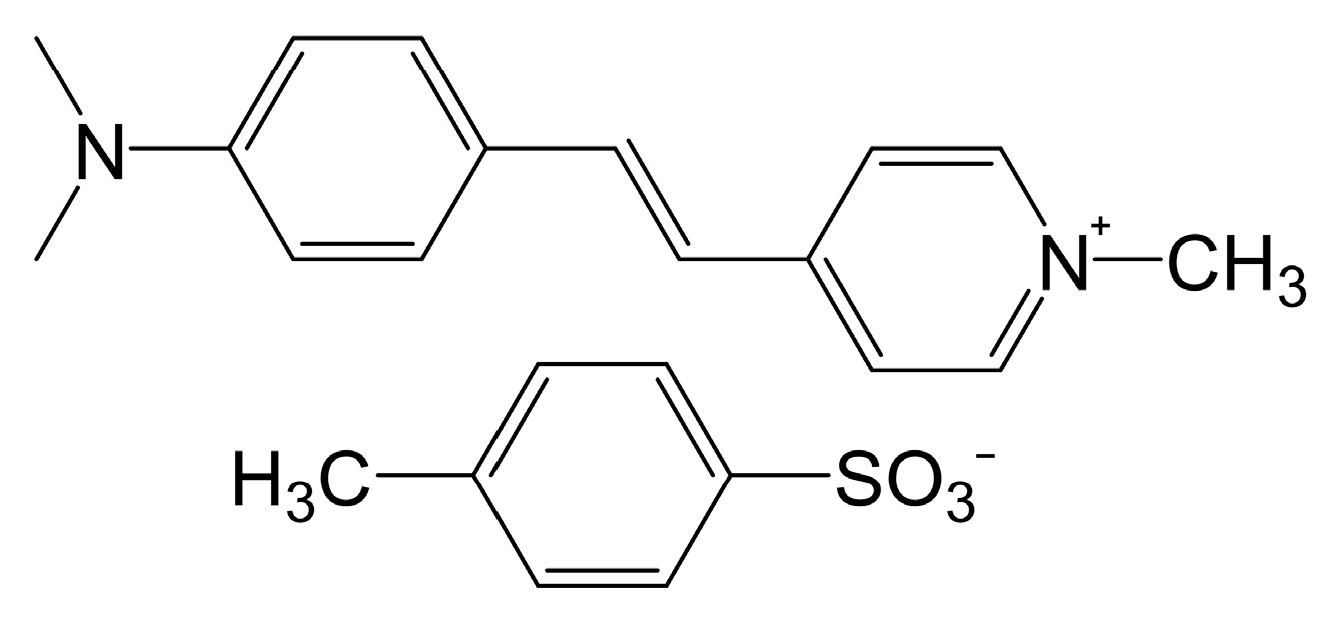
| DAST | Monoclinic Cc | 475 | 159 × 10−30 esu | d11 = 210 ± 55 pm/V at 1.9 µm | [20] |
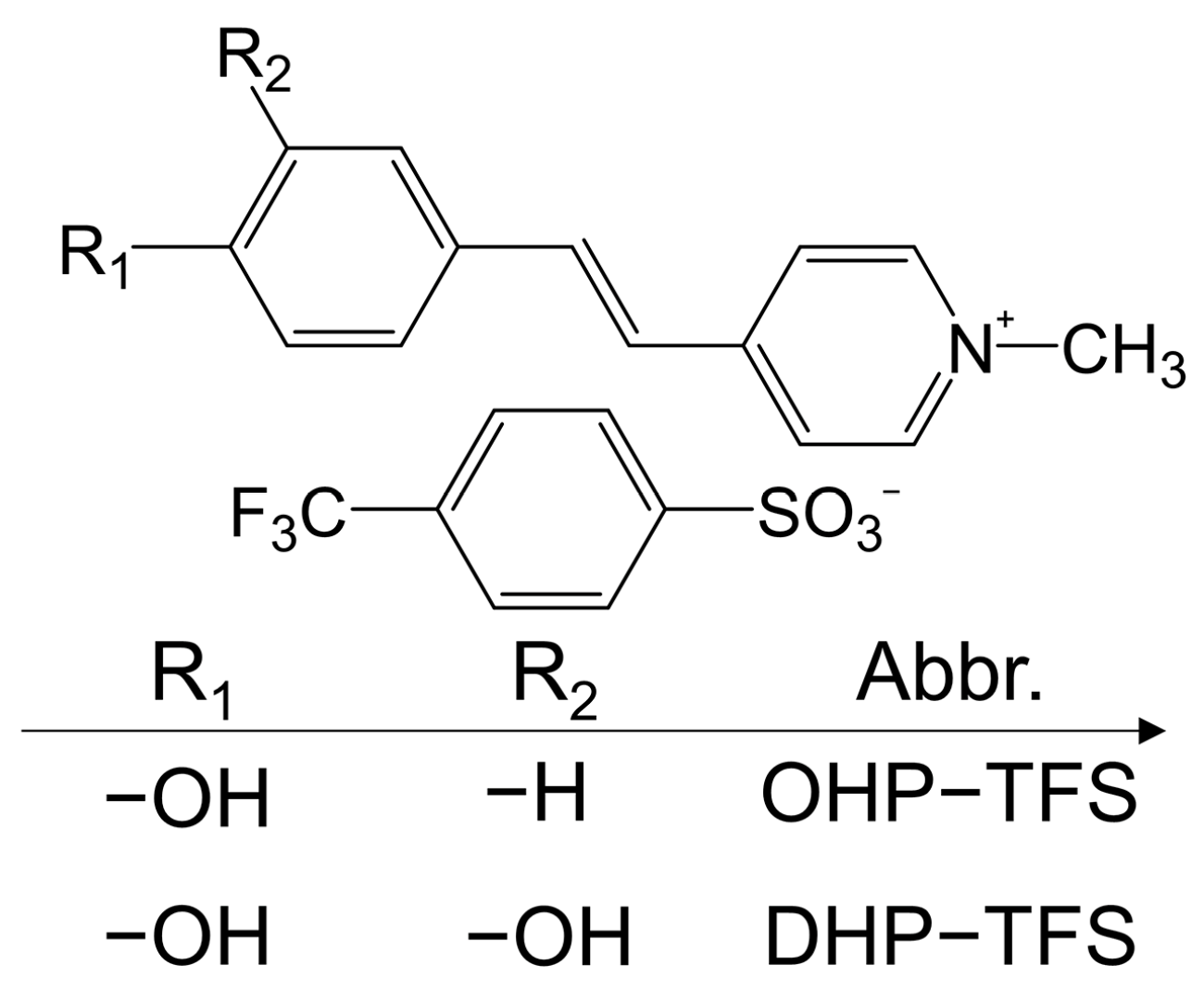
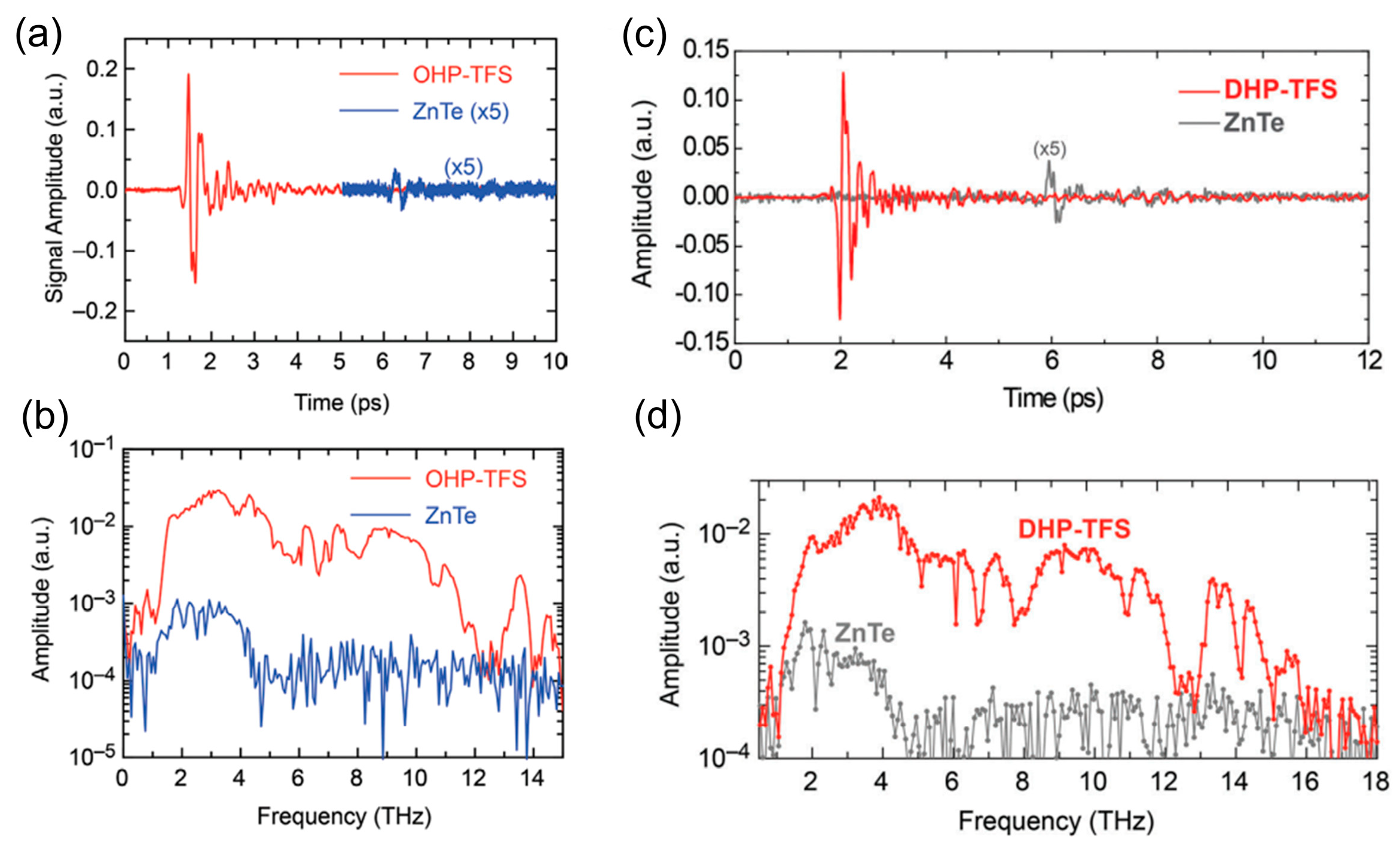
| OHP-TFS | Triclinic P1 | 392 | 125 × 10−30 esu | ~DAST | [28] |
| DHP-TFS | Triclinic P1 | 406 | 121 × 10−30 esu | ~0.49 × DAST, ≥1500 nm | [33] |
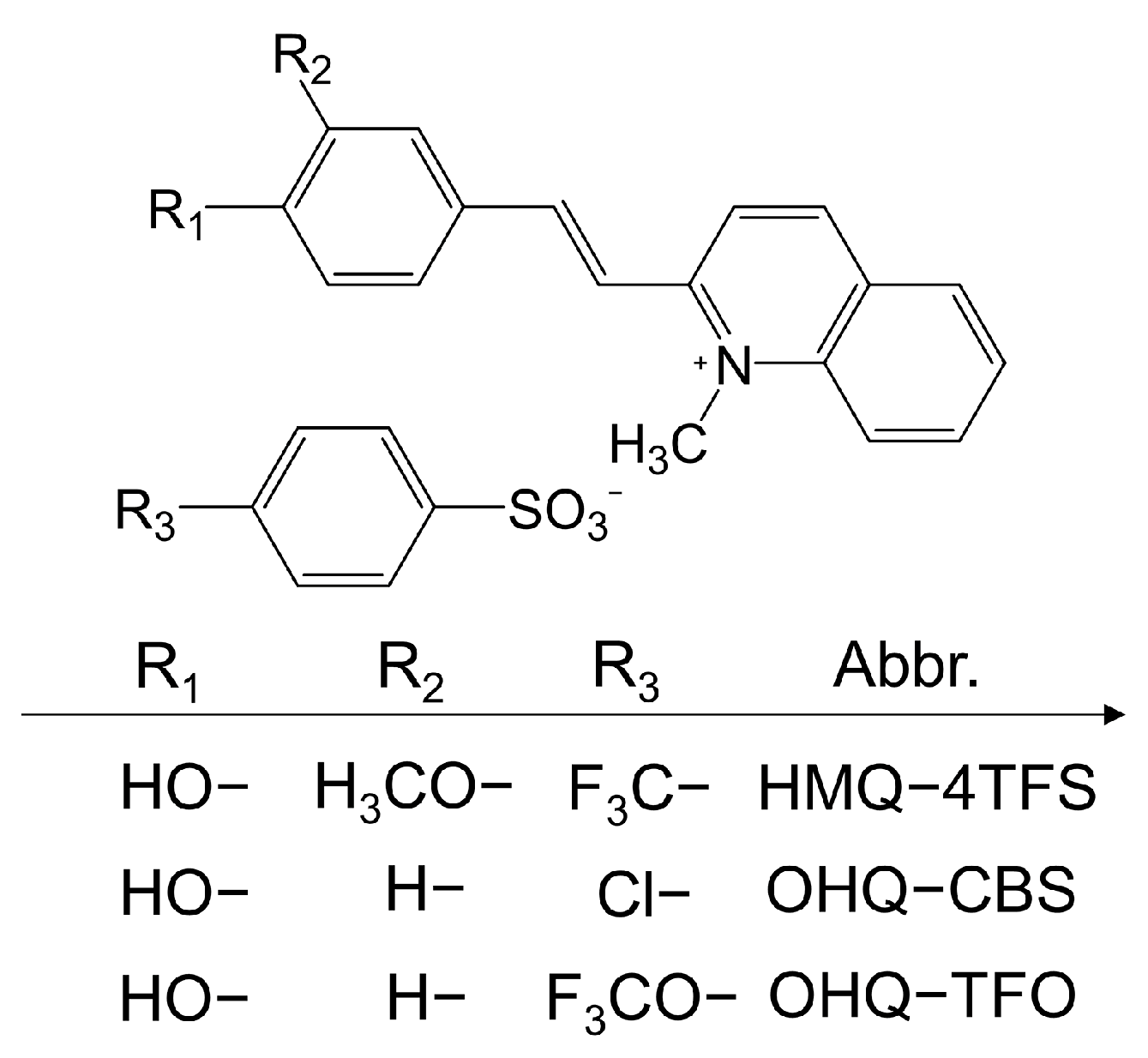
2.2. Quinolinium-Based THz Crystals
2.2.1. Halogen-Free Cations
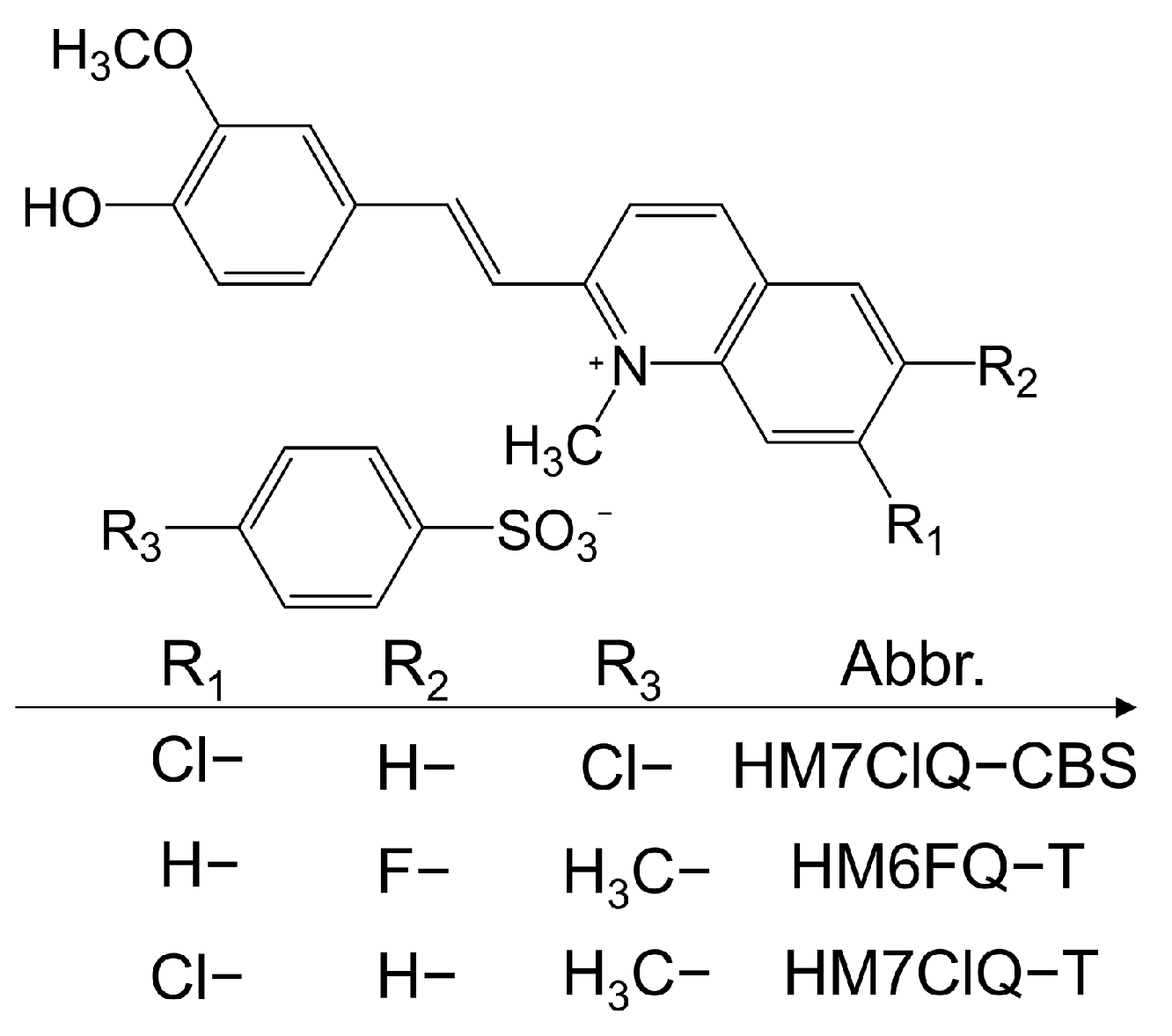
2.2.2. Halogen-Containing Cations
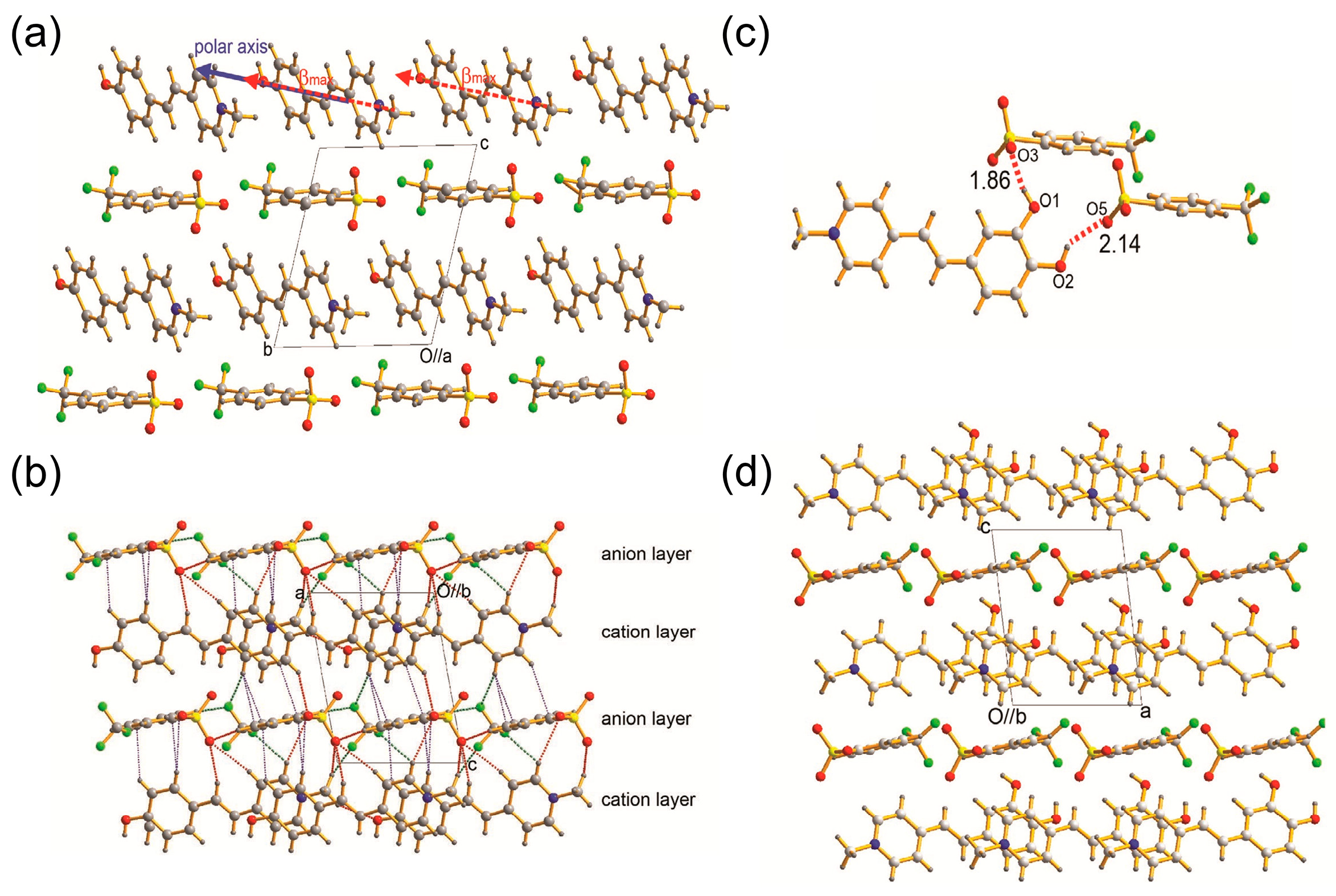
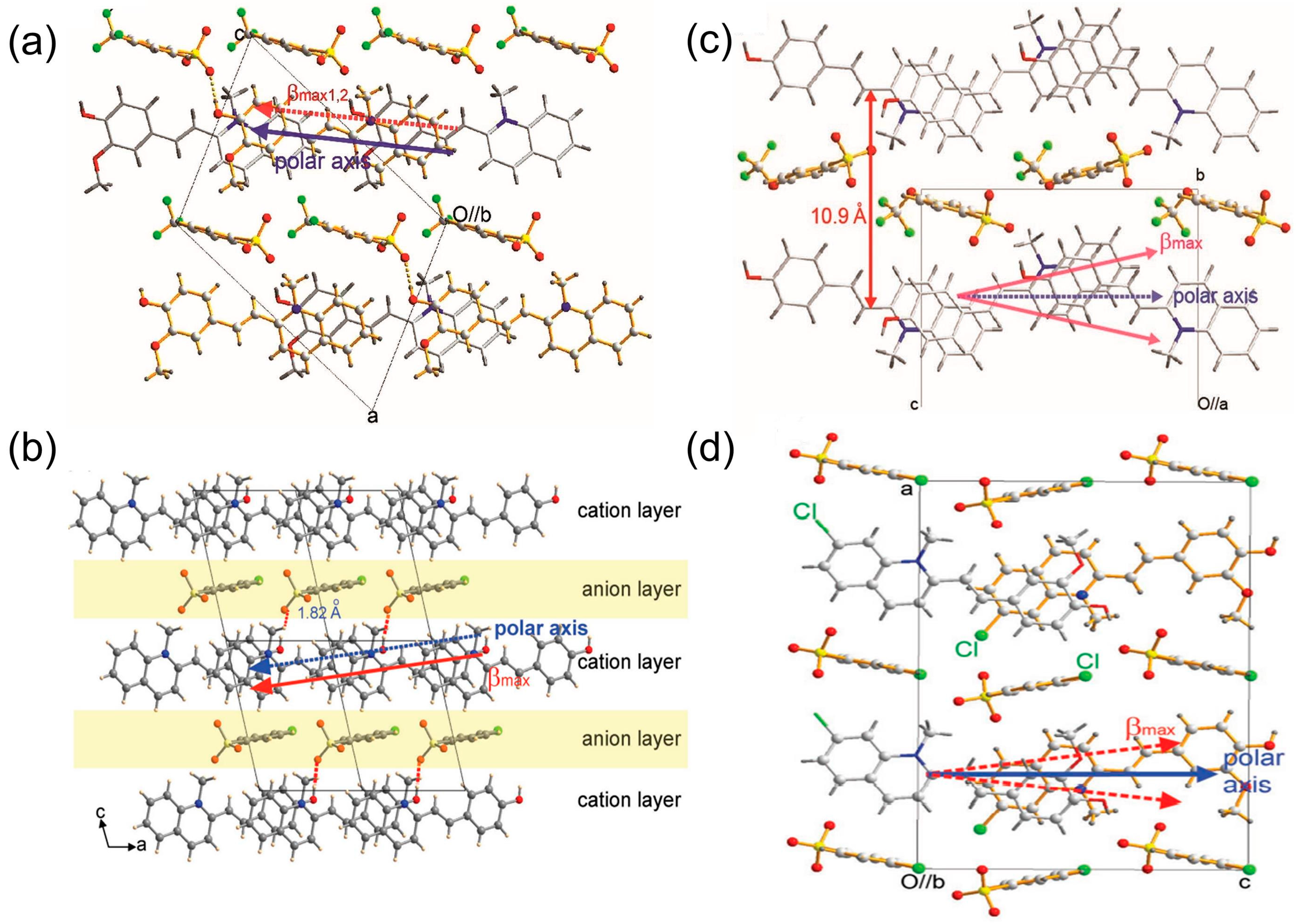
2.2.3. Effects of Halogen-Containing Anions on the Spatial Arrangement
2.2.4. Effects of Halogen-Free Anions on the Spatial Arrangement
| Crystal Name | Space Group | λmax(nm) in Methanol | βmax (Cation) | SHG Intensity | Ref. |
|---|---|---|---|---|---|
| HMQ-4TFS | Monoclinic Pn | 439 | 145 × 10−30 esu | ~DAST at 1250nm | [29] |
| OHQ-CBS | Triclinic P1 | 425 | ~118 × 10−30 esu | 1.2 × DAST at 1500 nm | [44] |
| OHQ-TFO | Monoclinic Pc | 425 | ~118 × 10−30 esu | ~2.3 × DAST at 1150 nm | [45] |
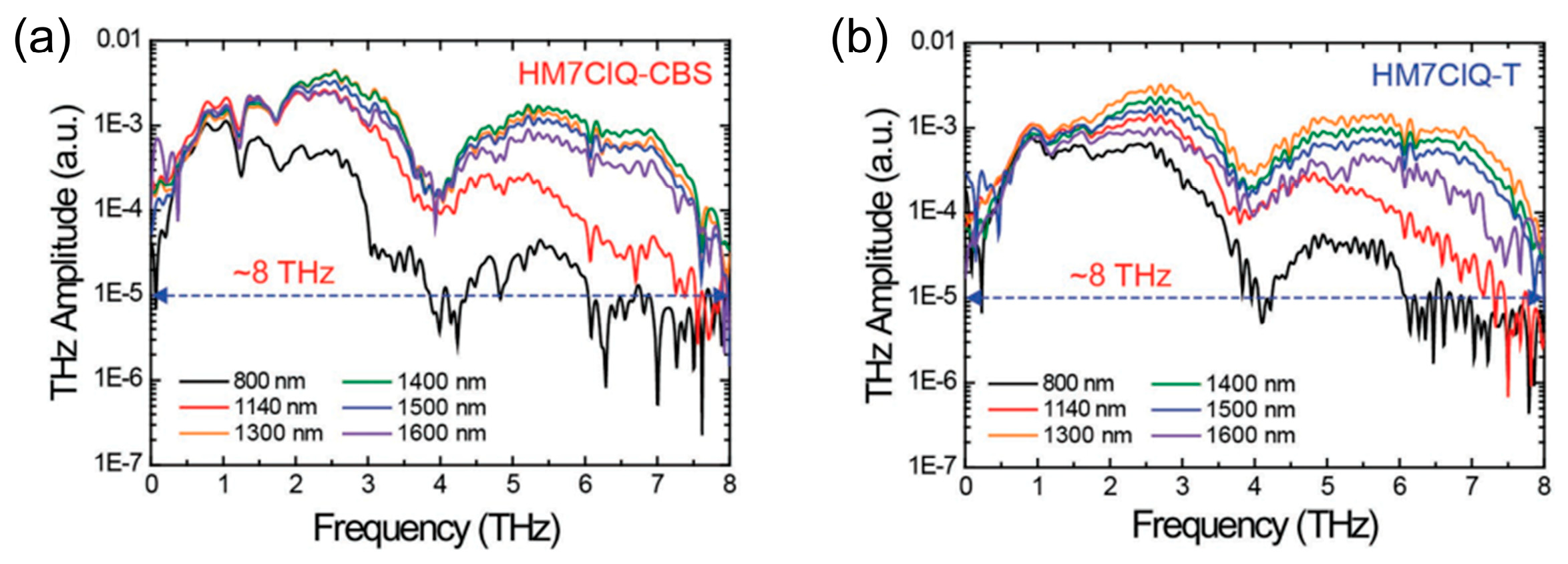
| HM7ClQ-CBS | Orthorhombic Pna21 | 450 | ~170 × 10−30 esu | ~1.2 × DAST at >1400 nm | [42] |
| HM6FQ-T | Monoclinic Pn | 444 | 140 × 10−30 esu | 0.63 × DAST at 1150–1900 nm | [43] |
| HM7ClQ-T | Orthorhombic Pna21 | 450 | ~170 × 10−30 esu | ~DAST at >1400 nm | [42] |
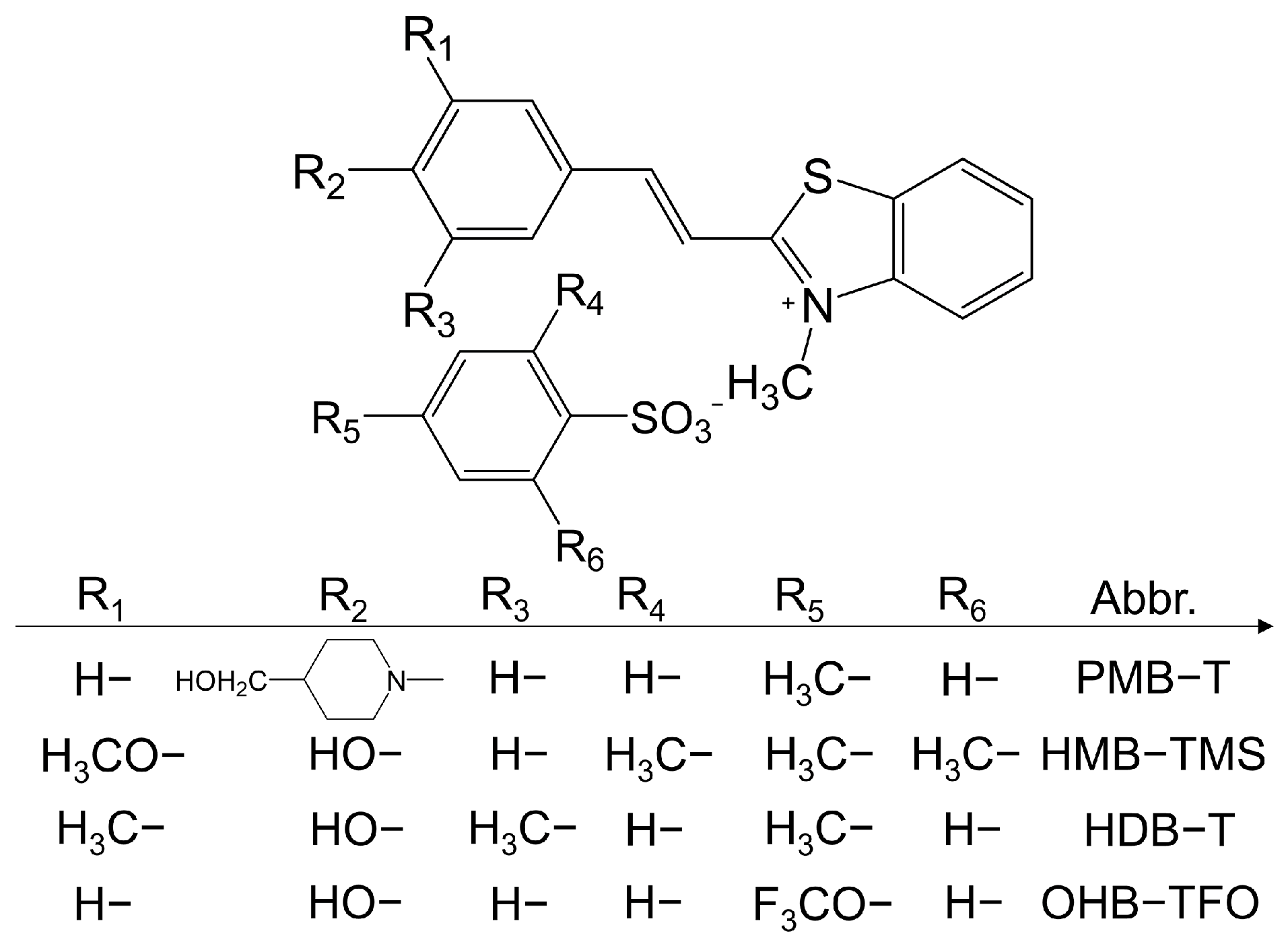
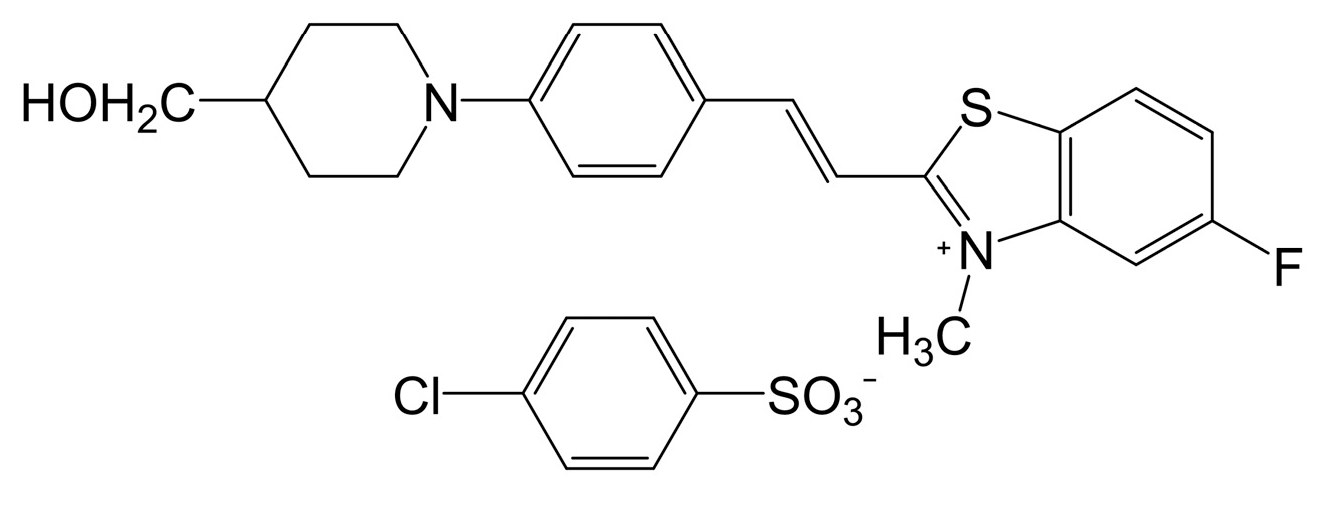
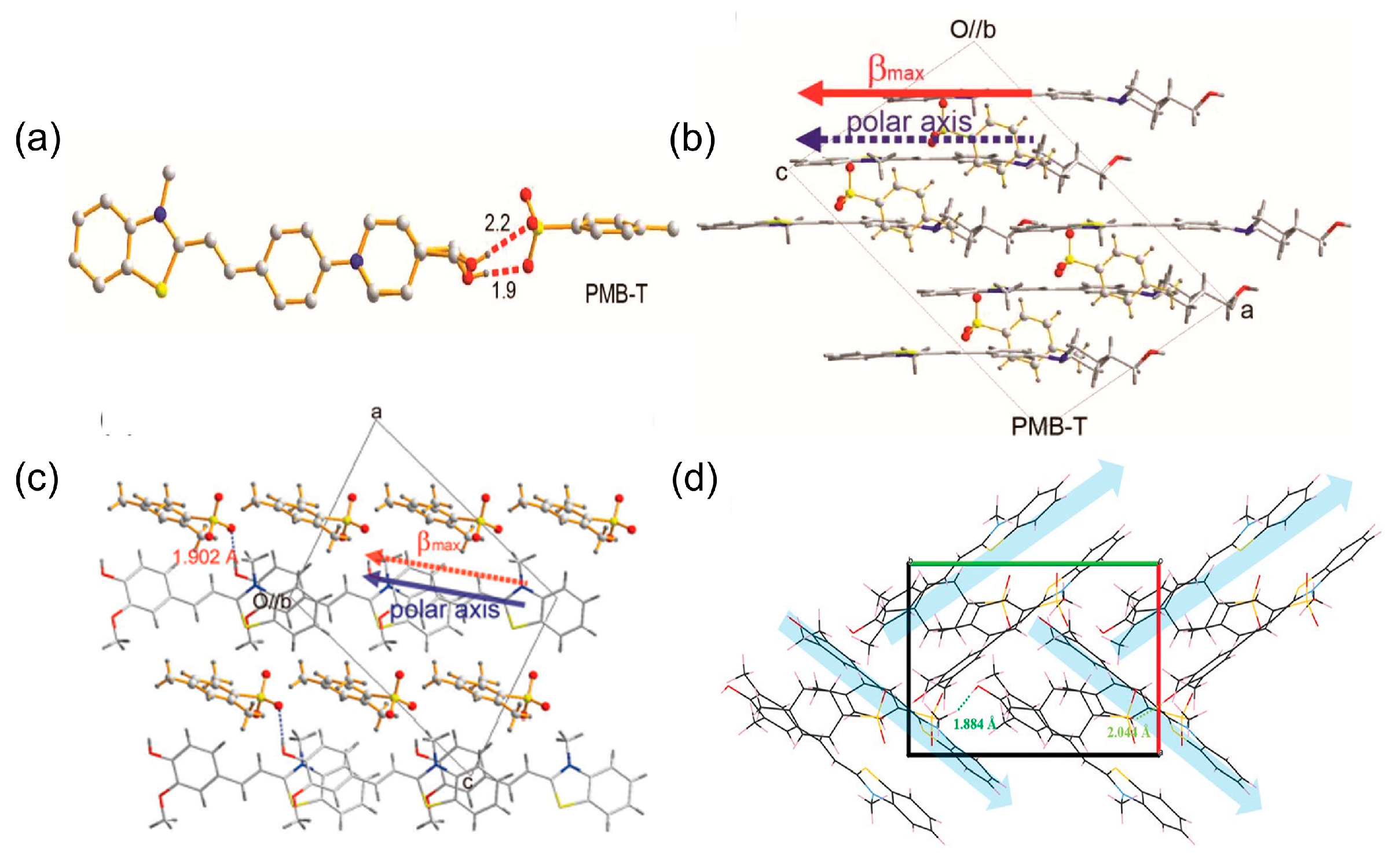
2.3. Benzothiazolium-Based THz Crystals
2.3.1. Halogen-Free Cations
2.3.2. Halogen-Containing Cations
2.3.3. Effects of Halogen-Free Anions on the Spatial Arrangement
2.3.4. Effects of Halogen-Containing Anions on the Spatial Arrangement
| Crystal Name | Space Group | λmax(nm) in Methanol | βmax (Cation) | SHG Intensity | Ref. |
|---|---|---|---|---|---|
| PMB-T | Monoclinic Cc | 523 | 274 × 10−30 esu | ~DAST at 1800 nm | [46] |
| HMB-TMS | Monoclinic Pn (m) | 438 | >162 × 10−30 esu | ~DAST at 1250 nm | [47] |
| HDB-T | Monoclinic P21 | 566 | - | 1.5 × OH1 at 2090 nm | [48] |
| OHB-TFO | Monoclinic Cc | 419 | 100 ± 7 × 10−30 esu | 0.3 × DAST at 1500 nm | [49] |
| PFB-CBS | Monoclinic Cc | 533 | ~274 × 10−30 esu | ~0.67 × DAST at 1800 nm | [50] |
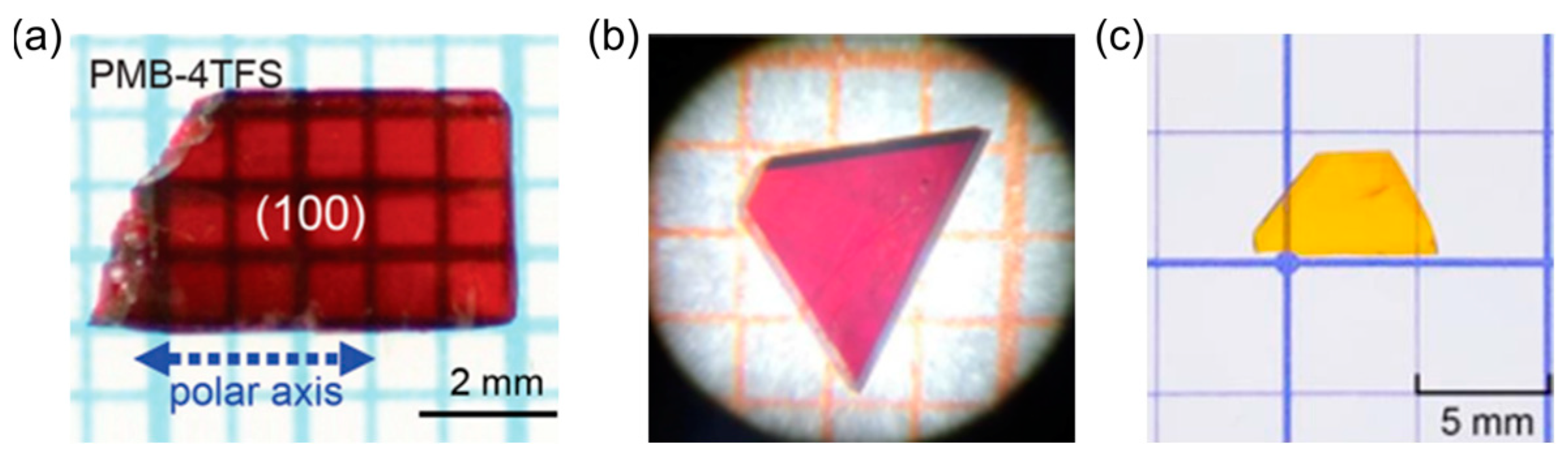
| PMB-4TFS | Monoclinic Cc | 523 | 274 × 10−30 esu | - | [27] |
| PMB-TFO | Monoclinic Cc | 523 | 274 × 10−30 esu | ~DAST at ≥1400 nm | [51] |
2.4. Indolium-Based THz Crystals
2.4.1. Classical Cations
2.4.2. Effect of Anions on the Spatial Arrangement
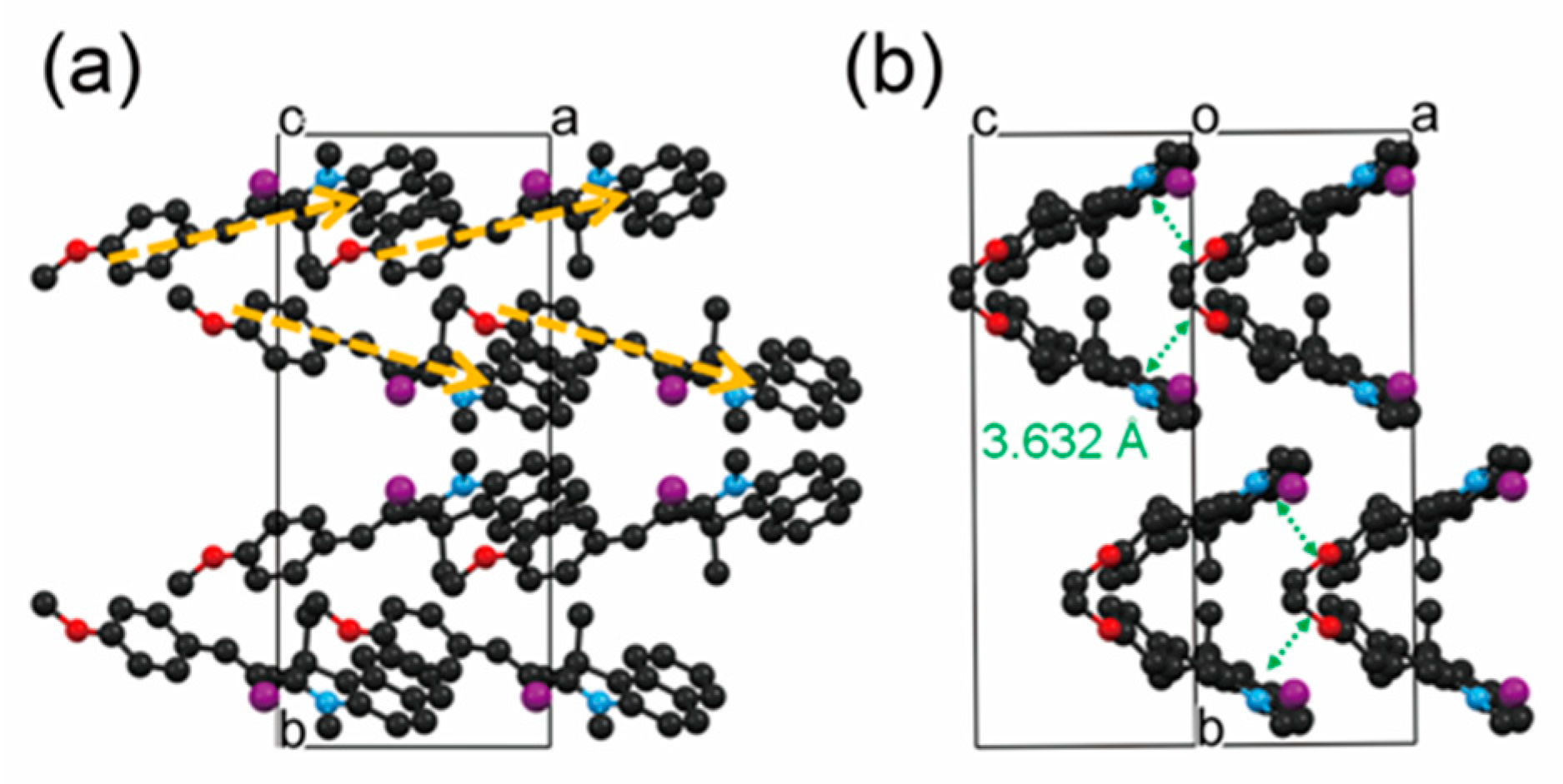
2.5. Benzoindolium-Based THz Crystals
2.5.1. Cations with Classical Electron Donor
| Crystal Name | Space Group | λmax(nm) in Methanol | βmax (Cation) | SHG Intensity | Ref. |
|---|---|---|---|---|---|
| EHPSI-4NBS | Monoclinic Pn | 549 | 123 × 10−30 esu | - | [52] |
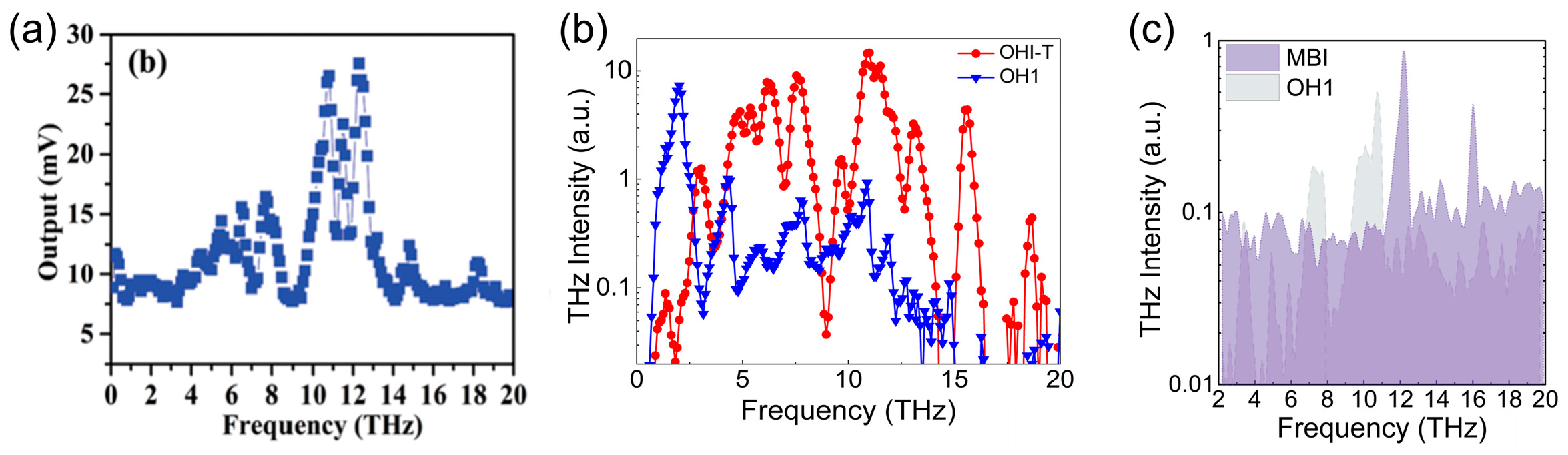
| OHI-T | Monoclinic Pn (m) | 437 | - | ~0.7 × OH1 at 2090 nm | [53] |
| MBI | Monoclinic Cc (m) | 443 | - | ~0.8 × OH1 at 2090 nm | [55] |
| HMI-TMS | Monoclinic Pn | 364 | ~76 × 10−30 esu | 0.06–0.12 × DAST at 1300–1800 nm | [56] |
2.5.2. Effect of the Iodide Anion on the Spatial Arrangement
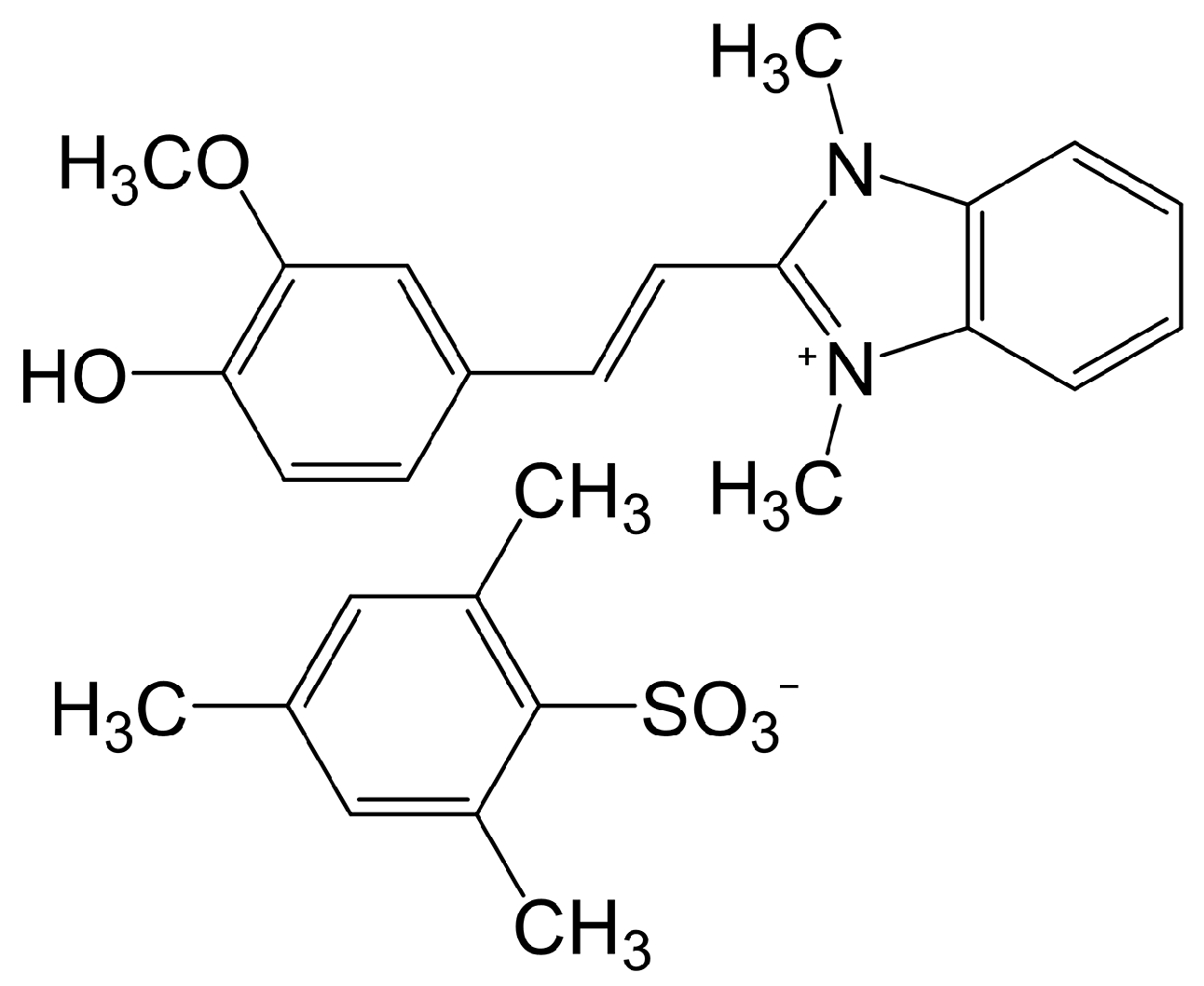
2.6. Benzimidazolium-Based THz Crystals
2.6.1. Benzimidazolium-Based Cation
2.6.2. Anion of HMI-TMS
2.7. Crystal Growth
2.7.1. Spontaneous Nucleation Method
Cooling Method
Slow Evaporation Method
2.7.2. Seed Method
2.8. THz Wave Generation
2.8.1. Optical Rectification (OR)
2.8.2. Difference Frequency Generation (DFG)
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, M.; Zhang, J.; Zhang, X.; Xu, D.; Hu, Z.; Yao, J.; Wu, Y. Steric Group Design for Enhancement of Optical Nonlinearity in Isoxazolone-Based Crystals and Terahertz-Wave Generation. Cryst. Growth Des. 2021, 21, 3153–3157. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, S.-I.; Jazbinsek, M.; Yoon, W.; Yun, H.; Kim, D.; Yu, I.C.; Rotermund, F.; Kwon, O.P. Design Strategy of Highly Efficient Nonlinear Optical Orange-Colored Crystals with Two Electron-Withdrawing Groups. Adv. Photonics Res. 2022, 3, 2100350. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, T.; Wang, G.; Xu, K.; Cui, Q.; Cao, L.; Teng, B. Synthesis, growth, structure analysis and performance characterization of DOST crystal: A nonlinear functional material with a wide range of applications. CrystEngComm 2020, 22, 8362–8373. [Google Scholar] [CrossRef]
- Jazbinsek, M.; Puc, U.; Abina, A.; Zidansek, A. Organic Crystals for THz Photonics. Appl. Sci. 2019, 9, 882. [Google Scholar] [CrossRef]
- Sinko, A.; Ozheredov, I.; Rudneva, E.; Manomenova, V.; Kozlova, N.; Lobova, N.; Voloshin, A.; Coutaz, J.-L.; Shkurinov, A. Perspective on Terahertz Applications of Molecular Crystals. Electronics 2022, 11, 2731. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Xu, F.; Cao, L.; Jiang, X.; Yang, S.; Sun, Y.; Zhao, X.; Lin, C.; Ye, N. Mg2In3Si2P7: A Quaternary Diamond-like Phosphide Infrared Nonlinear Optical Material Derived from ZnGeP2. J. Am. Chem. Soc. 2021, 143, 10309–10316. [Google Scholar] [CrossRef]
- Lu, W.; Gao, Z.; Liu, X.; Tian, X.; Wu, Q.; Li, C.; Sun, Y.; Liu, Y.; Tao, X. Rational Design of a LiNbO3-like Nonlinear Optical Crystal, Li2ZrTeO6, with High Laser-Damage Threshold and Wide Mid-IR Transparency Window. J. Am. Chem. Soc. 2018, 140, 13089–13096. [Google Scholar] [CrossRef]
- Liao, F.; Wang, Y.; Peng, T.; Peng, J.; Gu, Z.; Yu, H.; Chen, T.; Yu, J.; Gu, F. Highly Efficient Nonlinear Optical Conversion in Waveguiding GaSe Nanoribbons with Pump Pulses Down to a Femto-Joule Level. Adv. Opt. Mater. 2018, 6, 1701012. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Zhou, C.; Yang, W.; Liu, H.; Zhang, X.; Li, Z.; Zhang, B.; Jie, W.; Xu, Y. Enhanced Transmission from Visible to Terahertz in ZnTe Crystals with Scalable Subwavelength Structures. ACS Appl. Mater. Interfaces 2021, 13, 16997–17005. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.L.; Fang, Z.; Mao, J.G. K2Pb(H2C3N3O3)(4)(H2O)(4): A potential UV nonlinear optical material with large birefringence. Inorg. Chem. Front. 2021, 8, 3547–3555. [Google Scholar] [CrossRef]
- Guo, J.; Tudi, A.; Han, S.; Yang, Z.; Pan, S. α-SnF2: A UV Birefringent Material with Large Birefringence and Easy Crystal Growth. Angew. Chem. Int. Ed. Engl. 2021, 60, 3540–3544. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Mei, D.J.; Liang, F.; Zhao, J.; Wu, Y.D.; Lin, Z.S. Inherent laws between tetrahedral arrangement pattern and optical performance in tetrahedron-based mid-infrared nonlinear optical materials. Coord. Chem. Rev. 2020, 421, 213444. [Google Scholar] [CrossRef]
- Li, Y.; Yin, C.; Yang, X.; Kuang, X.; Chen, J.; He, L.; Ding, Q.; Zhao, S.; Hong, M.; Luo, J. A Nonlinear Optical Switchable Sulfate of Ultrawide Bandgap. CCS Chem. 2021, 3, 2298–2306. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Lin, Z.; Li, Y.; Ding, Q.; Chen, X.; Li, L.; Zhao, S.; Hong, M.; Luo, J. Nonpolar Na10Cd(NO3)4(SO3S)4 Exhibits a Large Second-Harmonic Generation. CCS Chem. 2022, 4, 526–531. [Google Scholar] [CrossRef]
- Ma, X.F.; Wang, C.; Zhang, J.; Wang, T.; Wang, A.Y.; Wang, S.Y.; Jia, Z.T.; Zhang, B.T.; He, J.L.; van Smaalen, S.; et al. Broadband BiOCl Nonlinear Saturable Absorber for Watt-Level Passively Q-Switched Yb:LuAG Single Crystal Fiber Laser. Adv. Opt. Mater. 2022, 10, 2201087. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Wang, D.; Cao, H. Molecular Structures and Second-Order Nonlinear Optical Properties of Ionic Organic Crystal Materials. Crystals 2016, 6, 158. [Google Scholar] [CrossRef]
- Li, B.; He, W.; Wang, L.; Xiao, X.; Yang, H. Effect of lateral fluoro substituents of rodlike tolanecyano mesogens on blue phase temperature ranges. Soft Matter 2013, 9, 1172–1177. [Google Scholar] [CrossRef]
- Rezzonico, D.; Kwon, S.J.; Figi, H.; Kwon, O.P.; Jazbinsek, M.; Gunter, P. Photochemical stability of nonlinear optical chromophores in polymeric and crystalline materials. J. Chem. Phys. 2008, 128, 124713. [Google Scholar] [CrossRef]
- Liu, J.; You, F.; Hu, C.; Ma, Y.; Teng, F.; Wang, T.; Tang, J.; Cao, L.; Teng, B. Growth and characterization of organic 4-chlorobenzaldehyde-N- methyl 4-stilbazolium tosylate crystal: A promising material for nonlinear optical device applications. Optik 2019, 178, 999–1009. [Google Scholar] [CrossRef]
- Pan, F.; Knöpfle, G.; Bosshard, C.; Follonier, S.; Spreiter, R.; Wong, M.S.; Günter, P. Electro-optic properties of the organic salt 4-N,N-dimethylamino-4′-N′-methyl-stilbazolium tosylate. Appl. Phys. Lett. 1996, 69, 13–15. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, X.; Wang, X.; Li, L.; Shi, X.; Li, S.; Ji, C.; Luo, J.; Hong, M. Engineering of Acentric Stilbazolium Salts with Large Second-Order Optical Nonlinearity and Enhanced Environmental Stability. Cryst. Growth Des. 2012, 12, 6181–6187. [Google Scholar] [CrossRef]
- Taniuchi, T.; Ikeda, S.; Mineno, Y.; Okada, S.; Nakanishi, H. Terahertz Properties of a New Organic Crystal, 4′-Dimethylamino-N-methyl-4-stilbazoliump-Chlorobenzenesulfonate. Jpn. J. Appl. Phys. 2005, 44, L932–L934. [Google Scholar] [CrossRef]
- Matsukawa, T.; Notake, T.; Nawata, K.; Inada, S.; Okada, S.; Minamide, H. Terahertz-wave generation from 4-dimethylamino-N′-methyl-4′-stilbazolium p-bromobenzenesulfonate crystal: Effect of halogen substitution in a counter benzenesulfonate of stilbazolium derivatives. Opt. Mater. 2014, 36, 1995–1999. [Google Scholar] [CrossRef]
- Matsukawa, T.; Yoshimura, M.; Takahashi, Y.; Takemoto, Y.; Takeya, K.; Kawayama, I.; Okada, S.; Tonouchi, M.; Kitaoka, Y.; Mori, Y.; et al. Bulk Crystal Growth of Stilbazolium Derivatives for Terahertz Waves Generation. Jpn. J. Appl. Phys. 2010, 49, 075502. [Google Scholar] [CrossRef]
- Wang, T.; Xu, K.; Wang, G.; Wang, Q.; Chen, R.; Ma, J.; Cao, L.; Teng, B. Growth, THz properties, and Hirshfeld surface studies of 4-N,N-dimethylamino-4-N-methyl-stilbazolium 2,4,6-trimethylbenzenesulfonate single crystal. J. Mater. Sci. Mater. El. 2022, 33, 4704–4711. [Google Scholar] [CrossRef]
- Matsukawa, T.; Mineno, Y.; Odani, T.; Okada, S.; Taniuchi, T.; Nakanishi, H. Synthesis and terahertz-wave generation of mixed crystals composed of 1-methyl-4-{2-[4-(dimethylamino)phenyl]ethenyl}pyridinium p-toluenesulfonate and p-chlorobenzenesulfonate. J. Cryst. Growth 2007, 299, 344–348. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.T.; Seok, J.-H.; Yu, I.C.; Jazbinsek, M.; Yoon, W.; Yun, H.; Kim, D.; Rotermund, F.; Kwon, O.P. Molecular salt crystals with bis(head-to-tail) interionic complementary assembly for efficient organic THz generators. J. Mater. Chem. C 2020, 8, 10078–10085. [Google Scholar] [CrossRef]
- Seok, J.H.; Puc, U.; Kim, S.J.; Yoon, W.; Yun, H.; Yu, I.C.; Rotermund, F.; Kim, D.; Jazbinsek, M.; Kwon, O.P. High-Density Organic Electro-Optic Crystals for Ultra-Broadband THz Spectroscopy. Adv. Opt. Mater. 2021, 9, 2100618. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, B.J.; Yoo, B.-W.; Lee, S.-C.; Lee, S.-J.; Jazbinsek, M.; Yun, H.; Rotermund, F.; Kwon, O.P. Terahertz Phonon Mode Engineering of Highly Efficient Organic Terahertz Generators. Adv. Funct. Mater. 2017, 27, 1605583. [Google Scholar] [CrossRef]
- Marder, S.R.; Perry, J.W.; Schaefer, W.P. Synthesis of organic salts with large second-order optical nonlinearities. Science 1989, 245, 626–628. [Google Scholar] [CrossRef]
- Walther, M.; Jensby, K.; Keiding, S.R. Far-infrared properties of DAST. Opt. Lett. 2000, 25, 911–913. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, C.; Xu, D.; Li, Y.; Shi, W.; Zhang, G.; Zhang, X.; Yao, J.; Wu, Y. Widely tunable and monochromatic terahertz difference frequency generation with organic crystal 2-(3-(4-hydroxystyryl)-5,5-dime-thylcyclohex-2-enylidene) malononitrile. Appl. Phys. Lett. 2016, 108, 011104. [Google Scholar] [CrossRef]
- Yoon, G.E.; Seok, J.H.; Puc, U.; Shin, B.R.; Yoon, W.; Yun, H.; Kim, D.; Yu, I.C.; Rotermund, F.; Jazbinsek, M.; et al. Phonon-Suppressing Intermolecular Adhesives: Catechol-Based Broadband Organic THz Generators. Adv. Sci. 2022, 9, e2201391. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.J.; Jeong, J.H.; Jazbinsek, M.; Kwon, S.J.; Yun, H.; Kim, J.T.; Lee, Y.S.; Baek, I.H.; Rotermund, F.; Günter, P.; et al. Acentric nonlinear optical N-benzyl stilbazolium crystals with high environmental stability and enhanced molecular nonlinearity in solid state. CrystEngComm 2011, 13, 444–451. [Google Scholar] [CrossRef]
- Lee, S.C.; Kang, B.J.; Koo, M.J.; Lee, S.H.; Han, J.H.; Choi, J.Y.; Kim, W.T.; Jazbinsek, M.; Yun, H.; Kim, D.; et al. New Electro-Optic Salt Crystals for Efficient Terahertz Wave Generation by Direct Pumping at Ti:Sapphire Wavelength. Adv. Opt. Mater. 2017, 5, 1600758. [Google Scholar] [CrossRef]
- Kang, B.J.; Baek, I.H.; Jeong, J.-H.; Kim, J.-S.; Lee, S.-H.; Kwon, O.P.; Rotermund, F. Characteristics of efficient few-cycle terahertz radiation generated in as-grown nonlinear organic single crystals. Curr. Appl. Phys. 2014, 14, 403–406. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kang, B.J.; Kim, J.S.; Jazbinsek, M.; Lee, S.H.; Lee, S.C.; Baek, I.H.; Yun, H.; Kim, J.; Lee, Y.S.; et al. High-power broadband organic THz generator. Sci. Rep. 2013, 3, 3200. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.J.; Jeong, J.H.; Jazbinsek, M.; Choi, S.B.; Baek, I.H.; Kim, J.T.; Rotermund, F.; Yun, H.; Lee, Y.S.; Günter, P.; et al. Highly Efficient Organic THz Generator Pumped at Near-Infrared: Quinolinium Single Crystals. Adv. Funct. Mater. 2012, 22, 200–209. [Google Scholar] [CrossRef]
- Kwon, O.P.; Kwon, S.J.; Jazbinsek, M.; Brunner, F.D.J.; Seo, J.I.; Hunziker, C.; Schneider, A.; Yun, H.; Lee, Y.S.; Günter, P. Organic Phenolic Configurationally Locked Polyene Single Crystals for Electro-optic and Terahertz Wave Applications. Adv. Funct. Mater. 2008, 18, 3242–3250. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, B.J.; Kim, J.S.; Yoo, B.W.; Jeong, J.H.; Lee, K.H.; Jazbinsek, M.; Kim, J.W.; Yun, H.; Kim, J.; et al. New Acentric Core Structure for Organic Electrooptic Crystals Optimal for Efficient Optical-to-THz Conversion. Adv. Opt. Mater. 2015, 3, 756–762. [Google Scholar] [CrossRef]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Kim, W.T.; Seok, J.H.; Jazbinsek, M.; Yoon, W.; Yu, I.C.; Yun, H.; Kim, D.; Rotermund, F.; Kwon, O.P. Organic σ-Hole Containing Crystals with Enhanced Nonlinear Optical Response and Efficient Optical-to-THz Frequency Conversion. Adv. Opt. Mater. 2020, 8, 1901840. [Google Scholar] [CrossRef]
- Kim, S.-I.; Kang, B.J.; Jeong, C.-U.; Shin, M.-H.; Kim, W.T.; Jazbinsek, M.; Yoon, W.; Yun, H.; Kim, D.; Rotermund, F.; et al. Fluorinated Organic Electro-Optic Quinolinium Crystals for THz Wave Generation. Adv. Opt. Mater. 2019, 7, 1801495. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kang, B.J.; Shin, M.-H.; Lee, S.-C.; Lee, S.-H.; Jazbinsek, M.; Yun, H.; Kim, D.; Rotermund, F.; Kwon, O.P. Efficient Optical-to-THz Conversion Organic Crystals with Simultaneous Electron Withdrawing and Donating Halogen Substituents. Adv. Opt. Mater. 2018, 6, 1700930. [Google Scholar] [CrossRef]
- Shin, M.H.; Kim, W.T.; Kim, S.I.; Lee, S.H.; Yu, I.C.; Jazbinsek, M.; Yoon, W.; Yun, H.; Kim, D.; Rotermund, F.; et al. Efficient Gap-Free Broadband Terahertz Generators Based on New Organic Quinolinium Single Crystals. Adv. Opt. Mater. 2019, 7, 1900953. [Google Scholar] [CrossRef]
- Lee, S.C.; Kang, B.J.; Lee, J.A.; Lee, S.H.; Jazbinsek, M.; Yoon, W.; Yun, H.; Rotermund, F.; Kwon, O.P. Single Crystals Based on Hydrogen-Bonding Mediated Cation-Anion Assembly with Extremely Large Optical Nonlinearity and Their Application for Intense THz Wave Generation. Adv. Opt. Mater. 2018, 6, 1701258. [Google Scholar] [CrossRef]
- Lee, S.H.; Lu, J.; Lee, S.J.; Han, J.H.; Jeong, C.U.; Lee, S.C.; Li, X.; Jazbinsek, M.; Yoon, W.; Yun, H.; et al. Benzothiazolium Single Crystals: A New Class of Nonlinear Optical Crystals with Efficient THz Wave Generation. Adv. Mater. 2017, 29, 1701748. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liang, F.; He, Y.; Zhang, X.; Lin, Z.; Xu, D.; Hu, Z.; Yao, J.; Wu, Y. Rational structural design of benzothiazolium-based crystal HDB-T with high nonlinearity and efficient terahertz-wave generation. Chem. Commun. 2019, 55, 7950–7953. [Google Scholar] [CrossRef]
- Shin, M.H.; Kim, W.T.; Kim, S.I.; Kim, S.J.; Yu, I.C.; Kim, S.W.; Jazbinsek, M.; Yoon, W.; Yun, H.; Rotermund, F.; et al. Organic Broadband THz Generators Optimized for Efficient Near-Infrared Optical Pumping. Adv. Sci. 2020, 7, 2001738. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, W.T.; Jazbinsek, M.; Kim, D.; Lee, S.H.; Yu, I.C.; Yoon, W.; Yun, H.; Rotermund, F.; Kwon, O.P. X-Shaped Alignment of Chromophores: Potential Alternative for Efficient Organic Terahertz Generators. Adv. Opt. Mater. 2020, 8, 1901921. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yu, I.C.; Lee, J.-A.; Kim, W.T.; Jazbinsek, M.; Yoon, W.; Yun, H.; Rotermund, F.; Kwon, O.P. New benzothiazolium crystals with very large off-diagonal optical nonlinearity. Dye. Pigment. 2021, 192, 109433. [Google Scholar] [CrossRef]
- Valdivia-Berroeta, G.A.; Jackson, E.W.; Kenney, K.C.; Wayment, A.X.; Tangen, I.C.; Bahr, C.B.; Smith, S.J.; Michaelis, D.J.; Johnson, J.A. Designing Non-Centrosymmetric Molecular Crystals: Optimal Packing May Be Just One Carbon Away. Adv. Funct. Mater. 2019, 30, 1904786. [Google Scholar] [CrossRef]
- Shi, J.; He, Y.; Liang, F.; Zhang, X.; Xu, D.; Yao, J.; Zhang, G.; Hu, Z.; Yao, J.; Wu, Y. Methyl substitution for noncentrosymmetric stacking: A promising organic single crystal for highly efficient terahertz-wave generation. J. Mater. Chem. C 2020, 8, 4226–4233. [Google Scholar] [CrossRef]
- Valdivia-Berroeta, G.A.; Heki, L.K.; Jackson, E.W.; Tangen, I.C.; Bahr, C.B.; Smith, S.J.; Michaelis, D.J.; Johnson, J.A. Terahertz generation and optical characteristics of P-BI. Opt. Lett. 2019, 44, 4279–4282. [Google Scholar] [CrossRef]
- Zhang, W.F.; Wang, Z.L.; Zhang, X.Y.; Wang, Y.Y.; Xu, D.G.; Hu, Z.G.; Yao, J.Q.; Wu, Y.C. Nonlinear Optical Benzoindolium-Based Single Crystals: H-Contacts Induced Noncentrosymmetric Alignment Approaching Efficient Terahertz-Wave Generation. Cryst. Growth Des. 2022, 22, 3311–3318. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.T.; Han, J.H.; Lee, J.A.; Lee, S.H.; Kang, B.J.; Jazbinsek, M.; Yoon, W.; Yun, H.; Kim, D.; et al. Wide-Bandgap Organic Crystals: Enhanced Optical-to-Terahertz Nonlinear Frequency Conversion at Near-Infrared Pumping. Adv. Opt. Mater. 2020, 8, 1902099. [Google Scholar] [CrossRef]
- Bharath, D.; Kalainathan, S. Dielectric, optical and mechanical studies of phenolic polyene OH1 organic electrooptic crystal. Opt. Laser Technol. 2014, 63, 90–97. [Google Scholar] [CrossRef]
- Yokoo, A.; Yokohama, I.; Takara, H.; Kaino, T. Growth method, optical properties, and application of organic nonlinear optical crystal 2-adamantylamino-5-nitropyridine. In Proceedings of the Nonlinear Optical Properties of Organic Materials X, San Diego, CA, USA, 30 July–1 August 1997. [Google Scholar]
- Adachi, H.; Takahashi, Y.; Yabuzaki, J.; Mori, Y.; Sasaki, T. Growth of high quality nonlinear optical crystal 4-dimethylamino-N-methyl-4-stilbazolium tosylate (DAST). J. Cryst. Growth 1999, 198, 568–571. [Google Scholar] [CrossRef]
- Ruiz, B.; Jazbinsek, M.; Günter, P. Crystal Growth of DAST. Cryst. Growth Des. 2008, 8, 4173–4184. [Google Scholar] [CrossRef]
- Wen, X.; Xu, X.; Zhou, H.; Hu, L.; Jing, Y.; Xu, J.; Cheng, X.; Shi, J.; Liu, X.; Fan, T.; et al. Growth of 4-N,N-Dimethylamino-4′-N′-methyl-stilbazolium Tosylate (DAST) Organic Single Crystals Controlled by Oleic Acid. Crystals 2019, 9, 494. [Google Scholar] [CrossRef]
- You, F.; Wang, T.; Wang, Q.; Cao, L.; Zhong, D.; Ji, S.; Hao, L.; Teng, B.; Tang, J. Stable growth of large-size organic 4-N,N-dimethylamino-4′-N′-methyl-stilbazolium tosylate crystal and its optical, dielectric and THz properties. J. Mol. Struct. 2021, 1236, 130360. [Google Scholar] [CrossRef]
- Jeong, C.-U.; Kang, B.J.; Lee, S.-H.; Lee, S.-C.; Kim, W.T.; Jazbinsek, M.; Yoon, W.; Yun, H.; Kim, D.; Rotermund, F.; et al. Yellow-Colored Electro-Optic Crystals as Intense Terahertz Wave Sources. Adv. Funct. Mater. 2018, 28, 1801143. [Google Scholar] [CrossRef]
- Suizu, K.; Miyamoto, K.; Yamashita, T.; Ito, H. High-power terahertz-wave generation using DAST crystal and detection using mid-infrared powermeter. Opt. Lett. 2007, 32, 2885–2887. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Neis, M.; Stillhart, M.; Ruiz, B.; Khan, R.U.A.; Günter, P. Generation of terahertz pulses through optical rectification in organic DAST crystals: Theory and experiment. J. Opt. Soc. Am. B 2006, 23, 1822–1835. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, X.; Hu, Z.; Wu, Y. Organic Nonlinear Optical Crystals for Highly Efficient Terahertz-Wave Generation. Crystals 2023, 13, 144. https://doi.org/10.3390/cryst13010144
Yang Y, Zhang X, Hu Z, Wu Y. Organic Nonlinear Optical Crystals for Highly Efficient Terahertz-Wave Generation. Crystals. 2023; 13(1):144. https://doi.org/10.3390/cryst13010144
Chicago/Turabian StyleYang, Ying, Xinyuan Zhang, Zhanggui Hu, and Yicheng Wu. 2023. "Organic Nonlinear Optical Crystals for Highly Efficient Terahertz-Wave Generation" Crystals 13, no. 1: 144. https://doi.org/10.3390/cryst13010144
APA StyleYang, Y., Zhang, X., Hu, Z., & Wu, Y. (2023). Organic Nonlinear Optical Crystals for Highly Efficient Terahertz-Wave Generation. Crystals, 13(1), 144. https://doi.org/10.3390/cryst13010144